Abstract
Purpose
To measure the association between patient financial strain and symptom burden and quality of life (QOL) for patients with new diagnoses of lung or colorectal cancer.
Patients and Methods
Patients participating in the Cancer Care Outcomes Research and Surveillance study were interviewed about their financial reserves, QOL, and symptom burden at 4 months of diagnosis and, for survivors, at 12 months of diagnosis. We assessed the association of patient-reported financial reserves with patient-reported outcomes including the Brief Pain Inventory, symptom burden on the basis of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30, and QOL on the basis of the EuroQoL-5 Dimension scale. Multivariable linear regression models were fit for each outcome and cancer type, adjusting for age, race/ethnicity, sex, income, insurance, stage at diagnosis, and comorbidity.
Results
Among patients with lung and colorectal cancer, 40% and 33%, respectively, reported limited financial reserves (≤ 2 months). Relative to patients with more than 12 months of financial reserves, those with limited financial reserves reported significantly increased pain (adjusted mean difference, 5.03 [95% CI, 3.29 to 7.22] and 3.45 [95% CI, 1.25 to 5.66], respectively, for lung and colorectal), greater symptom burden (5.25 [95% CI, 3.29 to .22] and 5.31 [95% CI, 3.58 to 7.04]), and poorer QOL (4.70 [95% CI, 2.82 to 6.58] and 5.22 [95% CI, 3.61 to 6.82]). With decreasing financial reserves, a clear dose-response relationship was present across all measures of well-being. These associations were also manifest for survivors reporting outcomes again at 1 year and persisted after adjustment for stage, comorbidity, insurance, and other clinical attributes.
Conclusion
Patients with cancer and limited financial reserves are more likely to have higher symptom burden and decreased QOL. Assessment of financial reserves may help identify patients who need intensive support.
INTRODUCTION
The financial reserves required to manage a cancer diagnosis are substantial.1 From diagnosis through survivorship to end-of-life care, the direct and indirect costs of cancer care can cause significant hardship for families without the financial resources to buffer the additional expenses.2,3 The influence of financial strain on the clinical outcomes and experiences of care in patients with cancer is an ongoing area of interest in health services research.4-9
Recent research suggests that families are not prepared to manage the expenses associated with cancer care10 and many continue to experience financial hardship during survivorship.11 Not surprisingly, these studies have demonstrated that financial hardship is positively associated with medication nonadherence and delaying medical care.12 Multiple studies demonstrate that patients living in regions with high levels of poverty delay medical care.13-20 However, most of these studies have relied on ecologic measures of socioeconomic status that are based on the attributes of the region of residence and thus may not reliably reflect individuals’ experiences.
A much smaller body of work has measured individual socioeconomic attributes, such as household income, and demonstrates a consistent association between financial hardship and inferior survival for patients with cancer.6,18,21,22 Personal and household income are also imperfect measures of financial hardship because they may not reflect assets, responsibilities, and debt. In contrast to income, the construct of financial strain is a subjective measure characterizing how an individual perceives his or her overall economic resources relative to obligations and needs. Observations from financial surveys indicate that approximately 34% of American adults lack enough emergency savings to cover living expenses for 90 days.23
Although it is clear that patients face financial strain as a result of a cancer diagnosis, and that such strain may influence treatment choices and adherence, there is limited understanding of how financial strain influences outcomes such as symptom burden and quality of life (QOL).24,25
To understand how individuals’ financial strain is associated with patient-reported health outcomes, we used data from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium, a large prospective population- and health system–based cohort composed of patients with recently diagnosed lung and colorectal cancer.26 We hypothesized that patients with financial strain would experience greater symptom burden and potentially inferior outcomes.
PATIENTS AND METHODS
The CanCORS study enrolled more than 10,000 patients with incident lung or colorectal cancer who had received the diagnosis between 2003 and 2005. Patients were enrolled from five geographic regions (Northern California, Los Angeles County, North Carolina, Iowa, and Alabama), five participating health maintenance organizations, and 15 sites within the Veteran’s Health Administration system.27,28 Each site identified incident cancer cases using a comprehensive, rapid case ascertainment protocol. The five regional participating sites used population-based cancer registries to identify eligible subjects, and the health maintenance organization and Veteran’s Health Administration sites identified eligible subjects from organizational cancer registries. The study was approved by the human subjects committees at all participating institutions.
Patients, or surrogates of patients who were deceased or too ill to participate, were interviewed approximately 4 months after diagnosis. Four versions of the baseline interview were available: a full patient interview; a brief patient interview for patients unable to complete the full interview; a surrogate interview for surrogates of deceased patients; and a surrogate interview for living patients too ill to complete the interview themselves. A follow-up patient or surrogate interview was performed approximately 12 months after diagnosis if the patient was alive at the time of the baseline interview. In accordance with the standards of the American Association of Public Opinion Research, where the denominator included both unsuccessful contacts and refusal/nonresponse, the response rate was 51.0%; the cooperation rate, assessing participation among patients contacted, was 59.9%28,29 For participants who gave consent, medical records from hospitals, radiation treatment facilities, and offices of medical oncologists, surgeons, gastroenterologists, pulmonologists, and primary care physicians were abstracted for the time period beginning 3 months before diagnosis until death or at least 15 months after diagnosis. The CanCORS study protocol and data collection have been described previously.29
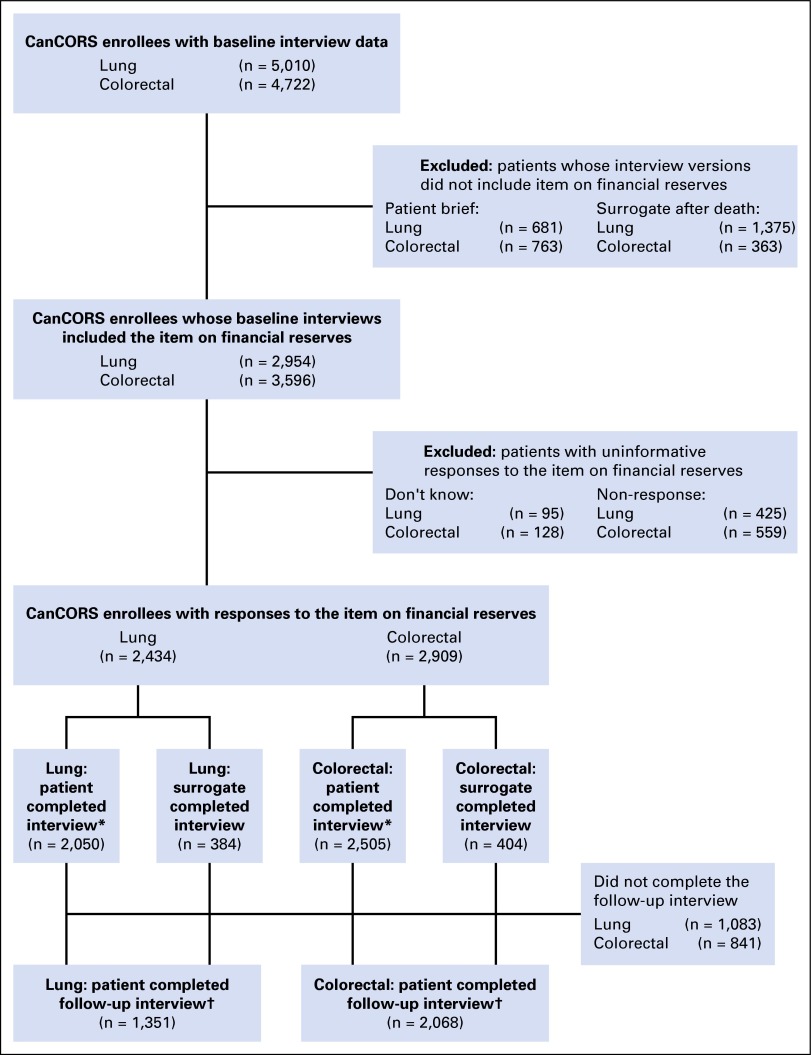
The primary explanatory variable of interest was patient-reported financial reserves.30-33 In the full interviews of patients and of surrogates of living patients, patients or their surrogates were asked, “If you lost all of your current sources of income (for example, your paycheck, Social Security or pension, public assistance) and had to live off of your savings, how long could you continue to live at your current address and standard of living?” Response options were less than 1 month, 1-2 months, 3-6 months, 7-12 months, more than 1 year, and don't know. This question was not included in the brief patient interview or surrogate interview for deceased patients, so these subjects were excluded from the cohort. Overall, 18% of patients with lung cancer and 19% of patients with colorectal cancer responded, “don't know” or did not answer the question about financial reserves; these individuals were also excluded from the primary analytic cohort. In general, patients with nonresponse to the question about financial reserves were older, were more likely to be Hispanic or Asian, and had lower income and education. Those who did not respond to the questions on income or education were also less likely to respond to the question on financial reserves (data not shown). Details of the cohort assembly are displayed in Figure 1.
Fig 1.
Flow diagram. *Subgroup with patient-reported quality of life, symptom burden, and physical function at baseline; †It was possible for patients to have completed the follow-up interview themselves, even when the baseline interview was completed by a surrogate. CanCORS, Cancer Care Outcomes Research and Surveillance.
The primary study outcome was patient-reported QOL across domains of physical and mental health. Overall QOL was measured using the 12-Item Short Form Health Survey (SF-12) physical and mental health scales.34 Patients also completed the five-item EuroQol-5 Dimension scale (EQ-5D), which provides a global measure of health-related QOL.35 Other measures were used to capture domains especially salient for patients with cancer. Pain was measured using the Brief Pain Inventory,36 symptom burden was measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 symptom inventory,37 and for patients with lung cancer, a measure of dyspnea was used.38 All these measures were patient reported at the time of the baseline interview, were phrased to address the patient's current health state, and were standardized to 100-point scales in which higher values represent worse outcomes. The QOL outcomes analyses were restricted to patients who completed the full patient interview, because these measures were truly patient reported (as opposed to being surrogate reports of the patient’s outcomes). The subset of patients who completed a follow-up interview (56% for lung, 71% for colorectal; Fig 1) reported on these same QOL domains again at approximately 12 months.
Univariable and multivariable linear regression analysis was used to calculate unadjusted and adjusted differences in mean QOL measures at baseline by financial reserves category, with 95% CIs. Adjusted results controlled for age, sex, race/ethnicity, income, education, insurance, cancer stage at diagnosis, and comorbidity at diagnosis. Comorbidity was ascertained from the medical record abstraction, using the Adult Comorbidity Evaluation 27, a validated medical record–based system that assigns each patient a comorbidity score on the basis of severity noted across multiple body systems39 and reflects comorbidities present from 3 months before diagnosis through initial treatment; patients who did not consent to a medical record abstraction were grouped in a separate category (ie, unknown comorbidity) in the models.
Univariable and multivariable ordinal logistic regression analysis was performed for the individual QOL items obtained at the follow-up survey. Regression analyses were performed on multiply imputed data sets to adjust for survey nonresponse40; however, imputed values were not used for the financial reserves variable, because this was the main predictor of interest.
To examine the potential for colinearity, the relationship between financial reserves and household income was summarized descriptively using Spearman's rank correlation coefficient, stratified by race/ethnicity. The strength of the association between these three measures (financial reserves, household income, and race/ethnicity) and the QOL measures was quantified using the R2 value from univariable linear regression. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 13.1 (STATA, College Station, TX), with CanCORS core data (version 1.18) and baseline and follow-up interview data (version 1.12).
RESULTS
Table 1 presents the distribution of patient financial strain as well as other sociodemographic and clinical characteristics. Among patients with lung and colorectal cancer, 40% and 33%, respectively, had limited financial reserves (< 2 months). When compared with patients with colorectal cancer, a higher percentage of patients with lung cancer had a household income less than $20,000 (34% v 29%), and fewer had completed a college degree (18% v 28%). The lung cancer cohort also had a higher percentage of stage IV patients. The colorectal cancer cohort had a higher percentage of patients younger than age 60 years (38% v 27%). Patients reporting low financial reserves were more likely to be insured by Medicaid or to have no insurance than were patients reporting reserves of more than 2 months. Among patients with lung cancer reporting low financial reserves, 19% had Medicaid, 10% had no insurance, and 71% had insurance other than Medicaid. In contrast, among patients with financial reserves of more than 2 months, 6% had Medicaid, 2% no insurance, and 91% non-Medicaid insurance. The pattern was similar for patients with colorectal cancer (Appendix Table A1, online only).
Table 1.
Characteristics of the CanCORS Study Cohort Stratified by Cancer Type
| Characteristic | Lung Cancer (n = 2,434), No. (%) | Colorectal Cancer (n = 2,909), No. (%) |
|---|---|---|
| Availability of financial reserves | ||
| ≤ 2 months | 966 (40) | 954 (33) |
| 3-6 months | 266 (11) | 382 (13) |
| 7-12 months | 186 (8) | 231 (8) |
| > 1 year | 1,016 (42) | 1,342 (46) |
| Age at diagnosis, years | ||
| < 59 | 658 (27) | 1,093 (38) |
| 60-64 | 375 (15) | 358 (12) |
| 65-69 | 412 (17) | 389 (13) |
| 70-74 | 406 (17) | 343 (12) |
| 75-79 | 328 (13) | 314 (11) |
| ≥ 80 | 255 (10) | 412 (14) |
| Sex | ||
| Male | 1,371 (56) | 1,636 (56) |
| Female | 1,063 (44) | 1,273 (44) |
| Race/ethnicity | ||
| White | 1,811 (74) | 1,901 (65) |
| Black | 277 (11) | 452 (16) |
| Hispanic | 127 (5) | 240 (8) |
| Asian | 81 (3) | 159 (5) |
| Other | 138 (6) | 157 (5) |
| Household income, $* | ||
| < 20,000 | 792 (34) | 794 (29) |
| 20,000-39,999 | 712 (31) | 744 (27) |
| 40,000-59,999 | 372 (16) | 458 (17) |
| ≥ 60,000 | 425 (18) | 763 (28) |
| Highest education level completed* | ||
| Less than high school | 495 (21) | 531 (18) |
| High school degree | 1,490 (62) | 1,548 (54) |
| College degree or higher | 426 (18) | 804 (28) |
| Health insurance | ||
| Other insurance | 2,024 (83) | 2,468 (85) |
| Medicaid or other low income | 276 (11) | 261 (9) |
| None | 134 (6) | 180 (6) |
| Stage at diagnosis | ||
| I | 719 (30) | 667 (23) |
| II | 231 (9) | 796 (27) |
| III | 689 (28) | 864 (30) |
| IV | 681 (28) | 436 (15) |
| Unknown | 114 (5) | 146 (5) |
| Comorbidity score at diagnosis† | ||
| None | 359 (15) | 640 (22) |
| Mild | 759 (31) | 910 (31) |
| Moderate | 418 (17) | 386 (13) |
| Severe | 402 (17) | 272 (9) |
| Unknown (patient did not consent to medical record abstraction) | 496 (20) | 701 (24) |
| Interview type | ||
| Full patient interview‡ | 2,050 (84) | 2,505 (86) |
| Surrogate (patient too sick) | 384 (16) | 404 (14) |
| Months from diagnosis to: | ||
| Baseline interview, median (interquartile range) | 4.2 (3.5-5.5) | 4.4 (3.5-5.7) |
| Follow-up interview, median (interquartile range)§ | 12.4 (11.7-14.0) | 13.4 (12.0-15.7) |
Abbreviation: CanCORS, Cancer Care Outcomes Research and Surveillance.
Household income was not reported by 283 patients (5%) and education was not reported by 49 patients (1%).
Medical records were abstracted for 1,938 patients with lung cancer (80%) and 2,208 patients with colorectal cancer (76%). The comorbidity score was defined using the Adult Comorbidity Evaluation 27, a validated medical record–based system that assigns each patient a four-category comorbidity score (none, mild, moderate, severe) that is based on severity noted across multiple body systems, as documented in the Cancer Care Outcomes Research and Surveillance medical record abstraction from 3 months before diagnosis through initial treatment.
Evaluable for patient-reported quality-of-life end points.
For 1,351 patients with lung cancer (56%) and 2,068 patients with colorectal cancer (71%) who completed the follow-up interview.
Financial Strain and QOL/Symptom Burden at 4 Months From Diagnosis
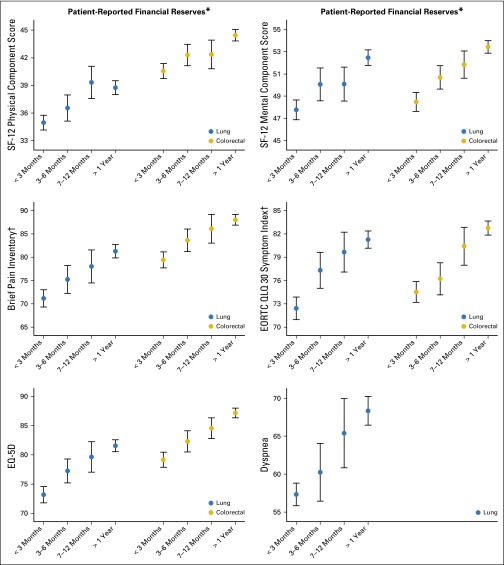
We found a strong, consistent association between greater financial strain and inferior QOL. This association was evident for physical and mental well-being as well as for each of the five QOL metrics examined. Figure 2 presents the association between financial strain and each of the QOL outcomes without adjustment for other patient attributes. These results demonstrate a remarkably consistent pattern associating low financial reserves with decreased well-being (all P < .001). Baseline QOL values reported by CanCORS corresponded to cancer-related QOL values reported in the literature.41-44
Fig 2.
Unadjusted means of baseline patient-reported quality-of-life measures by financial strain and cancer type. All measures are presented such that lower scores represent worse outcomes. Error bars represent 95% CIs. *P for trend < .001 for the association between financial strain and all quality-of-life measures, within both diseases; †Brief Pain Inventory (BPI) and EORTC symptom index were inverted so that lower scores represent worse outcomes. EORTC, European Organisation for Research and Treatment of Cancer; EQ-5D, EuroQoL-5 Dimension; QLQ 30, Quality of Life Questionnaire C30; SF-12, 12-Item Short Form Health Survey.
Appendix Table A2 (online only) details the relationship between financial strain and individual income stratified by race/ethnicity. It reveals that these two constructs are only modestly correlated (Spearman correlation ranging from 0.29 to 0.42 across all categories of race/ethnicity). For example, among white patients reporting household income below $20,000, 29% had financial reserves exceeding 1 year. In contrast, 14% of those with income above $60,000 reported financial strain on the basis of reserves of 2 months or less. This pattern was similar for other racial/ethnic groups.
The association between greater financial strain and inferior QOL was as strong, or stronger, than the association between either household income or race/ethnicity and these outcomes, with few exceptions. For example, for lung cancer, the variable financial reserves were more consistently associated with unadjusted predictor of SF-12 scores, EQ-5D, pain, and the European Organisation for Research and Treatment of Cancer symptom index than was household income. This pattern held true for colorectal cancer except that household income was more closely associated with low scores on the SF-12 physical function scale. Table 2 lists these associations for each QOL/symptom burden scale, as quantified by R2 values and the F statistic.
Table 2.
Comparison of the Statistical Association of Financial Strain, Household Income, and Race/Ethnicity With the QOL Measures, From Univariable Linear Regression
| Disease/Dependent Variable/Independent Variable | R2 | Mean Squared Error | F Statistic* |
|---|---|---|---|
| Lung cancer | |||
| SF-12 PCS | |||
| Financial reserves† | 0.027 | 11.05 | 17.4 |
| Household income† | 0.025 | 11.07 | 15.5 |
| Race/ethnicity | 0.005 | 11.18 | 2.3 |
| SF-12 MCS | |||
| Financial reserves† | 0.034 | 11.20 | 21.9 |
| Household income† | 0.023 | 11.27 | 14.3 |
| Race/ethnicity‡ | 0.008 | 11.35 | 3.9 |
| BPI | |||
| Financial reserves† | 0.039 | 23.70 | 26.0 |
| Household income† | 0.025 | 23.88 | 16.2 |
| Race/ethnicity† | 0.014 | 24.01 | 6.8 |
| EORTC QLQ 30 symptom index | |||
| Financial reserves† | 0.046 | 18.53 | 31.2 |
| Household income† | 0.023 | 18.75 | 15.4 |
| Race/ethnicity† | 0.017 | 18.82 | 8.2 |
| EQ-5D | |||
| Financial reserves† | 0.045 | 0.17 | 29.8 |
| Household income† | 0.030 | 0.18 | 19.8 |
| Race/ethnicity† | 0.011 | 0.18 | 5.5 |
| Dyspnea | |||
| Financial reserves† | 0.032 | 29.81 | 20.9 |
| Household income† | 0.041 | 29.66 | 27.8 |
| Race/ethnicity‡ | 0.007 | 30.19 | 3.6 |
| Colorectal cancer | |||
| SF-12 PCS | |||
| Financial reserves† | 0.021 | 10.93 | 16.4 |
| Household income† | 0.026 | 10.91 | 20.5 |
| Race/ethnicity | 0.002 | 11.04 | 1.3 |
| SF-12 MCS | |||
| Financial reserves† | 0.043 | 10.36 | 34.2 |
| Household income† | 0.010 | 10.53 | 8.1 |
| Race/ethnicity† | 0.021 | 10.48 | 12.4 |
| BPI | |||
| Financial reserves† | 0.029 | 22.14 | 23.3 |
| Household income† | 0.021 | 22.23 | 16.6 |
| Race/ethnicity† | 0.015 | 22.30 | 9.1 |
| EORTC QLQ 30 symptom index | |||
| Financial reserves† | 0.039 | 17.80 | 32.4 |
| Household income‡ | 0.006 | 18.10 | 5.1 |
| Race/ethnicity‡ | 0.004 | 18.13 | 2.4 |
| EQ-5D | |||
| Financial reserves† | 0.043 | 0.16 | 35.2 |
| Household income† | 0.021 | 0.16 | 16.8 |
| Race/ethnicity† | 0.012 | 0.16 | 7.3 |
NOTE. Higher values for R2 and F statistics, and lower values for mean squared error, correspond with higher statistical associations.
Abbreviations: BPI, Brief Pain Inventory; EORTC QLQ-30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; EQ-5D, EuroQol-5 Dimension; MCS, Mental Component Score; PCS, Physical Component Score; QOL, quality of life; SF-12, 12-Item Short Form Health Survey.
F statistics for financial reserves and income category are comparable because they have the same degrees of freedom; F statistics for race are not comparable with the others because they have different degrees of freedom.
P < .001.
P < .05.
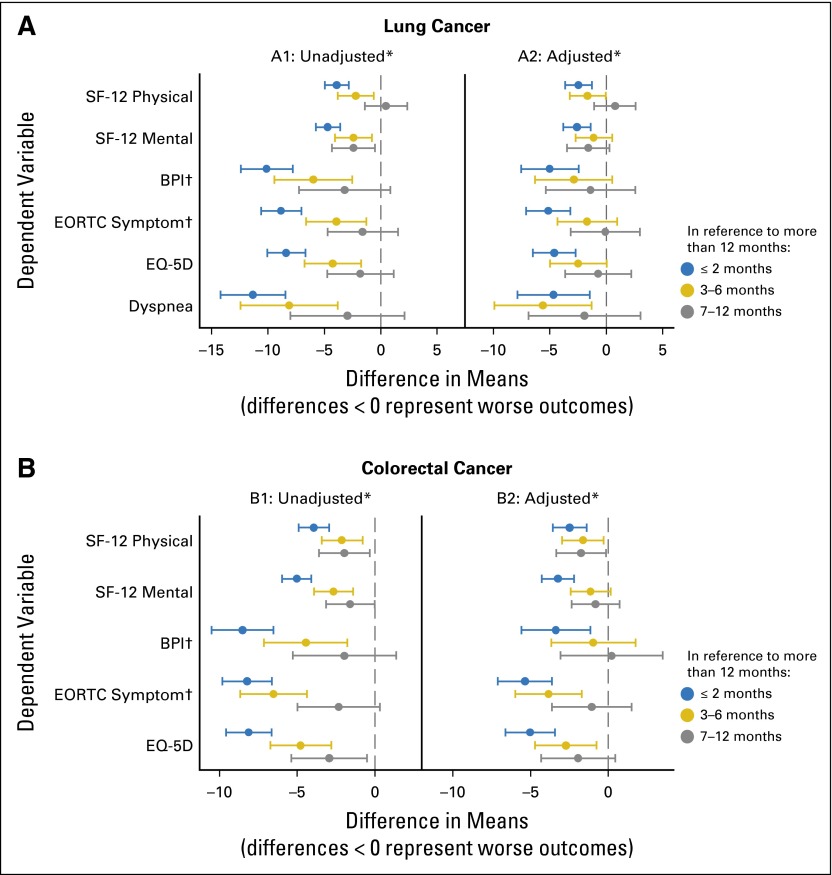
Figure 3 presents the unadjusted (A1 and B1) and adjusted (A2 and B2) differences in means for the dependent variables of interest according to the degree of patient-reported financial strain. For both patients with lung cancer and those with colorectal cancer, patients who had less than 2 months of financial reserve had significantly lower mean scores for all outcome measures compared with those with more than a year of financial reserve. Moreover, results demonstrate a clear dose-response relationship between financial strain and both lower QOL and greater symptom burden. These associations persisted after adjusting for other sociodemographic variables including household income, educational attainment, race/ethnicity, age, sex, stage at diagnosis, and comorbidity at diagnosis.
Fig 3.
Unadjusted and adjusted differences in patient-reported quality-of-life measures according to financial reserves, from linear regression models. (A) Lung cancer. (B) Colorectal cancer. Differences are in reference to financial reserves of more than 1 year. Adjusted differences control for age, sex, race/ethnicity, household income, education, health insurance, stage, and comorbidity. *For all measures, unadjusted P < .001 and adjusted P < .02 for the overall F test of an association between financial reserves and quality of life; †BPI and EORTC symptom index were inverted so that lower scores represent worse outcomes. BPI, Brief Pain Inventory; EORTC, European Organisation for Research and Treatment of Cancer; EQ-5D, EuroQoL-5 Dimension; SF-12, 12-Item Short Form Health Survey.
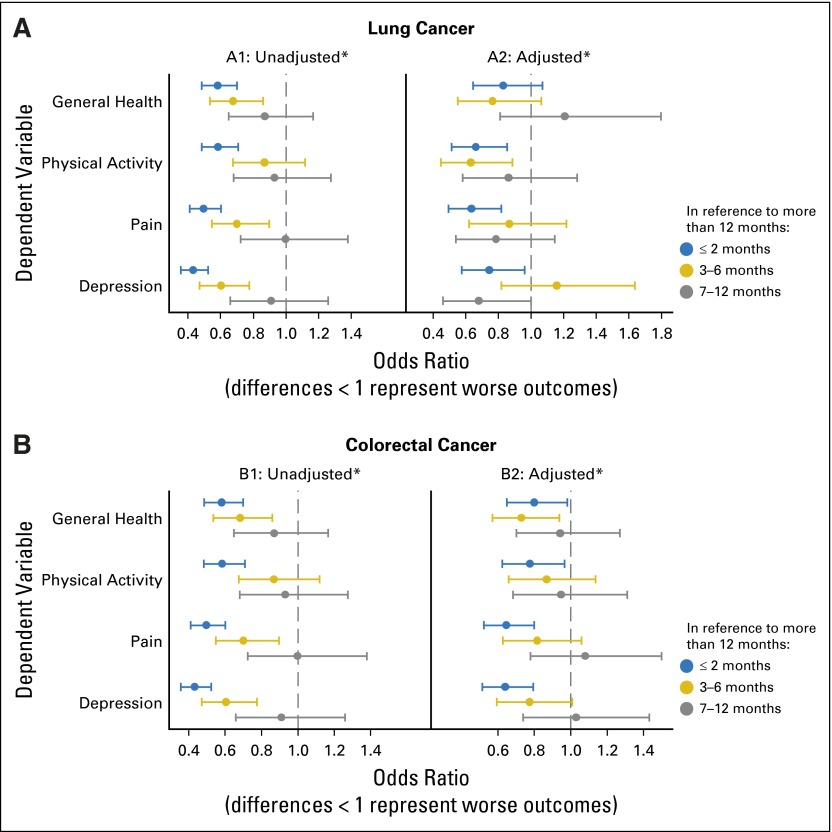
Financial Strain and Outcomes Approximately 1 Year From Diagnosis
Approximately 1 year after the baseline interview, a subset of patients and surrogates completed an abbreviated follow-up interview (Fig 1). Both patients with lung cancer and those with colorectal cancer who reported greater financial strain at diagnosis tended to have worse overall health, impairment in activity level, more pain, and more depression than did those with greater financial reserve. Multivariable analyses revealed that financial strain was also an independent predictor of impairment in the well-being of patients with cancer at follow-up interviews (Fig 4). Specifically, pain and depression were statically significant across both cancer types. Measures evaluating general health and physical activity followed the same pattern but were not significant predictors across both cancer types. In a sensitivity analysis, we examined the relationship between financial strain and the magnitude of change in patient-reported outcomes between the 4-month and 12-month interviews and found no consistent association (data not shown).
Fig 4.
Unadjusted and adjusted differences in patient-reported quality-of-life measures at the time of the follow-up survey according to financial reserves, from ordinal logistic regression. (A) Lung cancer. (B) Colorectal cancer. Differences are in reference to financial reserves of more than 1 year. Adjusted differences control for age, sex, race/ethnicity, household income, education, health insurance, stage, and comorbidity. *Overall F test of an association between financial reserves and quality of life: (1) unadjusted P < .001 for all measures and both cancer types, (2) lung: adjusted P = .13 for general health and adjusted P < .02 for other three measures, and (3) colorectal: adjusted P = .16 for physical activity and adjusted P < .05 for other three measures.
DISCUSSION
We found that financial strain at the time of diagnosis has a consistent independent association with the well-being of patients with cancer. Not surprisingly, patients who reported the greatest amount of financial strain were poorer, and a higher percentage were black or and Hispanic. Our analysis indicates an association between high levels of financial strain and increased symptom burden as well as a poorer QOL at the time of diagnosis.
We found that this relationship persisted even after adjusting for race/ethnicity, median household income, insurance status, stage at diagnosis, comorbidity, and other patient-level characteristics. Although related to other demographic attributes, financial strain had an independent association with both symptom burden and QOL Indeed, the impact was similar in magnitude to well-recognized associations between household income and race/ethnicity and these outcomes. Moreover, financial strain remained associated with changes in QOL and symptom burden values at follow-up interviews conducted 12 months after diagnosis.
The association between low financial reserves and high symptom burden/poor QOL was present in subgroup analyses that considered stage at diagnosis, the receipt of chemotherapy as a component of the initial treatment course, and comorbidity level (data not shown). The fact that self-reported low financial reserves coincided with higher symptom burden and worse QOL across these subgroups suggests that the association we describe is not simply confounded by baseline health status, disease severity, or treatment choice. The differences in EQ-5D and SF-12 according to degree of financial strain are clinically meaningful, although there is less information about the magnitude of meaningful differences for the Brief Pain Inventory and dyspnea metrics.42,45 Finally, given the representativeness of the CANCORS population and the fact that the QOL scores in our study are in line with the published accounts in the literature, our results should be generalizable to routine practice.41-44,46-48
More than 80% of patients were both willing and able to answer the single-item question about financial strain. This suggests the usefulness of this variable for possible use in routine clinical settings. Asking about financial reserves may be perceived as less intrusive than asking about household income and may also be a cognitively easier task. The independent association of this measure of financial stability with important patient-centered cancer outcomes suggests consideration for its inclusion with the common demographic information that patients are often asked to provide in health system interactions. Moreover, this metric should be prioritized in electronic medical records that allow for patient interaction.
The role of financial strain and its effects on health and health behaviors has been evaluated previously by Hudson et al,49 who examined the impact of race and socioeconomic position on self-rated health and depression and found that both racial discrimination and lower socioeconomic position over the life course were related to increased depressive symptoms and poorer self-reported health status. The increased pain and dyspnea we observed could be caused by worsening disease burden, even within stage, a lack of ability to access high-quality supportive care, or difficulty in obtaining the appropriate medications. Earlier work by Savoy et al50 has shown that financial strain alone can contribute to poor health and depressive symptoms, as well as increased cancer risk behaviors.
Our study is limited primarily by its observational nature, which precludes causal inference and a precise understanding of the mechanisms that account for these consistent associations. Ostensibly, financial strain could result in inferior QOL and increased symptom burden because of the inability to access needed care, poor social supports, or increased stress. Alternatively, the causal association may occur in the reverse direction; inferior outcomes and higher symptom burden may accelerate depletion of financial reserves and adversely affect work capacity. The relationship between financial strain and our outcomes of interest may indeed be bidirectional. However, from a health care delivery system perspective, the association is important irrespective of the directionality because it signals a vulnerable group of patients not easily identified through other metrics. Lastly, we used a validated single-item measure to assess financial reserves, but clearly, the construct of financial reserves is multidimensional. The single item we chose has been validated across multiple studies and diverse populations and is widely accepted in the public health arena.30,51,52
Using a range of well-validated metrics that characterize well-being in patients with cancer, we found that those with decreased financial reserves demonstrated an association between lower QOL scores and increased symptom burden scores. This effect manifests early in the course of the disease and continues over time. The strength of the association persists after adjustment for demographic variables and disease stage. Identifying which patients may be most in need of financial assistance could have a positive effect on patient outcomes and could allow for precision triaging of limited resources. Social workers are commonly called on for patient emotional support but, in many cases, are also able to help with identification of community resources for vulnerable patients. Often, this assistance is only given once there is an incident or problem that brings this to the attention of the clinician. The evaluation of financial strain could be performed by social workers, nurses, or physicians themselves, depending on the structure of the clinic.53
Our results support future research that should evaluate whether prospectively identifying patients experiencing financial strain and providing supportive interventions for these individuals is an effective strategy to improve outcomes and decrease suffering.
Acknowledgment
We thank Lin Ding for her data preparation and analysis.
GLOSSARY TERMS
- health-related quality of life (HRQoL):
a broad multidimensional concept that usually includes self-reported measures of physical and mental health.
Appendix
Table A1.
Crosstabulation of Health Insurance by Financial Reserves
| Availability of Financial Reserves (Months) | Health Insurance | ||
|---|---|---|---|
| Other | Medicaid or Other Low Income | None | |
| Lung cancer | |||
| ≤ 2 | 686 (71) | 182 (19) | 98 (10) |
| > 2 | 1,338 (91) | 94 (6) | 36 (2) |
| Colorectal cancer | |||
| ≤ 2 | 675 (71) | 159 (17) | 120 (13) |
| > 2 | 1,793 (92) | 102 (5) | 60 (3) |
NOTE. Data are presented as No. (%).
Table A2.
Relationship Between Financial Strain and Household Income, By Race-Ethnicity
| Race-Ethnicity/Household Income, $ | Financial Reserves | Spearman Correlation Coefficient | |||
|---|---|---|---|---|---|
| ≤ 2 Months | 3-6 Months | 7-12 Months | > 1 Year | ||
| Overall (N = 5,060) | |||||
| < 20,000 | 950 (60) | 143 (9) | 82 (5) | 411 (26) | 0.34 |
| 20,000-39,999 | 492 (34) | 194 (13) | 116 (8) | 654 (45) | |
| 40,000-59,999 | 193 (23) | 116 (14) | 82 (10) | 439 (53) | |
| ≥ 60,000 | 181 (15) | 164 (14) | 117 (10) | 726 (61) | |
| White (n = 3,543) | |||||
| < 20,000 | 527 (55) | 95 (10) | 57 (6) | 282 (29) | 0.31 |
| 20,000-39,999 | 318 (30) | 135 (13) | 94 (9) | 514 (48) | |
| 40,000-59,999 | 137 (22) | 78 (13) | 59 (10) | 346 (56) | |
| ≥ 60,000 | 130 (14) | 107 (12) | 78 (9) | 586 (65) | |
| Black (n = 692) | |||||
| < 20,000 | 225 (66) | 33 (10) | 17 (5) | 68 (20) | 0.29 |
| 20,000-39,999 | 81 (45) | 34 (19) | 11 (6) | 53 (30) | |
| 40,000-59,999 | 27 (30) | 20 (22) | 14 (16) | 29 (32) | |
| ≥ 60,000 | 18 (23) | 19 (24) | 9 (11) | 34 (43) | |
| Hispanic (n = 328) | |||||
| < 20,000 | 98 (75) | 5 (4) | 4 (3) | 23 (18) | 0.39 |
| 20,000-39,999 | 42 (49) | 9 (11) | 6 (7) | 28 (33) | |
| 40,000-59,999 | 13 (29) | 8 (18) | 3 (7) | 21 (47) | |
| ≥ 60,000 | 13 (19) | 18 (26) | 12 (18) | 25 (37) | |
| Asian (n = 215) | |||||
| < 20,000 | 42 (65) | 6 (9) | 1 (2) | 16 (25) | 0.42 |
| 20,000-39,999 | 13 (26) | 9 (18) | 2 (4) | 26 (52) | |
| 40,000-59,999 | 4 (12) | 6 (18) | 2 (6) | 21 (64) | |
| ≥ 60,000 | 8 (12) | 8 (12) | 6 (9) | 45 (67) | |
| Other (n = 282) | |||||
| < 20,000 | 58 (67) | 4 (5) | 3 (3) | 22 (25) | 0.33 |
| 20,000-39,999 | 38 (47) | 7 (9) | 3 (4) | 33 (41) | |
| 40,000-59,999 | 12 (29) | 4 (10) | 4 (10) | 22 (52) | |
| ≥ 60,000 | 12 (17) | 12 (17) | 12 (17) | 36 (50) | |
NOTE. Data are presented as No. (%). N = 5,060 patients who reported their income on the baseline interview.
Footnotes
Supported by grants from the National Cancer Institute (1P50CA148596 and K01 CA124581). The Cancer Care Outcomes Research and Surveillance Consortium was supported by grants from the National Cancer Institute to the Statistical Coordinating Center (U01 CA093344) and the National Cancer Institute–supported Primary Data Collection and Research Centers: Dana-Farber Cancer Institute/Cancer Research Network (U01 CA093332), Harvard Medical School/Northern California Cancer Center (U01 CA093324), RAND/UCLA (U01 CA093348), University of Alabama at Birmingham (U01 CA093329), University of Iowa (U01 CA093339), University of North Carolina (U01 CA093326) and by a Department of Veteran’s Affairs grant to the Durham VA Medical Center (CRS 02-164).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 1711
AUTHOR CONTRIBUTIONS
Conception and design: Christopher S. Lathan, John Z. Ayanian, Deborah Schrag
Financial support: Deborah Schrag
Administrative support: Deborah Schrag
Provision of study materials or patients: Deborah Schrag
Collection and assembly of data: Angel Cronin, John Z. Ayanian, Deborah Schrag
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association of Financial Strain With Symptom Burden and Quality of Life for Patients With Lung or Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Christopher S. Lathan
Honoraria: PER
Consulting or Advisory Role: Gerson Lehrman Group, Novartis, Eli Lilly
Research Funding: CVS Health
Angel Cronin
No relationship to disclose
Reginald Tucker-Seeley
No relationship to disclose
S. Yousuf Zafar
Employment: Novartis
Stock or Other Ownership: Novartis
Consulting or Advisory Role: Genentech
Travel, Accommodations, Expenses: Genentech
John Z. Ayanian
Stock or Other Ownership: Johnson & Johnson
Deborah Schrag
Consulting or Advisory Role: New Century Health, Ohio State University
Other Relationship: Journal of the American Medical Association
REFERENCES
- 1.Wagner L, Lacey MD. The hidden costs of cancer care: An overview with implications and referral resources for oncology nurses. Clin J Oncol Nurs. 2004;8:279–287. doi: 10.1188/04.CJON.279-287. [DOI] [PubMed] [Google Scholar]
- 2.Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32:1269–1276. doi: 10.1200/JCO.2013.53.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiarz P. RCA: Financial literacy and emergency savings. J Fam Econ Issues. 2014;35:40–50. [Google Scholar]
- 5.Palmer RCSE. Social disparites across the continuum of colorectal cancer: A systemic review. Cancer Causes Control. 2005;16:55–61. doi: 10.1007/s10552-004-1253-3. [DOI] [PubMed] [Google Scholar]
- 6.Weissman JS, Schneider EC. Social disparities in cancer: Lessons from a multidisciplinary workshop. Cancer Causes Control. 2005;16:71–74. doi: 10.1007/s10552-004-1255-1. [DOI] [PubMed] [Google Scholar]
- 7.Geyer S. Social inequalities in the incidence and case fatality of cancers of the lung, the stomach, the bowels, and the breast. Cancer Causes Control. 2008;19:965–974. doi: 10.1007/s10552-008-9162-5. [DOI] [PubMed] [Google Scholar]
- 8.Lynch J. Socioeconomic Position. New York, NY,: Oxford University Press; 2000. [Google Scholar]
- 9.Singh GK, Williams SD, Siahpush M, et al. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I-All cancers and lung cancer and Part II-Colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppercorn J. The financial burden of cancer care: Do patients in the US know what to expect? Expert Rev Pharmacoecon Outcomes Res. 2014;14:835–842. doi: 10.1586/14737167.2014.963558. [DOI] [PubMed] [Google Scholar]
- 11.Meneses K, Azuero A, Hassey L, et al. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124:437–443. doi: 10.1016/j.ygyno.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth P, Rodgers B, Windsor TD. Financial hardship, socio-economic position and depression: Results from the PATH Through Life Survey. Soc Sci Med. 2009;69:229–237. doi: 10.1016/j.socscimed.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: Sociological contributions. J Health Soc Behav. 2010;51(Suppl):S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DR, Kontos EZ, Viswanath K, et al. Integrating multiple social statuses in health disparities research: The case of lung cancer. Health Serv Res. 2012;47:1255–1277. doi: 10.1111/j.1475-6773.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DR. Race and health: Basic questions, emerging directions. Ann Epidemiol. 1997;7:322–333. doi: 10.1016/s1047-2797(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR. Missed opportunities in monitoring socioeconomic status. Public Health Rep. 1997;112:492–494. [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DL, Tortu S, Thomson J. Factors associated with delays to diagnosis and treatment of breast cancer in women in a Louisiana urban safety net hospital. Women Health. 2010;50:705–718. doi: 10.1080/03630242.2010.530928. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker KL, Good A, Miles A, et al. 2011. Socioeconomic inequalities in colorectal cancer screening uptake: does time perspective play a role? Health Psychol 30:702-709.
- 19.Bao Y, Fox SA, Escarce JJ. Socioeconomic and racial/ethnic differences in the discussion of cancer screening: "between-" versus "within-" physician differences. Health Serv Res. 2007;42:950–970. doi: 10.1111/j.1475-6773.2006.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee W, Nelson R, Mailey B, et al. 2012. Socioeconomic factors impact colon cancer outcomes in diverse patient populations. J Gastrointest Surg 16:692-704.
- 21.Ziehr DR, Mahal BA, Aizer AA, et al. 2015. Income inequality and treatment of African American men with high-risk prostate cancer. Urol Oncol 33:18.e7-18.e13, 2015.
- 22.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 23.Consumer Finance Survey Results NeighborWorks America. 2015. http://www.neighborworks.org/homes-finances/financial-security/survey-results.
- 24.Bradley CJ. Financial hardship: A consequence of survivorship? J Clin Oncol. 2012;30:1579–1580. doi: 10.1200/JCO.2011.40.7247. [DOI] [PubMed] [Google Scholar]
- 25.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Cancer Society . Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 27.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Catalano PJ, Ayanian JZ, Weeks JC, et al. Cancer Care Outcomes Research Surveillance Consortium Representativeness of participants in the Cancer Care Outcomes Research and Surveillance Consortium relative to the surveillance, epidemiology, and end results program. Med Care. 2013;51:e9–e15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Association for Public Opinion Research: Standard definitions: Final dispositions of case codes and outcome rates for surveys. http://www.aapor.org/AAPOR_Main/media/MainSiteFiles/Standard_Definitions_07_08_Final.pdf.
- 30.Stewart J. Economic Status. MacArthur Research Network on Socioeconomic Status and Health; 2009. [Google Scholar]
- 31.Tabnak F, Müller HG, Wang JL, et al. Timeliness and follow-up patterns of cervical cancer detection in a cohort of medically underserved California women. Cancer Causes Control. 2010;21:411–420. doi: 10.1007/s10552-009-9473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker-Seeley RD, Abel GA, Uno H, et al. Financial hardship and the intensity of medical care received near death. Psychooncology. 2015;24:572–578. doi: 10.1002/pon.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker-Seeley RD, Li Y, Sorensen G, et al. Lifecourse socioeconomic circumstances and multimorbidity among older adults. BMC Public Health. 2011;11:313. doi: 10.1186/1471-2458-11-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 35.EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 36.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 37.Hjermstad MJ, Fossa SD, Bjordal K, et al. Test/retest study of the European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995;13:1249–1254. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 38.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 39.Deyo RACD, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 40.He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickard AS, Wilke CT, Lin HW, et al. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 42.Bryant AS, Cerfolio RJ, Minnich DJ. Survival and quality of life at least 1 year after pneumonectomy. J Thorac Cardiovasc Surg. 2012;144:1139–1143. doi: 10.1016/j.jtcvs.2012.07.083. [DOI] [PubMed] [Google Scholar]
- 43.Färkkilä N, Sintonen H, Saarto T, et al. Health-related quality of life in colorectal cancer. Colorectal Dis. 2013;15:e215–e222. doi: 10.1111/codi.12143. [DOI] [PubMed] [Google Scholar]
- 44.Bennett L, Zhao Z, Barber B, et al. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer. 2011;105:1495–1502. doi: 10.1038/bjc.2011.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grutters JP, Joore MA, Wiegman EM, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax. 2010;65:903–907. doi: 10.1136/thx.2010.136390. [DOI] [PubMed] [Google Scholar]
- 47.Møller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167–177. doi: 10.2147/PROM.S81428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: Randomised controlled trial. Br J Cancer. 2006;94:1116–1121. doi: 10.1038/sj.bjc.6603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudson DL, Puterman E, Bibbins-Domingo K, et al. Race, life course socioeconomic position, racial discrimination, depressive symptoms and self-rated health. Soc Sci Med. 2013;97:7–14. doi: 10.1016/j.socscimed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Savoy EJ, Reitzel LR, Nguyen N, et al. Financial strain and self-rated health among black adults. Am J Health Behav. 2014;38:340–350. doi: 10.5993/AJHB.38.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gage-Bouchard EA, Devine KA. Examining parents’ assessments of objective and subjective social status in families of children with cancer. PLoS One. 2014;9:e89842. doi: 10.1371/journal.pone.0089842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez KA, Snyder CF, Malin JL, et al. Patient-reported quality of care and pain severity in cancer. Palliat Support Care. 2015;13:875–884. doi: 10.1017/S1478951514000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.John DA, Kawachi I, Lathan CS, et al. Disparities in perceived unmet need for supportive services among patients with lung cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Cancer. 2014;120:3178–3191. doi: 10.1002/cncr.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]