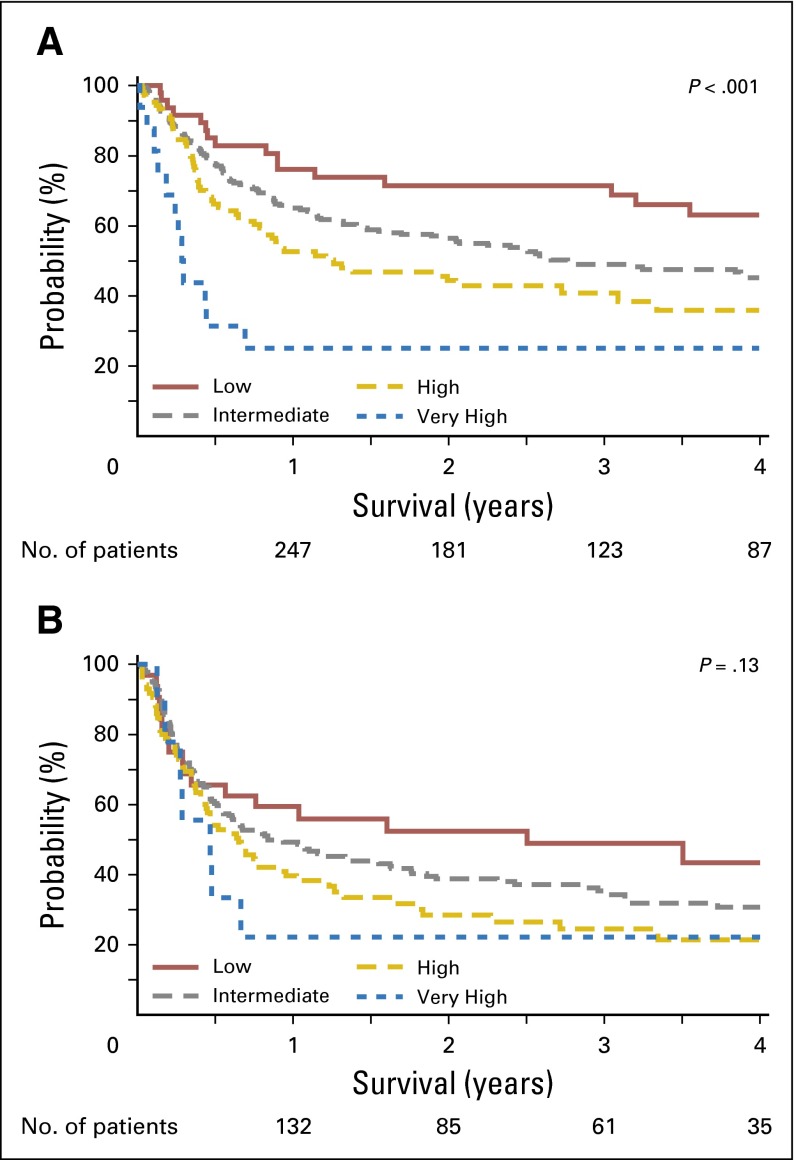
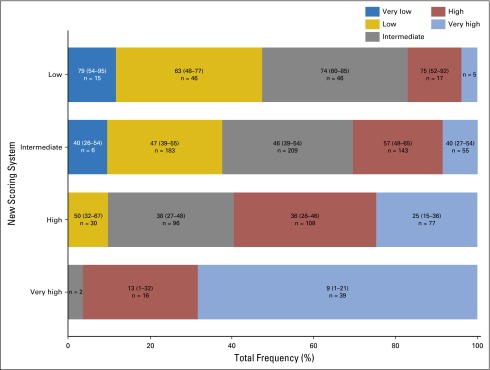
Brian C Shaffer
Brian C Shaffer
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,✉,
Kwang Woo Ahn
Kwang Woo Ahn
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Zhen-Huan Hu
Zhen-Huan Hu
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Taiga Nishihori
Taiga Nishihori
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Adriana K Malone
Adriana K Malone
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
David Valcárcel
David Valcárcel
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Michael R Grunwald
Michael R Grunwald
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Ulrike Bacher
Ulrike Bacher
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Betty Hamilton
Betty Hamilton
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Mohamed A Kharfan-Dabaja
Mohamed A Kharfan-Dabaja
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Ayman Saad
Ayman Saad
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Corey Cutler
Corey Cutler
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Erica Warlick
Erica Warlick
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Ran Reshef
Ran Reshef
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Baldeep Mona Wirk
Baldeep Mona Wirk
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Mitchell Sabloff
Mitchell Sabloff
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Omotayo Fasan
Omotayo Fasan
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Aaron Gerds
Aaron Gerds
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
David Marks
David Marks
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Richard Olsson
Richard Olsson
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
William Allen Wood
William Allen Wood
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Luciano J Costa
Luciano J Costa
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Alan M Miller
Alan M Miller
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Jorge Cortes
Jorge Cortes
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Andrew Daly
Andrew Daly
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Tamila L Kindwall-Keller
Tamila L Kindwall-Keller
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Rammurti Kamble
Rammurti Kamble
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
David A Rizzieri
David A Rizzieri
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Jean-Yves Cahn
Jean-Yves Cahn
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Robert Peter Gale
Robert Peter Gale
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Basem William
Basem William
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Mark Litzow
Mark Litzow
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Peter H Wiernik
Peter H Wiernik
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Jane Liesveld
Jane Liesveld
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Bipin N Savani
Bipin N Savani
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Ravi Vij
Ravi Vij
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Celalettin Ustun
Celalettin Ustun
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Edward Copelan
Edward Copelan
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Uday Popat
Uday Popat
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Matt Kalaycio
Matt Kalaycio
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Richard Maziarz
Richard Maziarz
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Edwin Alyea
Edwin Alyea
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Ron Sobecks
Ron Sobecks
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Steven Pavletic
Steven Pavletic
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Martin Tallman
Martin Tallman
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1,
Wael Saber
Wael Saber
1Brian C. Shaffer and Martin Tallman, Memorial Sloan Kettering Cancer Center; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai; Ran Reshef, Columbia University Medical Center, New York; Mark Litzow, Mayo Clinic Rochester; Jane Liesveld, University of Rochester Medical Center, Rochester; Peter H. Wiernik, Our Lady of Mercy Medical Center, Bronx, NY; Kwang Woo Ahn, Zhen-Huan Hu, and Wael Saber, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori and Mohamed A. Kharfan-Dabaja, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; David Valcárcel, Hospital Vall d’Hebron, Barcelona, Spain; Michael R. Grunwald, Omotayo Fasan, and Edward Copelan, Levine Cancer Institute, Carolinas HealthCare System, Charlotte; William Allen Wood, University of North Carolina at Chapel Hill, Chapel Hill; David A. Rizzieri, Duke University Medical Center, Durham, NC; Ulrike Bacher, University of Medicine Göttingen, Göttingen, Germany; Betty Hamilton and Aaron Gerds, Cleveland Clinic Taussig Cancer Institute; Matt Kalaycio and Ron Sobecks, Cleveland Clinic Foundation, Cleveland; Basem William, Ohio State University Medical Center, Columbus, OH; Ayman Saad and Luciano J. Costa, University of Alabama at Birmingham, Birmingham, AL; Corey Cutler and Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA; Erica Warlick and Celalettin Ustun, University of Minnesota Medical Center, Minneapolis, MN; Baldeep Mona Wirk, Seattle Cancer Care Alliance, Seattle, WA; Mitchell Sabloff, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario; Andrew Daly, Tom Baker Cancer Center, Calgary, Alberta, Canada; David Marks, University Hospitals Bristol National Health Service Trust, Bristol; Robert Peter Gale, Imperial College London, London, United Kingdom; Richard Olsson, Karolinska Institutet, Stockholm, Sweden; Alan M. Miller, Baylor University Medical Center; Rammurti Kamble, Baylor College of Medicine, Dallas; Jorge Cortes and Uday Popat, University of Texas MD Anderson Cancer Center, Houston, TX; Tamila L. Kindwall-Keller, University of Virginia Health System, Charlottesville, VA; Jean-Yves Cahn, University Hospital Grenoble, La Tronche, France; Bipin N. Savani, Vanderbilt University Medical Center, Nashville, TN; Ravi Vij, Washington University School of Medicine, St Louis, MO; Richard Maziarz, Oregon Health and Science University, Portland, OR; and Steven Pavletic, National Cancer Institute, Bethesda, MD.
1