Abstract
Background and Aims:
The advent of ultrasonographic-guided techniques has led to increased interest in femoro-sciatic nerve block (FSNB) for lower limb surgeries. α2-agonists have been used recently as adjuvants to local anaesthetics in nerve blocks. We aimed to compare equal doses of clonidine or dexmedetomidine as an adjuvant to levobupivacaine in FSNB for post-operative analgesia.
Methods:
Ninety patients scheduled to undergo below knee orthopaedic surgeries under subarachnoid block were divided into three groups: Group LL (n = 30) patients received 38 mL of 0.125% levobupivacaine with 2 mL normal saline, Group LD (n = 30) patients received 38 mL of 0.125% levobupivacaine with 0.5 μg/kg dexmedetomidine and Group LC (n = 30) received 38 mL of 0.125% levobupivacaine with 0.5 μg/kg clonidine in saline to make total drug volume of 40 mL. The primary and secondary outcome variables were duration of analgesia and rescue analgesic requirement, verbal rating score respectively. Continuous variables were analysed with analysis of variance or the Kruskal–Wallis test on the basis of data distribution. Categorical variables were analysed with the contingency table analysis and the Fisher's exact test.
Results:
Duration of analgesia was prolonged with dexmedetomidine (10.17 ± 2.40 h) and clonidine (7.31 ± 1.76 h) as compared to control (4.16 ± 1.04 h, P = 0.00). Significantly lower pain scores were observed in dexmedetomidine group as compared to clonidine up to 8 h post-operatively.
Conclusion:
Equal doses of clonidine or dexmedetomidine added to levobupivacaine prolonged the duration of analgesia, decreased requirement of rescue analgesia. Dexmedetomidine delays the requirement of rescue analgesics with better pain scores as compared to clonidine.
Keywords: Adjuvants, anaesthesia analgesia, dexmedetomidine hydrochloride, levobupivacaine, pain post-operative
INTRODUCTION
In patients undergoing orthopaedic surgeries, unrelieved post-operative pain not only results in discomfort to patients but predisposes to the development of chronic pain syndromes.[1] Regional anaesthetic techniques have an advantage over general anaesthesia such as excellent pain control, less adverse effects and shortened stay in the post-anaesthesia care unit.[2] Femoro-sciatic nerve block (FSNB) is a reliable, safe and effective method of providing analgesia in the immediate post-operative period.[3] The use of ultrasonographic (USG)-guided FSNB has various advantages including precise needle insertion, less block administration time, improves block quality and decreases dosage of local anaesthetic (LA).[4]
As relatively larger volume of LAs is administered in FSNB, ropivacaine and levobupivacaine[5] are preferred as they have greater margin of safety. The duration of sensory block is longer than the motor block with levobupivacaine,[5] as compared to ropivacaine, therefore, levobupivacaine appears to be the LA of choice, especially in orthopaedic surgeries.
However, these early advantages can be limited by the relatively brief duration of action of the LAs and in this regard, there is increased interest in clonidine and dexmedetomidine (α2-adrenoreceptor agonists) as adjuvants to LAs for increasing the duration of analgesia in the post-operative period. The α2-adrenoceptors are located on primary afferent terminals implicated in analgesia and therefore support the analgesic action at peripheral sites.[6] The efficacy of dexmedetomidine exceeds that of clonidine for peripheral nerve blocks.[7]
We hypothesised that equal doses of clonidine or dexmedetomidine (0.5 μg/kg) as adjuvants to levobupivacaine in USG-guided FSNB will have a beneficial effect in prolonging the duration of post-operative analgesia and improved verbal rating score (VRS) in patients scheduled for below knee surgery under subarachnoid block (SAB).
METHODS
The study was a prospective, randomised, double-blind, controlled clinical trial. The study protocol was approved by the Institutional Ethics Committee and written informed consent was obtained from all patients recruited from May 2014 to June 2015. This study was carried out on 90 American Society of Anesthesiologists’ (ASA) physical status 1-2 patients of both gender, in the age group of 20–60 years, scheduled for below knee surgery under SAB. Patient's refusal, neuropathy, treatment with β blockers or calcium channel blockers, hypersensitivity to LAs, allergy to study drugs and patients with known bradyarrhythmias formed the exclusion criteria.
During pre-anaesthetic visit, the patients were explained about the study purpose, advantages and risks of the procedure and instructed to demand analgesia as per requirement and informed written consent was obtained. Patients were educated about the 11 point VRS during the pre-operative assessment.
Randomisation was achieved by computer-generated random number table. Random group assigned was enclosed in a sealed opaque envelope to ensure concealment of allocation sequence. After shifting the patient inside operation theatre, sealed envelope was opened by anaesthesiologist not involved in the study to prepare the drug solution according to randomisation. All the patients were kept nil orally for 8 h before surgery and pre-medicated with tablet alprazolam 0.25 mg and tablet ranitidine 150 mg the night before surgery and 2 h before surgery. In the operation theatre, after securing 18-gauge intravenous (IV) cannula, 0.9% sodium chloride (normal saline [NS]) infusion was commenced. After establishing standard anaesthesia monitoring, baseline parameters such as heart rate (HR), non-invasive blood pressure and peripheral oxygen saturation were recorded.
All patients included in the study were given SAB in sitting position using 26 gauge Quincke spinal needle at L3–L4 interspace with 15 mg (3 mL) 0.5% hyperbaric bupivacaine after ensuring free flow of cerebrospinal fluid. After confirmation of adequate level (T6), surgery was allowed to proceed. After the surgery was over and the sensory level regressed to T10, following proper positioning of patients, USG-guided FSNB was given in allocated patients with respective drug solutions.
The femoral block was performed under ultrasound Sonosite Micromaxx® (Sonosite®, Bothell, WA, USA) guidance with patient supine and leg in neutral position. After skin disinfection with povidone iodine, sterile drapes were applied. Femoral nerve and vessels were identified (femoral nerve lies lateral to femoral artery in a groove formed by iliacus and psoas muscle) in short axis view using linear probe (8–12 Hz) covered with sterile plastic sheath and with sufficient application of sterilised gel. A 22 gauge echogenic needle (Stimuplex®, B. Braun, Melsungen, Germany) was used by an ultrasound-guided in-plane (lateral to medial) technique and positioned between the fascia iliaca and iliopsoas muscle near lateral corner of femoral nerve. After checking the exact location of the needle tip, 1 mL of NS was injected to open the plane and after confirmation of hypoechoic area on USG image, the injection of the 20 mL of drug solution was given.
Sciatic nerve block was given by popliteal approach with patient in supine position by same ultrasound probe mentioned above. Leg of the patient was elevated by putting sterile pillow between lower aspect of leg and table to allow the access of probe. After draping popliteal fossa and applying sufficient gel, a short axis view of popliteal neurovascular bundle was obtained. A 22 gauge echogenic needle was used by ultrasound-guided in-plane (lateral to medial) technique and under continuous ultrasound guidance, tip was placed between the tibial and common peroneal component of sciatic nerve near the division and 1 mL of NS was injected to open the plane and after confirmation of hypoechoic area on USG image, the injection of the 20 mL of drug solution was given.
The patients in Group LL (n = 30) received 38 mL of 0.125% levobupivacaine (Levoanawin™, Neon Laboratories, Limited, Mumbai 2.5 mg/mL, 10 mL) with 2 mL NS. Patients in Group LD (n = 30) received 38 mL of 0.125% levobupivacaine with 0.5 μg/kg dexmedetomidine (Dextomid™ 100 μg by Neon Laboratories, Mumbai, 100 μg/mL) and in Group LC (n = 30) received 38 mL of 0.125% levobupivacaine with 0.5 μg/kg clonidine (Cloneon™, Neon Laboratories, Limited 150 μg/mL, 1 mL) in saline to make total drug volume of 40 mL. In all patients, 20 mL of LA solution was injected around femoral nerve and 20 mL around sciatic nerve.
Block assessment was done at hourly interval up to 24 h by a blinded anaesthesiologist. Post-operatively, the patients were evaluated for pain, nausea or vomiting, sedation, haemodynamic parameters (HR, systolic blood pressure, diastolic blood pressure) and VRS in the post-anaesthesia care unit at 0, 2, 4, 8, 12 and 24 h by an investigator blinded to group assignment. For the first 24 h, the protocol for post-operative analgesia consisted of standard orders for intramuscular diclofenac 75 mg on demand for VRS >4. For breakthrough pain, patients were treated with IV tramadol 2 mg/kg as and when required.
Patients were asked to rate average pain they experience over a period of 24 h post-operatively by 11 point VRS,[8] where 0 was no pain and 10 was worst imaginable pain. Patients were asked to rate the severity of nausea, vomiting on a four-point scale:[9] none (1), mild (2), moderate (3), severe (4). Patient was evaluated for sedation using modified Ramsay sedation score:[10] (1) wide awake, (2) drowsy, (3) asleep but arousable with verbal stimulus, (4) arousable with mild physical stimulus, (5) not arousable with mild physical stimulus.
Duration of analgesia was the primary outcome of our study, whereas the rescue analgesics requirement, VRS scores, sedation score and haemodynamic parameters were the secondary outcomes. Duration of analgesia was taken as the outcome measure for the purpose of sample size calculation. It was estimated that 23 subjects would be required per group to detect a difference of 60 min in this parameter between the groups, with 80% power and 5% probability of Type 1 error. This calculation assumed a pooled standard deviation of 60 min for the duration of analgesia. To account for probable dropouts and block failure we included 30 patients in each group.
Data were collected and entered in MS Excel 2010. Statistical analysis was performed using SPSS software 17 (SPSS Inc., Chicago, IL, USA). Normal distribution of the collected data was first verified with the Kolmogorov-Smirnov test. Continuous variables were analysed with analysis of variance or the Kruskal–Wallis test on the basis of data distribution. Post hoc comparisons were performed with the unpaired Student's t-test or the Mann–Whitney U-test with Bonferroni's correction, as indicated. Categorical variables were analysed with the contingency table analysis and the Fisher's exact test. P ≤ 0.05 was considered significant. Continuous variables are presented as mean ± standard deviation or median (range) according to data distribution, whereas categorical variables are presented as number (percentage).
RESULTS
The total number of patients enrolled during the study were 106 for the three groups; seven patients were excluded for violating the inclusion criteria, while nine patients declined to participate in the study [Figure 1]. Thus, the total number of patients completing the study was 30 in each group. They were comparable to each other with respect to age, weight, body mass index (BMI), duration of surgery and ASA status [Table 1].
Figure 1.
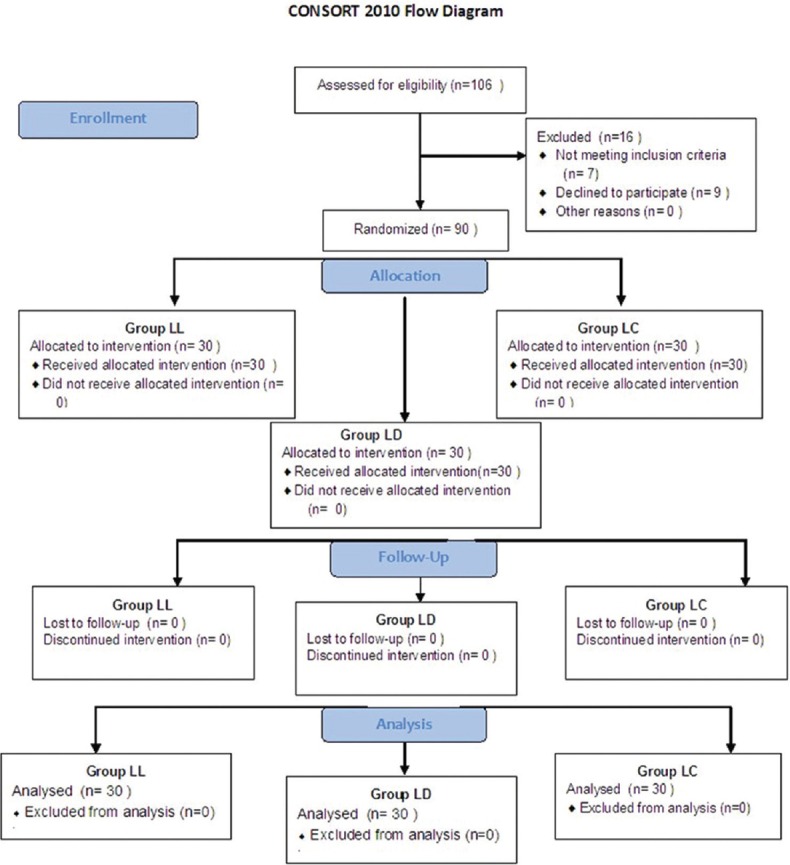
Flow chart of patients recruited and analysed in three groups. Group LL - Control GP; Group LD - Dexmedetomidine GP; Group LC - Clonidine GP
Table 1.
Demograhic data of patients in three groups
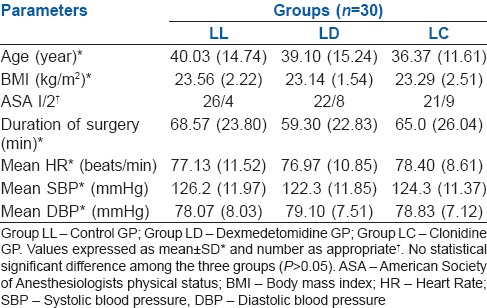
The total duration of analgesia defined as time interval between administration of FSNB and demand for first rescue analgesic was maximum in dexmedetomidine group as compared to clonidine and control group. In clonidine group, pain-free period was 7.31 ± 1.76 h, while block analgesia was 10.17 ± 2.40 h in dexmedetomidine group and 4.16 ± 1.04 h in control group (P < 0.000) [Figure 2].
Figure 2.
Group LL - Control group; Group LD - Dexmedetomidine group; Group LC - Clonidine group. Period of analgesia by block in three groups expressed as mean ± standard deviation (*Group LL and LD [P = 0.00], †Group LC and LL [P = 0.00] and ‡Group LD and LC [P = 0.00])
The Kaplan–Meier curves were used to generate a display of time to rescue analgesic among three groups [Figure 3]. The control group required rescue analgesic from 2 h up to 24 h with maximum boluses usage at 4 h and 24 h indicating shorter pain-free period and more requirement of analgesia post-operatively. The maximum bolus usage was at 12 h in clonidine and dexmedetomidine group, but bolus requirement in clonidine group started at 4 h, whereas majority of patients in the dexmedetomidine group required the first dose at 12 h indicating longer pain-free period as compared to clonidine group (P < 0.001).
Figure 3.
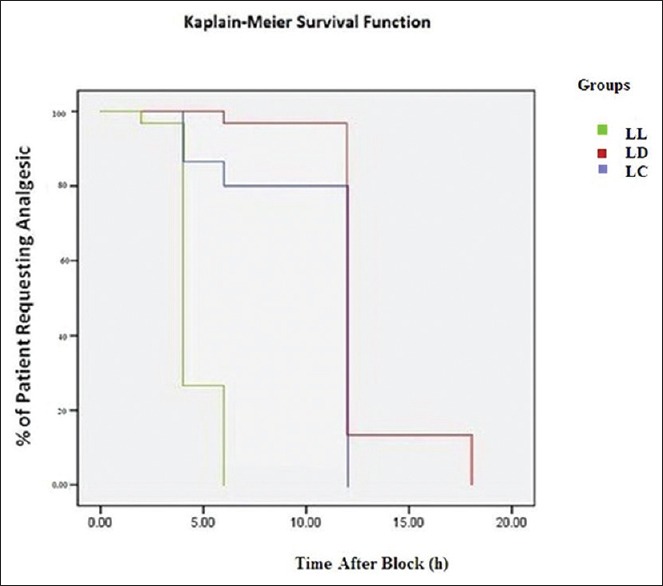
Group LL - Control GP; Group LD - Dexmedetomidine GP; Group LC - Clonidine GP. The survival curve displays the probability that the patients in three groups received rescue analgesics
There was statistically significant (P < 0.05) decrease in the VRS in clonidine and dexmedetomidine groups as compared to control group at various time intervals up to 24 h post-operatively except at 1 h [Figure 4]. The difference was statistically significant at 4 h and 8 h between clonidine and dexmedetomidine groups (P = 0.04 and 0.01), respectively.
Figure 4.
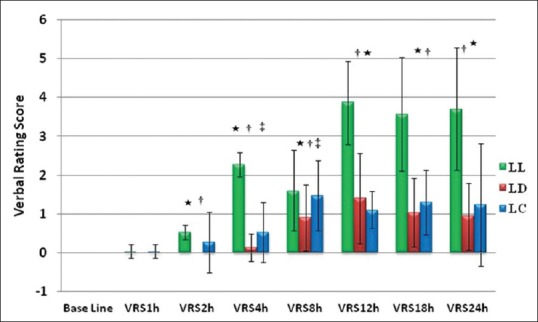
Group LL - Control group; Group LD - Dexmedetomidine group; Group LC - Clonidine group. Comparison of mean verbal rating scores over 24 h at different time intervals in three groups. Values expressed as mean ± standard deviation, *Group LL and LD (P < 0.05), †Group LL and LC (P < 0.05), ‡Group LD and LC (P < 0.05)
All patients in three groups were comfortable and responded to verbal commands in post-operative period. Patients in the clonidine and dexmedetomidine groups had higher sedation score at 1 h as compared to the control group (P < 0.001). However, there was no residual sedation in post-operative period after 1 h of performing block [Figure 5]. All the patients were haemodynamically stable at all time intervals in the post-operative period, and we did not observe any incidence of bradycardia in clonidine and dexmedetomidine group.
Figure 5.
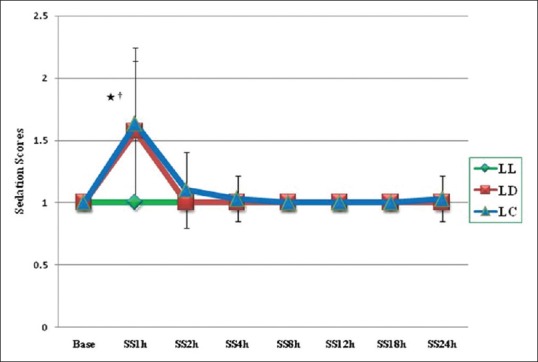
Group LL - Control GP; Group LD - Dexmedetomidine GP; Group LC - Clonidine GP. Graphical presentation of sedation scores in three groups over a period of 24 h and expressed as mean ± standard deviation *GP LD and LL (P = 0.00); †GP LC and LL (P = 0.00)
DISCUSSION
Moderate to severe post-operative pain is associated following lower limb orthopaedic surgical procedures and tends to last longer than 24 h, often resulting in immobility and a decrease in effective early mobilisation.[11] Therefore, it is important that effective post-operative pain management techniques should be used to decrease the incidence of the preventable post-operative complications. Peripheral nerve blocks have distinctly an edge over general anaesthesia and neuraxial blocks in this regard.
The analgesic efficacy of levobupivacaine in peripheral nerve blocks is already known, and this study also shows analgesic benefits of using levobupivacaine in FSNB. The efficacy of 0.2% ropivacaine, 0.2% levobupivacaine and 0.125% levobupivacaine was compared in continuous popliteal sciatic nerve block in a study involving sixty patients undergoing elective hallux valgus repair.[12] Post-operative analgesia was excellent in all three groups without motor block with 0.125% levobupivacaine. Therefore, for post-operative analgesia, 0.125% seems to be an ideal concentration of levobupivacaine, as it tends to avoid the motor block and thereby helps in early ambulation in the post-operative period especially after orthopaedic surgeries.
α2-adrenoceptor agonists, either alone or in combination with LAs, are highly effective in the treatment of short-term pain. The analgesic efficacy of clonidine is by virtue of its action on α2-adrenoceptors present on primary afferent terminals. We have used clonidine in the dose of 0.5 μg/kg in accordance with study.[13] Dexmedetomidine compared to clonidine is more selective α2-adrenoceptor agonist with seven times greater affinity.[14] Much like clonidine, dexmedetomidine has been shown to prolong the duration of analgesia by blocking the hyperpolarisation-activated cation current.[15]
In this study, mean duration of analgesia was significantly prolonged in dexmedetomidine group (10.17 ± 2.40 h) and clonidine group (7.31 ± 1.76 h) as compared to control group (4.16 ± 1.04 h) (P < 0.001). Clonidine (1 μg/kg) in FSNB[16] with 0.75% ropivacaine in patients undergoing hallux valgus repair was reported to increase the duration of post-operative analgesia by 3 h as compared to control group in accordance with the present study. However, the overall increase in duration of analgesia, up to 13 h in the control group may be attributed to the higher concentration of LA used in this study. We kept the sensory level higher (T6) because our intention was to give FSNB for post-operative analgesia under the effect of spinal so that patient does not experience pain during positioning for FSNB.
Dexmedetomidine (1 μg/kg) with 0.5% ropivacaine in posterior tibial nerve block[17] was found to increase duration of analgesia up to 21 h, however, 6 h gain in the post-operative analgesia over control group is consistent with the present study, whereas increased duration may be attributed to the higher concentration of LA. The present study found more consistent results with the study[18] comparing clonidine and dexmedetomidine added to 0.25% bupivacaine in supraclavicular block in a dose of 1 μg/kg and observed an increase of about 3 h in the duration of analgesia with the dexmedetomidine.
The LA-prolonging effect of α2-adrenoceptor agonists is probably mediated locally at the level of neurons. The effect appears to be more profound on C-fibers (pain fibres) than Aδ fibres (motor fibres),[6] thereby making the effects potentially more sensory specific.
A lot of heterogeneity was observed between the studies when comparing rescue analgesia. No differences were reported in total ketoprofen consumption or regarding the number of patients requesting post-operative analgesics between the clonidine and control groups in one of the studies.[16] Significantly less analgesic consumption in dexmedetomidine group was observed as compared to control in another study[15] which is consistent with this study.
Most of the studies[17,19] have observed sedation scores of grade 2 with clonidine and dexmedetomidine used as adjuvants irrespective of LA used, as was also observed in the present study, although the dose of adjuvant is less in present study.
More studies with the fixed doses and objectives are needed to evaluate this parameter in FSNB. All the patients were comfortable and responded to verbal command throughout the study period. No patient developed deep sedation, had airway compromise or desaturation.
We did not encounter any episode of bradycardia in the dose of 0.5 μg/kg clonidine or dexmedetomidine as compared to studies using doses of 1 μg/kg.
Our data supports specific action of α2-adrenoreceptors on peripheral nerves leading to better pain scores and decrease in post-operative analgesic requirement. To summarize, the addition of clonidine and dexmedetomidine in a dose of 0.5 μg/kg to 0.125% levobupivacaine decreases VRS scores post-operatively, prolongs duration of analgesia and decreases number of demands for rescue analgesics. Dexmedetomidine provides analgesia for longer duration as compared to clonidine; however, VRS scores in two groups were comparable beyond 8 h of the block.
The study had few limitations; the beneficial effects of α2-adrenoceptors could be because of systemic absorption rather than purely perineural effect. Also, the present study design was not meant to test the onset of sensory and motor blockades individually.
CONCLUSION
Dexmedetomidine or clonidine (0.5 μg/kg) used as adjuvants to levobupivacaine in USG-guided FSNB can prolong the duration of analgesia with reduced rescue analgesic requirement as compared to control in the post-operative period. Better pain scores were observed with use of dexmedetomidine as compared to clonidine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83:11–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu SS, Wu CL. The effect of analgesic technique on postoperative patient-reported outcomes including analgesia: A systematic review. Anesth Analg. 2007;105:789–808. doi: 10.1213/01.ane.0000278089.16848.1e. [DOI] [PubMed] [Google Scholar]
- 3.Casati A, Cappelleri G, Berti M, Fanelli G, Di Benedetto P, Torri G. Randomized comparison of remifentanil-propofol with a sciatic-femoral nerve block for out-patient knee arthroscopy. Eur J Anaesthesiol. 2002;19:109–14. doi: 10.1017/s0265021502000194. [DOI] [PubMed] [Google Scholar]
- 4.Oberndorfer U, Marhofer P, Bösenberg A, Willschke H, Felfernig M, Weintraud M, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. Br J Anaesth. 2007;98:797–801. doi: 10.1093/bja/aem092. [DOI] [PubMed] [Google Scholar]
- 5.Liisanantti O, Luukkonen J, Rosenberg PH. High-dose bupivacaine, levobupivacaine and ropivacaine in axillary brachial plexus block. Acta Anaesthesiol Scand. 2004;48:601–6. doi: 10.1111/j.0001-5172.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 7.Abosedira MA. Adding clonidine or dexmedetomidine to lidocaine during biers block: A comparative study. J Med Sci. 2008;8:660–4. [Google Scholar]
- 8.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. J Pain Symptom Manage. 2011;41:1073–93. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Joris JL, Poth NJ, Djamadar AM, Sessler DI, Hamoir EE, Defêchereux TR, et al. Supplemental oxygen does not reduce postoperative nausea and vomiting after thyroidectomy. Br J Anaesth. 2003;91:857–61. doi: 10.1093/bja/aeg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: A systematic review. Intensive Care Med. 2000;26:275–85. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- 11.Frost S, Grossfeld S, Kirkley A, Litchfield B, Fowler P, Amendola A. The efficacy of femoral nerve block in pain reduction for outpatient hamstring anterior cruciate ligament reconstruction: A double-blind, prospective, randomized trial. Arthroscopy. 2000;16:243–8. doi: 10.1016/s0749-8063(00)90047-1. [DOI] [PubMed] [Google Scholar]
- 12.Casati A, Vinciguerra F, Cappelleri G, Aldegheri G, Grispigni C, Putzu M, et al. Levobupivacaine 0.2% or 0.125% for continuous sciatic nerve block: A prospective, randomized, double-blind comparison with 0.2% ropivacaine. Anesth Analg. 2004;99:919–23. doi: 10.1213/01.ANE.0000129977.44115.93. [DOI] [PubMed] [Google Scholar]
- 13.Singelyn FJ, Dangoisse M, Bartholomée S, Gouverneur JM. Adding clonidine to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Reg Anesth. 1992;17:148–50. [PubMed] [Google Scholar]
- 14.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 15.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casati A, Magistris L, Fanelli G, Beccaria P, Cappelleri G, Aldegheri G, et al. Small-dose clonidine prolongs postoperative analgesia after sciatic-femoral nerve block with 0.75% ropivacaine for foot surgery. Anesth Analg. 2000;91:388–92. doi: 10.1097/00000539-200008000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 18.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (a2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty S, Chakrabarti J, Mandal MC, Hazra A, Das S. Effect of clonidine as adjuvant in bupivacaine-induced supraclavicular brachial plexus block: A randomized controlled trial. Indian J Pharmacol. 2010;42:74–7. doi: 10.4103/0253-7613.64498. [DOI] [PMC free article] [PubMed] [Google Scholar]


