Abstract
Purpose
The inclusion of metformin in the treatment arms of cancer clinical trials is based on improved survival that has been demonstrated in retrospective epidemiologic studies; however, unintended biases may exist when analysis is performed by using a conventional Cox proportional hazards regression model with dichotomous ever/never categorization. We examined the impact of metformin exposure definitions, analytical methods, and patient selection on the estimated effect size of metformin exposure on survival in a large cohort of patients with pancreatic ductal adenocarcinoma (PDAC).
Patients and Methods
Of newly diagnosed patients with PDAC with diabetes, 980 were retrospectively included, and exposure to metformin documented. Median survival was assessed by using Kaplan-Meier and log-rank methods. Hazard ratios (HR) and 95% CIs were computed to compare time-varying covariate analysis with conventional Cox proportional hazards regression analysis.
Results
Median survival of metformin users versus nonusers was 9.9 versus 8.9 months, respectively. By the time-varying covariate analysis, metformin use was not statistically significantly associated with improved survival (HR, 0.93; 95% CI, 0.81 to1.07; P = .28). There was no evidence of benefit in the subset of patients who were naïve to metformin at the time of PDAC diagnosis (most representative of patients enrolled in clinical trials; HR, 1.01; 95% CI, 0.80 to 1.30; P = .89); however, when the analysis was performed by using the conventional Cox model, an artificial survival benefit of metformin was detected (HR, 0.88; 95% CI, 0.77 to 1.01; P = .08), which suggested biased results from the conventional Cox analysis.
Conclusion
Our findings did not suggest the benefit of metformin use after patients are diagnosed with PDAC. We highlight the importance of patient selection and appropriate statistical analytical methods when studying medication exposure and cancer survival.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the third most common cause of cancer death in the United States,1 and the incidence is projected to increase by 55% from 2010 to 2030.1,2 Despite advances in surgical techniques and the availability of new chemotherapeutic agents, outcomes for patients with PDAC remain poor. The median survival is approximately 5 to 8 months, and the 5-year survival rate is only 8% for all stages combined.1 More effective treatments are critically needed.
Metformin is among the most commonly prescribed oral antidiabetic agents because it is relatively inexpensive, safe, and well tolerated. Epidemiologic studies have shown that metformin use was associated with an approximate 34% to 62% reduction in cancer-related mortality in various recalcitrant (5-year survival rate < 50%) malignancies, including lung, ovarian, and pancreas cancer.3,4 The potential benefit of metformin as a treatment of PDAC was suggested in a retrospective cohort of 302 patients with PDAC with diabetes.5 In the study, 117 patients who had ever used metformin had a significantly better survival than 185 patients who had never used metformin, with median survivals of 15.2 verus 11.1 months, respectively (P = .004). Metformin use was associated with a 32% reduction in mortality, although the benefit was observed only in patients with nonmetastatic disease. On the basis of the growing evidence of survival benefit from metformin use in retrospective cohort studies, along with preclinical data on biologically plausible mechanisms of metformin in the inhibition of cancer growth, metformin has been considered a useful adjunctive therapy.7 Currently, metformin is included in a treatment arm of at least 20 open clinical trials of recalcitrant cancers (Appendix Table A1, online only).8
As a result of the complexities of diabetes management, however, as well as the nature of retrospective studies, which are prone to incomplete information on medication use, including dosage, duration, and timing of medication initiation, metformin exposure is commonly categorized as ever/never use in epidemiologic retrospective cohort studies.3,9 This disregard of the time of initiation may introduce unintended biases, in particular, with regard to survival, which leads to the overestimation of drug effect.3,9-12
We examined the impact of metformin use classification, patient selection, and statistical analysis method on survival in a large retrospective cohort of patients with PDAC. Variation of estimated effects of metformin in our analyses and patient subsets highlight unintended biases in retrospective cohort studies of medication exposure on cancer survival.
PATIENTS AND METHODS
Study Population
This study was approved by the Mayo Clinic Institutional Review Board. A potential cohort of patients with diagnoses of both PDAC and diabetes was identified in three steps. First, we identified patients age > 18 who were diagnosed with PDAC between October 2000 and October 2011 through an ongoing prospective patient registry supported by the Mayo Clinic Specialized Program of Research Excellence in Pancreatic Cancer (N = 2,964).13 A detailed description of enrollment is given in the Appendix (online only).
Data Collection
Other variables included date of birth, sex, date of PDAC diagnosis, clinical cancer stage at diagnosis, that is, resectable, locally advanced, or metastatic disease, recurrence status, date of last visit, and vital status.
Detailed information on metformin exposure was abstracted from the medical record (MR). This process was performed in two steps. First, the medication data were electronically pulled from clinical notes, prescription records, and questionnaires by using key words (metformin, glucophage, glucophage XR, glumetza, riomet, fortamet, janumet, actoplus met, avandamet, avandaryl, prandiMet, metaglip, and glucovance). The three abstractors who verified the diagnosis of diabetes also performed a manual MR review to identify exposure to metformin in patients with diabetes. Second, after a list of patients with PDAC with diabetes who were potentially exposed to metformin was generated, metformin initiation date was manually abstracted. We were able to obtain the actual date of metformin initiation in 126 patients (23.9%). When the date of metformin initiation was ambiguous (n = 401), the date that metformin first appeared in the MR was used.
Statistical Analysis
All analyses were performed by using SAS (SAS/STAT User’s Guide, Version 9.3; SAS Institute, Cary, NC). Baseline characteristics were compared by using Student’s t test for continuous variables and the χ2 test for categorical variables. The primary end point was overall survival, which was calculated from the date of PDAC diagnosis until the earliest of the following: death date, last known alive date, or study end date (November 1, 2012). Survival was estimated by using the Kaplan-Meier method and compared by using the log-rank test. Median follow-up time of the entire cohort was 9.26 months.
Because metformin initiation date varied among patients, hazard ratios (HRs) and 95% CIs were computed by using a time-varying covariate Cox proportional hazards regression model, adjusting for age, sex, usual body mass index (BMI), PDAC diagnosis year group (≤ 2004, 2005 to 2008, and ≥ 2009), and stage of disease (resectable, locally advanced, or metastatic). In the time-varying covariate Cox model, metformin exposure was treated as a time-dependent variable.15 In brief, among metformin users, a patient was coded as nonuser before metformin initiation, then recoded to user on the date when metformin was initiated. Among patients who never used metformin, coding as nonuser was applied throughout. This analysis method is more accurate for defining metformin exposure status than the conventional Cox proportional hazards analysis because the analysis takes into account the variation in timing of metformin initiation among patients and considers the period of nonexposure to metformin (ie, coding as nonuser). As a result, the effect of metformin is less prone to overestimation.10
Next, the ideal design of a clinical trial to determine impact of medication exposure on cancer survival enrolls only patients who are naïve to medication exposure before cancer diagnosis. Thus, we analyzed this subgroup.
In addition, we performed three conventional Cox proportional hazards regressions models. In the first model, patients were initially categorized in two groups by status of metformin exposure as ever or never use, regardless of the timing of metformin initiation. Furthermore, patients were categorized in three and five groups to determine the impact of timing of metformin initiation on outcome. The three groups included never used metformin, metformin started before PDAC diagnosis, and metformin started post-PDAC diagnosis; and the five groups included never used metformin, metformin started > 1 year before PDAC diagnosis, metformin started within 1 year before PDAC diagnosis, metformin started ≤ 30 days post-PDAC diagnosis, and metformin started > 30 days post-PDAC diagnosis.
In the second model, patients for whom metformin was started after PDAC diagnosis were coded as never having used metformin, regardless of whether metformin was started immediately after PDAC diagnosis. In the third model, patients who started metformin after PDAC diagnosis were excluded.
RESULTS
Baseline Characteristics of the Study Cohort
Of 980 patients, 366 (37.3%) had a history of metformin use and 614 (62.7%) never used metformin. Table 1 displays baseline patient characteristics. A higher proportion of males (62.0% v 56.5%; P = .09) were metformin users; users had higher BMI than nonusers (mean ± standard deviation: 31.4 ± 6.2 v 30.3 ± 6.2; P = .007). Not surprisingly, a higher frequency of patients with long-standing diabetes, defined as having diabetes > 2 years before PDAC diagnosis, used metformin (55.5% v 32.1%; P < .001). There were 284, 354, and 341 patients with PDAC with resectable, locally advanced, and metastatic disease, respectively, with comparable tumor stages between both groups.
Table 1.
Baseline Characteristics of Patients With Pancreatic Adenocarcinoma With Diabetes
| Characteristic | Metformin Use | Total (N = 980) | P | |
|---|---|---|---|---|
| Ever (n = 366) | Never (n = 614) | |||
| Age, years | .18 | |||
| Mean (SD) | 66.8 (10.2) | 67.7 (10.6) | 67.4 (10.4) | |
| Median | 68.0 | 68.0 | 68.0 | |
| Q1, Q3 | 60.0, 74.0 | 60.0, 76.0 | 60.0, 75.0 | |
| Range | 37.0-92.0 | 29.0-93.0 | 29.0-93.0 | |
| Gender, No. (%) | .09 | |||
| Male | 227 (62.0) | 347 (56.5) | 574 (58.6) | |
| Female | 139 (38.0) | 267 (43.5) | 406 (41.4) | |
| Race, No. (%) | .10 | |||
| White | 338 (94.4) | 569 (96.6) | 907 (95.8) | |
| Non-White | 20 (5.6) | 20 (3.4) | 40 (4.2) | |
| Missing | 8 | 25 | 33 | |
| Usual BMI | .007 | |||
| No. | 325 | 539 | 864 | |
| Mean (SD) | 31.4 (6.2) | 30.3 (6.2) | 30.7 (6.2) | |
| Median | 30.4 | 29.3 | 29.7 | |
| Q1, Q3 | 27.0, 34.7 | 26.2, 33.5 | 26.5, 34.2 | |
| Range | 19.7-59.0 | 16.9-62.2 | 16.9-62.2 | |
| Duration of diabetes, years | < .001 | |||
| > 2 (long-standing DM) | 203 (55.5%) | 197 (32.1%) | 400 (40.8%) | |
| 0-2 (new-onset DM) | 163 (44.5%) | 417 (67.9%) | 580 (59.2%) | |
| Maximum tumor dimension, cm* | .90 | |||
| No. | 93 | 174 | 267 | |
| Mean (SD) | 3.8 (1.4) | 3.8 (1.5) | 3.8 (1.5) | |
| Median | 3.5 | 3.7 | 3.7 | |
| Q1, Q3 | 3.0, 4.5 | 2.8, 4.5 | 2.8, 4.5 | |
| Range | 0.0-8.4 | 0.3-9.0 | 0.0-9.0 | |
| Stage of disease, No. (%) | .30 | |||
| Resected | 97 (26.5) | 187 (30.5) | 284 (29.0) | |
| Locally Advanced | 132 (36.1) | 222 (36.2) | 354 (36.2) | |
| Metastatic | 137 (37.4) | 204 (33.3) | 341 (34.8) | |
| Missing | 0 | 1 | 1 | |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; SD, standard deviation, Q1, 25th percentile; Q3, 75th percentile.
Maximum tumor dimension measure is primarily available in patients with resected stage.
Metformin Use Is Associated With a Slight but Not Statistically Significant Prolongation of Survival in Patients With PDAC With Diabetes
There was no statistically significant difference in survival between metformin users versus nonusers, that is, 9.9 versus 8.9 months, respectively, with an HR of 0.93 (95% CI, 0.81 to 1.07; P = .28; Table 2, Fig 1A). The results remained similar when adjusted for age, sex, usual BMI, stage of cancer, and year of diagnosis (adjusted HR, 0.92; 95% CI, 0.79 to 1.08; P = .30; Table 3). When stratified by clinical stage, median survivals of metformin users versus nonusers were 22.8 versus 19.4, 10.2 versus 8.1, and 4.9 versus 5.3 for resectable, locally advanced, and metastatic groups, respectively (Table 2 and 3). Of interest, whereas statistical significance was achieved only in the locally advanced PDAC group (P = .03 in the adjusted model; Table 3), parameter estimates suggest a small beneficial effect of metformin in all but the patients with metastatic PDAC (adjusted HR, 0.84; 95% CI, 0.62 to 1.15]; adjusted HR, 0.75; 95% CI, 0.58 to 0.97; and adjusted HR, 1.19; 95% CI, 0.93 to 1.54; P = .29, .03, and .17, for resectable, locally advanced, and metastatic groups, respectively).
Table 2.
Unadjusted Analysis by Time-Varying Model and Conventional Cox Proportional Hazards Regression Analysis
| Metformin Use | Deaths/Total | Median Survival (95% CI)* | Time-Varying Model | Conventional Cox Model | ||
|---|---|---|---|---|---|---|
| HR (95% CI)† | P‡ | HR (95% CI)* | P‡ | |||
| Overall | .2819 | .0763 | ||||
| Never | 564/614 | 8.9 (8.0 to 9.6) | Ref | Ref | ||
| Ever | 311/366 | 9.9 (8.2 to 10.7) | 0.93 (0.81 to 1.07) | 0.88 (0.77 to 1.01) | ||
| Resectable | .2249 | .0908 | ||||
| Never | 153/187 | 19.4 (16.7 to 23.5) | Ref | Ref | ||
| Ever | 70/97 | 22.8 (18.6 to 30.9) | 0.84 (0.63 to 1.12) | 0.78 (0.59 to 1.04) | ||
| Locally advanced | .0093 | .0058 | ||||
| Never | 211/222 | 8.1 (6.9 to 9.5) | Ref | Ref | ||
| Ever | 111/132 | 10.2 (8.9 to 12.9) | 0.74 (0.58 to 0.93) | 0.72 (0.58 to 0.91) | ||
| Metastatic | .4370 | .7096 | ||||
| Never | 199/204 | 5.3 (4.6 to 6.1) | Ref | Ref | ||
| Ever | 130/137 | 4.9 (3.8 to 5.9) | 1.09 (0.88 to 1.36) | 1.04 (0.84 to 1.30) | ||
| Metformin naïve | .8889 | .5374 | ||||
| Never | 564/614 | 8.9 (8.0 to 9.6) | Ref | Ref | ||
| Ever | 72/85 | 10.2 (8.4 to 12.9) | 1.02 (0.80 to 1.30) | 0.93 (0.72 to 1.18) | ||
Abbreviations: HR, hazard ratio; Ref, reference.
Kaplan-Meier method.
Cox proportional hazards regression model.
Log-rank test.
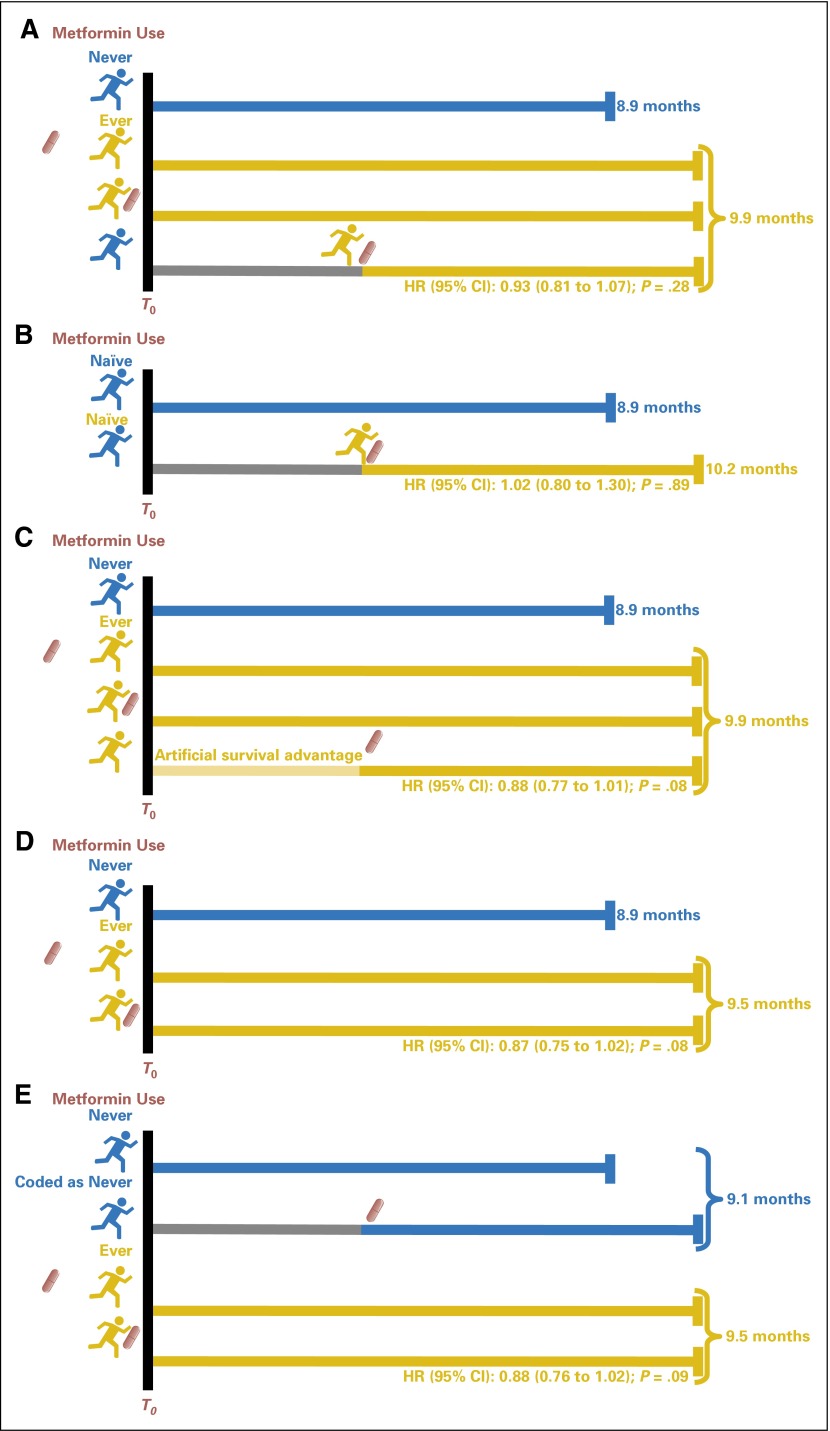
Fig 1.
Schema depicting analysis methods and exposure definitions. Hazard ratios (HRs), 95% CIs, and P values are from unadjusted analyses for all panels. (A) The concept of HR estimated by using the time-varying covariate analysis for the entire cohort. Unlike the conventional Cox proportional hazards regression analysis, metformin exposure is treated as a time-dependent variable. The survival clock of each patient is started at time T0 (time of PDAC diagnosis) as in other analyses, but the patient is not considered a metformin user until the time metformin use is initiated. Thus, this method controls for time of metformin initiation by eliminating the artificial survival advantage incurred during the period in which metformin has not yet been started after PDAC diagnosis; therefore, the effect size of metformin exposure is more reflective of a real effect of metformin use on survival of patients with PDAC. (B) HRs from a time-varying covariate analysis for the subgroup of patients who were naïve to metformin exposure by the time of PDAC diagnosis. (C) Concept of HRs calculated by using the conventional Cox proportional hazards regression analysis for the entire cohort and ignoring the time of metformin initiation. HR is computed by starting the survival clock (human figure represents the time when the clock is started) at T0 for all study patients, regardless of timing of metformin initiation (shown as red drug capsule). Each patient starts metformin at different time points, which can be either before PDAC diagnosis, at the time of PDAC diagnosis, or post-PDAC diagnosis. Patients who initiate metformin post-PDAC diagnosis had an artificial survival advantage (light yellow line) in this analysis because they must live long enough to start metformin after PDAC diagnosis, that is, their probability of metformin exposure is dependent on their survival. Thus, with this analysis, the estimated effect size of metformin use can be overestimated. (D) HRs from a conventional Cox proportional hazards regression analysis that excluded patients who initiated metformin post-PDAC diagnosis. (E) HRs from a conventional Cox proportional hazards regression analysis in which those who initiated metformin after PDAC diagnosis were coded as nonusers.
Table 3.
Adjusted Analysis by Time-Varying Model and Conventional Cox Proportional Hazards Regression Analysis
| Metformin Use | Deaths/Total | Median Survival (95% CI)* | Time-Varying Model | Conventional Cox Model | ||
|---|---|---|---|---|---|---|
| HR (95% CI)† | P‡ | HR (95% CI)* | P‡ | |||
| Overall | .2989 | .1026 | ||||
| Never | 564/614 | 8.9 (8.0 to 9.6) | Ref | Ref | ||
| Ever | 311/366 | 9.9 (8.2 to 10.7) | 0.92 (0.79 to 1.08) | 0.88 (0.76 to 1.03) | ||
| Resectable | .2873 | .1113 | ||||
| Never | 153/187 | 19.4 (16.7 to 23.5) | Ref | Ref | ||
| Ever | 70/97 | 22.8 (18.6 to 30.9) | 0.84 (0.62 to 1.15) | 0.78 (0.57 to 1.06) | ||
| Locally advanced | .0284 | .0198 | ||||
| Never | 211/222 | 8.1 (6.9 to 9.5) | Ref | Ref | ||
| Ever | 111/132 | 10.2 (8.9 to 12.9) | 0.75 (0.58 to 0.97) | 0.74 (0.57 to 0.95) | ||
| Metastatic | .1732 | .3100 | ||||
| Never | 199/204 | 5.3 (4.6 to 6.1) | Ref | Ref | ||
| Ever | 130/137 | 4.9 (3.8 to 5.9) | 1.19 (0.93 to 1.54) | 1.14 (0.88 to 1.48) | ||
| Metformin naïve | .7692 | .8377 | ||||
| Never | 564/614 | 8.9 (8.0 to 9.6) | Ref | Ref | ||
| Ever | 72/85 | 10.2 (8.4 to 12.9) | 1.04 (0.78 to 1.39) | 0.97 (0.73 to 1.30) | ||
NOTE. Adjusted for age, sex, usual adult body mass index, stage of disease, and year of diagnosis (≤ 2004, 2005-2008, ≥ 2009).
Abbreviations: HR, hazard ratio; Ref, reference.
Kaplan-Meier method.
Cox proportional hazards regression model.
Log-rank test.
Metformin Use After PDAC Diagnosis Does Not Confer Survival Benefit to Patients With PDAC With Diabetes Who Were Metformin-Naïve at the Time of Diagnosis
To assess the proposed inclusion of metformin as an adjunct chemotherapeutic agent in cancer therapy, the subset of 699 patients who had never used metformin at the time of PDAC diagnosis were included in further analyses (Fig 1B). Of 699 patients, 614 (87.8%) did not use metformin after PDAC diagnosis, whereas 85 (12.2%) were prescribed metformin during the follow-up period after diagnosis. The unadjusted and adjusted HRs (95% CI) of metformin initiation after PDAC diagnosis were 1.02 (0.80 to 1.30) and 1.04 (0.78 to 1.39; P = .89 and .77, respectively; Table 2 and 3), which indicates that starting metformin after PDAC diagnosis did not improve survival of patients with PDAC. This finding suggests that metformin was not beneficial when used as an adjuvant treatment of PDAC.
Results of Conventional Cox Proportional Hazards Regression Analyses
The conventional Cox analysis is commonly used to estimate the effect of drug exposure on survival by using an ever/never classification (Fig 1C); however, as this model disregards time of drug initiation, a survival advantage can be artificially detected as a result of an inherent survival bias. By univariable analysis, HR of metformin use was 0.88 (95% CI, 0.77 to 1.01; P = .08) for the entire cohort (Table 2). This estimate was smaller than the estimate calculated by the time-varying model (HR, 0.93; 95% CI, 0.81 to 1.07; P = .28), which suggests that the protective effect of metformin was inflated by the conventional Cox model. By multivariable analysis of the entire cohort, HRs remained the same and not statistically significant (adjusted HR, 0.88; 95% CI, 0.76 to 1.03; P = .10; Table 3). When stratified by clinical stage, metformin use was statistically significantly associated with survival in patients with locally advanced PDAC, with adjusted HR of 0.74 (95% CI, 0.57 to 0.95; P = .02; Table 3). There was no statistically significant difference in survival in the resectable and metastatic groups (Table 3).
To further dissect the finding of a statistically significant difference in survival among patients with locally advanced PDAC, patients were classified into three groups by timing of metformin initiation. Patients who started metformin post-PDAC diagnosis had longer survival than those who started metformin before PDAC diagnosis or those who never used metformin: median survival was 12.6 (95% CI, 9.7 to 15.5), 9.9 (95% CI, 8.0 to 12.8) and 8.1 (95% CI, 6.9 to 9.5) months, respectively. When patients with locally advanced PDAC were classified into five groups as described in Patients and Methods, patients for whom metformin was started > 30 days post-PDAC diagnosis survived longest. Median survival in months (95% CI) of these five groups—never used metformin, metformin started > 1 year before PDAC diagnosis, metformin started within 1 year before PDAC diagnosis, metformin started ≤ 30 days post-PDAC diagnosis, and metformin started > 30 days post-PDAC diagnosis—were: 8.1 (95% CI, 6.9 to 9.5; n = 222), 10.1 (95% CI, 6.2 to 16.5; n = 23), 9.9 (95% CI, 7.8 to 13.1; n = 71), 11.4 (95% CI, 8.0 to 15.5; n = 26), and 13.7 (95% CI, 11.0 to 21.4; n = 10), respectively (P = .07). The increased survival in the two groups of patients who started metformin post-PDAC diagnosis demonstrates the inherent survival bias of using the ever/never classification. That is, these patients lived long enough to receive metformin after PDAC diagnosis; however, metformin was not necessarily responsible for the increase in their survival (their classification as ever/never was determined by future exposure, which caused an inherent survival bias; Fig 1C). In other words, patients who live the longest have the largest chance of starting metformin use, and, thus, the longer survival causes the metformin use rather than the metformin use causing the longer survival.
Conventional Cox hazard analysis that excludes patients who initiated metformin post-PDAC diagnosis (Fig 1D) does not use all available data, whereas coding all patients who initiated metformin after PDAC diagnosis (Fig 1E) as nonusers may result in biased estimates for the never users. When we performed these analyses, HR estimates were smaller than those from the conventional Cox analysis including all patients, suggesting slightly more bias toward a protective effect of metformin use (Appendix Table A2, online only).
DISCUSSION
When appropriately accounting for the timing of metformin use post-PDAC diagnosis, our data do not support a benefit of metformin use when initiated after diagnosis. This study illustrates the fact that simple analysis by using a conventional Cox proportional hazards regression model with ever/never exposure classification gives biased results; in this study, approximately 3% too many person-years are attributed to the metformin group in the conventional Cox analysis. This bias will be greater particularly in cancers associated with longer survival (eg, breast or prostate cancer for which 5-year survival rates are 89% and 99%, respectively), and the bias will be less in cancers associated with shorter survival (eg, pancreatic or liver cancer for which 5-year survival rates are 7% and 17%, respectively). To avoid this bias, appropriate study design and analysis that accounts for time of drug initiation is crucial for future retrospective or prospective cohort studies of medication exposure and cancer survival.
Survival bias, immortal time bias, is not uncommon but is often unrecognized in epidemiologic studies of drug effect on cancer survival.11,16,17 When not properly controlled for, bias can potentially result in an overestimation of survival benefit with the drug.10 Survival bias occurs when the ever/never exposure to the drug variable is defined at the fixed starting time point of a follow-up period (ie, T0 or time of PDAC diagnosis in this study), and ever/never classification is dependent on future exposure (ie, at time of drug initiation after T0).18 Patients who die shortly after diagnosis will not have been exposed to the drug and are automatically classified never users, whereas patients who survive until the date of drug initiation after diagnosis are classified as ever exposed. The conventional Cox analysis does not accurately consider the survival time before drug initiation during which the patient was not yet exposed to the drug. Thus, patients in the ever exposed group have a survival advantage over patients in the never group by default because of the fact that patients in the ever exposed group simply live long enough to receive the drug. The magnitude of this bias depends on the number of patients who die early (ie, those classified as never users) and the number of patients who start the medication after T0. Thus, cancers associated with longer survival, such as breast and prostate cancers, are particularly susceptible to this bias.
Survival bias can be controlled for by using time-varying covariate analysis rather than fixed-time point analysis, that is, coding the drug exposure variable as a time-dependent variable instead of a categorical variable.10 The drug exposure variable is coded as never exposed until the timing of drug initiation when the variable is changed to ever; therefore, the period between T0 and timing of drug initiation when the patient is not exposed to the drug is not artificially counted as exposure time in the analysis. Time-varying covariate analysis, therefore, provides a more accurate estimate of drug effect than conventional Cox analysis.10
It is important to note that although time-varying covariate analysis is an appropriate method for the control of survival bias in retrospective cohort studies, it relies on some assumptions.19 First, the time-dependent variable of drug exposure initiation implies that the initiation of drug occurs randomly, that is, initiation is independent of other factors that may influence prescribing of the drug, which may not necessarily occur in practice.19 Patients with cancer who have been treated with metformin may have had disease characteristics that affected physician preferences for prescribing metformin (eg, less severe diabetes, fewer medical comorbidities, no contraindications to metformin, or living longer than expected survival from PDAC). Thus, being exposed to metformin may simply indicate better baseline health of patients, that is, less likelihood of death, rather than a real association of metformin use with improved cancer survival. In this study, the distributions of Karnofsky performance scores were similar between patients who never used metformin and those who had ever used metformin. Thus, we conclude that the chance of being prescribed metformin was independent of the general health of patients in our cohort. In addition, although the time of metformin initiation can be controlled for by a time-varying covariate Cox model, the treatment effect of metformin may be influenced by other unknown biases in cohort studies. Accordingly, results from retrospective cohort studies should be interpreted with caution. Given the lack of exact information on date of metformin initiation and discontinuation, we were not able to account for temporary cessation or discontinuation of metformin during the follow-up period or to determine whether the duration of metformin use was long enough to expect an effect.
To determine the effect of a drug on survival after cancer diagnosis, ideally, only patients without prior exposure to the drug at the time of cancer diagnosis would be included. Although such patients are more representative of those actually enrolled in clinical trials, retrospective cohort studies of such rare cancers as PDAC are often heterogeneous. Time-varying covariate Cox model allows for a more thorough analysis and maximizes the sample size, increases the power of the study, and permits more generalizable findings. Our large sample size allowed us to examine this effect. Our results suggest that proper study design and appropriate selection of study patient groups can minimize this type of survival bias.
Of interest, our findings indicate that there is a small protective effect of metformin in patients with locally advanced PDAC when received before PDAC diagnosis, which is similar to that observed previously.5 More studies are needed to confirm and elucidate this finding. Currently, there are > 20 ongoing clinical trials of metformin therapy for recalcitrant cancers (Appendix Table A1), many of which are currently recruiting patients. Two trials of PDAC have been completed, one study (NCTO1210911) reported that metformin did not improve survival of patients with locally advanced or metastatic PDAC.20 In addition, the extensive heterogeneity in study design, eligibility criteria that permits previous metformin use, and doses of metformin administered reflect inadequately informed designs.
In summary, retrospective studies of drug exposure and cancer survival require meticulous data collection, appropriate study design, and data analysis to prevent the overestimation of the effect of medication exposure on cancer survival. These factors should be considered in future epidemiologic studies of medication exposure and cancer survival. Lastly, when power calculations are done for prospective studies using an effect size from epidemiologic studies, one should be cognizant that the reported effect size may be larger than in reality.
Appendix
Study Population
The registry includes patients with pancreatic ductal adenocarcinoma (PDAC) who were recruited at the time of their visit for diagnosis and/or management. Participants in the registry completed baseline, 6-month, and 12-month health and risk factor questionnaires and gave authorization for the use of their biospecimens and medical records (MR) in research. PDAC diagnosis was confirmed in 93% of patients by histopathology.
Those patients who potentially had concurrent diabetes were identified (n = 1,690) through electronic MR abstraction on the basis of any of the following criteria: physician report of diabetes in the MR, patient self-report of diabetes in the baseline questionnaire, documentation of antidiabetic agent prescription in clinical notes and/or follow-up questionnaires; and fasting plasma glucose ≥ 126 mg/dL or HbA1c ≥ 6.5% in the laboratory record.14
Three physicians manually reviewed MRs of patients with PDAC who potentially had concurrent diabetes to verify diagnosis and date of diabetes diagnosis, which was defined by any of the following: physician report of diabetes, being prescribed antidiabetic agents, or meeting American Diabetes Association criteria, that is, fasting plasma glucose ≥ 126 mg/dL, HbA1c ≥ 6.5%, or random plasma glucose ≥ 200 mg/dL with presence of typical symptoms of hyperglycemia or hyperglycemic crisis. Patients who had transient elevation of plasma glucose as a result of stress, for example, during hospitalization or surgery-induced diabetes, or type I diabetes were excluded. To ensure the consistency of verification of diabetes diagnosis among the abstractors, all three abstractors reviewed the MRs of 10 patients until consensus was reached among abstractors and with the principal investigator. Exclusion criteria included PDAC diagnosed > 90 days before the first visit at Mayo Clinic, recurrent PDAC at the time of first visit at Mayo Clinic, post-PDAC diabetes, and unknown duration of diabetes. The final cohort comprised 980 patients with newly diagnosed PDAC and diabetes.
Table A1.
Summary of Clinical Trials of Recalcitrant Cancers, Defined as Cancers With a 5-Year Survival of < 50%, That Include Metformin in a Treatment Arm
| Study Characteristic | Cancer Type | |||||
|---|---|---|---|---|---|---|
| Pancreas (n = 11) | Non–Small-Cell Lung Cancer (n = 9) | Ovary (n = 3) | Liver Cancer (n = 1) | Brain (n = 3) | Acute Myeloid Leukemia (n=1) | |
| Status | ||||||
| Completed | 2 | 1 | ||||
| Recruiting | 6 | 8 | 2 | 2 | 1 | |
| Active, not recruiting | 0 | 1 | ||||
| Not yet recruiting | 1 | 1 | ||||
| Terminated | 1 | |||||
| Suspended participant recruitment | 1 | |||||
| Withdrawn before enrollment | 1 | |||||
| Cancer stage | Metastatic (8); metastatic or locally advanced (1); nonmetastatic or recurrent (1); and nonmetastatic (1) | Stage lb (1); stage lb-IIIa (1); stage Ilc/III/IV (1); stage IIIa/IIIb (2); stage IIIb/IV (2); and stage IV (2) | Recurrent (1); stage IIC, III, IV (1); and stage III-IV (1) | Not mentioned (1) | Stage IV astrocytoma (1); recurrent (1); and postcranial or cranial-spinal radiation (1) | Relapsed/refractory disease (1) |
| Primary outcome | ||||||
| Progression-free survival | 3 | 5 | 2 | |||
| Overall survival | 2 | |||||
| Recurrence-free survival | 1 | 1 | ||||
| Others | 5 | 4 | 1 | 3 | 1 | |
| Study phase | ||||||
| I | 3 | 1 | 1 | 2 | 1 | |
| II | 6 | 9 | 2 | |||
| I and II | 2 | |||||
| III | 1 | |||||
| Daily dose of metformin, mg | ||||||
| 2,550 (850 mg thrice daily) | 1 | |||||
| 2,000 | 1 | 6 | ||||
| 1,700 (850 mg twice daily) | 1 | 1 | ||||
| 1,500 | 1 | |||||
| 1,000 | 2 | |||||
| 500 | 2 | |||||
| Not mentioned | 4 | 1 | 3 | 1 | 2 | 1 |
| 1,000 mg/m2 | 1 | |||||
| Prior exposure to metformin | ||||||
| Not mentioned | 3 | 2 | 2 | 1 | ||
| Allowed | 1 | |||||
| Allowed if dose is < 1,000 mg daily | 1 | |||||
| Allowed if on metformin < 6months | 3 | |||||
| No metformin use in previous 6 months | 1 | 3 | 2 | |||
| No metformin use in previous 12 months | 1 | |||||
| Not allowed | 1 | 4 | 1 | 1 | ||
| No metformin use in previous 1 week | 1 | |||||
| History of diabetes | ||||||
| Not mentioned | 4 | 3 | 2 | |||
| Allowed if taking metformin < 6 months | 4 | |||||
| Allowed if not taking metformin | 1 | |||||
| Allowed if not taking metformin within 1 year | 1 | |||||
| Allowed if not taking metformin within 6 months | 1 | |||||
| Allowed | 1 | |||||
| Excluded | 5 | 3 | 1 | 1 | ||
| Included | 1 | |||||
NOTE. Data given as No. unless otherwise noted. Last access on June 1, 2015.
Table A2.
Comparison of Adjusted Hazard Ratios Estimated by Three Conventional Cox Analysis Models
| Group | Adjusted HR (95%CI) | ||
|---|---|---|---|
| Model A | Model B | Model C | |
| Overall | 0.880 (0.755 to 1.026) | 0.851 (0.722 to 1.003) | 0.855 (0.728 to 1.004) |
| Resectable | 0.778 (0.571 to 1.060) | 0.729 (0.521 to 1.021) | 0.736 (0.529 to 1.026) |
| Locally advanced | 0.736 (0.568 to 0.952) | 0.690 (0.522 to 0.912) | 0.703 (0.535 to 0.925) |
| Metastatic | 1.142 (0.884 to 1.475) | 1.125 (0.858 to 1.474) | 1.139 (0.877 to 1.479) |
NOTE: Models were adjusted for age, sex, usual body mass index, stage of disease, and year of diagnosis. Patients were simply coded as metformin users or nonusers. Model A included all patients in the analysis, whereas in Model B, patients who started metformin after the time of pancreatic ductal adenocarcinoma (PDAC) diagnosis were excluded from the analysis. In Model C, patients for whom metformin was started after PDAC diagnosis were coded as nonusers.
Abbreviation: HR, hazard ratio.
Footnotes
Supported by Mayo Clinic SPORE in pancreatic cancer (National Institutes of Health [NIH] Grant No. P50CA102701) and the Mayo Clinic Center for Translational Science Activities (NIH National Center for Research Resources Clinical and Translational Science Award Grant No. UL1-TR000135). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Roongruedee Chaiteerakij, Gloria M. Petersen, William R. Bamlet, Kari G. Chaffee, David B. Zhen, Lewis R. Roberts, Ann L. Oberg
Financial support: Gloria M. Petersen
Collection and assembly of data: Roongruedee Chaiteerakij, Gloria M. Petersen, William R. Bamlet, Kari G. Chaffee, David B. Zhen, Patrick A. Burch, Emma R. Leof, Lewis R. Roberts
Data analysis and interpretation: Gloria M. Petersen, William R. Bamlet, Kari G. Chaffee, Lewis R. Roberts, Ann L. Oberg
Manuscript writing: All authors:
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Roongruedee Chaiteerakij
No relationship to disclose
Gloria M. Petersen
No relationship to disclose
William R. Bamlet
No relationship to disclose
Kari G. Chaffee
No relationship to disclose
David B. Zhen
No relationship to disclose
Patrick A. Burch
No relationship to disclose
Emma R. Leof
No relationship to disclose
Lewis R. Roberts
Research Funding: Bristol-Myers Squibb (Inst), Gilead Sciences (Inst), Wako Diagnostics (Inst), Innova Diagnostics (Inst), Bayer AG (Inst), BTG (Inst), Ariad Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Fluorescence in situ hybridization probes for diagnosis of pancreatobiliary cancer
Ann L. Oberg
Consulting or Advisory Role: Procter & Gamble
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi N, Abbruzzese JL, Yeung SC, et al. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reference deleted.
- 7.Pollak M. Overcoming drug development bottlenecks with repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health ClinicalTrials.gov. https://clinicaltrials.gov/
- 9.Suissa S, Azoulay L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care. 2012;35:2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Rahme E, Abrahamowicz M, et al. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: A comparison of methods. Am J Epidemiol. 2005;162:1016–1023. doi: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
- 11.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 12.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 13.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 16.Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17–29. doi: 10.1146/annurev-med-062613-093128. [DOI] [PubMed] [Google Scholar]
- 17.van Walraven C, Davis D, Forster AJ, et al. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57:672–682. doi: 10.1016/j.jclinepi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Shariff SZ, Cuerden MS, Jain AK, et al. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19:841–843. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- 19.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute; 2000. Time dependent covariates, in; pp. 138–153. [Google Scholar]
- 20.Wilmink J, Kordes S, Zwinderman K, et al. A phase II randomized, placebo controlled study to evaluate the efficacy of the combination of gemcitabine, erlotinib, and metformin in patients with locally advanced or metastatic pancreatic cancer. J Clin Oncol. 2014;32(abstr 4021):5s. [Google Scholar]