Abstract
Purpose
Androgen deprivation therapy (ADT) may contribute to depression, yet several studies have not demonstrated a link. We aimed to determine whether receipt of any ADT or longer duration of ADT for prostate cancer (PCa) is associated with an increased risk of depression.
Methods
We identified 78,552 men older than age 65 years with stage I to III PCa using the SEER-Medicare–linked database from 1992 to 2006, excluding patients with psychiatric diagnoses within the prior year. Our primary analysis was the association between pharmacologic ADT and the diagnosis of depression or receipt of inpatient or outpatient psychiatric treatment using Cox proportional hazards regression. Drug data for treatment of depression were not available. Our secondary analysis investigated the association between duration of ADT and each end point.
Results
Overall, 43% of patients (n = 33,882) who received ADT, compared with patients who did not receive ADT, had higher 3-year cumulative incidences of depression (7.1% v 5.2%, respectively), inpatient psychiatric treatment (2.8% v 1.9%, respectively), and outpatient psychiatric treatment (3.4% v 2.5%, respectively; all P < .001). Adjusted Cox analyses demonstrated that patients with ADT had a 23% increased risk of depression (adjusted hazard ratio [AHR], 1.23; 95% CI, 1.15 to 1.31), 29% increased risk of inpatient psychiatric treatment (AHR, 1.29; 95% CI, 1.17 to 1.41), and a nonsignificant 7% increased risk of outpatient psychiatric treatment (AHR, 1.07; 95% CI, 0.97 to 1.17) compared with patients without ADT. The risk of depression increased with duration of ADT, from 12% with ≤ 6 months of treatment, 26% with 7 to 11 months of treatment, to 37% with ≥ 12 months of treatment (P trend < .001). A similar duration effect was seen for inpatient (P trend < .001) and outpatient psychiatric treatment (P trend < .001).
Conclusion
Pharmacologic ADT increased the risk of depression and inpatient psychiatric treatment in this large study of elderly men with localized PCa. This risk increased with longer duration of ADT. The possible psychiatric effects of ADT should be recognized by physicians and discussed with patients before initiating treatment.
INTRODUCTION
After multiple randomized controlled trials demonstrated improved survival with androgen deprivation therapy (ADT) alongside radiation therapy (RT) for high-risk and locally advanced prostate cancer (PCa),1-7 the proportion of patients with PCa given ADT within 12 months of diagnosis increased to nearly 50% by 2002.8 Although ADT has survival benefits, mounting evidence has demonstrated extensive adverse effects, including metabolic, cardiovascular, bone, and cognitive.9 There is reason to believe that ADT may negatively impact mood, causing clinically significant depression. Although several small studies have not identified any association between ADT and clinical depression,10-15 possibly as a result of inadequate power, others have found a significant association16-18; yet there remains no consensus on the subject. To investigate this further, we used a large population-based data set to measure the association between pharmacologic ADT and diagnosis of depression and increased psychiatric use in men with localized PCa, as well as to measure the effect of ADT duration on these outcomes.
METHODS
Data Source
Data were abstracted from the SEER-Medicare–linked database. SEER is a population-based registry sponsored by the National Cancer Institute including 18 regions, or 28% of the US population.19 Medicare provides federal health insurance for approximately 97% of individuals age 65 years and older, and Medicare to SEER linkage is complete for 93% of eligible patients.20
Study Cohort
The initial cohort of 172,733 patients included men age 66 years and older diagnosed with clinically localized PCa between 1992 and 2006 who had no other malignancy in SEER, had both Medicare Part A and B claims available from 1 year before through 36 months after diagnosis, and were not enrolled in a health maintenance organization from 1992 to 2006. Patients diagnosed at autopsy or by death certificate (n = 5,315) or who died within 6 months of diagnosis (n = 21) were excluded (Appendix Table A1, online only). Patients with orchiectomy were excluded (n = 2,986) to avoid confounding from effects that physical and anatomic changes from castration may have on depression. Additionally, to reduce confounding of advanced or recurrent disease, men with stage IV or unknown stage PCa (n = 68,133) were excluded, as were men started on ADT beyond 6 months from PCa diagnosis, because this could be salvage ADT (n = 10,255). Finally, patients with a diagnosis of depression from 12 months before diagnosis through time 0 (t0; defined as 6 months after diagnosis) were excluded (n = 7,511). These patients were identified if International Classification of Diseases codes, ninth revision (ICD-9), (296.2, 296.3, 296.5, 296.6, 296.7, 298.0, 301.10, 301.12, 301.13, 309.0, 309.1, and 311; further described in Appendix Table A2, online only).21-23 The final cohort included 78,552 patients with localized PCa.
Patient Sociodemographic and Clinical Characteristics
Each patient’s age at diagnosis, race, marital status, and county-level population density were extracted from SEER. Zip code–level median income and education (percentage with college diploma) were identified from census-linked data. Comorbidity status was assigned using the Klabunde modification24 of the Charlson comorbidity index (CCI) as 0, 1, or ≥ 2 from Medicare claims data 1 year before PCa diagnosis.
Clinically, overall stage was recorded as stage I, II, or III. Gleason score was recorded from SEER grade where, before 2003, well differentiated was defined as a Gleason score of 2 to 4, moderately differentiated was defined as a Gleason score of 5 or 6, and poorly differentiated was defined as a Gleason score of 7 to 10. After 2004, Gleason score was recorded, and we categorized patients similarly. Three risk groups were defined by modification of the risk groups identified by Lu-Yao et al,25 with risk group A as stage I to II and Gleason score of 2 to 6, risk group B as stage I to II and Gleason score of 7 to 10, and risk group C as stage III with any grade.25
Primary Treatment Type
Patients were categorized as receiving ADT (ADT+) if ADT was the primary therapy or if ADT was given alongside radical prostatectomy (RP) or RT before t0. Patients were categorized as not receiving ADT (ADT−) if ADT was not started from diagnosis through the follow-up period of 36 months after diagnosis. Primary treatment was defined as RP with or without ADT, RT with or without ADT, or ADT alone if initiated before t0 as identified through SEER-Medicare claims files, as previously described.25,26 Primary treatment was assigned as observation if there was an absence of active treatment codes. ADT+ was stratified by duration, defined as 1 to 6, 7 to 11, and ≥ 12 months.
End Points
The primary end point of interest was a diagnosis of depression, defined as the presence of depression codes (Appendix Table A2), in Medicare claims data, including physician, outpatient, and inpatient claims, from t0 through 36 months after PCa diagnosis. Secondary end points included any outpatient psychiatric or inpatient psychiatric use.27-29 Psychiatric use was measured by depression diagnosis code or psychiatric procedure code and categorized as inpatient or outpatient treatment (Appendix Table A2). The ICD-9 and procedure codes used to identify the end points in this study have not been formally validated but have been used in prior studies.27-29
Statistical Analysis
Baseline demographic, clinical, and treatment characteristics were described for the overall cohort, stratified by ADT+ and ADT− and compared using χ2 test for categorical variables and Wilcoxon rank sum test for ordinal variables. The incidence of and time to primary and secondary end points were measured and compared by χ2 analysis and Wilcoxon rank sum test. A number needed to harm was also calculated. Cox proportional hazards analyses were performed to compare the possible effect of any ADT versus no ADT, controlling for demographic and clinical factors, on primary and secondary end points. The analysis was repeated for an association with duration of ADT (1 to 6, 7 to 11, and ≥ 12 months). Finally, propensity-matched sensitivity analyses, with 1:1 matching without replacement, were conducted for each end point as a sensitivity analysis (Appendix, online only).30 Demographic covariates included age at diagnosis, marital status, race, county-level population density, zip code income and education quartiles, and year of diagnosis. Clinical variables included modified CCI, PCa risk group, and receipt of primary RP or RT. An interaction term was measured between year of diagnosis (1992 to 1999 v 2000 to 2006) and receipt of ADT on a diagnosis of depression to consider homogeneity of the effect of ADT over the study duration. Finally, unadjusted cumulative incidence function graphs were created comparing time to depression and inpatient psychiatric treatment by receipt of ADT.
Statistical testing was two-sided with a level of significance set at P = .05. Analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC). The Dana-Farber Cancer Institute’s Institutional Review Board provided a waiver of informed consent for this study.
RESULTS
Baseline Patient Demographics and Treatment
The study cohort included 78,552 men with localized PCa, of whom 33,882 (43.1%) received ADT within 6 months of diagnosis (ADT+). Baseline demographic and clinical characteristics compared between patients who were ADT+ and those who were ADT− are listed in Table 1. To summarize, the ADT+ cohort was older at diagnosis, had more comorbidities, had shorter life expectancies, and had more high-grade disease (all P < .001).
Table 1.
Basic Demographic, Clinical, Treatment, and Psychiatric Outcomes, Compared by Receipt of ADT
| Characteristic | No ADT | ADT | |||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | P | |
| Age, years | < .001 | ||||
| Median | 72.6 | 75.2 | |||
| IQR | 69.2-76.7 | 71.3-79.4 | |||
| Mean | 73.5 | 75.7 | |||
| Race | < .001 | ||||
| White | 37,781 | 84.6 | 28,015 | 82.7 | |
| Black | 4,471 | 10.0 | 3,532 | 10.4 | |
| Other | 2,418 | 5.0 | 2,335 | 7.0 | |
| Risk group | < .001 | ||||
| A | 29,839 | 66.8 | 17,202 | 50.8 | |
| B | 9,489 | 21.2 | 13,800 | 40.7 | |
| C | 5,342 | 12.0 | 2,880 | 8.5 | |
| CCI | < .001 | ||||
| 0 | 29,546 | 66.1 | 21,814 | 64.4 | |
| 1 | 11,678 | 26.2 | 8,266 | 24.4 | |
| 2 | 3,446 | 7.7 | 3,802 | 11.2 | |
| Primary treatment | < .001 | ||||
| ADT | 0 | 0.0 | 9,849 | 29.1 | |
| Prostatectomy | 11,324 | 25.3 | 2,048 | 6.0 | |
| RT or BT | 19,736 | 44.2 | 21,985 | 64.9 | |
| Observation | 13,610 | 30.5 | 0 | 0.0 | |
| ADT in first 2 years, months | < .001 | ||||
| None | 44,670 | 100.0 | 0 | 0.0 | |
| 1-6 | 0 | 0.0 | 15,350 | 45.3 | |
| 7-11 | 0 | 0.0 | 7,474 | 22.1 | |
| ≥12 | 0 | 0.0 | 11,058 | 32.6 | |
| Depression after t0 | < .001 | ||||
| No | 42,369 | 94.9 | 31,495 | 93.0 | |
| Yes | 2,301 | 5.2 | 2,387 | 7.1 | |
| Inpatient psychiatric treatment after t0 | < .001 | ||||
| No | 43,826 | 98.1 | 32,923 | 97.2 | |
| Yes | 844 | 1.9 | 959 | 2.8 | |
| Outpatient psychiatric treatment after t0 | < .001 | ||||
| No | 43,536 | 97.5 | 32,746 | 96.7 | |
| Yes | 1,134 | 2.5 | 1,136 | 3.4 | |
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; CCI, Charlson comorbidity index; IQR, interquartile range; RT, radiation therapy; t0, time 0 (defined as 6 months after diagnosis).
Of the 44,670 patients categorized as ADT−, 25.3% had RP, 44.2% had RT, and 30.5% received no treatment. Of the 33,882 patients categorized as ADT+, 6.0% had RP plus ADT, 64.9% had RT plus ADT, and 29.1% had ADT alone. In the 2 years after diagnosis, 45.3% of the ADT+ cohort received ≤ 6 months of ADT, 22.1% received 7 to 11 months of ADT, and 32.6% received ≥ 12 months of ADT. In this last strata, 40.8% of patients (n = 4,515) received ≥ 18 months of ADT.
Risk of Depression and Psychiatric Treatment Associated With Any ADT
The cumulative incidence of new depression from 6 to 36 months after PCa diagnosis was higher in the ADT+ than the ADT− cohorts (7.1% v 5.2%, respectively; P < .001; Table 1). The number needed to harm was 53 patients. More patients who were ADT+ had inpatient (2.8% v 1.9%, respectively) and outpatient psychiatric treatment (3.4% v 2.5%, respectively; all P < .001; Table 1).
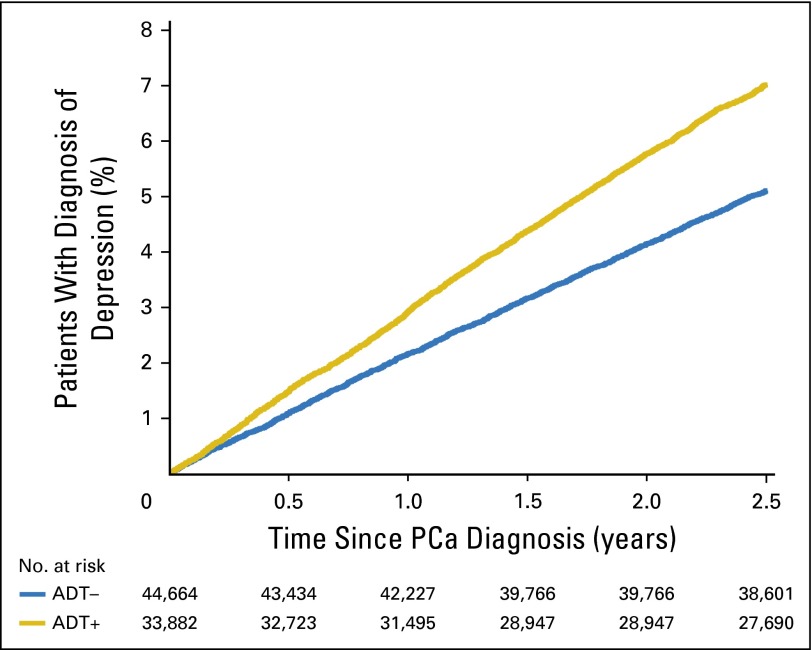
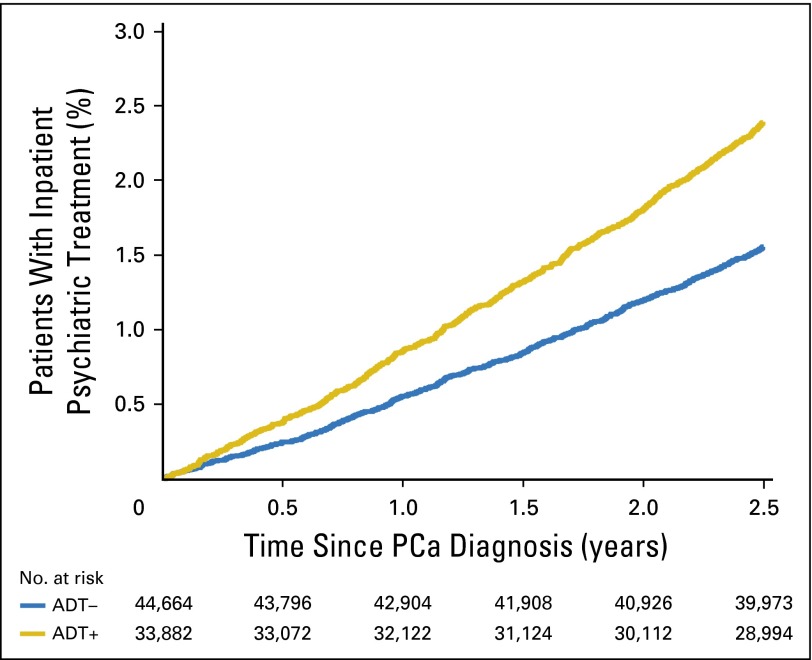
An adjusted Cox proportional hazards analysis showed that, compared with ADT–, there was a 23% increased risk of depression associated with the ADT+ cohort (adjusted hazard ratio [AHR], 1.23; 95% CI, 1.15 to 1.31; P < .001). Similarly, the ADT+ cohort had a 29% increased risk, compared with the ADT– cohort, of inpatient psychiatric treatment (AHR, 1.29; 95% CI, 1.17 to 1.43; P < .001) and a 7% increased risk of outpatient psychiatric treatment (AHR, 1.07; 95% CI, 0.97 to 1.17; P = .17). These associations were strengthened when using propensity matching in sensitivity analyses (Appendix Table A3, online only). Older age, being unmarried, and having greater comorbidity (CCI > 0) were also associated with increased risk of depression (all P < .001). An interaction term between the era of PCa diagnosis (1992 to 1999 v 2000 to 2006) and the receipt of ADT on depression was not significant (P = .306), suggesting that the effect of receiving ADT on a diagnosis of depression was not significantly different before or after the year 2000. Figures 1 and 2 show the unadjusted cumulative incidence function of time to depression and inpatient psychiatric treatment, respectively, demonstrating that the incidence was increased almost immediately after initiating ADT therapy.
Fig 1.
Unadjusted cumulative incidence plot of time to diagnosis of depression by receipt of any androgen deprivation therapy (ADT), with number at risk. PCa, prostate cancer.
Fig 2.
Unadjusted cumulative incidence plot of time to inpatient psychiatric treatment by receipt of any androgen deprivation therapy (ADT), with number at risk. PCa, prostate cancer.
Risk of Depression and Psychiatric Treatment Associated With Duration of ADT
The ADT+ cohort was stratified by duration of therapy, defined as ≤ 6, 7 to 11, and ≥ 12 months. The cumulative incidence of depression increased by strata, from 6.1% to 7.6% to 8.0% with ≤ 6, 7 to 11, and ≥ 12 months of ADT, respectively, compared with an incidence of 5.2% in the ADT− cohort (P < .001). The incidence of inpatient psychiatric treatment increased by strata, from 2.4% to 3.0% to 3.3%, respectively, compared to 1.9% in the ADT− cohort (P < .001), as did the incidence of outpatient psychiatric treatment, from 2.8% to 3.5% to 4.1%, respectively, compared to 2.5% in the ADT− cohort (P < .001).
Table 2 shows the adjusted Cox proportional hazards analysis of the association between ADT duration and each end point, controlling for demographic and clinical variables. The risk for depression among the ADT+ cohort compared with the ADT− cohort increased with ADT duration, from a 12% higher risk with ≤ 6 months (AHR, 1.12; 95% CI, 1.03 to 1.21) to 26% with 7 to 11 months (AHR, 1.26; 95% CI, 1.15 to 1.39) and 37% with ≥ 12 months of ADT (AHR, 1.37; 95% CI, 1.26 to 1.49; P trend < .001). Similarly, the risk of inpatient psychiatric treatment increased with ADT duration, compared with no ADT, from 16% higher risk with ≤ 6 months (AHR, 1.16; 95% CI, 1.02 to 1.32) to 28% with 7 to 11 months (AHR, 1.28; 95% CI, 1.10 to 1.50) and 47% with ≥ 12 months of ADT (AHR, 1.47; 95% CI, 1.29 to 1.68; P trend < .001). The risk for outpatient psychiatric treatment increased slightly with duration of ADT from 3% lower risk with ≤ 6 months (AHR, 0.97; 95% CI, 0.87 to 1.10) to 3% higher risk with 7 to 11 months (AHR, 1.03; 95% CI, 0.90 to 1.19) and 20% higher risk with ≥ 12 months of ADT (AHR, 1.20; 95% CI, 1.07 to 1.35; P trend = .04).
Table 2.
Cox Proportional Hazards Regression Analysis: Association Between Duration of ADT and Each Psychiatric End Point
| Factor | Depression | Outpatient Psychiatric Treatment | Inpatient Psychiatric Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AHR* | 95% CI | P | AHR* | 95% CI | P | AHR* | 95% CI | P | |
| Months of ADT | |||||||||
| None | Ref | Ref | Ref | ||||||
| 1-6 | 1.12 | 1.03 to 1.21 | .008 | 0.97 | 0.87 to 1.10 | .66 | 1.16 | 1.02 to 1.32 | .02 |
| 7-11 | 1.26 | 1.15 to 1.39 | < .001 | 1.03 | 0.90 to 1.19 | .65 | 1.28 | 1.10 to 1.50 | .002 |
| ≥12 | 1.37 | 1.26 to 1.49 | < .001 | 1.20 | 1.07 to 1.35 | .002 | 1.47 | 1.29 to 1.68 | < .001 |
| P trend | 1.11 | 1.08 to 1.14 | < .001 | 1.06 | 1.02 to 1.10 | .004 | 1.14 | 1.09 to 1.19 | < .001 |
| Age at diagnosis | 1.04 | 1.03 to 1.04 | < .001 | 1.05 | 1.04 to 1.06 | < .001 | 1.04 | 1.03 to 1.05 | < .001 |
| Race | |||||||||
| Black v white | 0.74 | 0.66 to 0.82 | < .001 | 1.11 | 0.96 to 1.27 | .15 | 0.58 | 0.48 to 0.70 | < .001 |
| Other v white | 0.71 | 0.61 to 0.82 | < .001 | 0.65 | 0.52 to 0.82 | < .001 | 0.64 | 0.49 to 0.82 | < .001 |
| Unmarried v married | 1.23 | 1.15 to 1.32 | < .001 | 1.44 | 1.30 to 1.58 | < .001 | 1.37 | 1.23 to 1.53 | < .001 |
| CCI | |||||||||
| 1 v 0 | 1.23 | 1.15 to 1.32 | < .001 | 1.22 | 1.11 to 1.35 | < .001 | 1.32 | 1.18 to 1.47 | < .001 |
| ≥ 2 v 0 | 1.92 | 1.77 to 2.09 | < .001 | 2.01 | 1.79 to 2.25 | < .001 | 2.36 | 2.08 to 2.68 | < .001 |
| Risk group | |||||||||
| B v A | 1.09 | 1.02 to 1.17 | .009 | 1.14 | 1.04 to 1.25 | .007 | 1.04 | 0.93 to 1.15 | .52 |
| C v A | 1.08 | 0.96 to 1.20 | .20 | 1.07 | 0.91 to 1.26 | .40 | 1.00 | 0.83 to 1.20 | .97 |
Abbreviations: ADT, androgen deprivation therapy; AHR, adjusted hazard ratio; CCI, Charlson comorbidity index; Ref, reference.
Additional adjustments included education level, income, population density, year of diagnosis, primary treatment with radiation therapy, and primary treatment with radical prostatectomy.
DISCUSSION
Patients who received any ADT had a 23% increased risk of new depression compared with patients who did not receive ADT when adjusting for demographic and clinical variables in this large, population-based study of 78,000 US men with localized PCa age ≥ 66 years. We also observed a significant trend between an increased duration of ADT and increased risk of depression, finding that patients receiving ≤ 6 months of ADT had a 12% increased risk of depression compared with no ADT versus a 37% increased risk with ≥ 12 months of ADT. This evidence strongly suggests an association between receiving ADT and developing new depression in men age ≥ 66 years with localized PCa.
The impact of ADT on depression may plausibly occur via deregulation of neurochemicals, such as serotonin, in addition to the well-described physical effects.31,32 Although a direct mechanism linking ADT to depression has yet to be identified, studies in mice have demonstrated that enzymes responsible for maintaining the neurochemical balance are hormonally responsive and that gonectamized mice have higher activity of monoamine oxidase A in the prefrontal cortex compared with controls.31 This increased activity of monoamine oxidase A could be responsible for deregulation of serotonin and thus increased rates of depression. Additionally, low testosterone has been associated with increased depression in otherwise healthy men,33-35 although the literature is somewhat inconsistent.12,36 Additionally, the many known adverse effects of ADT have been shown to negatively impact quality of life,32 including physical effects, such as erectile dysfunction, shortening of penile length, vasomotor flushing, weight gain, and gynecomastia, as well as cognitive effects, including insomnia and a decline in verbal and executive function.9,36
Because data from physician claims have been shown to underestimate disease incidence37 and can be subject to coding errors, we chose two additional measures of clinically significant psychiatric disease—inpatient and outpatient psychiatric treatment. A similar measurement was used in a population-based study of PCa in Sweden.29 We again found the risk for inpatient psychiatric treatment increased with ADT duration, from 16% for ≤ 6 months of ADT (AHR, 1.16; 95% CI, 1.02 to 1.32) to 47% with ≥ 12 months of ADT (AHR, 1.47; 95% CI, 1.29 to 1.68; P trend < .001) compared with no ADT. The P trend values for both inpatient and outpatient psychiatric treatment were significant, demonstrating that with increasing duration of ADT the relative risk of these outcomes increased compared with no ADT. The association of ADT with psychiatric treatment provides further evidence that ADT may have a clinically significant effect on psychiatric health.
There are multiple implications of our findings. First, we contribute to mounting evidence around the extensive adverse effect profile of ADT,9,36 which should include risk of clinically significant psychiatric disease. Physicians should note psychiatric illness as a possible adverse effect when discussing ADT and consider including such effects in consent forms. Second, judicious use of ADT is warranted to avoid unnecessary adverse effects. ADT should not be used in low-risk patients, because it does not provide benefit.38 Among patients with intermediate-risk PCa, the potential benefits of ADT must be weighed against the potential harms, including increased depression risk.
Third, further work should identify what patient subgroups are at greatest risk of psychiatric adverse effects with ADT and what interventions may reduce this risk. In this study, older age, being unmarried, and having more comorbidity were associated with increased depression and psychiatric treatment. Although patients with recent psychiatric disease were excluded from the current study, other work has identified increased rates of depression among similar patients.12,39 These populations may be appropriate to target for early depression screening or intervention after ADT initiation.
Depression in PCa also impacts health care use,21,40,41 including increased emergency room visits and hospitalizations,40 and patient outcomes, including increased suicide and overall mortality.42 Therefore, identification of and support for men at risk for depression may have broad effects on quality of life, health care use, and survival. Prior work identifying interventions to reduce the risk of depression with ADT has suggested a role for exercise,9,37,41 although two reviews have concluded that the current evidence is lacking.41,43 High-quality research on interventions to mitigate depressive effects of ADT is needed. Prevention may be especially important because little is known about whether ADT use modifies the efficacy of commonly used treatments for depression in these patients.44,45 Prior work has demonstrated that further characterization of depressive symptoms in men receiving ADT may also guide identification and treatment.46
Published literature considering the association between ADT and depression has been highly inconsistent.10-18,40,47 Many of these studies were likely underpowered, failing to find an association between ADT and depression, whereas previous population-based studies have used a broad definition of depression47 or considered only primary rather than adjuvant ADT,29 limiting the generalizability of their results. Our findings show that any ADT increases the risk of depression using a more specific definition of clinical depression, demonstrate an association between ADT and increased psychiatric treatment, and show that ADT is associated with these outcomes in the adjuvant setting. Furthermore, we demonstrated a dose-response relationship with ADT duration, which may reflect a direct biologic effect of increased exposure to ADT, an indirect biologic effect as a result of other symptoms (eg, decreased libido), or a negative emotional response to being told that one needs a longer course of ADT. ADT duration has only been previously considered in three small studies, none of which demonstrated different risk with duration of ADT or between long- and short-course ADT.12,32,37
There are some potential limitations of this study. First, on the basis of the eligibility for Medicare, the cohort was limited to patients age ≥ 66 years at diagnosis. Therefore, these results may not reflect the psychiatric effects of ADT given to younger men, who account for approximately one third of patients with PCa.48 Second, the outcome of depression was based on diagnosis, rather than symptoms, which relied on physician recognition and reporting.49 Older men are less likely to report depressive symptoms and often present with somatic rather than emotional symptoms12,50,51; therefore, it is possible that many symptomatic men were undiagnosed and therefore not measured in this study. This should have affected each cohort similarly. Additionally, patients receiving ADT likely had more appointments than the cohort that did not receive ADT, and physicians may have been more sensitive to symptoms of depression in patients receiving ADT or with more aggressive disease, increasing the likelihood of patients who received ADT being diagnosed with depression compared with patients who did not receive ADT.
We attempted to account for this by also analyzing end points such as inpatient and outpatient psychiatric treatment, which may be less likely to be affected by this potential bias. The secondary end point in this study was an exploratory analysis inspired by prior studies conducted outside of SEER-Medicare considering psychotherapy. Many codes included for this secondary end point come from the psychotherapy literature as well as from identification of depression ICD-9 codes associated with hospitalization (Appendix Table A2). The codes were carefully and thoughtfully chosen to most accurately reflect psychiatric use by patients.
Third, we could not control for the use of psychotropic medication before PCa diagnosis but did exclude patients based on history of depressive diagnosis or psychiatric treatment. Data on prescription of psychiatric medication were not available in SEER-Medicare for use as an end point.
Fourth, increased depression associated with ADT may be secondary to confounding, because these patients were older, had more comorbidity, had more advanced disease, and experienced physical effects of ADT. These additional symptoms and comorbidity could have led to an overdiagnosis of depression in the ADT+ cohort. However, all multivariable analyses were adjusted for demographic and clinical variables, including age, comorbidity (CCI), and PCa risk group, and found a consistent increased risk with duration of ADT across all three end points. In addition, a greater proportion of the ADT+ cohort, compared to the ADT– cohort, was composed of ethnic minorities (17.3% v 15.4%, respectively) and had low comorbidity (CCI = 0; 66.2% v 64.5%, respectively), which were both variables independently associated with lower rates of depression in multivariable analyses. Fifth, we cannot control for disease progression in patients receiving ADT, which may contribute to increased incidence of depression. Finally, this study is nonrandomized and shares the general limitations associated with retrospective, observational studies.
In conclusion, we observed a significantly increased risk of depression and inpatient psychiatric treatment in men treated with ADT for PCa, as well as a duration-response effect such that more ADT was linked to an increasing risk of depression and inpatient and outpatient psychiatric treatment. The possible psychiatric effects of ADT should be recognized by physicians and discussed with patients before initiating treatment.
Acknowledgment
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute, the Office of Research, Development and Information, Centers for Medicare and Medicaid Services, Information Management Services, and the SEER Program tumor registries in the creation of the SEER-Medicare database.
GLOSSARY TERMS
- androgen deprivation therapy (ADT):
therapy which results in a reduction of circulating testosterone levels, which may be achieved by surgical castration, by LHRH agonists or antagonists, by androgen receptor blockers, or by androgen synthesis blockers.
- Charlson comorbidity index:
a weighted index that takes into account the number and seriousness of 19 comorbid diseases to categorize comorbidity burden. The Charlson comorbidity index has prognostic significance in assessing disease outcomes and health resource use and has been validated in the cancer population.
- Gleason score:
a pathologic description of prostate cancer grade on the basis of the degree of abnormality in the glandular architecture. Gleason patterns 3, 4, and 5 denote low, intermediate, and high levels of histologic abnormality and tumor aggressiveness, respectively. The score assigns primary and secondary numbers on the basis of the most common and second most common patterns identified.
Appendix
Supplemental Methods
For the propensity-matched sensitivity analyses, nearest neighbor 1:1 matching was used—the preferred method for selecting patients intended for follow-up.30 This method allowed patients in the ADT− cohort who were not matched to patients in the ADT+ cohort to be discarded while maintaining the size of the ADT+ group. This method has also been shown to increase the overall power of the comparison.30 The groups were also matched without replacement, because the ADT− cohort was sufficiently large enough to provide matches for each member of the ADT+ cohort. The propensity-matched cohorts were used to conduct regression analyses considering the effect of any ADT on the incidence of depression, inpatient psychiatric treatment, and outpatient psychiatric treatment.
Table A1.
Inclusion and Exclusion Criteria
| Criteria | No. of Excluded Patients | No. of Patients |
|---|---|---|
| Inclusion criteria | 0 | 172,773 |
| ≥ 66 years of age; diagnosed with PCa from 1992 to 2006; no other malignancy in SEER; Medicare Part A and B claims available from 1 year before diagnosis through 60 months after diagnosis; not enrolled in an HMO at any point during the study; had complete claims data | ||
| Exclusion criteria | ||
| PCa diagnosis from autopsy or death certificate only | 5,315 | |
| Died between diagnosis and t0 | 21 | |
| Stage IV or unknown stage | 68,133 | |
| Received orchiectomy during study period | 2,986 | |
| Started on ADT beyond t0 | 10,255 | |
| Psychiatric use in the 12 months before diagnosis through t0 | 7,511 | |
| Final cohort | 78,552 |
Abbreviations: ADT, androgen deprivation therapy; HMO, health maintenance organization; PCa, prostate cancer; t0, time 0 (defined as 6 months after diagnosis).
Table A2.
ICD-9 and CPT Codes Used to Identify Depression and Psychiatric Treatment Outcomes
| Description | ICD-9 or CPT Code |
|---|---|
| ICD-9 codes for diagnosis of depression | |
| Major depressive disorder, initial diagnosis | 296.2 |
| Major depressive disorder, recurrent | 296.3 |
| Depression associated with bipolar I disorder | 296.5-296.7 |
| Depressive psychoses | 298.0 |
| Depression associated with personality disorder | 301.10, 301.12, 301.13 |
| Adjustment disorder with depression | 309.0, 309.1 |
| Depressive disorder, not elsewhere classified | 311 |
| ICD-9 codes for inpatient psychiatric use | |
| General psychiatric evaluation | V70.2 |
| Psychologic evaluation and testing | 94.02, 94.09 |
| Psychiatric interview, consultation, and evaluation | 94.12 |
| Psychotherapy, individual | 94.3 |
| Psychotherapy, group | 94.42, 94.44 |
| CPT codes for outpatient psychiatric use | |
| Diagnostic interview examination | 90801 |
| Outpatient psychotherapy, individual | 90804-90807 |
| Outpatient psychotherapy, group | 90847, 90853 |
| Psychopharmacology appointment | 90862 |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, ninth revision.
Table A3.
Propensity-Matched Analyses
| Outcome | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Depression | 1.436 | 1.353 to 1.523 | < .001 |
| Inpatient psychiatric treatment | 1.519 | 1.381 to 1.671 | < .001 |
| Outpatient psychiatric treatment | 1.260 | 1.158 to 1.372 | < .001 |
Footnotes
Supported by Fitz’s Cancer Warriors, David and Cynthia Chapin, the Prostate Cancer Foundation, Hugh Simons in honor of Frank and Anne Simons, Scott Forbes and Gina Ventre, Campbell family in honor of Joan Campbell, and a grant from an anonymous family foundation. Also supported by the Professor Walter Morris-Hale Distinguished Chair in Urologic Oncology at Brigham and Women’s Hospital (Q.-D.T.) and an unrestricted educational grant from the Vattikuti Urology Institute (Q.-D.T.). The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute’s SEER Program under Contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California, Contract No. HHSN261201000035C awarded to the University of Southern California, and Contract No. HHSN261201000034C awarded to the Public Health Institute, and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under Agreement No. U58DP003862-01 awarded to the California Department of Public Health.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Kathryn T. Dinh, Brandon A. Mahal, Paul L. Nguyen
Financial support: Paul L. Nguyen
Collection and assembly of data: Kathryn T. Dinh, Gally Reznor, Paul L. Nguyen
Data analysis and interpretation: Kathryn T. Dinh, Gally Reznor, Vinayak Muralidhar, Brandon A. Mahal, Michelle D. Nezolosky, Toni K. Choueiri, Karen E. Hoffman, Jim C. Hu, Christopher J. Sweeney, Quoc-Dien Trinh, Paul L. Nguyen
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association of Androgen Deprivation Therapy With Depression in Localized Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kathryn T. Dinh
No relationship to disclose
Gally Reznor
No relationship to disclose
Vinayak Muralidhar
No relationship to disclose
Brandon A. Mahal
No relationship to disclose
Michelle D. Nezolosky
No relationship to disclose
Toni K. Choueiri
Honoraria: National Comprehensive Cancer Network, UpToDate
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol-Myers Squibb (Inst)
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst)
Karen E. Hoffman
No relationship to disclose
Jim C. Hu
No relationship to disclose
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix, BIND Biosciences
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, BIND Biosciences, Genentech, AstraZeneca
Research Funding: Janssen Biotech (Inst), Exelixis (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide; Exelixis: Abiraterone plus cabozantinib combination
Quoc-Dien Trinh
Speakers' Bureau: Intuitive Surgical
Paul L. Nguyen
Consulting or Advisory Role: Medivation, GenomeDx, Ferring
REFERENCES
- 1.D’Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 6.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 7.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841–850. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2011;29:647–653. doi: 10.1016/j.urolonc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. doi: 10.1016/j.eururo.2014.07.010. Nguyen PL, Alibhai SM, Basaria S, et al: Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 67:825-836, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Pirl WF, Greer JA, Goode M, et al. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psychooncology. 2008;17:148–153. doi: 10.1002/pon.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hervouet S, Savard J, Ivers H, et al. Depression and androgen deprivation therapy for prostate cancer: A prospective controlled study. Health Psychol. 2013;32:675–684. doi: 10.1037/a0031639. [DOI] [PubMed] [Google Scholar]
- 13.Timilshina N, Breunis H, Alibhai S. Impact of androgen deprivation therapy on depressive symptoms in men with nonmetastatic prostate cancer. Cancer. 2012;118:1940–1945. doi: 10.1002/cncr.26477. [DOI] [PubMed] [Google Scholar]
- 14.Cary KC, Singla N, Cowan JE, et al. Impact of androgen deprivation therapy on mental and emotional well-being in men with prostate cancer: Analysis from the CaPSURE™ registry. J Urol. 2014;191:964–970. doi: 10.1016/j.juro.2013.10.098. [DOI] [PubMed] [Google Scholar]
- 15.Wiechno PJ, Sadowska M, Kalinowski T, et al. Does pharmacological castration as adjuvant therapy for prostate cancer after radiotherapy affect anxiety and depression levels, cognitive functions and quality of life? Psychooncology. 2013;22:346–351. doi: 10.1002/pon.2095. [DOI] [PubMed] [Google Scholar]
- 16.van Tol-Geerdink JJ, Leer JW, van Lin EN, et al. Depression related to (neo)adjuvant hormonal therapy for prostate cancer. Radiother Oncol. 2011;98:203–206. doi: 10.1016/j.radonc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Saini A, Berruti A, Cracco C, et al. Psychological distress in men with prostate cancer receiving adjuvant androgen-deprivation therapy. Urol Oncol. 2013;31:352–358. doi: 10.1016/j.urolonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Psychooncology. 2015;24:472–477. doi: 10.1002/pon.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Cancer Institute: SEER*Stat Database: Incidence–SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (1973-2011 varying)–Linked to County Attributes–Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission. www.seer.cancer.gov.
- 20. doi: 10.1097/01.MLR.0000020942.47004.03. Warren JL, Klabunde CN, Schrag D, et al: Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3-IV-18, 2002 (suppl 8) [DOI] [PubMed] [Google Scholar]
- 21.Prasad SM, Eggener SE, Lipsitz SR, et al. Effect of depression on diagnosis, treatment, and mortality of men with clinically localized prostate cancer. J Clin Oncol. 2014;32:2471–2478. doi: 10.1200/JCO.2013.51.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. The effect of depression on stage at diagnosis, treatment, and survival in pancreatic adenocarcinoma. Surgery. 2012;152:403–413. doi: 10.1016/j.surg.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Lu-Yao GL, Albertsen PC, Li H, et al. Does primary androgen-deprivation therapy delay the receipt of secondary cancer therapy for localized prostate cancer? Eur Urol. 2012;62:966–972. doi: 10.1016/j.eururo.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sammon JD, Abdollah F, Reznor G, et al. Patterns of declining use and the adverse effect of primary androgen deprivation on all-cause mortality in elderly men with prostate cancer. Eur Urol. 2015;68:32–39. doi: 10.1016/j.eururo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325–335. doi: 10.1037/a0027957. [DOI] [PubMed] [Google Scholar]
- 28.Crystal S, Sambamoorthi U, Walkup JT, et al. Diagnosis and treatment of depression in the elderly Medicare population: Predictors, disparities, and trends. J Am Geriatr Soc. 2003;51:1718–1728. doi: 10.1046/j.1532-5415.2003.51555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bill-Axelson A, Garmo H, Nyberg U, et al. Psychiatric treatment in men with prostate cancer: Results from a nation-wide, population-based cohort study from PCBaSe Sweden. Eur J Cancer. 2011;47:2195–2201. doi: 10.1016/j.ejca.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers B, D’Agostino A, Walker J, et al. Gonadectomy and hormone replacement exert region- and enzyme isoform-specific effects on monoamine oxidase and catechol-O-methyltransferase activity in prefrontal cortex and neostriatum of adult male rats. Neuroscience. 2010;165:850–862. doi: 10.1016/j.neuroscience.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54:85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: The Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 34.McIntyre RS, Mancini D, Eisfeld BS, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31:1029–1035. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Shores MM, Moceri VM, Sloan KL, et al. Low testosterone levels predict incident depressive illness in older men: Effects of age and medical morbidity. J Clin Psychiatry. 2005;66:7–14. doi: 10.4088/jcp.v66n0102. [DOI] [PubMed] [Google Scholar]
- 36.Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7:S37–S43. (Suppl 5) [PMC free article] [PubMed] [Google Scholar]
- 37.Chipperfield K, Fletcher J, Millar J, et al. Predictors of depression, anxiety and quality of life in patients with prostate cancer receiving androgen deprivation therapy. Psychooncology. 2013;22:2169–2176. doi: 10.1002/pon.3269. [DOI] [PubMed] [Google Scholar]
- 38.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 39.Pirl WF, Siegel GI, Goode MJ, et al. Depression in men receiving androgen deprivation therapy for prostate cancer: A pilot study. Psychooncology. 2002;11:518–523. doi: 10.1002/pon.592. [DOI] [PubMed] [Google Scholar]
- 40.Jayadevappa R, Malkowicz SB, Chhatre S, et al. The burden of depression in prostate cancer. Psychooncology. 2012;21:1338–1345. doi: 10.1002/pon.2032. [DOI] [PubMed] [Google Scholar]
- 41.Chipperfield K, Brooker J, Fletcher J, et al. The impact of physical activity on psychosocial outcomes in men receiving androgen deprivation therapy for prostate cancer: A systematic review. Health Psychol. 2014;33:1288–1297. doi: 10.1037/hea0000006. [DOI] [PubMed] [Google Scholar]
- 42.Llorente MD, Burke M, Gregory GR, et al. Prostate cancer: A significant risk factor for late-life suicide. Am J Geriatr Psychiatry. 2005;13:195–201. doi: 10.1176/appi.ajgp.13.3.195. [DOI] [PubMed] [Google Scholar]
- 43.Parahoo K, McDonough S, McCaughan E, et al. Psychosocial interventions for men with prostate cancer. Cochrane Database Syst Rev. 2013;12:CD008529. doi: 10.1002/14651858.CD008529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irani J, Salomon L, Oba R, et al. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: A double-blind, randomised trial. Lancet Oncol. 2010;11:147–154. doi: 10.1016/S1470-2045(09)70338-9. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadi-Davis S, Velasco R, Stewart JT. Goserelin-induced depression in a man with prostate cancer. Psychosomatics. 2014;55:720–722. doi: 10.1016/j.psym.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Sharpley CF, Bitsika V, Wootten AC, et al. Differences in major depressive disorder and generalised anxiety disorder symptomatology between prostate cancer patients receiving hormone therapy and those who are not. Psychooncology. 2014;23:1350–1355. doi: 10.1002/pon.3566. [DOI] [PubMed] [Google Scholar]
- 47.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–471. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Cancer Society: What are the key statistics about prostate cancer? http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics.
- 49.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40::IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. (Suppl 8) [DOI] [PubMed] [Google Scholar]
- 50.Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: Findings from a nationally representative survey. World Psychiatry. 2015;14:74–81. doi: 10.1002/wps.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharpley CF, Bitsika V, Christie DR. Diagnosing ‘male’ depression in men diagnosed with prostate cancer: The next step in effective translational psycho-oncology interventions? Psychooncology. 2014;23:1042–1048. doi: 10.1002/pon.3530. [DOI] [PubMed] [Google Scholar]