Abstract
Hypertriglyceridemia is an independent risk factor for cardiovascular disease, and plasma triglycerides (TGs) correlate strongly with plasma apolipoprotein C-III (ApoC-III) levels. Antisense oligonucleotides (ASOs) for ApoC-III reduce plasma TGs in primates and mice, but the underlying mechanism of action remains controversial. We determined that a murine-specific ApoC-III–targeting ASO reduces fasting TG levels through a mechanism that is dependent on low-density lipoprotein receptors (LDLRs) and LDLR-related protein 1 (LRP1). ApoC-III ASO treatment lowered plasma TGs in mice lacking lipoprotein lipase (LPL), hepatic heparan sulfate proteoglycan (HSPG) receptors, LDLR, or LRP1 and in animals with combined deletion of the genes encoding HSPG receptors and LDLRs or LRP1. However, the ApoC-III ASO did not lower TG levels in mice lacking both LDLR and LRP1. LDLR and LRP1 were also required for ApoC-III ASO–induced reduction of plasma TGs in mice fed a high-fat diet, in postprandial clearance studies, and when ApoC-III–rich or ApoC-III–depleted lipoproteins were injected into mice. ASO reduction of ApoC-III had no effect on VLDL secretion, heparin-induced TG reduction, or uptake of lipids into heart and skeletal muscle. Our data indicate that ApoC-III inhibits turnover of TG-rich lipoproteins primarily through a hepatic clearance mechanism mediated by the LDLR/LRP1 axis.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in Western societies and are predicted to become a prominent problem worldwide (1). Most current therapeutic interventions are aimed at lowering low-density lipoprotein (LDL) cholesterol. Yet patients with a substantial reduction in LDL cholesterol still have a residual cardiovascular risk that persists and increases incrementally with each additional feature of the metabolic syndrome (1–3). This residual risk has shifted attention to elevated plasma triglyceride (TG) levels, which constitute an independent risk factor for coronary artery disease. Hypertriglyceridemia results from the accumulation of TG-rich lipoproteins (TRLs) in the circulation. The concentration of plasma TRLs reflects a balance between de novo synthesis in the liver (very low-density lipoproteins [VLDLs]), intestinal absorption of dietary fats (chylomicrons), lipoprotein lipase–mediated (LPL-mediated) lipolysis in the peripheral circulation, and hepatic TRL clearance.
The liver and, to a lesser extent, the intestine produce apolipoprotein C-III (ApoC-III), an 8.8-kDa glycoprotein associated with TRLs, LDL, and high-density lipoproteins (HDLs) (4). It was shown more than 35 years ago that plasma TGs and ApoC-III levels are highly correlated over a wide range of plasma TG levels (5). But the importance of ApoC-III in TRL metabolism did not became fully apparent until it was shown that transgenic expression of APOC3 in mice resulted in hypertriglyceridemia (6), whereas a null mutation in the murine gene decreased plasma TG levels (7). Similarly, inactivating mutations affecting the expression of APOC3 in humans led to reduced plasma TG levels (8) and protection against CVD (9–11).
Evidence supports the existence of multiple potential mechanisms by which ApoC-III could affect plasma TG levels, including inhibition of LPL-mediated lipolysis (12, 13), promotion of hepatic VLDL secretion (and/or chylomicron formation) (4, 14), and suppression of TRL remnant clearance in the liver (Figure 1A). Graham et al. showed that lowering ApoC-III using antisense oligonucleotides reduces plasma TGs in rodents, nonhuman primates, and humans (15). The prevailing thought has been that ApoC-III inhibits LPL activity. However, a recent clinical study showed that lowering plasma ApoC-III levels with volanesorsen, a generation 2.0+ ApoC-III–specific antisense oligonucleotide (ASO), dramatically lowered elevated plasma TG levels in patients who have genetic defects in LPL (familial chylomicronemia syndrome [FCS]) (16). Despite the lack of a placebo control group in this study, this finding suggests that ApoC-III impairs TG clearance predominantly in an LPL-independent fashion. In the current study, we evaluated the relative contribution of the various pathways in mice. We show that an ApoC-III ASO–mediated reduction of plasma TGs is mediated predominantly through inhibition of hepatic TRL clearance via the LDL/LRP1 axis.
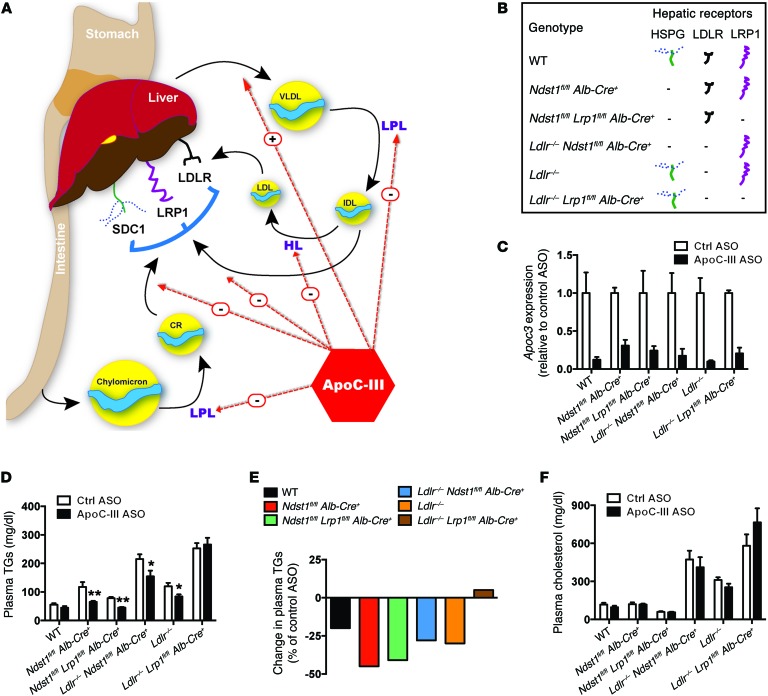
Figure 1. ApoC-III ASO–mediated plasma TG reduction in mice on a chow diet depends on hepatic LDLR and LRP1.
(A) Schematic overview of the possible effects of ApoC-III on TRL metabolism. (B) Mutant mice and their representative expression of hepatic TRL-clearance receptors HSPG, LDLR, and LRP1. (C) Liver expression of Apoc3 in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 3–7/group). (D) Fasting plasma TG levels in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 5–10/group). (E) Relative changes in plasma TG levels induced by ApoC-III ASO in mutant mice. (F) Fasting plasma cholesterol in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 5–10/group). Values represent the mean ± SEM. *P < 0.05 **P < 0.01 compared with control ASO-treated mice. ANOVA with Bonferroni’s post-hoc test. Ctrl, control; HL, Hepatic Lipase.
Results
ApoC-III ASO requires hepatic LDLR and LRP1 expression to lower plasma TG levels.
In the liver, low-density lipoprotein receptors (LDLRs), LDLR-related protein 1 (LRP1), and heparan sulfate proteoglycan receptors (HSPGs), predominantly syndecan-1 (SDC1), mediate the endocytic clearance of TRL remnants (17, 18). To examine whether hepatic clearance via these receptors contributed to an ApoC-III ASO–mediated reduction of plasma TGs, we administered the ApoC-III ASO for 4 weeks to mice lacking LDLR (Ldlr–/–), HSPGs (Ndst1fl/fl Alb-Cre+), or hepatic LRP1 (Lrp1fl/fl Alb-Cre+), and to mice lacking various pairs of these receptors (depicted in Figure 1B). Administration of the ApoC-III ASO reduced hepatic Apoc3 mRNA expression by 70% to 94% in the various mutants (Figure 1C). In chow-fed animals, ApoC-III ASO reduced fasting plasma TG levels by 30% to 45% in animals defective in hepatic Ndst1 and Ldlr and in animals with combined deletions of Ndst1 and Lrp1 or Ldlr (Figure 1, D and E). However, the ASO had no effect on plasma TG levels in mice lacking both Lrp1 and Ldlr (Ldlr–/– Lrp1fl/fl Alb-Cre+) (Figure 1, D and E). ApoC-III ASO treatment reduced TG levels in chylomicrons, VLDL, and remnant particles in all of the mutants as measured by size-exclusion fast protein liquid chromatography (FPLC) (Figure 2, A–E), but had no effect in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (Figure 2F). In a parallel approach, LDLR and LRP1 expression was reduced in adult mice using LDLR and LRP1 ASOs targeted to the liver (Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/JCI86610DS1). Simultaneous treatment with Apoc3 ASO significantly reduced hepatic Apoc3 mRNA in all models (Supplemental Figure 1C) and reduced plasma TGs by 20% to 35% in mice treated with saline, LDLR ASO, or LRP1 ASO (Supplemental Figure 1, D and E). Again, plasma TGs did not change when LDLR, LRP1, and ApoC-III were suppressed simultaneously (Supplemental Figure 1, D and E). These findings suggest that an ASO-mediated reduction of plasma TG levels depends on LDLR and LRP1.
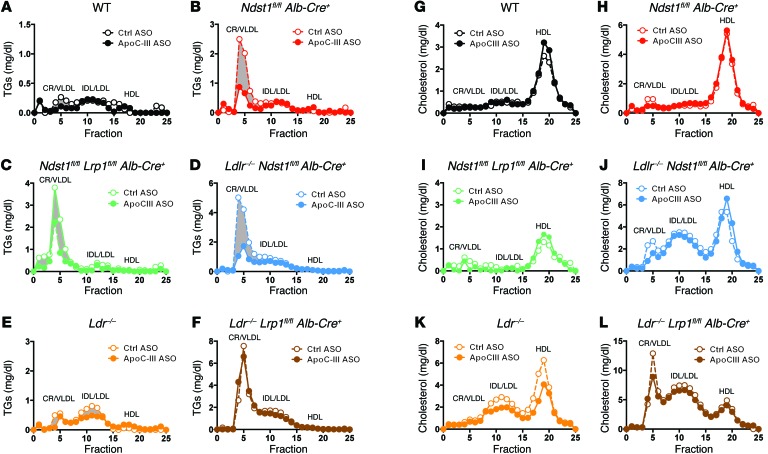
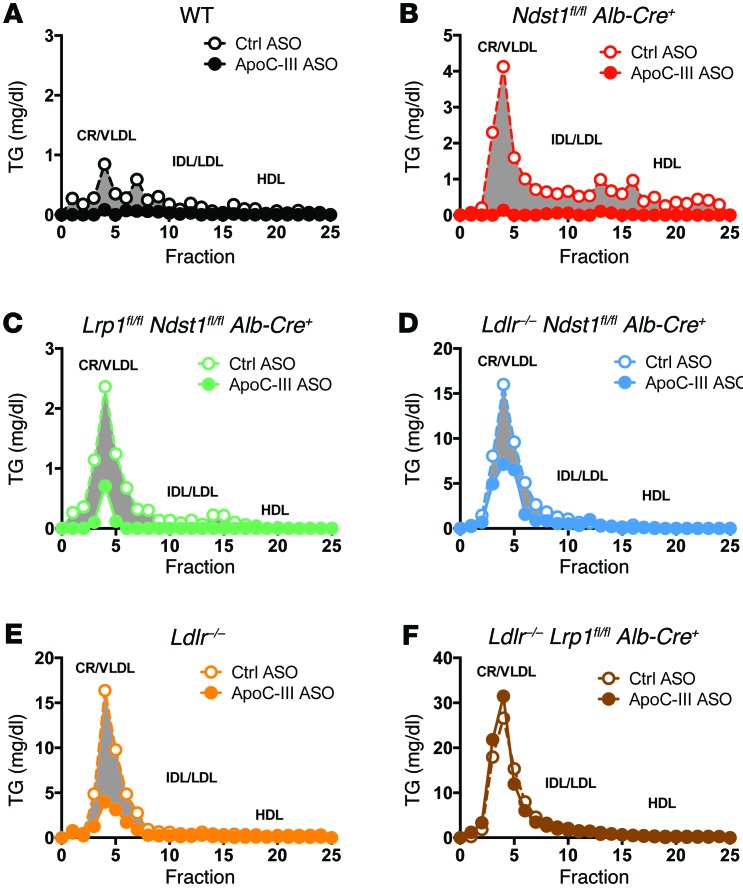
Figure 2. ApoC-III ASO decreases TRL TGs in mice expressing hepatic LDLR or LRP1.
(A–F) Mutant mice of the indicated genotype were treated for 4 weeks with control or ApoC-III ASO, and pooled plasma lipoproteins from fasted animals were analyzed by size-exclusion FPLC (n = 3/pool). The amount of TG in each fraction was measured. (G–L) Lipoprotein cholesterol profiles in plasma from pooled, fasted mutant mice treated for 4 weeks with control or ApoC-III ASO were analyzed by FPLC (n = 3/pool). The elution positions of CR/VLDL, intermediate-density lipoprotein (IDL), LDL, and HDL are indicated.
ApoC-III ASO treatment did not alter fasting plasma cholesterol levels (Figure 1F and Supplemental Figure 1F) or lipoprotein cholesterol levels in chow-fed animals (Figure 2, G–L). A reduction in TG levels also did not cause hepatic steatosis (Figure 3, A and B) or increase the production of ketone bodies (Figure 3C), and at most had only a modest effect on circulating nonesterified free fatty acid (NEFA) levels (Figure 3D). Furthermore, administration of the ApoC-III ASO did not induce weight gain or loss in the mice (Supplemental Figure 2).
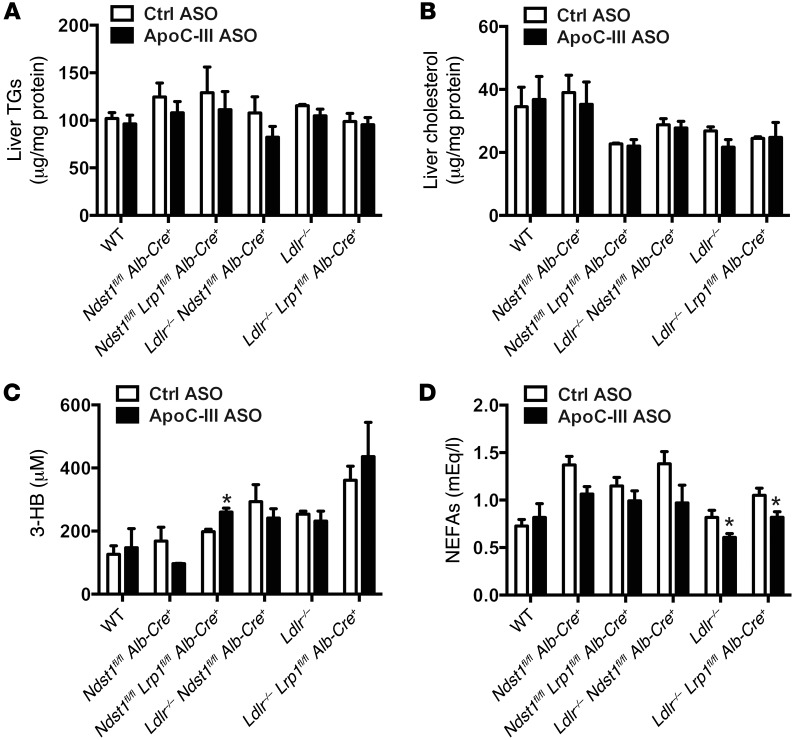
Figure 3. ApoC-III ASO does not induce liver steatosis or production of ketone bodies.
(A) Liver TG levels in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 3–5/group). (B) Liver cholesterol levels in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 3–5/group). (C) Fasting plasma levels of 3-hydroxybutyrate (3-HB) in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 3–9/group). (D) Fasting plasma NEFA levels in mutant mice treated for 4 weeks with control or ApoC-III ASO (n = 3–9/group). Values represent the mean ± SEM. *P < 0.05 compared with control ASO-treated mice. ANOVA with Bonferroni’s post-hoc test.
Hepatic ApoC-III reduction enhances postprandial TG clearance via LDLR and LRP1.
To measure the impact of ApoC-III suppression on the clearance of dietary TGs in the circulation, we administered a bolus of corn oil by oral gavage to mice treated with ApoC-III ASO and sampled their blood at various time points. Treatment with ApoC-III ASO reduced postprandial plasma TGs in all of the mutants except in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (Figure 4, A and B). Similar results were obtained when overnight-fasted mice were orally gavaged with a bolus of corn oil containing [3H]retinol (the disappearance of [3H]retinol from plasma is a direct measure of hepatic clearance of intestinally derived lipoproteins) (Figure 4, C–I). Clear improvement in retinol ester clearance from the circulation occurred in mice treated with ApoC-III ASO, with the exception of Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (Figure 4, C and I).
Figure 4. ApoC-III ASO accelerates TRL turnover in mice expressing LDLR or LRP1.
(A) Postprandial TG clearance in fasted mutant and WT mice treated for 4 weeks with control or ApoC-III ASO after an oral gavage of corn oil (250 μl). TG levels were measured 0, 1, 2, and 3 hours after gavage (n = 3–5/group). AUC values are shown for each of the genotypes and their relative treatment. (B) Relative changes in the area under the curve (AUC) are shown. (C–I) Chylomicron clearance was measured by retinyl ester excursion. Mutant and WT mice were treated for 4 weeks with control or ApoC-III ASO, fasted for 4 hours, and orally gavaged with corn oil containing [3H]retinol. (D–I) Blood samples were taken at the indicated times, and radioactivity remaining in the plasma samples (10 μl) was determined by liquid scintillation counting (n = 3–4/group). (C) Relative changes in the AUC are shown. Values represent the mean ± SEM. *P < 0.05 and **P < 0.01 compared with control ASO-treated mice, #P < 0.05 and ##P < 0.01 compared with RE AUC of control ASO-treated mice. ANOVA with Bonferroni’s post-hoc test. FTT, fat tolerance test; RE, retinyl ester.
ApoC-III reduction enhances TG clearance via LDLR and LRP1 in animals fed a high-fat diet.
To determine whether ApoC-III reduction had similar effects in mice fed a high-fat diet, the various mutant mice were fed a diet containing 21% fat for 6 weeks and administered a control or ApoC-III ASO for the last 5 weeks. The high-fat diet raised plasma TG levels in Ldlr–/–, Ldlr–/– Ndst1fl/fl Alb-Cre+, and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice, reaching values of 515 to 1,045 mg/dl (Figure 5A). Under these conditions, administration of the ApoC-III ASO reduced hepatic Apoc3 mRNA expression in the various mutants by 74% to 99% (Figure 5B) and reduced plasma TGs by 35% to 57% (Figure 5, A and C). Again, ApoC-III ASO treatment had no effect on plasma TG levels in mice lacking both Lrp1 and Ldlr (Figure 5, A and C), as observed with animals fed a standard chow diet (Figure 1) and under postprandial conditions (Figure 3). FPLC analysis showed that ASO treatment reduced TGs in VLDL and chylomicron remnant (CR) particles in all of the mutants (Figure 6, A–E), but had no effect in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (Figure 6F).
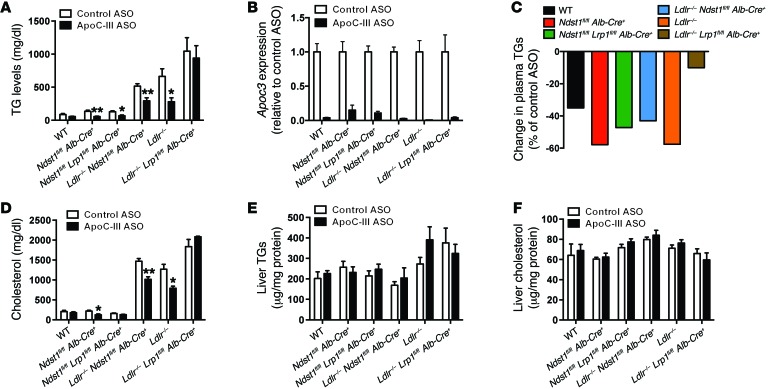
Figure 5. ApoC-III ASO–mediated plasma TG reductions in mice on a high-fat diet depends on hepatic LDLR and LRP1.
(A) Fasting plasma TG levels in mutant mice on a high-fat diet treated for 5 weeks with control or ApoC-III ASO (n = 5–10/group). (B) Liver expression of Apoc3 mRNA levels in mutant mice on a high-fat diet for 6 weeks and treated for the last 5 weeks with control or ApoC-III ASO (n = 3–7/group). (C) Relative changes in plasma TG levels induced by ApoC-III ASO in mutant mice on a high-fat diet. (D) Fasting plasma cholesterol levels in mutant mice on a high-fat diet treated for 5 weeks with control or ApoC-III ASO (n = 5–10/group). (E) Liver TG levels in mutant mice on a high-fat diet treated for 5 weeks with control or ApoC-III ASO (n = 3–5/group). (F) Liver cholesterol levels in mutant mice on a high-fat diet treated for 5 weeks with control or ApoC-III ASO (n = 3–5/group). Values represent the mean ± SEM. *P < 0.05 and **P < 0.01 compared with control ASO-treated mice. ANOVA with Bonferroni’s post-hoc test.
Figure 6. ApoC-III ASO decreases TRL TGs in mice expressing hepatic LDLR or LRP1 in mice on a high-fat diet.
(A–F) Mutant mice of the indicated genotypes that were fed a high-fat diet for 6 weeks were treated for the last 5 weeks with control or ApoC-III ASO, and pooled plasma lipoproteins from fasted animals were analyzed by size-exclusion FPLC (n = 3/pool). The amount of TGs in each fraction was measured. The elution positions of CR/VLDL, IDL, LDL, and HDL are indicated.
As expected, animals fed a high-fat diet had elevated plasma cholesterol levels (Figure 5D). Interestingly, a reduction of ApoC-III had variable effects on plasma cholesterol levels, reducing levels in Ndst1fl/fl Alb-Cre+, Ldlr–/–, and Ldlr–/– Ndst1fl/fl Alb-Cre+ mice by 30% to 40% (Supplemental Figure 3). The cause of the variation in cholesterol reduction across the strains remains unknown. Nevertheless, the reduction in plasma TG and cholesterol levels induced by ApoC-III ASO did not enhance hepatic steatosis (Figure 5, E and F) or induce weight gain or loss in the mice (Supplemental Figure 4).
Administration of the ApoC-III ASO improves plasma TG independently of changes in LPL activity or VLDL secretion.
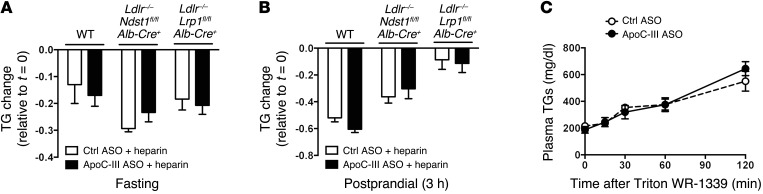
Previous studies have suggested that ApoC-III can inhibit the action of LPL on TRL substrates (12, 19, 20). To explore this possibility in vivo, fasted and fed mice were injected with heparin to mobilize LPL into the circulation (21). Administration of heparin resulted in a rapid reduction of plasma TGs in WT, Ldlr–/– Ndst1fl/fl Alb-Cre+, and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice under fasting conditions (Figure 7A). A comparable reduction in plasma TG levels occurred after heparin injection of mice treated with ApoC-III ASO (Figure 7A). Similar results were obtained under postprandial conditions (Figure 7B). These findings show that suppression of ApoC-III did not alter heparin-induced lipolysis, suggesting that the reduction of ApoC-III did not affect LPL hydrolysis of TRL substrates. Administration of the ApoC-III ASO did not affect VLDL-TG secretion (Figure 7C) or chylomicron formation in vivo in mice as reported previously (15).
Figure 7. ApoC-III ASO treatment does not alter in vivo LPL activity or VLDL production.
(A and B) Plasma TGs were measured before and after heparin injection in WT, Ldlr–/– Ndst1fl/fl Alb-Cre+, and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice treated for 4 weeks with control or ApoC-III ASO (A) after an overnight fast or (B) 3 hours after a fat challenge. Heparin was injected i.v. (50 U/mouse), and blood was sampled via tail-vein bleeding 10 minutes after injection (n = 3–5/group). (C) Hepatic VLDL production was determined in overnight-fasted Ldlr–/– Lrp1fl/fl Alb-Cre+ mice after injection with tyloxapol to inhibit lipolysis. Blood samples were collected at the indicated time points and processed to measure plasma TG accumulation and VLDL production rates (n = 3/group). Values represent the mean ± SEM. ANOVA with Bonferroni’s post-hoc test.
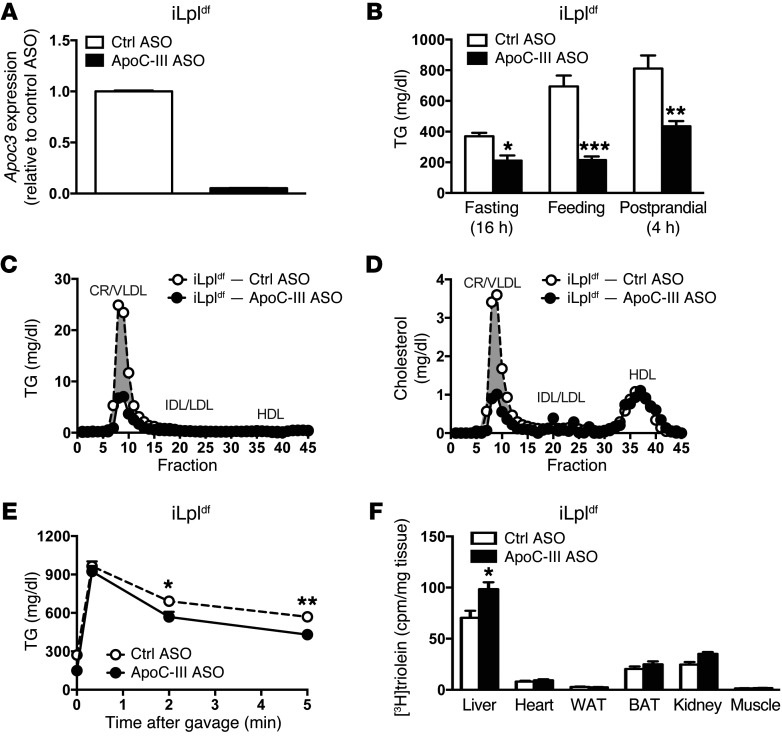
We used a previously described tamoxifen-inducible, Lpl-deficient mouse model (Lplfl/fl Actb-MerCreMer+, referred to herein as iLpldf mice) to further assess the in vivo contribution of LPL to the reduction of plasma TGs by ApoC-III ASO (22). Administration of tamoxifen to LPL-deficient mice resulted in a significant reduction in Lpl mRNA expression in liver, muscle, heart, and adipose tissue (Supplemental Figure 5A) and in LPL activity in plasma (after i.v. heparin administration), muscle, heart, and adipose tissue (Supplemental Figure 5B). The iLpldf mice presented with elevated plasma TG and cholesterol levels (Supplemental Figure 5, C and D, respectively), along with a dramatic increase in VLDL TGs and a reduction in HDL cholesterol levels (Supplemental Figure 5, E and F), as observed in patients with LPL deficiency (16). Treatment of iLpldf mice with ApoC-III ASO decreased Apoc3 mRNA levels (Figure 8A) and protein expression (Supplemental Figure 6A) and reduced plasma TGs (Figure 8B) and TGs in CR/VLDL particles (Figure 8C). Moreover, HDL cholesterol levels did not increase, as might be expected if LPL-mediated lipolysis were modulated by ApoC-III (Figure 8D and ref. 22). Repetitive treatment with the ApoC-III ASO progressively decreased plasma TGs (Supplemental Figure 6B) and increased TG clearance after oral corn oil gavage (Supplemental Figure 6C) or after injection of [3H]triolein-labeled Liposyn particles (Figure 8E). Lowering of plasma TG levels after injection of [3H]triolein-labeled Liposyn particles was associated with a greater uptake of [3H]triolein in the liver, but not in organs such as the heart or skeletal muscle (Figure 8F), as would be expected if LPL activity were enhanced. Together, these findings suggest that the major mechanism by which ApoC-III affects plasma TG levels functions independently of LPL.
Figure 8. TG levels are reduced in an inducible LPL-deficient mouse model after ApoC-III ASO administration.
(A) Liver Apoc3 mRNA expression in iLpldf mice treated with control or ApoC-III ASO (n = 8/group). (B) Fasting, fed, and postprandial plasma TG levels in iLpldf mice treated for 4 weeks with control or ApoC-III ASO (n = 5–6/group). (C) Lipoprotein TG profiles in plasma from pooled, fasted iLpldf mice treated with tamoxifen to induce inactivation of LPL expression (iLpldf) (n = 3 pooled mice/group). (D) Lipoprotein cholesterol profiles in plasma from fasted iLpldf mice treated with control ASO or ApoC-III ASO (4 weeks, n = 3 mice/pool). The elution positions of CR/VLDL, IDL/LDL, and HDL are indicated. (E) Plasma TG levels of fasted mice before and after injection of 100 μl 20% Liposyn II particles at the indicated time points. (F) Tissue distribution of [3H]triolein 5 minutes after injection of 100 μl [3H]triolein-labeled 20% Liposyn II particles (n = 8/group). BAT, brown adipose tissue; WAT, white adipose tissue. Values represent the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control ASO-treated mice. ANOVA with Bonferroni’s post-hoc test.
Absence of ApoC-III on TRLs improves hepatic clearance via LDLR and LRP1.
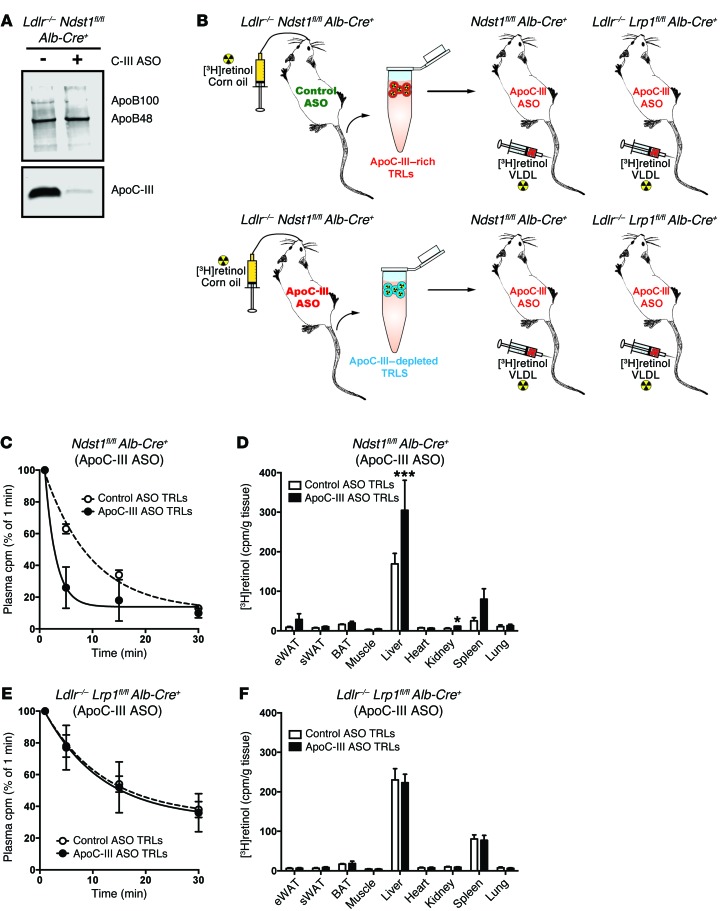
Given these findings, we proposed that the dominant mode of action of the ApoC-III ASO in mice is to remove the inhibitory effect of ApoC-III on the clearance of TRLs through LDLR and LRP1, which act in a redundant manner with respect to TRL clearance (17, 23). Additional supporting evidence was obtained by measuring the clearance rate of ApoC-III–rich and ApoC-III–depleted [3H]TRLs (24). Donor [3H]retinol-labeled TRLs were collected from ApoC-III ASO–treated and control ASO-treated Ldlr–/– Ndstfl/fl Alb-Cre+ mice 3 hours after a fat load to generate ApoC-III–depleted TRLs and ApoC-III–bearing TRLs, respectively (Figure 9A). Both ApoC-III–depleted TRLs and ApoC-III–bearing TRLs had equal amounts of TGs, free cholesterol, cholesteryl esters, and [3H]retinol when normalized to protein (Supplemental Figure 7). This suggested that the TRL preparations showed no significant differences in particles size or retinol composition, except for the reduction in ApoC-III content. Equal amounts of [3H]TRLs (30,000 cpm/mouse) were injected into recipient mice treated with ApoC-III ASO to minimize the association of endogenously produced ApoC-III with the injected [3H]TRLs (Figure 9B). Although TRL clearance measured in this way is a function of both LPL activity and hepatic receptors, only the receptors in the liver differed across the different mouse strains; hence, any difference in catabolic rates presumably arose from differences in receptor-mediated clearance in the liver. ApoC-III–depleted TRLs were cleared much faster than were ApoC-III–rich TRLs in Ndst1fl/fl Alb-Cre+ mice (t1/2 = 2.8 min vs. 8.2 min, respectively) (Figure 9C). In contrast, no difference in the catabolic rate between ApoC-III–depleted and ApoC-III–bearing TRLs was observed when the particles were injected into Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (t1/2 = 16.2 min vs. 16.8 min, respectively) (Figure 9E). Note that the clearance of both types of TRL particles was much slower in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice than what was observed when these 2 receptors were present; the presence or absence of ApoC-III did not influence this rate. The accelerated catabolic rate of ApoC-III–depleted TRLs in Ndst1fl/fl Alb-Cre+ mice was associated with increased [3H]TRL uptake in the liver (Figure 9D), whereas liver accumulation did not differ when ApoC-III–bearing [3H]TRLs were injected into Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (Figure 9F). In a second approach, [3H]retinol-radiolabeled ApoC-III–depleted and ApoC-III–bearing TRLs (Figure 9A and Supplemental Figure 6) were evaluated for their uptake by primary hepatocytes isolated from Ndst1fl/fl Alb-Cre+ and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice. Overall binding of both [3H]TRL preparations was significantly reduced in hepatocytes isolated from Ldlr–/– Lrp1fl/fl Alb-Cre+ mice compared with hepatocytes isolated from Ndst1fl/fl Alb-Cre+ mice (Figure 10). Binding and uptake of radiolabeled ApoC-III–depleted TRLs was significantly increased in hepatocytes isolated from Ndst1fl/fl Alb-Cre+ mice (i.e., in mice expressing both LDLR and LRP1) when compared with ApoC-III–bearing TRLs (Figure 10). In contrast, primary mouse hepatocytes from Ldlr–/– Lrp1fl/fl Alb-Cre+ mice did not show a significant difference in binding or uptake between ApoC-III–depleted and ApoC-III–bearing TRLs (Figure 10). These results support the notion that the increased clearance of ApoC-III–depleted [3H]TRLs in Ndst1fl/fl Alb-Cre+ mice, and not in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice, is a consequence of increased uptake by LDLR/LRP1 on hepatocytes.
Figure 9. ApoC-III interferes with hepatic TRL clearance via LRP1 and LDLR.
(A) Western blot analysis for ApoB and ApoC-III in pooled TRLs (5 μg) isolated from fasted Ldlr–/– Ndst1fl/fl Alb-Cre+ mice treated for 4 weeks with control or ApoC-III ASO (n = 3–4/pool). (B) Schematic overview of [3H]TRL clearance experiments. (C) Isolated [3H]TRLs from control ASO- and ApoC-III ASO–treated Ldlr–/– Ndst1fl/fl Alb-Cre+ mice were injected i.v. into ApoC-III ASO–treated Ndst1fl/fl Alb-Cre+ mice (n = 3). Clearance of [3H]TRLs was assessed by measuring the counts remaining in the plasma relative to the counts recovered 1 minute after injection. (D) Mice were euthanized and the indicated tissues were dissected, homogenized, and assayed for radioactivity 20 minutes after injection. Counts per gram wet weight are reported. (E) [3H]TRLs isolated from control ASO-treated (white circles) and ApoC-III ASO–treated (black circles) Ldlr–/– Ndst1fl/fl Alb-Cre+ mice were injected i.v. into ApoC-III ASO–treated Ldlr–/– Lrp1fl/fl Alb-Cre– mice (n = 3). Clearance of [3H]TRLs was assessed by measuring the counts remaining in the plasma relative to the counts recovered 1 minute after injection. (F) Mice were euthanized and the indicated tissues were dissected, homogenized, and assayed for radioactivity 20 minutes after injection. Counts per gram wet weight are reported. (C–F) Values represent the mean ± SEM. *P < 0.05 and ***P < 0.001 compared with control ASO-treated TRLs. ANOVA with Bonferroni’s post-hoc test. eWAT, epididymal WAT; sWAT, subcutaneous WAT.
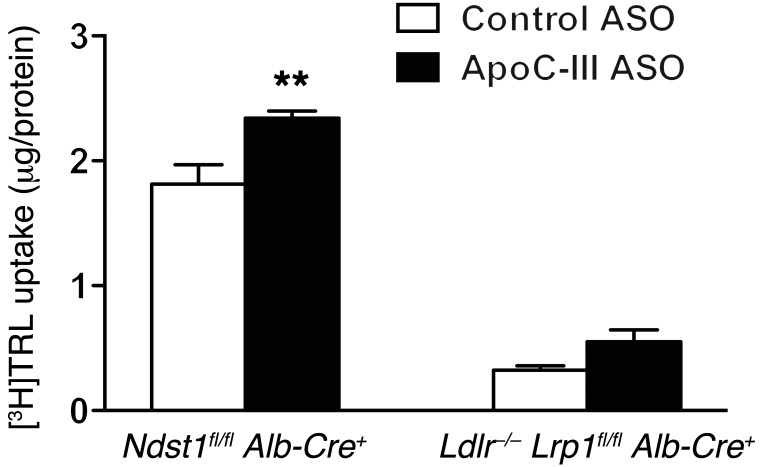
Figure 10. ApoC-III depletion improves TRL clearance in primary hepatocytes expressing LRP1 and LDLR.
Binding and uptake of isolated [3H]TRLs (50 μg/ml) from control ASO- and ApoC-III ASO–treated Ldlr–/– Ndst1fl/fl Alb-Cre+ mice in primary hepatocytes isolated from Ndst1fl/fl Alb-Cre+ and Ldlr–/– Lrp1fl/fl Alb-Cre– mice after a 4-hour incubation at 37°C (n = 3/condition). Values represent the mean ± SEM. **P < 0.01 compared with control ASO-treated TRLs. ANOVA with Bonferroni’s post-hoc test.
Discussion
Our results support a model in which ApoC-III on TRLs contributes to hypertriglyceridemia, primarily by preventing TRL hepatic clearance via both LDLR and LRP1. We also present evidence that TRLs with reduced ApoC-III content do not affect lipolysis and that a reduction in LPL expression and activity does not suppress the impact of ASO inhibition on lowering plasma TG levels. These results are consistent with the recent observation that targeting of ApoC-III effectively reduces plasma TGs in LPL-deficient patients (16). In agreement with Graham et al. (15), we confirm that an antisense reduction of hepatic ApoC-III does not affect hepatic VLDL production (Figure 7C).
LPL is a pivotal player in TG metabolism, as mutations in LPL or in its cofactors (ApoC-II, glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1, lipase maturation factor 1) account for the majority of monogenic hypertriglyceridemia cases (25). Prior studies suggesting that ApoC-III may predominantly affect LPL-mediated TG hydrolysis were based on fractional clearance rates of ApoB in patients lacking ApoC-III and ApoA-I (13) and in vitro studies showing that excess ApoC-III (or ApoC-I) blocks LPL activity in lipid droplets or emulsions possibly due to competition for the lipid-water interface (12, 19). Transgenic overexpression of APOC3 in mice also led to hypertriglyceridemia that was likely due to defective lipolysis (20), but it should be noted that an excess of any apolipoprotein has a similar effect (26–29), probably by shielding the core TG and preventing LPL access. Larsson and colleagues suggested that increasing the ratio of ApoC-III to ApoC-II by 5 or more was required to displace LPL from the TRL lipid surface, leading to subsequent inactivation by angiopoietin-like protein 4 (19). These ratios of ApoC-III to ApoC-II exceed the normal physiological range of these apolipoproteins present on human VLDL particles, even in patients with hypertriglyceridemia (0.7 to 2) (30). Thus, under physiological conditions, we believe that ApoC-III exerts its primary effect through inhibition of hepatic clearance rather than through lipolysis.
This conclusion is supported by our data. First, a reduction in ApoC-III failed to reduce plasma TGs in Ldlr–/– Lrp1fl/fl Alb-Cre+ mice, a murine model with normal LPL activity levels. Second, heparin injection, which releases LPL into the circulation, reduced plasma TG levels independently of ApoC-III ASO treatment. Third, a reduction of ApoC-III did not increase lipid uptake into the muscles, as would be expected if LPL inhibition was reversed. Finally, the ApoC-III effect was also manifest in LPL-deficient animals. The clinical definition of LPL deficiency is a greater than 90% loss of LPL activity. The subjects studied by Gaudet et al. had reduced LPL activity due to the production of a mutated protein (16), unlike the mice that had a partial, but marked, reduction in LPL production. Both situations might lead to a small amount of residual LPL activity. At these levels, the residual activity could be due to other lipases that cross-react in the TG hydrolysis assay (e.g., hepatic lipase, hormone-sensitive lipase, or adipose TG lipase). While LPL-deficient patients had higher TG levels than were detected in the LPL-deficient mice, the TG levels in the mice were not dissimilar from those seen in patients who strictly adhered to a low-fat diet that might be viewed as the equivalent of mouse chow. It is also important to realize that studying a truly systemic LPL–KO mouse is difficult, as 97% of the mice die shortly (15 days) after birth, unless they receive adenoviral rescue of LPL expression in the heart, in which case the animals have residual post-heparin LPL activity (31).
A usual consequence of enhanced lipolysis via LPL and TG reduction is an increase in HDL cholesterol levels. The observation that HDL cholesterol did not increase in ApoC-III–treated mice would seem to support an LPL-independent mechanism as well, although the absence of cholesterol ester transfer protein in mice confounds this finding. We also cannot exclude the possibility that in murine models, a complete absence of ApoC-III production might be required to observe effects on LPL-mediated lipolysis, although we think this is unlikely, given the dramatic reduction of ApoC-III levels in several of the models (20). And although the heparin-induced lipolysis experiments suggest that ApoC-III depletion does not affect LPL activity, we cannot fully exclude the possibility that ApoC-III depletion might differentially affect LPL activity in different tissues.
Although the impact of ApoC-III on LPL-mediated hydrolysis will likely remain controversial, the impact of ApoC-III depletion on clearance by LDLR/LRP1 is clearly demonstrated. The mechanism by which ApoC-III blocks hepatic clearance via LDLR and LRP1 may involve displacement of ApoE from TRLs or by direct interference with ApoE binding (32–34). However, an increase in plasma TG levels also occurs in ApoC-III–transgenic mice that are deficient in ApoE (20, 35). Furthermore, we did not observe any change in ApoE content on TRLs after ApoC-III depletion in any of the mouse models (Supplemental Figure 8A). An earlier study by Kowal et al. suggested that a mixture of ApoCs of undefined composition modulated ApoE-enriched β-VLDL binding to LRP1, but it was thought that clearance through the LDLR was not affected (34). A follow-up study showed that ApoC-III did not significantly inhibit ApoE-mediated VLDL binding to LRP1 (36). An alternate mechanism based on interference with ApoB-48 recognition (37) could explain why ApoC-III reduces TRL clearance only in mice deficient in both LRP1 and LDLR. Interestingly, ApoC-III does not seem to interfere with clearance mediated by hepatic HSPG receptors, which occurs via binding of ApoE and ApoA-V to heparan sulfate (24). The accumulation of ApoC-III in TRLs from all mouse strains lacking N-deacetylase/N-sulfotransferase-1 (NDST1) (Supplemental Figure 8B) suggests the possibility that ApoC-III–bearing particles may be preferentially cleared through HSPG receptors.
In conclusion, our genetic studies establish the fact that ApoC-III ASO reduces hypertriglyceridemia in mice by reducing plasma ApoC-III levels, which in turn enhances clearance of TRLs through LDLR family members. It is not clear what selective advantage may be gained by a mechanism that leads to delayed clearance of TRLs. Perhaps blocking the rapid clearance of TRL remnants through LDLR and LRP1 has an adaptive role in shunting fatty acids liberated from plasma TGs to the heart and skeletal muscle when food is less plentiful. ApoC-III accumulation in all mouse strains lacking hepatic HSPGs also suggests that shunting a subfraction of particles to these receptors may have a functional role.
A reduction of ApoC-III levels by ASO therapy could lead to a reduction of CVD. Elevated plasma TG levels represent a risk factor for CVD in humans, and an adjusted analysis of patients in the Framingham Heart Study suggests that each decrease of 1 mg/dl in plasma ApoC-III levels is associated with a 4% decrease in CVD risk (11), further supporting the potential therapeutic benefit of suppressing plasma ApoC-III levels in humans to reduce CVD. A number of correlative studies showed a greater incidence of CVD in persons with ApoC-III–rich LDL (38). These correlations using ApoC-III associated with LDL are more complex, as it was also reported that increased ApoE content in LDL fractions with ApoC-III is associated with a lower risk of CVD (39). Nevertheless, the use of an antisense-mediated strategy to target hepatic ApoC-III levels in order to effectively lower plasma TG levels (16, 40) could prove to be a valid therapeutic approach for treating subjects with FCS, for which there is now no effective therapy. Further research is warranted to determine whether, in fact, the antisense strategy to target hepatic ApoC-III will result in the lowering of CVD risk in patients with mild-to-moderate hypertriglyceridemic, who comprise approximately 28% of the general population (41).
Methods
Mice.
Lrp1fl/fl, Ldlr–/–, and Alb-Cre+ mice were purchased from The Jackson Laboratory. Ndst1fl/fl Alb-Cre+ mice were generated and genotyped as described previously (22, 42). Inducible LPL–deleted mice were created by crossing floxed Lpl (Lplfl/fl) mice with β-actin–driven tamoxifen-inducible Cre (Mer/Cre/Mer) transgenic mice to obtain the Lplfl/fl Actb3-MerCreMer+ offspring designated as iLpldf mice, as previously described (22). Both Lplfl/fl and iLpldf mice were given i.p. injections of 4-hydroxytamoxifen (Sigma-Aldrich) in corn oil at a dose of 40 mg/kg BW/day for 5 consecutive days. Plasma TG levels were determined 2 weeks after the last tamoxifen injection. All animals were fully backcrossed onto a C57Bl/6 background. Mice were weaned at 3 weeks, maintained on a 12-hour light cycle, and had ad libitum access to water and a standard rodent chow diet (PicoLab Rodent Diet 20, 5053; LabDiet) or a high-fat diet (TD.88137; Harlan Teklad). Mice received i.p. injections of ION 440726 (murine ApoC-III ASO) or ION 141923 (murine control ASO) at 50 mg/kg/week and ION 793588 (murine LRP1 ASO) and ION 713852 (murine LDLR ASO) at 5 mg/kg/week (Supplemental Table 1). As reported by Noh et al. (43), tamoxifen transiently increases plasma TG levels, which return to normal 2 weeks after the last administration. Therefore, ASO administration to iLpldf mice was initiated 2 weeks after the last tamoxifen injection.
RNA analysis.
Total RNA was isolated in TRIzol from homogenized tissue and cells and purified using RNeasy columns and RNase-Free DNase digestion according to the manufacturer’s instructions (QIAGEN). The quality and quantity of total RNA were monitored and measured with a NanoDrop (NanoDrop Technologies) following the manufacturer’s instructions. For quantitative PCR analysis, 1 μl cDNA was used for real-time PCR with gene-specific primers (primer sequences are indicated in Supplemental Table 2). Quantitative PCR (SYBR Green) analysis was performed on an Applied Biosystems 7300 Real-Time PCR System (Invitrogen).
Lipid analysis.
Blood was drawn via the tail vein from mice that had been fasted for 5 hours. Total cholesterol and TG levels in plasma were determined using kits from Genzyme or Thermo Fisher Scientific. NEFA and 3-hydroxybutyrate levels were determined using enzymatic kits (Wako Chemicals).
LPL activity assay.
LPL activity was determined as described previously (44, 45). Post-heparin plasma was obtained from fasted mice 5 minutes after retro-orbital injection of 5 units per mouse of heparin. To measure total lipase activity, plasma samples were incubated with 10% Intralipid/[3H]TG emulsion (Hospira) as a substrate and human serum as the source of ApoC-III (24). The contribution of hepatic lipase was determined by including 1 M NaCl in the assay, and the values were subtracted from the total lipase activity to estimate the activity attributed to LPL. Heparin-releasable LPL activity in heart, skeletal muscle, and white adipose tissue was measured using 50–80 mg of snap-frozen tissues. The tissue was minced in 0.6 ml PBS with 2 mg/ml BSA in the presence of 5 U/ml heparin. Minced tissues were incubated for 1 hour in a 37°C shaker and subsequently centrifuged at 1,000 g for 15 minutes. The supernatant was collected for LPL assay. A 100-μl aliquot of the buffer was used for the lipase assay in combination with 100 μl of a 10% Intralipid/[3H]triolein emulsion for 1 hour at 25°C. The LPL activity measurements were normalized for the initial tissue weights.
Ultracentrifugation.
Plasma was pooled from several mice (70 μl/mouse, n = 3–5 mice/genotype). Lipoprotein fractions were separated by buoyant density ultracentrifugation according to established methods (46). Briefly, 150 μl pooled plasma was loaded into micro-ultracentrifuge tubes (Beckman Coulter). The samples were centrifuged for 12 hours in a 42.2 Ti rotor at 100,000 g at 4°C. The top 50-μl fraction containing VLDL and chylomicron remnants (δ <1.006 g/ml) was removed and used for analysis. TRLs were analyzed by SDS-PAGE on 4% to 12% Bis-Tris gradient gels (NuPage, Invitrogen). Proteins were visualized by silver staining (Pierce, Thermo Fisher Scientific) or after transfer to an Immobilon-FL PVDF membrane (EMD Millipore). Membranes were blocked with Odyssey Blocking Buffer (LI-COR Biosciences) for 1 hour and incubated overnight at 4°C with the respective Abs. Rabbit and mouse Abs were incubated with secondary Odyssey IR dye Abs (1:15,000) and visualized with an Odyssey IR Imaging system (LI-COR Biosciences). Western blot primary Abs included rabbit anti-mouse ApoB (Abcam; catalog ab20737; 1:1,000) and anti-mouse ApoC-III (Ionis Pharmaceuticals; 1:2,000) (15).
Fast-performance liquid chromatography.
Pooled plasma samples were separated by gel-filtration fast-performance liquid chromatography (FPLC). Samples were loaded on a GE Superose 6 10/30 GL column in 0.15 M sodium chloride containing 1 mM ethylenediaminetetraacetic acid and 0.02% sodium azide, pH 7.4. Fractions (0.5-ml) were collected (0.5 ml/min). Total cholesterol and TG levels were determined enzymatically as described above.
Postprandial clearance studies.
Mice were fasted for 4 (iLpldf ) or 5 (other models) hours. At 1 pm, they were given a 200-μl bolus of corn oil (Sigma-Aldrich) by oral gavage. At the indicated time points, mice were bled via the tail vein. TG and cholesterol levels were measured as described above.
[3H]TG tissue uptake.
Mice were fasted overnight and then injected i.v. with 100 μl Liposyn emulsion (Hospira) containing 2 μCi [3H]triolein (New England Nuclear). Blood was obtained over a 5-minute period, during which time the mice were exsanguinated and perfused with 10 ml of 4°C PBS. After the tissues blanched, the organs were harvested. Each organ was weighed, either the whole or partial organ was homogenized in 1 ml PBS, 100-μl aliquots (in triplicate) were counted, and the total organ uptake was calculated.
Retinyl ester excursion.
Clearance of chylomicrons derived from dietary TGs was measured by vitamin A excursion essentially as described previously (18). Briefly, 25 μCi [11,12-3H]-retinol (PerkinElmer, 44 Ci/mmol) in ethanol was mixed with 1 ml corn oil (Sigma-Aldrich) and 200 μl was administered by gavage to mice fasted from 4 am until 9 am. Blood was obtained every 2 hours via the tail vein and 8 hours after gavage by cardiac puncture. [3H]Counts remaining in the serum were measured by liquid scintillation counting.
Clearance of [3H]TRLs.
Ldlr–/–Ndst1fl/flAlb-Cre+ mice were treated for 4 weeks with a control ASO or ApoC-III ASO. Mice were fasted for 5 hours and subjected to oral gavage (200 μl/mouse) with 5 μCi [11,12-3H]retinol in corn oil (Sigma-Aldrich). Blood was collected 3 hours after gavage by cardiac puncture. [3H]TRLs were isolated by buoyant density ultracentrifugation as described above. Next, Ndst1fl/fl Alb-Cre+ and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice (n = 3) were treated with ApoC-III ASO for 4 weeks, fasted for 5 hours, and then injected with purified [3H]TRLs via the tail vein (30,000 cpm/mouse). Serial tail-vein blood samples were taken at the indicated times (Figure 9C and Figure 3E). Radioactivity in serial plasma samples was determined by liquid scintillation counting and expressed relative to the number of counts in the circulation 1 minute after injection.
Binding and uptake of [3H]TRLs.
Ldlr–/–Ndst1fl/flAlb-Cre+ mice were treated for 4 weeks with a control ASO or ApoC-III ASO. Mice were fasted for 5 hours and subjected to oral gavage (200 μl/mouse) with 5 μCi [11,12-3H]retinol in corn oil (Sigma-Aldrich). Blood was collected 3 hours after gavage by cardiac puncture. [3H]TRLs were isolated by buoyant density ultracentrifugation as described above. Next, primary hepatocytes were isolated from Ndst1fl/fl Alb-Cre+ and Ldlr–/– Lrp1fl/fl Alb-Cre+ mice by perfusion of the liver with EDTA to dissociate the cells, followed by Percoll density gradient centrifugation as described previously (47). Hepatocytes were cultured in DMEM containing 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin for 36 hours prior to in vitro experiments. Uptake experiments were performed in hepatocytes seeded into collagen-coated, 6-well plates (Nalge Nunc International) at 500,000 cells per well. Purified [3H]TRLs were added at a concentration of 50 μg/ml in DMEM containing 10% lipoprotein-deficient serum (LPDS). After a 4-hour incubation at 37ºC, the cells were washed 4 times with PBS. Hepatocytes were solubilized in 0.1 M NaOH. Finally, total radioactivity and total cellular protein of the lysate were determined. All uptake data were obtained in triplicate.
Hepatic VLDL-TG secretion and intestinal lipid absorption.
Mice were fasted for 5 hours prior to tail-vein injection of tyloxapol (10% solution in PBS, Sigma-Aldrich) at a dose of 0.5 mg/g BW. Plasma was collected by tail bleeding at 1, 15, 30, 60, and 120 minutes after injection. Plasma TG levels were measured as described above.
Statistics.
Data were analyzed by 2-tailed Student’s t test or 2-way ANOVA and are presented as the mean ± SEM. Statistical analyses were performed using Prism software (version 5, GraphPad Software). A P value of less than 0.05 was considered significant.
Study approval.
All animals were housed and bred in vivaria approved by the Association for Assessment and Accreditation of Laboratory Animal Care located in the School of Medicine of UCSD and at Columbia University and New York University, following standards and procedures approved by the IACUC of UCSD.
Author contributions
PLSMG designed research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript. RN, NHS, BR, IL, JCG, BET, DB, and AEM conducted experiments and acquired data. RGL designed the research studies, conducted experiments, and acquired data. MJG, IJG, and RMC designed the research studies, provided reagents, and analyzed data. JLW designed research studies. JDE designed research studies, analyzed data, and wrote the manuscript.
Supplementary Material
Acknowledgments
This work was supported by Ionis Pharmaceuticals Inc.; NIH grants GM33063 and P01 HL107150 (to J.D. Esko); a European Community FP7 Award (PIOF-GA-2010-273994); and AHA grants 15BGIA25550111 (to P.L.S.M. Gordts), HL45095 and 73029 (to I.J. Goldberg), and HL088093 (to J.L. Witztum).
Footnotes
Conflict of interest: R.G. Lee, A.E. Mullick, M.J. Graham, and R.M. Crooke are employees of Ionis Pharmaceuticals Inc. J.L. Witztum is a consultant to Ionis Pharmaceuticals Inc., Cymabay Therapeutics, Intercept Pharmaceuticals, Inc., and Prometheus Laboratories Inc. J.D. Esko and I.J. Goldberg have received a grant from Ionis Pharmaceuticals.
Reference information:J Clin Invest. 2016;126(8):2855–2866. doi:10.1172/JCI86610.
Contributor Information
Philip L.S.M. Gordts, Email: pgordts@ucsd.edu.
Ryan Nock, Email: rnock@ucsd.edu.
Ni-Huiping Son, Email: Ni-huiping.Son@nyumc.org.
Bastian Ramms, Email: bramms@ucsd.edu.
Irene Lew, Email: ilew@ucsd.edu.
Debapriya Basu, Email: debapriya.basu@nyumc.org.
Ira J. Goldberg, Email: ira.goldberg@nyumc.org.
Jeffrey D. Esko, Email: jesko@ucsd.edu.
References
- 1.Chapman MJ, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen M, Varbo A, Tybjaerg-Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin Chem. 2014;60(5):737–746. doi: 10.1373/clinchem.2013.219881. [DOI] [PubMed] [Google Scholar]
- 4.Yao Z. Human apolipoprotein C-III - a new intrahepatic protein factor promoting assembly and secretion of very low density lipoproteins. Cardiovasc Hematol Disord Drug Targets. 2012;12(2):133–140. doi: 10.2174/1871529X11202020133. [DOI] [PubMed] [Google Scholar]
- 5.Schonfeld G, George PK, Miller J, Reilly P, Witztum J. Apolipoprotein C-II and C-III levels in hyperlipoproteinemia. Metabolism. 1979;28(10):1001–1010. doi: 10.1016/0026-0495(79)90004-0. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249(4970):790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- 7.Maeda N, Li H, Lee D, Oliver P, Quarfordt SH, Osada J. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem. 1994;269(38):23610–23616. [PubMed] [Google Scholar]
- 8.Norum RA, et al. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary-artery disease. N Engl J Med. 1982;306(25):1513–1519. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- 9.Pollin TI, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 11.Crosby J, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972;46(2):375–382. doi: 10.1016/S0006-291X(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg HN, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78(5):1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaram M, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51(1):150–161. doi: 10.1194/M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham MJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112(11):1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 16.Gaudet D, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371(23):2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 17.Foley EM, et al. Hepatic remnant lipoprotein clearance by heparan sulfate proteoglycans and low-density lipoprotein receptors depend on dietary conditions in mice. Arterioscler Thromb Vasc Biol. 2013;33(9):2065–2074. doi: 10.1161/ATVBAHA.113.301637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanford KI, et al. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119(11):3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson M, Vorrsjo E, Talmud P, Lookene A, Olivecrona G. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288(47):33997–34008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jong MC, Rensen PC, Dahlmans VE, van der Boom H, van Berkel TJ, Havekes LM. Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J Lipid Res. 2001;42(10):1578–1585. [PubMed] [Google Scholar]
- 21.Weinstein MM, et al. Abnormal patterns of lipoprotein lipase release into the plasma in GPIHBP1-deficient mice. J Biol Chem. 2008;283(50):34511–34518. doi: 10.1074/jbc.M806067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharadwaj KG, et al. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285(49):37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101(3):689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales JC, Gordts PLM, Foley EM, Esko JD. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. J Clin Invest. 2013;123(6):2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahm AJ, Hegele RA. Chylomicronaemia — current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11(6):352–362. doi: 10.1038/nrendo.2015.26. [DOI] [PubMed] [Google Scholar]
- 26.Shachter NS, et al. Overexpression of apolipoprotein CII causes hypertriglyceridemia in transgenic mice. J Clin Invest. 1994;93(4):1683–1690. doi: 10.1172/JCI117151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shachter NS, et al. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein Cl. J Clin Invest. 1996;98(3):846–855. doi: 10.1172/JCI118857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kypreos KE, Li X, van Dijk KW, Havekes LM, Zannis VI. Molecular mechanisms of type III hyperlipoproteinemia: The contribution of the carboxy-terminal domain of ApoE can account for the dyslipidemia that is associated with the E2/E2 phenotype. Biochemistry. 2003;42(33):9841–9853. doi: 10.1021/bi0271796. [DOI] [PubMed] [Google Scholar]
- 29.Merkel M, et al. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280(22):21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 30.Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010;121(15):1722–1734. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss JG, et al. Adenovirus-mediated rescue of lipoprotein lipase-deficient mice. Lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J Biol Chem. 2001;276(39):36083–36090. doi: 10.1074/jbc.M104430200. [DOI] [PubMed] [Google Scholar]
- 32.Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985;26(5):556–565. [PubMed] [Google Scholar]
- 33.Aalto-Setala K, et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992;90(5):1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowal RC, Herz J, Weisgraber KH, Mahley RW, Brown MS, Goldstein JL. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265(18):10771–10779. [PubMed] [Google Scholar]
- 35.Ebara T, Ramakrishnan R, Steiner G, Shachter NS. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J Clin Invest. 1997;99(11):2672–2681. doi: 10.1172/JCI119456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisgraber KH, Mahley RW, Kowal RC, Herz J, Goldstein JL, Brown MS. Apolipoprotein C-I modulates the interaction of apolipoprotein E with β-migrating very low density lipoproteins (β-VLDL) and inhibits binding of β-VLDL to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265(36):22453–22459. [PubMed] [Google Scholar]
- 37.Veniant MM, et al. Lipoprotein clearance mechanisms in LDL receptor-deficient “Apo-B48-only” and “Apo-B100-only” mice. J Clin Invest. 1998;102(8):1559–1568. doi: 10.1172/JCI4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyler von Ballmoos MC, Haring B, Sacks FM. The risk of cardiovascular events with increased apolipoprotein CIII: A systematic review and meta-analysis. J Clin Lipidol. 2015;9(4):498–510. doi: 10.1016/j.jacl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Mendivil CO, Rimm EB, Furtado J, Sacks FM. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J Am Heart Assoc. 2013;2(3): doi: 10.1161/JAHA.113.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudet D, et al. Antisense Inhibition of Apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438–447. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 41.Hegele RA, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2(8):655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacArthur JM, et al. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117(1):153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noh HL, Okajima K, Molkentin JD, Homma S, Goldberg IJ. Acute lipoprotein lipase deletion in adult mice leads to dyslipidemia and cardiac dysfunction. Am J Physiol Endocrinol Metab. 2006;291(4):E755–E760. doi: 10.1152/ajpendo.00111.2006. [DOI] [PubMed] [Google Scholar]
- 44.Hocquette JF, Graulet B, Olivecrona T. Lipoprotein lipase activity and mRNA levels in bovine tissues. Comp Biochem Physiol B Biochem Mol Biol. 1998;121(2):201–212. doi: 10.1016/S0305-0491(98)10090-1. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Arcos I, et al. Adipose-specific lipoprotein lipase deficiency more profoundly affects brown than white fat biology. J Biol Chem. 2013;288(20):14046–14058. doi: 10.1074/jbc.M113.469270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley JL, Kruski AW. Density gradient ultracentrifugation of serum lipoproteins in a swinging bucket rotor. Methods Enzymol. 1986;128:170–181. doi: 10.1016/0076-6879(86)28067-2. [DOI] [PubMed] [Google Scholar]
- 47.Gordts PL, et al. Impaired LDL receptor-related protein 1 translocation correlates with improved dyslipidemia and atherosclerosis in apoE-deficient mice. PLoS One. 2012;7(6): doi: 10.1371/journal.pone.0038330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.