Abstract
Purpose
To evaluate for an association between 25-hydroxyvitamin D levels (vitamin D) and outcome measures in patients with melanoma after evaluation is controlled for systemic inflammatory response (SIR) on the basis of simultaneous C-reactive protein (CRP) measurement.
Materials and Methods
Plasma samples from 1,042 prospectively observed patients with melanoma were assayed for vitamin D and CRP. The associations of demographics and CRP with vitamin D were determined, followed by a determination of the association between vitamin D and stage and outcome measures from the date of blood draw. The vitamin D level was considered sufficient if it was 30 to 100 ng/mL. Kaplan-Meier and Cox regression analyses were performed.
Results
The median vitamin D level was 25.0 ng/mL. The median follow-up time was 7.1 years. A lower vitamin D was associated with the blood draw during fall/winter months (P < .001), older age (P = .001), increased CRP (P < .001), increased tumor thickness (P < .001), ulcerated tumor (P = .0105), and advanced melanoma stage (P = .0024). On univariate analysis, lower vitamin D was associated with poorer overall (OS; P < .001), melanoma-specific survival (MSS; P = .0025), and disease-free survival (DFS; P = .0466). The effect of vitamin D on these outcome measures persisted after adjustment for CRP and other covariates. Multivariable hazards ratios per unit decrease of vitamin D were 1.02 for OS (95% CI, 1.01 to 1.04; P = .0051), 1.02 for MSS (95% CI, 1.00 to 1.04; P = .048), and 1.02 for DFS (95% CI, 1.00 to 1.04; P = .0427).
Conclusion
Lower vitamin D levels in patients with melanoma were associated with poorer outcomes. Although lower vitamin D was strongly associated with higher CRP, the associations of lower vitamin D with poorer OS, MSS, and DFS were independent of this association. Investigation of mechanisms responsible for these associations may be of value to patients with melanoma.
INTRODUCTION
Vitamin D deficiency has been associated with risks of morbidity and mortality from diabetes and cardiovascular disease, as well as with several cancers, including melanoma.1-4 Vitamin D has anti-inflammatory properties, has antiproliferative effects on melanoma cells, can inhibit tumor growth5 and tumor invasiveness,6 and promotes melanoma cell DNA repair.7 However, investigations of dietary vitamin D intake or blood levels of vitamin D with melanoma risk have yielded inconsistent results.8-11 Vitamin D deficiency has been associated with advanced melanoma stage12; conversely, elevated vitamin D has been associated with thinner tumors and longer survival.13 These findings are intriguing but require validation.
Factors that influence blood vitamin D levels have also been associated with melanoma risk or patient outcome. For example, although sun exposure promotes vitamin D synthesis14,15 it conversely increases the risk of developing melanoma.16 Markers of the systematic inflammatory response (SIR), specifically C-reactive protein (CRP), have been associated with survival in patients with melanoma12,17; our recent investigation has provided validated evidence that elevated CRP independently predicts poorer melanoma-specific survival (MSS)18; in that investigation, we did not evaluate vitamin D levels. Because CRP levels are inversely associated with vitamin D levels in blood, and because levels of vitamin D, an acute-phase reactant, decline with inflammation,19 measured vitamin D levels in patients with melanoma could reflect SIR. Coordinated investigation of vitamin D and CRP in outcomes of patients with melanoma has not been undertaken previously. We therefore conducted a hospital-based investigation in which blood samples were collected after diagnosis to examine the relationship between blood vitamin D levels and outcomes in patients with melanoma, and we accounted for important confounders, including SIR as assessed by simultaneous CRP measurement.
MATERIALS AND METHODS
Study Design
This study is part of an ongoing prospective investigation that includes patients with all stages of invasive cutaneous melanoma. Individuals gave written informed consent, and the protocol was approved by the institutional review board at The University of Texas MD Anderson Cancer Center. Peripheral blood samples were collected at study entry from 3,189 non-Hispanic white patients with melanoma and cancer-free controls recruited between August 1997 and August 2009.
Data were collected from patient records and maintained in the Melanoma Informatics, Tissue Resource, and Pathology Core Resource. Disease stage was determined according to the 2009 edition of the American Joint Committee on Cancer Cancer Staging Manual.20 Primary outcome measures were overall survival (OS; time from date of blood draw to date of death, or censored as date of last follow-up if still alive at conclusion of follow-up), MSS (time from date of blood draw to date of death as a result of melanoma, or censored as date of last follow-up/death if death not a result of melanoma), and disease-free survival (DFS; time from date of blood draw to date of relapse of melanoma, or censored as date of last follow-up if without relapse).
Experiments
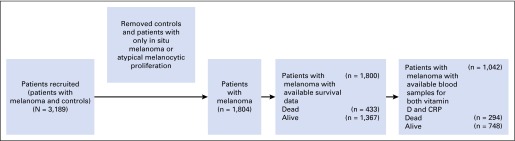
Heparinized plasma samples were stored at −80°C. A total of 1,042 patients had sufficient plasma stocks for testing (Appendix Fig A1, online only). Sequential blood draws were performed in 66 patients at follow-up. Vitamin D levels were tested as 25-hydroxyvitamin D (25-OH D, including 25-OH D2 and 25-OH D3) and were measured via enzyme immunoassay (AC-57F1; Immunodiagnostic Systems, Gaithersburg, MD). The minimum detection level was 2 ng/mL. CRP was tested via enzyme-linked immunosorbent assay (catalog no. DCRP00; R&D Systems, Minneapolis, MN). The minimum detection level was 0.010 ng/mL. All tests were batch processed in the same laboratory. Determinations of vitamin D and CRP levels were performed by personnel blinded to the clinical status of the patient. Among 1,042 patients, 44 (4.2%) had blood drawn before definitive locoregional surgery, 84 (8.1%) had blood drawn within 2 weeks after surgery, and 914 (87.7%) had blood drawn more than 2 weeks after surgery. All blood samples were drawn before initiation of systemic therapy.
Statistical Analysis
To determine whether patient demographics, including blood draw season, affected vitamin D, we performed Spearman’s ρ test and analysis of variance for correlations between continuous variables and vitamin D, and we used the Wilcoxon rank-sum test to assess associations between dichotomous categorical variables and vitamin D. We included standard clinical features associated with patient outcome. In addition, we investigated the potential influence of storage time on vitamin D. Consistent with standard clinical practice and prior reports,13,21 we used raw vitamin D as a predictor to investigate the relationship between continuous vitamin D and OS, MSS, and DFS. In contrast, because of the highly skewed nature of the distribution of CRP and to be consistent with our previous analysis,22 we used log-transformed CRP (log CRP) as a predictor in the multivariable model. We dichotomized vitamin D at 20 ng/mL (by convention, levels < 20 ng/mL were considered deficient). We also classified patients (again, by convention) into levels of less than 20 ng/mL (deficiency), 20 to 30 ng/mL (insufficiency), or at least 30 ng/mL23,24 (sufficiency) and evaluated—with or without adjustment for age at blood draw, sex, disease stage at blood draw, log CRP, and blood draw season (spring/summer [May to October], fall/winter, [November to April]) —whether these categories were associated with clinical outcomes. To determine the best threshold of vitamin D for predicting OS, we performed recursive partitioning of vitamin D and recorded the split that led to the smallest P values in the log-rank test by using the party module in R software v 2.15.0.24a Survival analysis was executed with the functions coxph and survfit from the R package survival.
All analyses (except for recursive partitioning) were performed using SAS Enterprise Guide 4.3 (SAS Institute, Cary, NC). Kaplan-Meier survival analysis was performed, Cox regression models were built to estimate hazard ratios (HRs), and adjusted survival curves were plotted.25 All P values were two sided. P values less than .05 were considered statistically significant.
RESULTS
Demographic and Clinical Data
To determine whether vitamin D levels changed with time from diagnosis, we examined for an association between vitamin D and time from diagnosis to blood draw. Our data demonstrated a small, nonsignificant decrease in vitamin D with increasing time from diagnosis (Pearson correlation test r2 = −0.01145 [P = .712]; Spearman correlation test r2 = −0.05302 [P = .087]). To evaluate whether vitamin D levels changed over time in individual patients with melanoma, 66 patients for whom serial blood samples were available underwent testing. There was no association between vitamin D levels and time between blood draws (median, 11.4 months apart; range, 0.3 to 65.8 months apart; Spearman correlation test r2 = −0.00434 [P = .9724]). The median blood draw time from diagnosis was longer in patients with stage III/IV disease (5.3 months with stage I/II v 12 months with stage III/IV; P < .001; data not shown), which reflected the referral nature of this population in particular, and median vitamin D levels were lower in patients with stage III/IV disease (25.58 ng/mL with stage I/II v 23.94 ng/mL with stage III/IV; P = .002; Appendix Table A1, online only). However, the lack of dependence of vitamin D on time from diagnosis to blood draw and the lack of association of vitamin D with interval between blood draws suggested that lower vitamin D levels in patients with stage III/IV disease were related to the disease stage itself rather than to the longer blood draw interval. Because vitamin D levels did not seem to decline significantly over time after a diagnosis of melanoma, we did not include time from diagnosis to blood draw as a covariate in our analyses. In addition, because we found no relationship between the length of time from surgery to blood draw and vitamin D level (Spearman correlation test r2 = −0.02444; P = .43), we did not include time from surgery to blood draw as a covariate in our analyses.
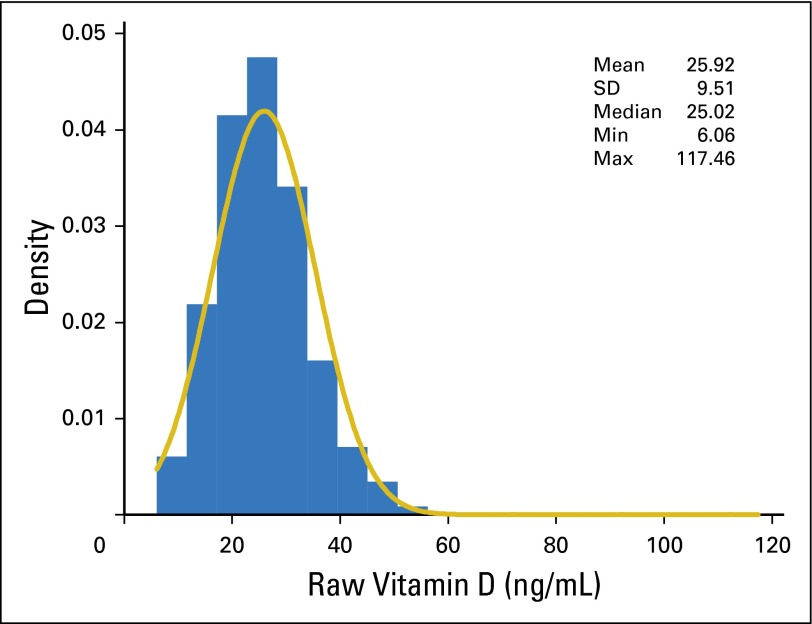
Demographic and clinical information is provided in Table 1. Blood was stored for a median of 5.1 years before testing. The distribution of raw vitamin D levels was slightly skewed (skewness = 1.80, kurtosis = 11.03; Appendix Fig A2, online only), whereas the distribution of raw CRP levels was highly skewed (skewness = 8.23, kurtosis = 101.70; data not shown). The median vitamin D level was 25.0 ng/mL, and 24.6% of patients had a vitamin D level less than 20 ng/mL. The median follow-up time from blood draw to last contact or death was 7.1 years (interquartile range, 3.8 to 9.0 years).
Table 1.
Demographic and Clinical Characteristics in 1,042 Patients With Melanoma
| Characteristic | Data Set (N = 1,042) |
|---|---|
| Age at blood draw, years | |
| Median | 54.8 |
| Interquartile range | 44.2-66.0 |
| No. (%) female | 452 (43.4) |
| Tumor thickness, mm | |
| Median | 1.2 |
| Interquartile range | 0.7-2.4 |
| No./No. evaluated (%) with ulceration | 176/840 (21.0) |
| No./No. evaluated (%) with ≥ 1 mitosis per mm2 | 455/654 (69.6) |
| No. (%) with SLN biopsy performed | 695 (66.7) |
| No./No. with biopsy performed (%) with positive SLN | 135/695 (19.4) |
| No. (%) with stage III/IV at blood draw, | 349 (33.5) |
| No. (%) by spring/summer season | 515 (49.4) |
| Time from diagnosis to blood draw, years | |
| Median | 0.6 |
| Interquartile range | 0.1-2.4 |
| Storage time from blood draw to Vitamin D testing, years | |
| Median | 5.1 |
| Interquartile range | 3.7-6.7 |
| Vitamin D level, ng/mL | |
| Median | 25.0 |
| Interquartile range | 20.1-30.6 |
| No. (%) with vitamin D < 20 ng/mL | 256 (24.6) |
| No. (%) with vitamin D ≥ 20 and < 30 ng/mL | 497 (47.7) |
| No. (%) with vitamin D ≥ 30 ng/mL | 289 (27.7) |
| CRP, mg/L | |
| Median | 1.7 |
| Interquartile range | 0.7-4.4 |
| Follow-up time from blood draw to disease relapse or censoring, years | |
| Median | 7.2 |
| Interquartile range | 4.4-9.1 |
| Follow-up time from blood draw to death or censoring, years | |
| Median | 7.1 |
| Interquartile range | 3.8-9.0 |
| No./No. evaluated (%) with recurrence among all patients | 148/881(16.8) |
| No./No. evaluated (%) with recurrence among patients with stage I/II disease | 71/692 (10.3) |
| No. (%) of deaths | 294 (28.2) |
| No. (%) of deaths as a result of melanoma | 191 (18.3) |
Abbreviations: CRP, C-reactive protein; SLN, sentinel lymph node.
Correlations Between Clinical Factors and Vitamin D Levels
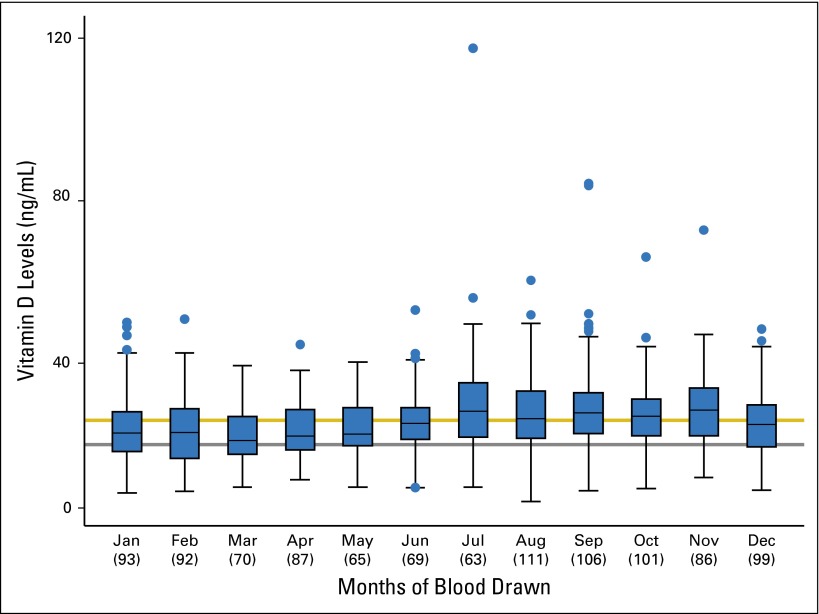
The median vitamin D level was higher in blood drawn in spring/summer than in fall/winter (26.39 ng/mL v 23.59 ng/mL; nonparametric P < .001; Appendix Table A1; Appendix Fig A3, online only). Consistent with other reports,26,27 decreased vitamin D was not significantly associated with blood storage time (nonparametric r2 = −0.06; P = .0581) but was associated with higher patient age (r2 = −0.10; P = .001), increased primary tumor thickness (r2 = −0.15; P < .001), and increased CRP (r2 = −0.19; P < .001). Patients with ulcerated primary tumors had lower vitamin D levels than those without ulcerated tumors (P = .0105), and patients with advanced-stage disease at blood draw had lower vitamin D levels than those with earlier-stage disease (P = .0024; Appendix Table A1). We included these factors in the multivariable model.
Association of Vitamin D Levels With Patient Outcome
Univariate analysis demonstrated an association between lower vitamin D and poorer OS (HR, 1.03 per unit decrease of vitamin D; 95% CI, 1.01 to 1.04; P < .001), MSS (HR, 1.03 per unit decrease of vitamin D; 95% CI, 1.01 to 1.04; P = .0025), and DFS (HR, 1.02 per unit decrease of vitamin D; 95% CI, 1.00 to 1.04; P = .0466; Table 2; Appendix Table A2, online only). Older age at blood draw, male sex, advanced stage at blood draw, and higher log-transformed CRP were also associated with poorer OS, MSS, and DFS (all P < .05; Appendix Table A2). Although blood draw season was not associated with OS (P = .7468) or MSS (P = .2957), it was associated with DFS on univariate analysis (P = .0367; Appendix Table A2) and multivariable analysis (P = .0139; Table 2). After adjustment for age, sex, disease stage, blood draw season, and log-transformed CRP, vitamin D levels remained significantly associated with outcome measures of OS (HR, 1.02 per unit decrease of vitamin D; 95% CI, 1.01 to 1.04; P = .005) MSS (HR, 1.02 per unit decrease of vitamin D; 95% CI, 1.00 to 1.04; P = .048), and DFS (HR, 1.02 per unit decrease of vitamin D; 95% CI, 1.00 to 1.04; P = .04327; Table 2). In addition to the evaluation of DFS in patients with all stages of disease (similar to the approach reported previously by Newton-Bishop et al13), we performed DFS subset analyses in patients with stage I/II disease and stage III/IV disease for completeness (Appendix Tables A3 and A4, online only). These stratified analyses show borderline effects or nonsignificant results for early- or late-stage cancers. This likely reflects the smaller number of patients and events in these subsets, so the effect estimates and significance of findings were more variable. One additional possibility considered was a nonlinear relationship of vitamin D on risk, which might confound the relationships of vitamin D with outcomes according to stage; we had assumed that vitamin D risk increases linearly with hazard for recurrence or death. To evaluate potential nonlinear effects of vitamin D on the three outcomes, we also modeled quadratic terms (data not shown). In all cases, the quadratic terms provided no significant or substantial improvement to the linear hazard models.
Table 2.
Association Between Blood Levels of Vitamin D and OS, MSS, and DFS in 1,042 Patients With Melanoma
| Variable by survival and analysis type | Analysis by Vitamin D Level (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Continuous (per-unit decrease) | < 16 v ≥ 16 | < 20 v ≥ 20 | < 20 v 20-30 v ≥ 30* | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| OS (n = 1,042) | ||||||||
| Univariate analysis | ||||||||
| Vitamin D | 1.03 (1.01 to 1.04) | < .001 | 2.00 (1.50 to 2.66) | < .001 | 1.44 (1.13 to 1.85) | .004 | 1.69 (1.23 to 2.33) | .0013 |
| 1.27 (0.95 to 1.71) | .1127 | |||||||
| Multivariate analysis | ||||||||
| Vitamin D | 1.02 (1.01 to 1.04) | .0051 | 1.85 (1.38 to 2.48) | < .001 | 1.40 (1.08 to 1.81) | < .001 | 1.46 (1.05 to 2.04) | .0247 |
| < .001 | 1.07 (0.79 to 1.44) | .6788 | ||||||
| Logarithmic CRP | 1.17 (1.07 to 1.27) | < .001 | 1.16 (1.07 to 1.27) | < .001 | 1.18 (1.08 to 1.28) | < .001 | 1.18 (1.08 to 1.28) | < .001 |
| Age in 5-year units | 1.22 (1.17 to 1.27) | < .001 | 1.22 (1.17 to 1.27) | < .001 | 1.22 (1.17 to 1.27) | < .001 | 1.22 (1.17 to 1.27) | < .001 |
| Male v female sex | 1.75 (1.36 to 2.26) | < .001 | 1.76 (1.36 to 2.27) | < .001 | 1.76 (1.37 to 2.28) | < .001 | 1.76 (1.37 to 2.28) | < .001 |
| Stage III/IV v I/II | 3.68 (2.90 to 4.67) | < .001 | 3.71 (2.92 to 4.70) | < .001 | 3.69 (2.91 to 4.68) | < .001 | 3.68 (2.90 to 4.67) | < .001 |
| Spring/summer v fall/winter | 1.10 (0.87 to 1.39) | .4048 | 1.09 (0.87 to 1.37) | .4651 | 1.10 (0.87 to 1.40) | .4104 | 1.11 (0.88 to 1.40) | .3902 |
| MSS (n = 1,042) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.03 (1.01-1.04) | .0025 | 1.76 (1.22 to 2.53) | .0026 | 1.37 (1.00 to 1.87) | .048 | 1.71 (1.14 to 2.56) | .0097 |
| 1.39 (0.96 to 2.02) | .0794 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.02 (1.00-1.04) | .048 | 1.52 (1.04 to 2.22) | .029 | 1.31 (0.94 to 1.81) | .111 | 1.40 (0.91 to 2.14) | .1265 |
| 1.10 (0.75 to 1.60) | .6284 | |||||||
| Logarithmic CRP | 1.22 (1.10 to 1.35) | < .001 | 1.22 (1.10 to 1.36) | < .001 | 1.23 (1.11 to 1.36) | < .001 | 1.23 (1.10 to 1.36) | < .001 |
| Age in 5-year units | 1.08 (1.02 to 1.34) | .0046 | 1.08 (1.02 to 1.34) | .0039 | 1.08 (1.02 to 1.34) | .005 | 1.08 (1.02 to 1.34) | .0048 |
| Male v female sex | 1.57 (1.15 to 2.13) | .0043 | 1.56 (1.15 to 2.12) | .0047 | 1.57 (1.15 to 2.15) | .004 | 1.57 (1.15 to 2.15) | .0043 |
| Stage III/IV v I/II | 8.36 (5.89 to 11.9) | < .001 | 8.41 (5.93 to 11.9) | < .001 | 8.43 (5.94 to 12.0) | < .001 | 8.39 (5.91 to 11.9) | < .001 |
| Spring/summer v fall/winter | 1.16 (0.87 to 1.55) | .3202 | 1.13 (0.85 to 1.51) | .4056 | 1.15 (0.86 to 1.54) | .356 | 1.16 (0.86 to 1.56) | .3299 |
| DFS (n = 881) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.02 (1.00 to 1.04) | .0466 | 1.62 (1.04 to 2.53) | .0335 | 1.12 (0.77 to 1.62) | .564 | 1.42 (0.89 to 2.28) | .1427 |
| 1.44 (0.96 to 2.16) | .0791 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.02 (1.00 to 1.04) | .0427 | 2.00 (1.27 to 3.16) | .003 | 1.29 (0.87 to 1.90) | .204 | 1.53 (0.94 to 2.49) | .0857 |
| 1.29 (0.86 to 1.94) | .2255 | |||||||
| Logarithmic CRP | 1.02 (0.90 to 1.16) | .711 | 1.03 (0.91 to 1.16) | .6616 | 1.04 (0.92 to 1.18) | .555 | 1.03 (0.91 to 1.17) | .6303 |
| Age in 5-year units | 1.12 (1.05 to 1.19) | < .001 | 1.12 (1.06 to 1.19) | < .001 | 1.12 (1.05 to 1.19) | < .001 | 1.12 (1.05 to 1.19) | < .001 |
| Male v female sex | 1.42 (1.00 to 2.03) | .0502 | 1.44 (1.01 to 2.05) | .0449 | 1.43 (1.00 to 2.04) | .052 | 1.42 (0.99 to 2.03) | .0553 |
| Stage III/IV v I/II | 4.77 (3.43 to 6.64) | < .001 | 4.94 (3.55 to 6.89) | < .001 | 4.79 (3.44 to 6.67) | < .001 | 4.74 (3.40 to 6.60) | < .001 |
| Spring/summer v fall/winter | 1.51 (1.09 to 2.10) | .0139 | 1.50 (1.08 to 2.08) | .0154 | 1.49 (1.07 to 2.08) | .018 | 1.51 (1.09 to 2.11) | .0144 |
Abbreviations: CRP, C-reactive protein; DFS, disease-free survival; MSS, melanoma-specific survival; OS, overall survival.
*Single Cox regression analysis using three categories of vitamin D levels, with ≥ 30 ng/dL as the reference group.
Because vitamin D levels less than 20 ng/mL are considered deficient, we evaluated this level as a clinical cutoff. On univariate analysis, patients with vitamin D levels less than 20 ng/mL had poorer OS (HR, 1.44; 95% CI, 1.13 to 1.85; P = .0036) and MSS (HR, 1.37; 95% CI, 1.00 to 1.87; P = .0475) than those with a vitamin D level of 20 ng/mL or greater, but the DFS in patients with vitamin D levels less than 20 ng/mL (HR, 1.12; 95% CI, 0.77 to 1.62; P = .5644) was similar to that in patients with a vitamin D of 20 ng/mL or greater (Table 2; Appendix Table A2). In multivariable analysis, we observed similar significance patterns (Table 2), as we did in univariate and multivariable analyses when we stratified vitamin D levels into three standard, clinically defined groups (deficiency, insufficiency, and sufficiency; Table 2).
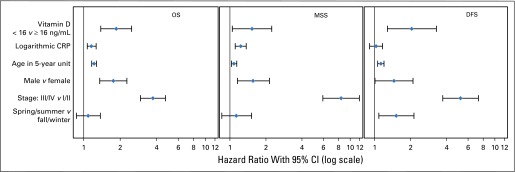
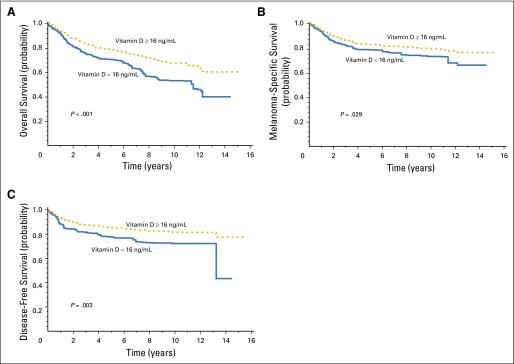
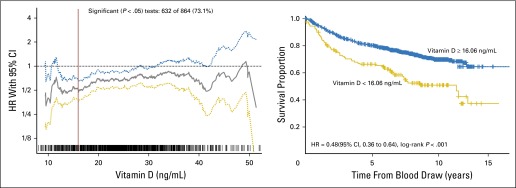
To determine the optimal cutoff value of vitamin D for predicting OS in patients with melanoma, we applied recursive partition in R using the party function and obtained the smallest P value in log-rank testing (Appendix Fig A4, online only) at an optimal cutoff value of 16 ng/mL. We used this cutoff point in the analysis of MSS and DFS. Compared with patients who had a vitamin D level of 16 ng/mL or greater, patients who had a vitamin D level less than 16 ng/mL were 2.0 times more likely to die as a result of all-cause disease (OS; 95% CI, 1.50 to 2.66; P < .001), 1.76 times more likely to die as a result of melanoma (MSS; 95% CI, 1.22 to 2.53; P = .0026), and 1.62 times more likely to have disease recurrence (DFS; 95% CI, 1.04 to 2.53; P = .0335) on univariate analysis. All three associations remained significant on multivariable analysis (Table 2; Figs 1 and 2).
Fig 1.
Multivariable-adjusted forest plots of hazard ratios of vitamin D (dichotomized at 16 ng/mL) and significant covariates for overall survival (OS), melanoma-specific survival (MSS), and disease-free survival (DFS) in 1,042 patients with melanoma. CRP, C-reactive protein.
Fig 2.
Multivariable-adjusted survival probabilities by vitamin D levels dichotomized at 16 ng/mL, with adjustment for logarithmic C-reactive protein, age, sex, stage, and blood draw season. Vitamin D effects on overall survival (A); melanoma-specific survival (B); and disease-free survival (C).
To determine if vitamin D and CRP have joint effects on melanoma outcomes, we tested for an interaction between logarithmic CRP and continuous or categorical vitamin D, with or without adjustment for other covariates, on OS, MSS, and DFS. No significant joint effects were found, with the exception of an effect on OS by categorical vitamin D at a cutoff of 16 ng/mL on univariate analysis (P = .048 for the interaction; additional data not shown).
DISCUSSION
In this study, we simultaneously examined vitamin D and CRP levels in a hospital-based cohort of patients with melanoma in relation to demographics, tumor histopathology, disease stage, and clinical outcome measures. We found that a lower level of vitamin D was associated with higher CRP, fall/winter months of blood draw, thicker and more ulcerated primary tumors, and advanced melanoma stage. We also found that a lower level of vitamin D was independently associated with poorer OS, MSS, and DFS. We confirmed in a population of patients with melanoma a finding from other populations19,28: vitamin D levels are inversely correlated with CRP levels. Therefore, we included CRP as a measure of SIR and as a potential confounder of associations between vitamin D and patient outcome measures.29 To our knowledge, this is the first evaluation to link coordinated measurements of these two important inflammatory biomarkers with long-term clinical outcomes in a large population of patients with cancer. Newton-Bishop et al13 observed a significant association between higher vitamin D level and lower Breslow primary tumor thickness as well as lower rates of relapse and death in a two-stage study; however, no evaluation of the contribution of SIR to these associations was included.13 The current study identified associations between vitamin D levels and melanoma disease severity and clinical outcomes similar to those reported by Newton-Bishop et al13; we extend their findings by reporting an independent association of low vitamin D with an increased risk of melanoma-related death (ie, MSS). Although our results demonstrated significant associations between vitamin D and OS, MSS, and DFS in the combined population of patients with all disease stages, we found borderline effects or nonsignificant associations on stratified stage-specific subgroup analyses. This likely reflects the smaller number of patients and events in these smaller subsets, so the effect estimates and significance were more variable. When we investigated alternative hypotheses, we found no evidence for a nonlinear relationship of vitamin D on risk, and we also observed no interaction between stage and vitamin D levels. These findings emphasize that additional confirmation should be sought in large, prospective series.
We found no significant association between time from diagnosis to blood draw and vitamin D levels. Furthermore, serial blood draw data showed no association between consecutive blood draw interval and vitamin D level. We concluded that there was no demonstrable direct evidence for reverse causality in this patient population (that is, that sun-avoiding behavior in patients diagnosed with melanoma led to a decline in vitamin D levels after diagnosis).
Importantly, after adjustment for CRP, vitamin D remained an independent predictor of OS, MSS, and DFS. This suggests that, although blood levels of vitamin D and CRP are highly correlated with each other, each independently predicts clinical outcome in patients with melanoma. Therefore, an investigation of the roles of each of these biomarkers is important in clinical studies of outcomes in patients with melanoma and in clinical trials of novel therapies. Furthermore, these data suggest that interventions to increase vitamin D or to reduce SIR and CRP could ultimately benefit patients with melanoma.
Our study has several limitations. First, our patients were recruited from a population referred to a large cancer hospital and might not be representative. Second, we did not collect patient-specific sun exposure data. Although blood draw season provides an indirect measure of UV radiation exposure proximate to blood draw, it does not accurately estimate long-term sun exposure. We adjusted for blood draw season in the analyses, as has been done in other similar studies.8,30 Third, we did not include measures of vitamin D intake. Few studies have assessed the correlation between vitamin D intake and melanoma risk or mortality.26,31 The potential influence of vitamin D supplementation on melanoma risk remains controversial,14 although vitamin D levels are correlated with vitamin D intake.32,33 We believe that investigations of the potential role of vitamin D supplementation in melanoma risk are reasonable to pursue but were beyond the scope of this study. Last, we did not include additional potential confounders, such as body mass index (BMI), details of surgery, or systematic treatment variables, in our analyses. We excluded BMI, because systematic review has indicated only a weak inverse link between serum 25-OH D levels and BMI in adults.34 We did not adjust for timing of surgery, because our data revealed no relationship between time from surgery to blood draw and vitamin D levels. We did not attempt to adjust for systemic therapies, because of the following: (1) patients underwent initial blood draw before administration of any systemic therapy; (2) multiple different systemic therapies were provided to patients with advanced disease, which made analysis of small subsets problematic; (3) treatments available during the study period had limited efficacy; and (4) patients in this study were treated before introduction of more effective, modern targeted therapies or immune checkpoint inhibitors. We recognize that it will be important to include systemic treatment variables, for example, in future investigations.
In summary, the current investigation is the first, to our knowledge, to report a significant, independent association between lower vitamin D levels and poorer melanoma survival after adjustment for the influence of the SIR through simultaneous measurement of CRP. A coordinated investigation of the mechanisms responsible for the independent association of these two important inflammatory biomarkers with outcomes in patients with melanoma is likely to be clinically relevant and may have implications for other cancers. Additional investigation is needed to determine whether supplementation of vitamin D and/or interventions to reduce maladaptive systemic inflammation could be beneficial to patients with melanoma.
Supplementary Material
Appendix
Fig A1.
Flow diagram of population data sets used in this study. CRP, C-reactive protein.
Fig A2.
Distribution of raw vitamin D levels in 1,042 patients with melanoma. Max, maximum; Min, minimum; SD, standard deviation.
Fig A3.
Vitamin D levels by month of blood collection in 1,042 patients with melanoma. Numbers in parentheses under the month of blood collection indicate the number of patients. The horizontal line in each month box indicates the median vitamin D value. The lower limit of the box represents the first quartile (Q1), and the upper limit of the box represents the third quartile (Q3) vitamin D value. The lower bar represents the lower limit (Q1 − 1.5 [Q3-Q1]), and the upper bar represents the upper limit (Q3 + 1.5 [Q3-Q1]). Blue dots represent outliers. The gray line signifies a vitamin D level of 20 ng/ML, and the gold line is the mean vitamin D level of 25.92 ng/mL.
Fig A4.
Recursive partitioning was performed to obtain the optimal vitamin D level cutoff to predict overall survival. Hazard ratios (HRs; solid line) and 95% CIs (dotted line) were calculated for each vitamin D level association with overall survival. The optimal cutoff at 16.06 ng/mL (rounded to 16 ng/mL in the text) was defined as the vitamin D level with the most significant split on log-rank test (left); a Kaplan curve was plotted based on this split (right).
Table A1.
Association Between Blood Level of Vitamin D and Demographic and Clinical Characteristics in 1,042 Patients With Melanoma
| Clinical characteristic | No. of Patients | Vitamin D Level (ng/mL) | P* | |
|---|---|---|---|---|
| Median | Interquartile Range | |||
| Storage time, years | 1,042 | 25.03 | 20.10-30.65 | .0581† |
| Season | < .001 | |||
| Fall/winter | 527 | 23.59 | 18.7-29.4 | |
| Spring/summer | 515 | 26.39 | 21.6-31.9 | |
| Sex | .1846 | |||
| Female | 452 | 24.94 | 18.9-30.6 | |
| Male | 590 | 25.09 | 20.8-30.8 | |
| Age at blood draw, years | 1,042 | 25.03 | 20.10-30.65 | .001† |
| Tumor thickness | 928 | 25.09 | 20.16-30.82 | < .001† |
| Tumor thickness level, mm | < .001 | |||
| ≤ 1 | 409 | 26.51 | 21.0-32.2 | |
| 1-2 | 251 | 25.20 | 20.4-29.9 | |
| 2-4 | 176 | 23.89 | 18.6-28.3 | |
| > 4 | 92 | 21.86 | 16.0-30.1 | |
| Ulceration | .0105 | |||
| Absent | 664 | 25.49 | 20.6-31.3 | |
| Present | 176 | 24.32 | 17.8-29.5 | |
| Mitotic rate | .2704 | |||
| 0 | 199 | 25.63 | 20.3-31.7 | |
| > 0 | 455 | 25.11 | 20.2-30.8 | |
| Positive SLN | .4922 | |||
| No | 560 | 25.33 | 20.3-30.9 | |
| Yes | 135 | 25.06 | 20.2-29.8 | |
| Stage at blood draw | .0024 | |||
| I, I/II, or II | 693 | 25.58 | 20.4-31.3 | |
| III or IV | 349 | 23.94 | 19.1-29.3 | |
| Logarithmic CRP | 1,042 | 25.03 | 20.10-30.65 | < .001* |
Abbreviations: CRP, C-reactive protein; SLN, sentinel lymph node.
Spearman's ρ test for continuous variables; Wilcoxon test for categorical variables.
Negative coefficients (storage time, −0.06; age, −0.10; thickness, −0.15; logarithmic CRP, −0.19).
Table A2.
Univariable Analysis of Clinical Factors Associated With OS, MSS, and DFS in 1,042 Patients With Melanoma
| Associated variable | OS | MSS | DFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Deaths | Univariate P | Hazard Ratio (95% CI) | No. of Melanoma-Specific Deaths | Univariate P | Hazard Ratio (95% CI) | No. of Patients | Recurrence | Univariate P | Hazard Ratio (95% CI) | |
| Age at blood draw | 1,042 | 294 | < .001 | 1.04 (1.03 to 1.05) | 191 | < .001 | 1.02 (1.01 to 1.03) | 881 | 148 | < .001 | 1.02 (1.01 to 1.03) |
| Tumor thickness | 1,042 | 294 | < .001 | 1.11 (1.09 to 1.13) | 191 | < .001 | 1.11 (1.09 to 1.14) | 881 | 148 | < .001 | 1.11 (1.08 to 1.14) |
| Sex | |||||||||||
| Female | 452 | 87 | — | 1.00 | 62 | — | 1.00 | 383 | 47 | — | 1.00 |
| Male | 590 | 207 | < .001 | 1.99 (1.55 to 2.55) | 129 | < .001 | 1.71 (1.27 to 2.32) | 498 | 101 | < .0017 | 1.74 (1.23 to 2.46) |
| Stage | |||||||||||
| I/II | 693 | 117 | — | 1.00 | 41 | — | 1.00 | 692 | 71 | — | 1.00 |
| III/IV | 349 | 177 | < .001 | 4.15 (3.28 to 5.25) | 150 | < .001 | 9.59 (6.79 to 13.60) | 189 | 77 | < .001 | 4.88 (3.53 to 6.74) |
| Mitotic rate | |||||||||||
| 0 | 199 | 25 | — | 1.00 | 11 | — | 1.00 | 196 | 13 | — | 1.00 |
| ≥ 1 | 455 | 144 | < .001 | 2.78 (1.82 to 4.25) | 100 | < .001 | 4.33 (2.32 to 8.08) | 394 | 86 | < .001 | 3.54 (1.97 to 6.34) |
| Ulceration | |||||||||||
| Absent | 664 | 125 | — | 1.00 | 61 | — | 1.00 | 615 | 64 | — | 1.00 |
| Present | 176 | 91 | < .001 | 3.82 (2.92 to 5.01) | 71 | < .001 | 5.78 (4.10 to 8.14) | 143 | 57 | < .001 | 4.94 (3.46 to 7.07) |
| Season | |||||||||||
| Fall/winter | 527 | 148 | — | 1.00 | 90 | — | 1.00 | 452 | 65 | — | 1.00 |
| Spring/summer | 515 | 146 | .7468 | 1.04 (0.83 to 1.31) | 101 | .2927 | 1.16 (0.88 to 1.55) | 429 | 83 | .0367 | 1.41 (1.02 to 1.96) |
| Continuous vitamin D | 1,042 | 294 | < .001 | 1.03 (1.01 to 1.04) | 191 | .0025 | 1.03 (1.01 to 1.04) | 881 | 148 | .0466 | 1.02 (1.00 to 1.04) |
| Logarithmic CRP | 1,042 | 294 | < .001 | 1.31 (1.20 to 1.43) | 191 | < .001 | 1.42 (1.27 to 1.58) | 881 | 148 | .0385 | 1.14 (1.01 to 1.30) |
| Two categories of vitamin D, ng/mL | |||||||||||
| ≥ 20 | 786 | 202 | — | 1.00 | 134 | — | 1.00 | 679 | 111 | — | 1.00 |
| < 20 | 256 | 92 | .0036 | 1.44 (1.13 to 1.85) | 57 | .0475 | 1.37 (1.00 to 1.85) | 202 | 37 | .5644 | 1.12 (0.77 to 1.62) |
| Three categories of vitamin D, ng/mL | |||||||||||
| ≥ 30 | 289 | 64 | — | 1.00 | 40 | — | 1.00 | 257 | 33 | — | 1.00 |
| 20-30 | 497 | 138 | .1127 | 1.27 (0.95 to 1.71) | 94 | .0794 | 1.39 (0.96 to 2.02) | 422 | 78 | .0791 | 1.44 (0.96 to 2.16) |
| < 20 | 256 | 92 | .0013 | 1.69 (1.23 to 2.33) | 57 | .00970 | 1.71 (1.14 to 2.56) | 202 | 37 | .1427 | 1.42 (0.89 to 2.28) |
Abbreviations: CRP, C-reactive protein
Table A3.
Association Between Blood Levels of Vitamin D and OS, MSS, and DFS in Patients With Stage I/II Disease
| Variable by survival and analysis type | Analysis by Vitamin D Level (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Continuous (per-unit decrease) | < 16 v ≥ 16 | < 20 v ≥ 20 | < 20 v 20-30 v ≥ 30* | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| OS (n = 693) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.03 (1.01 to 1.05) | .0041 | 2.50 (1.61 to 3.86) | < .001 | 1.66 (1.13 to 2.44) | .010 | 1.25 (0.78 to 2.01) | .3489 |
| 1.92 (1.17 to 3.15) | .01 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.02 (0.99 to 1.04) | .1393 | 1.89 (1.19 to 3.03) | .0074 | 1.32 (0.88 to 1.98) | .174 | 1.20 (0.75 to 1.93) | .4462 |
| 1.49 (0.89 to 2.50) | .1272 | |||||||
| Logarithmic CRP | 1.11 (0.95 to 1.30) | .1846 | 1.09 (0.93 to 1.27) | .3011 | 1.12 (0.96 to 1.31) | .164 | 1.11 (0.95 to 1.30) | .175 |
| Age in 5-year units | 1.38 (1.28 to 1.48) | < .001 | 1.38 (1.28 to 1.48) | < .001 | 1.38 (1.28 to 1.48) | < .001 | 1.38 (1.28 to 1.48) | < .001 |
| Male v female sex | 2.17 (1.41 to 3.33) | < .001 | 2.19 (1.43 to 3.37) | < .001 | 2.20 (1.43 to 3.38) | < .001 | 2.20 (1.43 to 3.38) | < .001 |
| Stage I/II v I | 2.13 (1.10 to 4.13) | .0246 | 2.20 (1.14 to 4.23) | .0189 | 2.22 (1.15 to 4.28) | .018 | 2.19 (1.13 to 4.23) | .0201 |
| Stage II v I | < .001 | 2.13 (1.43 to 3.15) | < .001 | 2.21 (1.49 to 3.27) | < .001 | 2.19 (1.48 to 3.25) | < .001 | |
| Spring/summer v fall/winter | 2.22 (1.50 to 3.28) | .7319 | 0.97 (0.67 to 1.42) | .8865 | 0.94 (0.64 to 1.37) | .739 | 0.94 (0.65 to 1.38) | .7662 |
| MSS (n = 693) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.01 (0.98 to 1.05) | .5128 | 0.95 (0.34 to 2.67) | .9223 | 0.93 (0.44 to 1.95) | .843 | 1.66 (0.77 to 3.58) | .1984 |
| 1.30 (0.51 to 3.28) | .5808 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.00 (0.97 to 1.04) | .9423 | 0.68 (0.23 to 1.97) | .4734 | 0.70 (0.32 to 1.53) | .375 | 1.51 (0.69 to 3.29) | .3016 |
| 0.94 (0.36 to 2.49) | .901 | |||||||
| Logarithmic CRP | 0.99 (0.76 to 1.30) | .9652 | 1.02 (0.77 to 1.34) | .8983 | 1.02 (0.78 to 1.35) | .872 | 1.01 (0.76 to 1.33) | .9519 |
| Age in 5-year units | 1.11 (0.99 to 1.24) | .0758 | 1.11 (0.99 to 1.24) | .072 | 1.11 (0.99 to 1.24) | .070 | 1.11 (0.99 to 1.24) | .0687 |
| Male v female sex | 1.78 (0.87 to 3.62) | .1141 | 1.77 (0.87 to 3.60) | .1165 | 1.73 (0.85 to 3.54) | .131 | 1.70 (0.83 to 3.48) | .1458 |
| Stage I/II v I | 2.85 (0.80 to 10.2) | .1069 | 2.86 (0.80 to 10.2) | .1066 | 2.82 (0.79 to 10.1) | .111 | 2.78 (0.78 to 9.94) | .1166 |
| Stage II v I | 6.43 (3.18 to 13.0) | < .001 | 6.60 (3.27 to 13.3) | < .001 | 6.70 (3.30 to 13.6) | < .001 | 6.58 (3.25 to 13.4) | < .001 |
| Spring/summer v fall/winter | 1.20 (0.64 to 2.23) | .569 | 1.18 (0.64 to 2.19) | .595 | 1.17 (0.63 to 2.17) | .628 | 1.21 (0.65 to 2.25) | .5546 |
| DFS (n = 692) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.01 (0.98 to 1.03) | .4776 | 1.58 (0.83 to 3.00) | .165 | 1.11 (0.64 to 1.90) | .716 | 1.25 (0.71 to 2.21) | .436 |
| 1.28 (0.66 to 2.47) | .4674 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.00 (0.97 to 1.02) | .8973 | 1.27 (0.65 to 2.51) | .4868 | 0.92 (0.52 to 1.64) | .787 | 1.22 (0.69 to 2.17) | .4994 |
| 1.06 (0.52 to 2.13) | .878 | |||||||
| Logarithmic CRP | 1.04 (0.85 to 1.27) | .7307 | 1.02 (0.83 to 1.25) | .8393 | 1.05 (0.85 to 1.28) | .665 | 1.04 (0.85 to 1.27) | .7207 |
| Age in 5-year units | 1.05 (0.97 to 1.14) | .2395 | 1.05 (0.97 to 1.14) | .2448 | 1.05 (0.97 to 1.14) | .228 | 1.05 (0.97 to 1.14) | .2274 |
| Male v female sex | 1.87 (1.10 to 3.19) | .0216 | 1.89 (1.10 to 3.22) | .0202 | 1.86 (1.09 to 3.18) | .024 | 1.84 (1.08 to 3.15) | .026 |
| Stage I/II v I | 2.60 (1.05 to 6.40) | .0382 | 2.58 (1.05 to 6.34) | .0391 | 2.61 (1.06 to 6.41) | .037 | 2.58 (1.05 to 6.35) | .0392 |
| Stage II v I | 4.72 (2.83 to 7.88) | < .001 | 4.62 (2.76 to 7.74) | <.001 | 4.79 (2.86 to 8.02) | < .001 | 4.77 (2.84 to 7.99) | < .001 |
| Spring/summer v fall/winter | 1.76 (1.08 to 2.85) | .0225 | 1.77 (1.09 to 2.86) | .02 | 1.74 (1.07 to 2.81) | .025 | 1.77 (1.09 to 2.87) | .0209 |
Abbreviations: CRP, C-reactive protein; HR, hazard ratio.
*Single Cox regression analysis using three categories of vitamin D levels, with ≥ 30 ng/dL as the reference group.
Table A4.
Association Between Blood Levels of Vitamin D and OS, MSS, and DFS in Patients With Stage III/IV Disease
| Variable by survival and analysis type | Analysis by Vitamin D Level (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Continuous (per-unit decrease) | < 16 v ≥ 16 | < 20 v ≥ 20 | < 20 v 20-30 v ≥ 30* | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| OS (n = 349) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.01 (1.00 to 1.03) | .1582 | 1.43 (0.98 to 2.09) | .0647 | 1.17 (0.85 to 1.62) | .338 | 1.03 (0.70 to 1.51) | .8862 |
| 1.19 (0.79 to 1.81) | .406 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.01 (0.99 to 1.03) | .1841 | 1.30 (0.87 to 1.92) | .2006 | 1.20 (0.85 to 1.70) | .295 | 0.99 (0.67 to 1.46) | .9511 |
| 1.19 (0.76 to 1.87) | .4459 | |||||||
| Logarithmic CRP | 1.12 (1.01 to 1.25) | .0339 | 1.13 (1.01 to 1.26) | .0265 | 1.13 (1.02 to 1.26) | .024 | 1.13 (1.02 to 1.26) | .0241 |
| Age in 5-year units | 1.11 (1.05 to 1.17) | < .001 | 1.11 (1.05 to 1.17) | < .001 | 1.11 (1.05 to 1.17) | < .001 | 1.11 (1.05 to 1.17) | < .001 |
| Male v female sex | 1.42 (1.03 to 1.96) | .0347 | 1.40 (1.01 to 1.93) | .0407 | 1.41 (1.02 to 1.96) | .039 | 1.41 (1.02 to 1.96) | .0393 |
| Stage IV v III | 2.60 (1.85 to 3.67) | < .001 | 2.54 (1.79 to 3.59) | < .001 | 2.60 (1.84 to 3.66) | < .001 | 2.60 (1.84 to 3.66) | < .001 |
| Spring/summer v fall/winter | 1.13 (0.83 to 1.53) | .4431 | 1.10 (0.82 to 1.49) | .5218 | 1.12 (0.82 to 1.52) | .480 | 1.12 (0.82 to 1.52) | .4914 |
| MSS (n = 349) | ||||||||
| Univariate analysis | ||||||||
| Vitamin D | 1.02 (1.00 to 1.04) | .0494 | 1.62 (1.09 to 2.40) | .0174 | 1.33 (0.94 to 1.87) | .105 | 1.03 (0.68 to 1.57) | .8874 |
| 1.36 (0.86 to 2.13) | .1863 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.02 (1.00 to 1.04) | .1026 | 1.35 (0.89 to 2.06) | .1604 | 1.3 (0.90 to 1.89) | .162 | 1.00 (0.65 to 1.54) | .987 |
| 1.31 (0.80 to 2.14) | .2842 | |||||||
| Logarithmic CRP | 1.16 (1.03 to 1.30) | .0151 | 1.16 (1.03 to 1.31) | .0114 | 1.16 (1.04 to 1.31) | .010 | 1.16 (1.04 to 1.31) | .0105 |
| Age in 5-year units | 1.06 (1.00 to 1.12) | .0631 | 1.0 (1.00 to 1.12) | .061 | 1.06 (1.00 to 1.12) | .066 | 1.06 (1.00 to 1.12) | .066 |
| Male v female sex | 1.41 (1.00 to 2.01) | .0517 | 1.39 (0.98 to 1.96) | .0657 | 1.41 (0.99 to 2.01) | .054 | 1.42 (0.99 to 2.02) | .0542 |
| Stage IV v III | 2.91 (2.02 to 4.19) | < .001 | 2.83 (1.95 to 4.10) | < .001 | 2.90 (2.01 to 4.17) | < .001 | 2.90 (2.01 to 4.17) | < .001 |
| Spring/summer v fall/winter | 1.11 (0.79 to 1.55) | .5472 | 1.07 (0.77 to 1.49) | .6761 | 1.10 (0.79 to 1.54) | .578 | 1.10 (0.78 to 1.55) | .5813 |
| DFS (n = 189) | ||||||||
| Univariable analysis | ||||||||
| Vitamin D | 1.03 (1.00 to 1.06) | .0931 | 1.88 (1.01 to 3.48) | .0448 | 1.12 (0.67 to 1.88) | .665 | 1.29 (0.72 to 2.32) | .3968 |
| 1.35 (0.68 to 2.65) | .3909 | |||||||
| Multivariable analysis | ||||||||
| Vitamin D | 1.03 (1.00 to 1.08) | .0408 | 2.46 (1.28 to 4.72) | .0068 | 1.23 (0.70 to 2.16) | .481 | 1.33 (0.74 to 2.41) | .3409 |
| 1.51 (0.73 to 3.11) | .2622 | |||||||
| Logarithmic CRP | 1.03 (0.87 to 1.23) | .7106 | 1.06 (0.89 to 1.26) | .4941 | 1.07 (0.90 to 1.27) | .442 | 1.06 (0.89 to 1.26) | .4941 |
| Age in 5-year units | 1.15 (1.05 to 1.27) | .0025 | 1.15 (1.05 to 1.27) | .0015 | 1.15 (1.05 to 1.27) | .005 | 1.15 (1.05 to 1.27) | .0044 |
| Male v female sex | 1.04 (0.64 to 1.68) | .8777 | 1.01 (0.63 to 1.64) | .9521 | 0.98 (0.60 to 1.61) | .936 | 0.97 (0.59 to 1.60) | .9158 |
| Stage IV v III | 0.66 (0.15 to 2.84) | .5804 | 0.64 (0.15 to 2.73) | .5448 | < .001 | .560 | 0.63 (0.15 to 2.70) | .5294 |
| Spring/summer v fall/winter | 1.26 (0.80 to 1.98) | .3251 | 1.19 (0.76 to 1.88) | .4418 | 1.22 (0.77 to 1.93) | .406 | 1.23 (0.77 to 1.95) | .3857 |
Abbreviations: CRP, C-reactive protein; DFS, disease-free survival; HR, hazard ratio; MSS, melanoma-specific survival; OS, overall survival.
*Single Cox regression analysis using three categories of vitamin D levels, with ≥ 30 ng/dL as the reference group.
Footnotes
Supported by the National Cancer Institute Specialized Programs of Research Excellence (SPORE) Grant No. P50 CA093459, philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program, the Miriam and Jim Mulva Research Fund, the McCarthy Skin Cancer Research Fund, and the Marit Peterson Fund for Melanoma Research.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 1713
AUTHOR CONTRIBUTIONS
Conception and design: John D. Reveille, Christopher I. Amos, Jeffrey E. Lee
Financial support: Jeffrey E. Lee
Administrative support: Jeffrey E. Lee
Provision of study materials or patients: Merrick I. Ross, Jeffrey E. Gershenwald, Qingyi Wei, Jeffrey E. Lee
Collection and assembly of data: Yuling Wang, Huey Liu, Merrick I. Ross, Jeffrey E. Gershenwald, Janice N. Cormier, Richard E. Royal, Anthony Lucci, Jennifer Wargo, Qingyi Wei, Christopher I. Amos, Jeffrey E. Lee
Data analysis and interpretation: Shenying Fang, Dawen Sui, Yuling Wang, Yi-Ju Chiang, Janice N. Cormier, Mimi I. Hu, Julie M. Gardner, Roland L. Bassett, Christopher I. Amos, Jeffrey E. Lee
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association of Vitamin D Levels With Outcome in Patients With Melanoma After Adjustment For C-Reactive Protein
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Shenying Fang
No relationship to disclose
Dawen Sui
No relationship to disclose
Yuling Wang
No relationship to disclose
Huey Liu
No relationship to disclose
Yi-Ju Chiang
No relationship to disclose
Merrick I. Ross
Honoraria: Merck, Amgen, Caladrius, GlaxoSmithKline
Consulting or Advisory Role: Merck, Amgen, GlaxoSmithKline
Speakers' Bureau: Merck
Research Funding: Amgen, GlaxoSmithKline
Travel, Accommodations, Expenses: Merck, Amgen, Provectus
Jeffrey E. Gershenwald
Consulting or Advisory Role: Merck
Patents, Royalties, Other Intellectual Property: Mercator Therapeutics
Janice N. Cormier
No relationship to disclose
Richard E. Royal
Consulting or Advisory Role: Delcath Systems
Anthony Lucci
Speakers' Bureau: Genomic Health
Jennifer Wargo
Honoraria: Dava Oncology, H. Lee Moffitt Cancer Center and Research Institute
Consulting or Advisory Role: Genentech, GlaxoSmithKline, Novartis
Speakers' Bureau: Illumina, Bristol-Myers Squibb, Dava Oncology
Research Funding: Genentech, GlaxoSmithKline, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb, JP Morgan
Mimi I. Hu
No relationship to disclose
Julie M. Gardner
No relationship to disclose
John D. Reveille
No relationship to disclose
Roland L. Bassett
No relationship to disclose
Qingyi Wei
No relationship to disclose
Christopher I. Amos
No relationship to disclose
Jeffrey E. Lee
No relationship to disclose
REFERENCES
- 1.Al Mheid I, Patel RS, Tangpricha V, et al. Vitamin D and cardiovascular disease: Is the evidence solid? Eur Heart J. 2013;34:3691–3698. doi: 10.1093/eurheartj/eht166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kienreich K, Tomaschitz A, Verheyen N, et al. Vitamin D and cardiovascular disease. Nutrients. 2013;5:3005–3021. doi: 10.3390/nu5083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality: A review of recent evidence. Autoimmun Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Vuolo L, Di Somma C, Faggiano A, et al. Vitamin D and cancer. Front Endocrinol (Lausanne) 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichrath J, Rech M, Moeini M, et al. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther. 2007;6:48–55. doi: 10.4161/cbt.6.1.3493. [DOI] [PubMed] [Google Scholar]
- 6.Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res. 1987;47:21–25. [PubMed] [Google Scholar]
- 7.Reichrath J, Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: An update. Adv Exp Med Biol. 2014;810:208–233. doi: 10.1007/978-1-4939-0437-2_12. [DOI] [PubMed] [Google Scholar]
- 8.Major JM, Kiruthu C, Weinstein SJ, et al. Pre-diagnostic circulating vitamin D and risk of melanoma in men. PLoS One. 2012;7:e35112. doi: 10.1371/journal.pone.0035112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyskens FL, Jr, Farmer PJ, Anton-Culver H. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:293. [PubMed] [Google Scholar]
- 10.Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–1051. [PubMed] [Google Scholar]
- 11.Veierød MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: A prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71:600–604. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Nürnberg B, Gräber S, Gärtner B, et al. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res. 2009;29:3669–3674. [PubMed] [Google Scholar]
- 13.Newton-Bishop JA, Beswick S, Randerson-Moor J, et al. Serum 25-hydroxyvitamin D3 levels are associated with Breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berwick M, Erdei EO. Vitamin D and melanoma incidence and mortality. Pigment Cell Melanoma Res. 2013;26:9–15. doi: 10.1111/pcmr.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field S, Davies J, Bishop DT, et al. Vitamin D and melanoma. Dermatoendocrinol. 2013;5:121–129. doi: 10.4161/derm.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YM, Barrett JH, Bishop DT, et al. Sun exposure and melanoma risk at different latitudes: A pooled analysis of 5,700 cases and 7,216 controls. Int J Epidemiol. 2009;38:814–830. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findeisen P, Zapatka M, Peccerella T, et al. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol. 2009;27:2199–2208. doi: 10.1200/JCO.2008.18.0554. [DOI] [PubMed] [Google Scholar]
- 18.Fang S, Wang Y, Sui D, et al. C-reactive protein as a marker of melanoma progression. J Clin Oncol. 2015;33:1389–1396. doi: 10.1200/JCO.2014.58.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghashut RA, Talwar D, Kinsella J, et al. The effect of the systemic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumin. PLoS One. 2014;9:e92614. doi: 10.1371/journal.pone.0092614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly JL, Salles G, Goldman B, et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: A prospective evaluation in SWOG and LYSA studies. J Clin Oncol. 2015;33:1482–1490. doi: 10.1200/JCO.2014.57.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsilidis KK, Branchini C, Guallar E, et al. C-reactive protein and colorectal cancer risk: A systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 24a. The R Project for Statistical Computing. https://www.r-project.org/
- 25.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Asgari MM, Maruti SS, Kushi LH, et al. A cohort study of vitamin D intake and melanoma risk. J Invest Dermatol. 2009;129:1675–1680. doi: 10.1038/jid.2008.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: Challenges and needs. Am J Clin Nutr. 2008;88:507S–510S. doi: 10.1093/ajcn/88.2.507S. [DOI] [PubMed] [Google Scholar]
- 28.Duncan A, Talwar D, McMillan DC, et al. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95:64–71. doi: 10.3945/ajcn.111.023812. [DOI] [PubMed] [Google Scholar]
- 29.Conway FJ, McMillan DC. Plasma vitamin D concentration and survival in colorectal cancer: Potential confounding by the systemic inflammatory response. J Clin Oncol. 2015;33:224. doi: 10.1200/JCO.2014.59.2386. [DOI] [PubMed] [Google Scholar]
- 30.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32:2430–2439. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- 31.Tang JY, Fu T, Leblanc E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: Post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29:3078–3084. doi: 10.1200/JCO.2011.34.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland CF, French CB, Baggerly LL, et al. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011;31:607–611. [PubMed] [Google Scholar]
- 33.Sullivan SS, Rosen CJ, Halteman WA, et al. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105:971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes Rev. 2013;14:393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.