Abstract
Purpose
Patients with human papillomavirus (HPV)–related oropharyngeal cancer (OPC) generally present with more advanced disease but have better survival than patients with HPV-unrelated OPC. The current American Joint Commission on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system for OPC was developed for HPV-unrelated OPC. A new staging system is needed to adequately predict outcomes of patients with HPV-related OPC.
Patients and Methods
Patients with newly diagnosed HPV-positive OPC (by p16 immunohistochemistry or in situ hybridization) treated at our institution from January 2003 through December 2012 were included. By using recursive partitioning analysis (RPA), we developed new stage groupings with both traditional OPC regional lymph node (N) categories and nasopharyngeal carcinoma (NPC) N categories. Survival was estimated by the Kaplan-Meier method, and the relationship between stage and survival was examined by using Cox proportional hazards regression analysis.
Results
A total of 661 patients with HPV-positive OPC met the inclusion criteria. With the traditional TNM staging system, there was no difference in survival between stages (P = .141). RPA with NPC N categories resulted in more balanced stage groups and better separation between groups for 5-year survival than RPA with traditional OPC N categories. With the stage groupings that were based in part on NPC N categories, the risk of death increased with increasing stage (P for trend < .001), and patients with stage III disease had five times the risk of death versus patients with stage IA disease.
Conclusion
New stage groupings that are based on primary tumor (T) categories and NPC N categories better separate patients with HPV-positive OPC with respect to survival than does the current AJCC/UICC TNM staging system. Although confirmation of our findings in other patient populations is needed, we propose consideration of NPC N categories as an alternative to the traditional OPC N categories in the new AJCC/UICC TNM staging system that is currently being developed.
INTRODUCTION
Oropharyngeal carcinoma (OPC) is increasing in incidence, particularly in the developed world, and a substantial and increasing proportion of OPC is attributable to human papillomavirus (HPV) infection.1-3 HPV-related OPC is biologically and clinically distinct from HPV-unrelated OPC (commonly caused by tobacco and alcohol use); HPV-related OPC is associated with notably better survival than HPV-unrelated OPC, even though HPV-related OPC often is diagnosed at later stages.4-7
In patients with head and neck cancer, the regional lymph node (N) category has traditionally been considered the most important prognostic indicator, and cervical lymph node metastases are associated with a 50% reduction in 5-year overall survival (OS).8 However, recent studies have found that, for OPC, HPV status and, to a lesser extent, tobacco smoking are the most important prognostic factors.4,9 The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC), based the current TNM staging system for OPC on tobacco-related/HPV-unrelated OPC,10 and TNM stages correspond well with outcomes for patients with tobacco- and alcohol-related OPC. We and others have shown that the current AJCC/UICC TNM staging system is not adequate for HPV-related OPC10-13; therefore, there is a need for a separate staging system for HPV-related OPC. Recently, Huang et al12 proposed a new staging system for HPV-related OPC that is based on recursive partitioning analysis (RPA) that performed better than the seventh-edition AJCC/UICC TNM staging system among a cohort of patients in Canada.
The goal of this study was to compare current TNM stage groupings and alternative stage groupings that are based on RPA in terms of their abilities to separate patients with HPV-related OPC treated at our institution with respect to survival.
PATIENTS AND METHODS
Study Population
This study was a retrospective database abstraction that included all patients with newly diagnosed, histopathologically confirmed, nonmetastatic (M0) squamous cell carcinoma of the oropharynx (OPC) who were definitively treated with intensity-modulated radiation therapy at The University of Texas MD Anderson Cancer Center during the period from January 1, 2003, through December 31, 2012. Less than 15% of patients with newly diagnosed OPC who presented to the institution during the study period received treatment elsewhere, and less than 2% were treated with primary surgery; therefore, virtually all patients were treated with definitive intensity-modulated radiation therapy. Demographic, exposure, and clinical data were collected prospectively as part of routine clinical care; they were not collected specifically for this project. Each imaging study was reviewed by a radiation oncologist for verification of nodal status, including bilaterality and low-neck disease. Disease progression was confirmed by biopsy. The study protocol was approved by the institutional review board, and all patients provided written informed consent.
In Situ Hybridization and p16 Immunohistochemistry
Patients were tested for tumor HPV status upon diagnosis. They were considered to have HPV-positive OPC if their disease was positive for HPV by in situ hybridization (ISH), p16 immunohistochemistry (IHC), or both methods. Both assays were performed using paraffin-embedded tumor tissue, and methods have been described elsewhere.14,15 HPV ISH was performed using the ISH-catalyzed signal-amplification method for biotinylated probes for types 16, 18, 31, 33, and 51 (Enzo, Farmingdale, NY). An HPV-positive tumor was defined as a tumor for which there was specific staining of tumor-cell nuclei for HPV. Tumor p16 expression was evaluated by immunohistochemical analysis with the CINtec histology kit (Ventana Medical Systems, Tucson, AZ). Positive p16 expression was defined as strong and diffuse nuclear and cytoplasmic staining in 70% or more of the tumor cells.
Statistical Methods
Stata 12.0 (StataCorp, College Station, TX) was used for all statistical analyses. All tests were two sided, and a P value of < .05 was considered statistically significant. Standard descriptive statistics were used to describe the study population. Statistical significance of differences between groups was determined by the χ2 test for categorical variables and by the t test with adjustment for unequal variances where indicated or by the nonparametric equality-of-medians test for continuous variables.
For the survival analysis, OS was defined as time from diagnosis to death as a result of any cause, and progression-free survival (PFS) was defined as time from diagnosis to first clinically detectable recurrence or death as a result of any cause. For OS, patients who were alive at the end of the study period or lost to follow up were considered censored; for PFS, patients who were alive and recurrence free at the end of the study period or lost to follow up were considered censored. The Kaplan-Meier method with the log-rank test was used to evaluate differences in survival between groups. Cox proportional hazards regression was used to calculate hazard ratios (HRs) with 95% CIs. On the basis of statistical significance in univariate analysis, age, pack-years of smoking, and the use of chemotherapy (induction and/or concurrent with intensity-modulated radiation therapy) were included in the final multivariable model. Because none of the interaction terms for stage and age, stage and pack-years, and stage and chemotherapy was significant, they were not included in the final model. We quantified the predictive discrimination of the models by using the concordance probability estimate (CPE), which takes into account censored outcomes.16
We used the program STREE (http://c2s2.yale.edu/software/stree/) to perform RPA to determine stage grouping for patients with HPV-positive OPC.17 Only primary tumor (T) and N categories were included in the RPA models. We entered T and N categories for OPC into the model as ordinal variables (T1/T2/T3/T4 and N0/N1/N2a/N2b/N2c/N3). We also performed a separate RPA, in which we entered T categories for OPC and N categories for NPC (N0/N1/N2/N3) into the model to determine whether this made a difference in staging. NPC N categories are based on the number of positive nodes as well as on laterality of positive nodes and whether supraclavicular nodes are involved. Although the AJCC/UICC system for NPC uses a clinical descriptor for supraclavicular nodes, we captured radiographic nodal locations. Hence, we defined involvement of supraclavicular nodes as level-4 involvement (nodes below the level of the cricoid cartilage) but otherwise adhered to the AJCC definitions for NPC nodal groupings. Appendix Table A1 (online only) shows the detailed definitions of the AJCC/UICC N categories.18
Multiple imputation with logistic regression was used to impute HPV status for the 789 patients for whom HPV status was unknown. We created 50 data sets and included age, sex, pack-years of smoking, chemotherapy status, T category, NPC N category, and the Kaplan-Meier survivor function. We calculated HRs with 95% CIs by using Rubin’s combination rules for the subset of patients who were HPV positive by imputation.
RESULTS
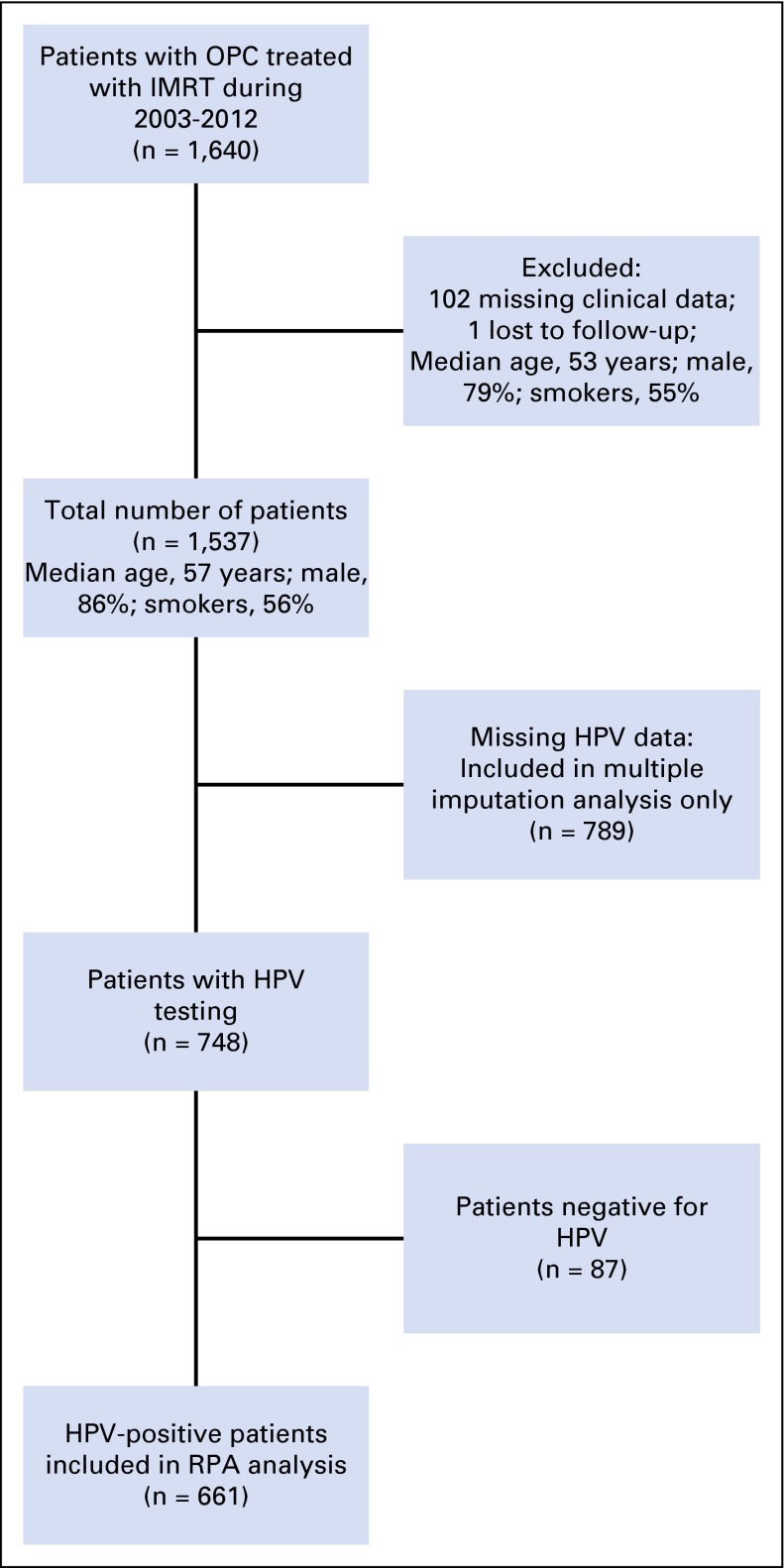
A total of 1,640 patients with OPC were treated with definitive intensity-modulated radiation therapy at our institution during the period from January 1, 2003, through December 31, 2012. Appendix Figure A1 (online only) shows the patient selection diagram. One hundred three patients were excluded because of missing clinical or follow-up data, which resulted in a final sample size of 1,537 patients. Of these, 748 patients (48.7%) had known HPV status by ISH and/or p16 IHC, 661 of whom (88%) had HPV-positive disease. Median follow-up time for patients with HPV-positive OPC who were alive at last follow up was 41 months (range, 3 to 122 months). Patients with known HPV status and those with unknown HPV status were similar with respect to age and sex, but patients with known HPV status were more likely to be never smokers, to present with a lower T category, and to have nodal metastases (Appendix Table A2). Demographic, exposure, and clinical data for patients with HPV-positive OPC are listed in Table 1. Among patients with HPV-positive OPC, 64% had tumors positive by both p16 IHC and ISH, 12% had tumors positive by p16 IHC only, and less than 1% had tumors positive by ISH only (Table 1). Eighty-seven percent of patients with HPV-positive OPC were men, and 51% were never smokers (Table 1). Furthermore, approximately 70% of patients with HPV-positive OPC had T1 or T2 tumors, and 93% had positive nodal status (Table 1). Only 12 patients had oncologic surgery at their primary tumor sites before radiation.
Table 1.
Demographic, Smoking Exposure, and Clinical Characteristics of 661 Patients With HPV-Positive OPC
| Variable | No. (%) of Patients |
|---|---|
| Age, years | |
| Mean (SD) | 57.3 (8.9) |
| Median (IQR) | 56 (52-63) |
| Sex | |
| Male | 574 (86.8) |
| Female | 87 (13.2) |
| Smoking status | |
| Never | 335 (50.7) |
| Former | 210 (31.8) |
| Current | 116 (17.6) |
| Smoking exposure, pack-years* | |
| Mean (SD) | 25.9 (22.1) |
| Median (IQR) | 20 (9-36) |
| p16/ISH HPV status | |
| p16+/ISH+ | 423 (64.0) |
| p16+/ISH− | 81 (12.3) |
| p16−/ISH+ | 4 (.6) |
| p16+/ISH missing | 80 (12.1) |
| p16 missing/ISH+ | 73 (11.0) |
| T category | |
| T1 | 212 (32.1) |
| T2 | 251 (38.0) |
| T3 | 122 (18.5) |
| T4 | 76 (11.5) |
| N category† | |
| N0 | 47 (7.1) |
| N1 | 82 (12.4) |
| N2a | 66 (10.0) |
| N2b | 293 (44.4) |
| N2c | 133 (20.2) |
| N3 | 39 (5.9) |
| NPC N category | |
| N0 | 47 (7.1) |
| N1 | 411 (62.2) |
| N2 | 106 (16.0) |
| N3 | 97 (14.7) |
| Chemotherapy‡ | |
| No | 124 (18.8) |
| Yes | 537 (81.2) |
Abbreviations: HPV, human papillomavirus; IQR, interquartile range; ISH, in situ hybridization; NPC, nasopharyngeal cancer; OPC, oropharyngeal cancer; SD, standard deviation.
Former and current smokers only; four patients were missing pack-year data.
N category could not be determined for one patient.
Chemotherapy included induction and/or concurrent therapy (n = 117 patients received induction only, n = 233 patients received concurrent only, and n = 187 patients received both).
OS According to Current AJCC/UICC TNM Stages and Proposed New Stage Groupings for HPV-Positive OPC
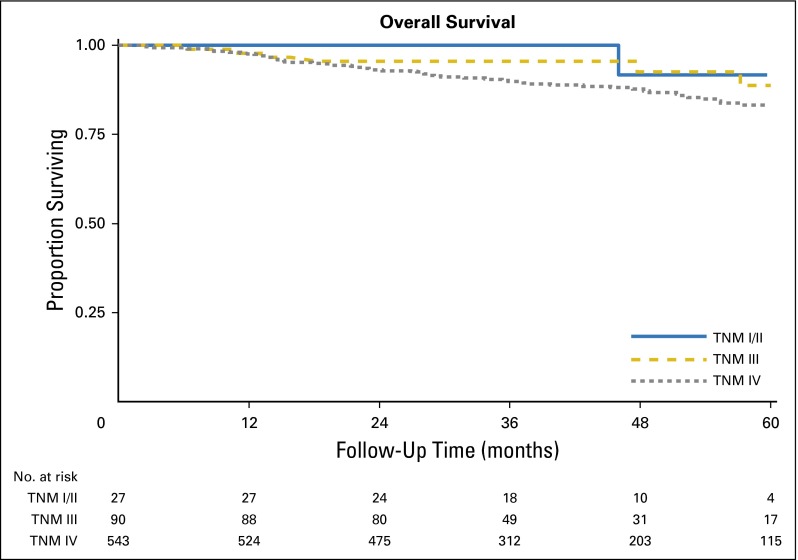
There was no significant difference in OS among patients with HPV-positive OPC across TNM stages, as defined according to the seventh edition of the AJCC/UICC system (P = .141; Appendix Fig A2). Moreover, the distribution of patients between stages was imbalanced, because 82% of patients had stage IV disease.
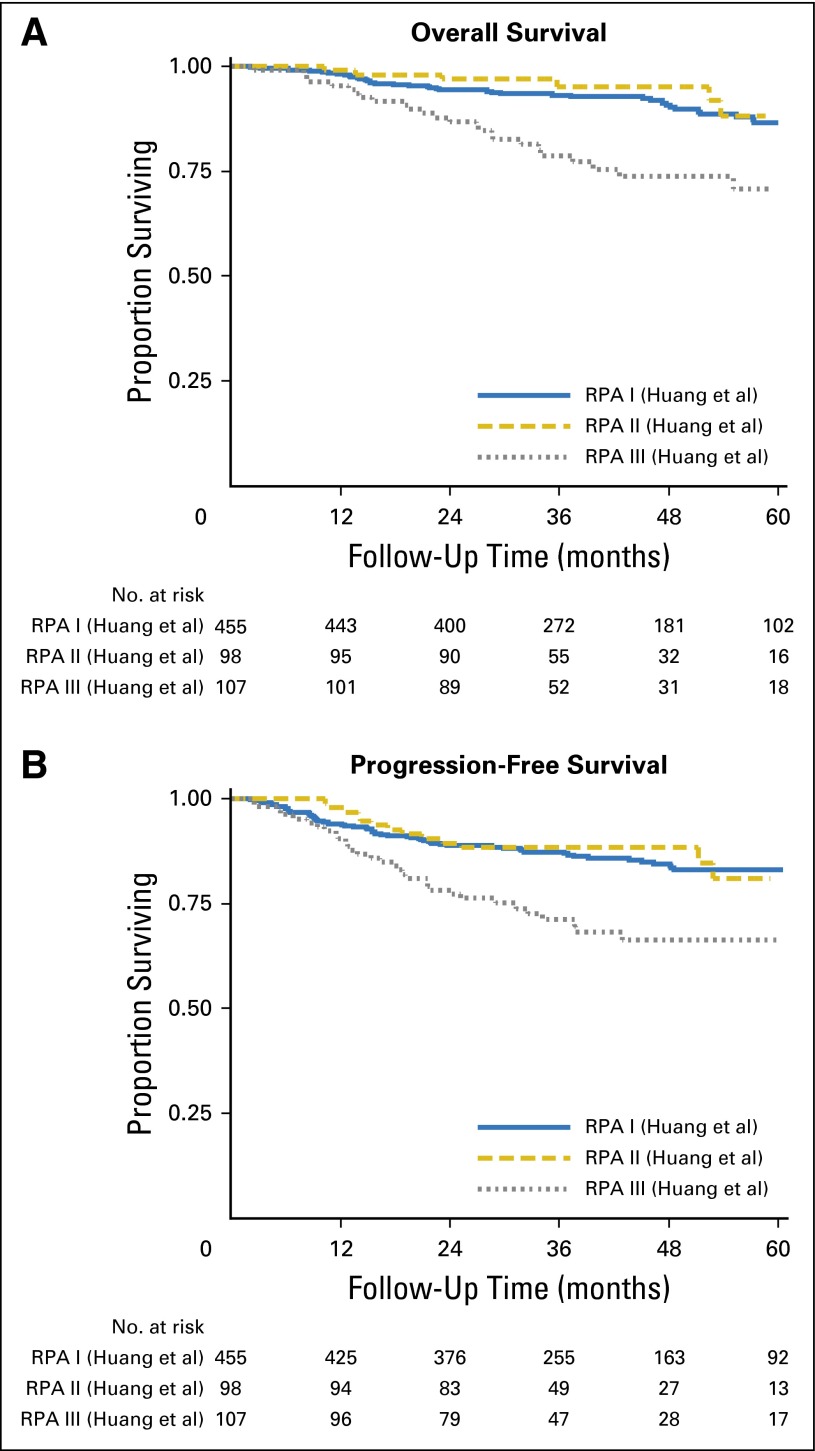
When we used the stage grouping for HPV-positive OPC recently suggested by Huang et al12 (stage I: T1-3, N0-N2b; II: T1-3, N2c; III: T4 or N3), we found no significant difference in OS or PFS between patients with stage I and stage II disease (P = .807 for OS and P = .929 for PFS), although there was a significant difference in survival between patients with stage II and stage III disease (P = .001 for OS and P = .003 for PFS; Appendix Fig A3A and A3B).12 Interestingly, patients with stage II disease had nonsignificantly better OS than those with stage I disease (5-year OS, 87% for stage I and 88% for stage II). Similar to the AJCC/UICC stage grouping, the distribution between stages was imbalanced; 69% of patients had stage I disease.
RPA
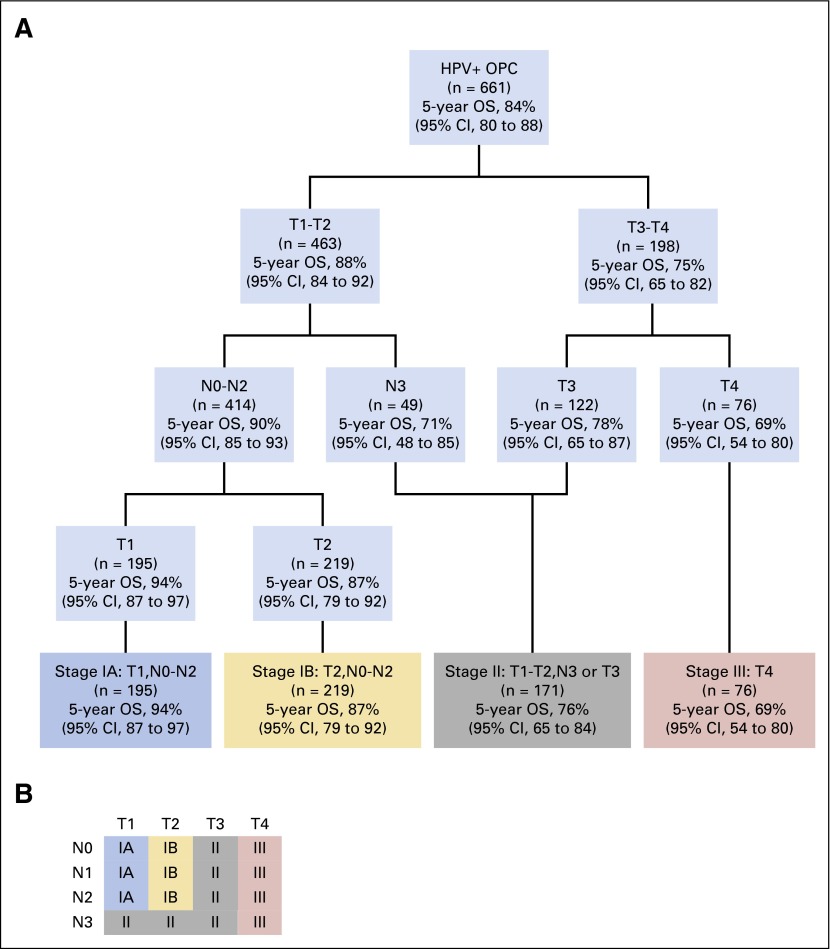
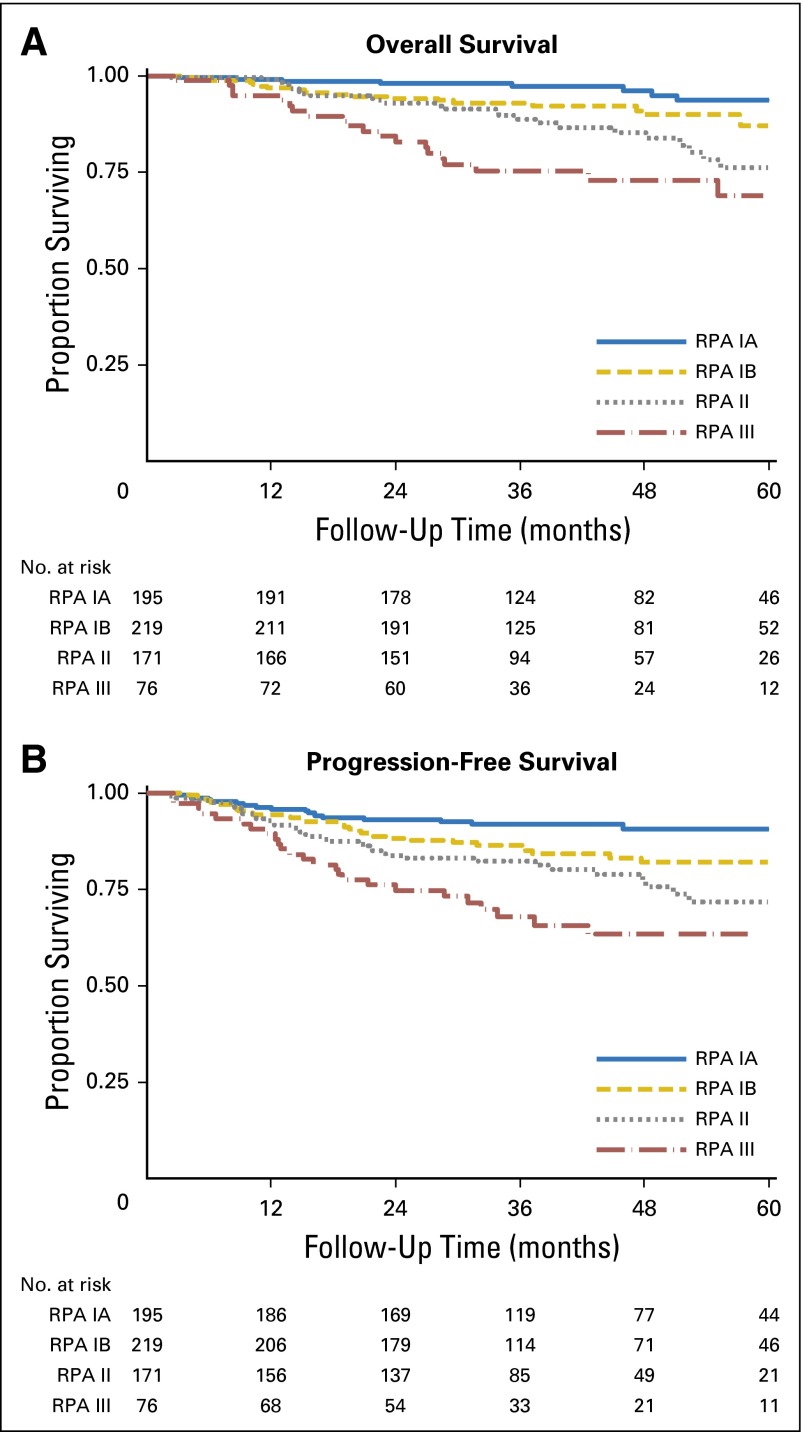
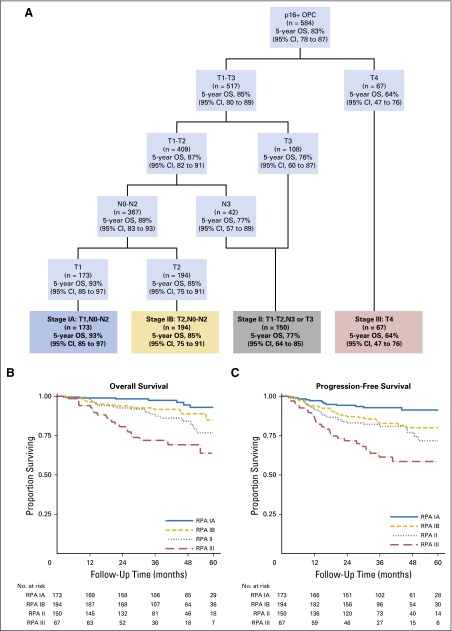
In RPA with the traditional T and N categories for OPC, we found that T category was most important and that N category had no effect on stage grouping (results not shown). When we replaced OPC N categories with NPC N categories, T category was still most important, but N category became significant among patients who had HPV-positive OPC with T1 and T2 tumors. Figure 1A shows stage groups that are based on RPA that used NPC N categories with 5-year OS estimates, and Figure 1B shows the T and N categories for each stage. We propose division of patients with stage I disease into two groups: stage IA, defined as T1, N0-N2; and stage IB, defined as T2, N0-N2. Stage II would be defined as T1-T2, N3 or T3, N0-N3; stage III would be defined as T4 regardless of nodal involvement, and stage IV would be all M1 tumors (Fig 1A and 1B). These groups are well balanced with respect to the number of patients in each group and are significantly different from each other with respect to both OS and PFS; absolute differences in survival rates are at least 7% between groups (Fig 2A and 2B, respectively). Moreover, this stage grouping has greater predictive power than either the AJCC/UICC system or that proposed by Huang et al12 (CPE for our stage grouping, .670; AJCC/UICC, .555; Huang et al,12 .575).
Fig 1.
Stage groups for human papillomavirus –positive (HPV+) oropharyngeal cancer (OPC) that are based on recursive partitioning analysis (RPA) with nasopharyngeal cancer N categories. (A) Stage groups and 5-year overall survival (OS) estimates. (B) Diagram of proposed stages (stage IV would be reserved for patients with M1 disease).
Fig 2.
Kaplan-Meier curves for overall survival (A) and progression-free survival (B) among patients with human papillomavirus (HPV) –positive oropharyngeal cancer by using proposed stage groups that are based on recursive partitioning analysis (RPA) with nasopharyngeal cancer regional lymph node categories.
Next we calculated 5-year survival rates by RPA stage and RPA stage stratified by smoking and chemotherapy use (Table 2). For all patients regardless of stage, the 5-year OS rate was 84% (95% CI, 80% to 88%), and the 5-year PFS rate was 80% (95% CI, 76% to 83%). Overall, patients with greater than 10 pack-years of smoking had significantly worse OS and PFS than those with 10 or fewer pack-years (P = .011 and P = .008, respectively; Table 2); however, there was no significant difference in survival between these groups when stratified by stage (data not shown). As shown in Table 2, when patients were stratified by pack-years (≤ 10 v > 10), the RPA stage grouping performed equally well in both groups. Stage groups were significantly different from each other for both OS and PFS. When patients were stratified by whether treatment included chemotherapy (induction and/or concurrent with intensity-modulated radiation therapy), stage groups were significantly different from each other except for OS among patients who received no chemotherapy (Table 2). Within stage groups, there were no significant differences in OS or PFS between patients who were and those who were not treated with chemotherapy (data not shown).
Table 2.
5-Year OS and PFS Rates of Patients With HPV-Positive OPC by RPA Stage and RPA Stage Stratified by Smoking Exposure and Chemotherapy Use
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| No. of Events/Total No. | % 5-Year Survival (95% CI) | P | No. of Events/Total No. | % 5-Year Survival (95% CI) | P | |
| All patients | 79/661 | 84 (80 to 88) | 115/661 | 80 (76 to 83) | ||
| RPA stage | ||||||
| IA | 8/195 | 94 (87 to 97) | 16/195 | 91 (85 to 94) | ||
| IB | 21/219 | 87 (79 to 92) | 34/219 | 82 (75 to 87) | ||
| II | 28/171 | 76 (65 to 84) | 37/171 | 72 (62 to 80) | ||
| III | 22/76 | 69 (54 to 80) | < .001 | 28/76 | 64 (50 to 74) | < .001 |
| Smoking* | ||||||
| Patients with ≤ 10 pack-years | 41/427 | 86 (81 to 90) | 62/427 | 83 (78 to 87) | ||
| RPA stage | ||||||
| IA | 5/144 | 95 (88 to 98) | 12/144 | 91 (85 to 95) | ||
| IB | 12/140 | 89 (81 to 94) | 20/140 | 83 (74 to 89) | ||
| II | 16/110 | 76 (61 to 86) | 21/110 | 75 (62 to 84) | ||
| III | 8/33 | 68 (37 to 86) | < .001 | 9/33 | 71 (49 to 85) | .018 |
| Patients with > 10 pack-years | 37/230 | 80 (72 to 86) | 52/230 | 75 (68 to 81) | ||
| RPA stage | ||||||
| IA | 3/51 | 90 (71 to 97) | 4/51 | 90 (74 to 96) | ||
| IB | 9/76 | 81 (63 to 91) | 14/76 | 80 (67 to 88) | ||
| II | 12/61 | 76 (58 to 87) | 16/61 | 67 (49 to 80) | ||
| III | 13/42 | 71 (54 to 82) | .006 | 18/42 | 60 (43 to 73) | < .001 |
| Chemotherapy use | ||||||
| Patients with no chemotherapy | 6/124 | 92 (83 to 97) | 13/124 | 88 (80 to 93) | ||
| RPA stage | ||||||
| IA | 4/88 | 92 (79 to 97) | 7/88 | 90 (80 to 95) | ||
| IB | 1/32 | 94 (80 to 100) | 4/32 | 86 (65 to 94) | ||
| II | 1/4 | 75 (13 to 96) | 2/4 | 50 (6 to 84) | ||
| III | 0 | [em] | .197 | 0 | [em] | .009 |
| Patients with chemotherapy | 73/537 | 82 (77 to 86) | 102/537 | 78 (74 to 82) | ||
| RPA stage | ||||||
| IA | 4/107 | 96 (89 to 98) | 9/107 | 91 (84 to 95) | ||
| IB | 20/187 | 85 (76 to 91) | 30/187 | 81 (74 to 87) | ||
| II | 27/167 | 76 (64 to 84) | 35/167 | 72 (62 to 80) | ||
| III | 22/76 | 69 (54 to 80) | < .001 | 28/76 | 64 (50 to 74) | < .001 |
Abbreviations: HPV, human papillomavirus; OPC, oropharyngeal cancer; OS, overall survival; PFS, progression-free survival; RPA, recursive partitioning analysis.
Four patients were missing pack-year data.
Survival Analysis Using Staging Groups Derived From RPA
In multivariable Cox regression, there was a significant trend for increased risk of death among patients in each successive stage group after adjustment for age, pack-years of smoking, and chemotherapy use (P for trend < .001; Table 3; Appendix Table A3). Compared with patients who had stage IA disease, those who had stage III disease had five times the risk of death (HR, 5.0; 95% CI, 2.0 to 12.2). These results were consistent in an expanded cohort after multiple imputation of HPV status with and without multivariable adjustment (P for trend < .001; Table 3).
Table 3.
Hazard Ratios for Patients With OPC Known to be HPV Positive and With Imputed HPV-Positive Status by RPA Stage Adjusted for Age, Pack-Years, and Chemotherapy Use
| RPA stage | No. (%) of Patients | Analysis by Type of HPV-Positive Status | |||
|---|---|---|---|---|---|
| Known | Imputed | ||||
| Crude HR (95% CI) | Adjusted* HR (95% CI) | Crude HR (95% CI) | Adjusted* HR (95% CI) | ||
| IA | 195 (29.5) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| IB | 219 (33.1) | 2.5 (1.1 to 5.6) | 2.1 (.9 to 4.8) | 1.4 (.9 to 2.3) | 1.4 (.8 to 2.2) |
| II | 171 (25.9) | 4.4 (2.0 to 9.8) | 3.4 (1.5 to 7.8) | 2.8 (1.8 to 4.3) | 2.6 (1.6 to 4.3) |
| III | 76 (11.5) | 8.2 (3.7 to 18.5) | 5.0 (2.0 to 12.2) | 6.4 (4.1 to 9.9) | 5.2 (3.0 to 8.9) |
Abbreviations: HPV, human papillomavirus; HR, hazard ratio; OPC, oropharyngeal cancer; RPA, recursive partitioning analysis.
Adjusted for age, smoking pack-years, and chemotherapy use.
DISCUSSION
HPV status is a strong independent predictor of prognosis for patients with OPC; thus, it has been suggested by our group and others that a new staging system for HPV-related OPC is urgently needed.10-12,19,20 Here, we used RPA to explore new stage groupings for HPV-positive OPC and found that incorporation of NPC N categories resulted in better distribution of patients between groups than did use of the traditional OPC N categories.
Groome et al20 identified four characteristics of useful stage groupings: similar survival rates for patients within groups defined by T and N category; significantly different survival rates across groups; accurate prediction of outcome; and balanced distribution of patients between groups. When we applied the AJCC/UICC TNM staging criteria to our patients with HPV-positive OPC, we did not find differences in survival between stage groups, and the distribution of patients was unbalanced: the majority of patients had stage IV disease. The lack of prognostic differences and the unbalanced distribution between groups highlight the need for a revised staging system. Huang et al12 recently proposed a new stage grouping for HPV-positive OPC; however, when we applied this grouping to our patient population, we found that most patients had stage I disease; there were no significant differences in survival between stage I and II; and, although stage III was significantly different, only about 16% of patients was in this group. Therefore, we performed RPA with different N categories for our patient population to determine whether alternate stage groupings performed better.
When we used traditional OPC N categories in the RPA analysis, T category was the most important factor, whereas N category had a negligible effect. Others have similarly found that T category but not N category predicts survival for patients with HPV-positive OPC.10,19,21,22 In an analysis of Surveillance, Epidemiology, and End Results data, Keane et al13 found that the effect of T category on head and neck cancer–specific mortality increased between 1997 and 2008 (P for interaction = .01), whereas the effect of N category declined during this same period (P for interaction < .001), and overall stage was not predictive of outcome.13 Similarly, Ward et al10 retrospectively analyzed 266 patients with OPC and found that the TNM system adequately staged patients with HPV-negative but not HPV-positive disease. Among patients with HPV-positive disease, only T category was prognostic; there was no significant difference in survival according to N category or TNM stage.10 Conversely, among patients with HPV-negative disease, T category was not prognostic, whereas N category was.10 These results and ours demonstrate the inadequacy of the current N classification for HPV-positive OPC.
Although tumors of the oropharynx have the same N classification as tumors of the other major head and neck cancer sites (oral cavity, hypopharynx, larynx, and paranasal sinuses), tumors of the nasopharynx have a different N classification because of their different risk factors (eg, Epstein-Barr virus is a risk factor for NPC but not other head and neck cancers) and natural history.8 The fact that HPV-related OPC is another virally induced cancer with a natural history different from that of tumors at other head and neck sites indicates the need for different staging criteria for HPV-positive OPC as well. Consequently, we applied NPC N categories in our RPA to determine the usefulness of these categories in staging HPV-positive OPC. To our knowledge, no other studies have applied the NPC N categories to HPV-positive OPC, although revision of N categories to take into account nodal size, bilaterality of nodes, and matted nodes has been suggested.23
Inclusion of NPC N categories in our RPA resulted in well-differentiated stage groups with respect to both OS and PFS (P < .001 for both). There was an increasing risk of death with increasing stage, and the risk of death was five times as high in patients with stage III disease as in patients with stage IA disease. When we stratified patients on the basis of smoking history (≤ 10 v > 10 pack-years), we found that our RPA stage groups separated never/light smokers and heavy smokers well with respect to both 5-year OS and 5-year PFS. However, when these groups were compared within each stage group, we did not find significant differences in survival. Thus, although smoking is an important prognostic factor for HPV-positive OPC, smoking may not need to be included in a new staging system if we assume that TNM stages adequately separate patients.
A limitation of this study was the lack of knowledge of HPV status for greater than half of all patients in the cohort, because patients who have their diagnoses confirmed via fine-needle aspiration are not routinely tested for HPV status. However, we restricted our stage analysis to HPV-positive patients, and our proposed staging system worked similarly in patients with unknown HPV status when we imputed HPV status and controlled for multiple variables. We considered patients to have HPV-positive OPC if their disease was positive for HPV by either p16 IHC or ISH, and there may have been some false positives. However, the number of false positives is likely small, because less than 13% of patients had disease that was positive by only one method, and both methods are now well established for determining tumor HPV DNA status.24-27 Furthermore, repeat analysis restricted to patients who were p16 positive resulted in the same RPA groups as shown in Appendix Figure A4. We acknowledge that this analysis is limited to our institutional experience and that survival outcomes for our patients and patients at other institutions may differ. Therefore, additional studies with other patient populations are needed to confirm our findings.
In this single-center cohort study, we demonstrated that the current AJCC/UICC TNM staging system is inadequate for predicting survival in patients with HPV-positive OPC. Moreover, we used RPA to identify new staging categories and found that incorporation of NPC N categories resulted in better separation of stage groups with respect to both OS and PFS and provided more even distribution of patients between groups. Although the results of this study require confirmation in other populations of patients with HPV-positive OPC, we propose that NPC N categories be considered alternatives to traditional OPC N categories in the new staging system for HPV-positive OPC currently being developed by the AJCC/UICC.
Acknowledgment
The authors thank Stephanie Deming for manuscript editing.
GLOSSARY TERMS
- American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging:
a cancer staging system that describes the extent of cancer in a patient’s body. “T” describes the size of the tumor and whether it has invaded nearby tissue; “N” describes regional lymph nodes that are involved; “M” describes distant metastasis (spread of cancer from one body part to another). The TNM Classification of Malignant Tumours was developed and maintained by the UICC to achieve consensus on one globally recognized standard for classifying the extent of spread of cancer. The TNM classification was also used by the AJCC. In 1987,: the UICC and AJCC staging systems were unified into a single staging system. Prognosis of a patient is defined by TNM classification.
- human papillomavirus (HPV):
a double-stranded DNA virus from the papillomaviridae family. Human papillomavirus is a cause of cervical cancer as well as of a subset of cancers of the anus, oropharynx, penis, vagina, and vulva.
- oropharyngeal carcinoma:
a carcinoma arising from the middle part of the pharynx behind the mouth and includes the back one third of the tongue, the soft palate, the side and back walls of the throat, and the tonsils. This typically includes the base of tongue, soft palate, uvula, tonsils, and pharyngeal walls.
- overall survival:
the duration between random assignment and death.
- progression-free survival:
time from random assignment until death or first documented relapse, categorized as either locoregional (primary site or regional nodes) failure or distant metastasis or death.
- recursive partitioning:
multivariable analysis that generates a clinically intuitive decision tree model in which the population is divided into prognostic subgroups. This is achieved through multiple dichotomous divisions on the basis of a set of independent variables.
Appendix
Fig A1.
Patient selection diagram that shows the median age, percentage who were men, and percentage of ever-smokers for patients excluded from and included in the analysis. HPV, human papillomavirus; IMRT, intensity-modulated radiation therapy; OPC, oropharyngeal cancer.
Fig A2.
Kaplan-Meier curve for overall survival among patients with human papillomavirus (HPV) –positive oropharyngeal cancer (OPC) by current American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM stage groups.
Fig A3.
Kaplan-Meier curves for overall survival (A) and progression-free survival (B) among patients with human papillomavirus (HPV) –positive oropharyngeal cancer (OPC) by stage groups that are based on recursive partitioning analysis (RPA) proposed by Huang et al12 (stage I: T1-3, N0-N2b; II: T1-3, N2c; III: T4 or N3).
Fig A4.
Stage groups for p16-positive oropharyngeal cancer (OPC) that are based on recursive partitioning analysis (RPA) with nasopharyngeal cancer regional lymph node categories. (A) Stage groups and 5-year overall survival (OS) estimates. Kaplan-Meier curves for overall survival (B) and progression-free survival (C) among patients with p16-positive OPC by using proposed stage groups that are based on RPA with nasopharyngeal cancer N categories.
Table A1.
AJCC (ed 7) Regional Lymph Node (N) Classification for Nasopharynx and Oropharynx/Hypopharynx Cancer
| Classification | Description by Cancer Type | |
|---|---|---|
| Nasopharynx Cancer | Oropharynx/Hypopharynx Cancer* | |
| NX | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Unilateral metastasis in cervical lymph node(s), ≤ 6 cm in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral, retropharyngeal lymph nodes, ≤ 6 cm in greatest dimension† | Metastasis in a single ipsilateral lymph node, ≤ 3 cm in greatest dimension |
| N2 | Bilateral metastasis in cervical lymph node(s), ≤ 6 cm in greatest dimension, above the supraclavicular fossa† | Metastasis in a single ipsilateral lymph node, > 3 cm but not > 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension |
| N2a | Not specified | Metastasis in a single ipsilateral lymph node > 3 cm but not > 6 cm in greatest dimension |
| N2b | Not specified | Metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension |
| N2c | Not specified | Metastasis in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension |
| N3 | Metastasis in a lymph node(s)† > 6 cm and/or to supraclavicular fossa | Metastasis in a lymph node > 6 cm in greatest dimension |
| N3a | > 6 cm in dimension | Not specified |
| N3b | Extension to the supraclavicular fossa‡ | Not specified |
NOTE. Used with permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this information is the AJCC Cancer Staging Manual (ed 7), published in 2010 by Springer.18
Metastases at level VII are considered regional lymph node metastases, originally described by Ho (Ho JHC: IARC Scientific Publication 20, 1978, pp 99–113). It is defined by three points: the superior margin of the sternal end of the clavicle, the superior margin of the lateral end of the clavicle, and the point where the neck meets the shoulder. Note that this definition would include caudal portions of levels IV and VB. All cases with lymph nodes (whole or part) in the fossa were considered N3b.
Midline nodes are considered ipsilateral nodes.
Supraclavicular zone or fossa is relevant to the staging of nasopharyngeal carcinoma and is the triangular region.
Table A2.
Demographics, Smoking Exposure, and Clinical Characteristics of Patients With OPC by Known and Unknown HPV Status
| Characteristic | No. (%) of Patients by HPV Status | P | |
|---|---|---|---|
| Known (n = 748) | Unknown (n = 789) | ||
| Age, years | |||
| Mean (SD) | 57.4 (9.0) | 57.7 (9.9) | .568* |
| Median (IQR) | 56.5 (52-63) | 57 (51-64) | .720 |
| Sex | .475 | ||
| Male | 640 (85.6) | 685 (86.8) | |
| Female | 108 (14.4) | 104 (13.2) | |
| Smoking status | .013 | ||
| Never | 357 (47.7) | 321 (40.7) | |
| Former | 246 (32.9) | 279 (35.4) | |
| Current | 145 (19.4) | 189 (24.0) | |
| T category | .001 | ||
| T1 | 236 (31.6) | 198 (25.1) | |
| T2 | 269 (36.0) | 275 (34.9) | |
| T3 | 142 (19.0) | 159 (20.2) | |
| T4 | 101 (13.5) | 157 (19.9) | |
| N category† | .002 | ||
| N0 | 55 (7.4) | 95 (12.1) | |
| N1 | 90 (12.1) | 111 (14.1) | |
| N2a | 71 (9.5) | 72 (9.1) | |
| N2b | 332 (44.4) | 284 (36.0) | |
| N2c | 156 (20.9) | 166 (21.1) | |
| N3 | 43 (5.8) | 60 (7.6) | |
Abbreviations: HPV, human papillomavirus; IQR, interquartile range; NPC, nasopharyngeal cancer; OPC, oropharyngeal cancer; SD, standard deviation.
Adjusted for unequal variances.
N category could not be determined in two patients (n = 1 with known and n = 1 with unknown HPV status).
Table A3.
HRs for Patients With OPC Known to Be HPV Positive and With Imputed HPV-Positive Status by RPA Stage Adjusted for Age, Pack Years, and Chemotherapy Use
| Variable | Analysis by Type of HPV-Positive Status | |||||||
|---|---|---|---|---|---|---|---|---|
| Known | Imputed | |||||||
| Crude | Adjusted | Crude | Adjusted | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| RPA stage | ||||||||
| IA | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| IB | 2.47 (1.09 to 5.58) | .030 | 2.07 (.89 to 4.84) | .092 | 1.44 (.90 to 2.31) | .130 | 1.37 (.84 to 2.23) | .209 |
| II | 4.44 (2.03 to 9.76) | < .001 | 3.37 (1.45 to 7.82) | .005 | 2.79 (1.80 to 4.34) | < .001 | 2.62 (1.58 to 4.33) | < .001 |
| III | 8.24 (3.67 to 18.51) | < .001 | 5.00 (2.04 to 12.18) | < .001 | 6.39 (4.10 to 9.94) | < .001 | 5.17 (3.01 to 8.89) | < .001 |
| Age | 1.04 (1.01 to 1.06) | .004 | 1.03 (1.00 to 1.05) | .030 | 1.04 (1.03 to 1.06) | < .001 | 1.03 (1.01 to 1.05) | < .001 |
| Smoking pack-years | 1.02 (1.01 to 1.03) | < .001 | 1.01 (1.01 to 1.02) | .001 | 1.01 (.99 to 1.03) | .285 | 1.00 (.99 to 1.02) | .309 |
| Chemotherapy | 3.18 (1.38 to 7.32) | .006 | 1.74 (.70 to 4.35) | .236 | 1.96 (1.36 to 2.81) | < .001 | 1.03 (.66 to 1.62) | .893 |
Abbreviations: HPV human papillomavirus; HR, hazard ratio; OPC, oropharyngeal cancer; RPA, recursive partitioning analysis.
Footnotes
Processed as a Rapid Communication manuscript.
Supported in part by the National Institutes of Health through The MD Anderson Cancer Center Support Grant No. CA016672. The research was accomplished within the Oropharynx Program at The University of Texas MD Anderson Cancer Center and funded in part through the Stiefel Oropharyngeal Research Fund.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 1833
AUTHOR CONTRIBUTIONS
Conception and design: Adam S. Garden, Erich M. Sturgis
Financial support: Erich M. Sturgis
Administrative support: Erich M. Sturgis
Provision of study materials or patients: Adam S. Garden, Erich M. Sturgis
Collection and assembly of data: Kristina R. Dahlstrom, Adam S. Garden, Ming Yann Lim
Data analysis and interpretation: Kristina R. Dahlstrom, Adam S. Garden, William N. William Jr, Erich M. Sturgis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Proposed Staging System for Patients With HPV-Related Oropharyngeal Cancer Based on Nasopharyngeal Cancer N Categories
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kristina R. Dahlstrom
No relationship to disclose
Adam S. Garden
No relationship to disclose
William N. William Jr
No relationship to disclose
Ming Yann Lim
No relationship to disclose
Erich M. Sturgis
No relationship to disclose
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117:2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 3.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–992. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 7.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roland N. 2012. Assessment and staging, in Watkinson J, Gilbert RW (eds): Stell and Maran's Textbook of Head and Neck Surgery and Oncology (ed 5). Boca Raton, FL, CRC Press, 2012, pp 38–56. [Google Scholar]
- 9.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward MJ, Mellows T, Harris S, et al. Staging and treatment of oropharyngeal cancer in the human papillomavirus era. Head Neck. 2015;37:1002–1013. doi: 10.1002/hed.23697. [DOI] [PubMed] [Google Scholar]
- 11.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: A staging system in need of repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus–related oropharyngeal carcinomas. J Clin Oncol. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 13.Keane FK, Chen YH, Neville BA, et al. Changing prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer. 2015;121:2594–2602. doi: 10.1002/cncr.29402. [DOI] [PubMed] [Google Scholar]
- 14.Guo M, Baruch AC, Silva EG, et al. Efficacy of p16 and ProExC immunostaining in the detection of high-grade cervical intraepithelial neoplasia and cervical carcinoma. Am J Clin Pathol. 2011;135:212–220. doi: 10.1309/AJCP1LLX8QMDXHHO. [DOI] [PubMed] [Google Scholar]
- 15.Guo M, Gong Y, Deavers M, et al. Evaluation of a commercialized in situ hybridization assay for detecting human papillomavirus DNA in tissue specimens from patients with cervical intraepithelial neoplasia and cervical carcinoma. J Clin Microbiol. 2008;46:274–280. doi: 10.1128/JCM.01299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–970. [Google Scholar]
- 17. Zhang H, Singer B: Analysis of censored data: Survival trees, in Zhang H, Singer B (eds): Recursive Partitioning in the Health Sciences. New York, NY: Springer, 1999, pp 93-103. [Google Scholar]
- 18. Edge S, Byrd DR, Compton CC. Pharynx, in AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010, pp 41-56. [Google Scholar]
- 19.Fischer CA, Kampmann M, Zlobec I, et al. p16 expression in oropharyngeal cancer: Its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21:1961–1966. doi: 10.1093/annonc/mdq210. [DOI] [PubMed] [Google Scholar]
- 20.Groome PA, Schulze KM, Mackillop WJ, et al. A comparison of published head and neck stage groupings in carcinomas of the tonsillar region. Cancer. 2001;92:1484–1494. doi: 10.1002/1097-0142(20010915)92:6<1484::aid-cncr1473>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Hong AM, Martin A, Armstrong BK, et al. Human papillomavirus modifies the prognostic significance of T stage and possibly N stage in tonsillar cancer. Ann Oncol. 2013;24:215–219. doi: 10.1093/annonc/mds205. [DOI] [PubMed] [Google Scholar]
- 22.Klozar J, Koslabova E, Kratochvil V, et al. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 2013;107:625–633. doi: 10.1002/jso.23292. [DOI] [PubMed] [Google Scholar]
- 23.Spector ME, Gallagher KK, Bellile E, et al. Patterns of nodal metastasis and prognosis in human papillomavirus–positive oropharyngeal squamous cell carcinoma. Head Neck. 2014;36:1233–1240. doi: 10.1002/hed.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 25.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010;17:394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]
- 26.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin-embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 27.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]