Abstract
Background and Aims:
Three-in-one and femoral nerve blocks are proven modalities for postoperative analgesia following anterior cruciate ligament (ACL) reconstruction. The aim of this study was to evaluate the efficacy of magnesium (Mg) as an adjuvant to bupivacaine in 3-in-1 block for ACL reconstruction.
Methods:
Sixty patients undergoing arthroscopic ACL reconstruction were randomly allocated to Group I (3-in-1 block with 30 ml of 0.25% bupivacaine preceded by 1.5 ml of intravenous [IV] saline), Group II (3-in-1 block with 30 ml of 0.25% bupivacaine preceded by 1.5 ml of solution containing 150 mg Mg IV) or Group III (3-in-1 block with 30 ml containing 0.25% bupivacaine and 150 mg of Mg as adjuvant preceded by 1.5 ml of IV saline). Post-operatively, patients received morphine when visual analogue scale (VAS) score was ≥4. Quantitative parameters were compared using one-way ANOVA and Kruskal–Wallis test and qualitative data were analysed using Chi-square test.
Results:
Demographics, haemodynamic parameters, intra-operative fentanyl requirement, post-operative VAS scores and total morphine requirement were comparable between groups. Time to first analgesic requirement was significantly prolonged in Group III (789 ± 436) min compared to Group I (466 ± 290 min) and Group II (519 ± 274 min), (P = 0.02 and 0.05). Significantly less number of patients in Group III (1/20) received morphine in the first 6 h post-operatively, compared to Group I (8/20) and Group II (6/20) (P = 0.008 and 0.03). No side effects were observed.
Conclusion:
Mg as an adjuvant to bupivacaine in 3-in-1 block for ACL reconstruction significantly prolongs the time to first analgesic requirement and reduces the number of patients requiring morphine in the immediate post-operative period.
Keywords: Adjuvants, bupivacaine, magnesium, three in one block
INTRODUCTION
Arthroscopic anterior cruciate ligament (ACL) repair, although a minimally invasive procedure, is associated with significant post-operative pain impairing early post-operative mobilisation.[1] Three-in-one block and femoral nerve blocks have been proven as effective modalities for post-operative analgesia following knee surgeries.[2] Various adjuvants have been used with local anaesthetics to prolong the duration of analgesia.[3] Magnesium (Mg) has been used in intravenous (IV), intrathecal, epidural/caudal routes to enhance analgesia. Its role in peripheral nerve blocks is still under investigation, and available literature has shown mixed results.[4,5] Thus, this study was designed to evaluate the efficacy of Mg as an adjuvant in 3-in-1 blocks for arthroscopic knee surgeries.
METHODS
After institutional ethics committee approval, sixty patients of American Society of Anesthesiologists physical status 1 or 2, aged between 18 and 60 years, who were scheduled for arthroscopic ACL repair were included in this prospective randomised study. An informed written consent was obtained from all the participants. Patients who refused regional anaesthesia, had an infection at the block site or allergy to study drugs, coagulation disorders, peripheral neuropathies, femoral bypass or those who received calcium channel blockers were excluded from the study.
Since no previous study was available using Mg as an adjuvant in 3-in-1 block, sixty patients were considered. The primary objective was to find a difference in time to first analgesic request. The patients were divided into three groups of twenty each, using computer-generated table of random numbers in a double-blind model and allocation concealment was done by sealed envelope technique. The study solution was prepared by a person who did not participate in the conduct of the study. In Group I, patients received 30 ml bupivacaine 0.25% for 3-in-1 block preceded by 1.5 ml of saline IV. Patients in Group II received 30 ml of bupivacaine 0.25% for 3-in-1 block preceded by 1.5 ml solution containing 150 mg Mg IV. In Group III, patients received 30 ml volume of solution containing bupivacaine 0.25% with 150 mg of Mg for 3-in-1 block preceded by 1.5 ml saline IV.
Patients were pre-medicated with midazolam 1–2 mg IV before induction and their baseline haemodynamic parameters were noted. The airway was secured with an appropriate size laryngeal mask airway ProSeal™ after induction with 2 μg/kg fentanyl and propofol. A volume of 1.5 ml of solution containing either normal saline or Mg was given IV just before the start of the block, as per the group allocation, by the same person who prepared the drug and did not participate in the study any further.
The single shot 3-in-1 block was given using a B. Braun Stimuplex® nerve stimulator needle under strict aseptic precautions. After negative aspiration, 30 ml of the study solution was injected when patellar movements were elicited with a stimulation current of 0.5 mA. The distal pressure was held at the time of injection till 5 min after completion of the block, to facilitate the cephalad spread of the anaesthetic to the level of the lumbar plexus.
Following the block, muscle relaxation was provided with vecuronium bromide (0.08 mg/kg IV) and anaesthesia was maintained using O2 and N2O (500 ml: 500 ml), end-tidal isoflurane (0.8–1%) with closed circuit. Supplemental analgesia was provided with fentanyl (1 μg/kg) on the basis of any one of the parameters-rise in heart rate (HR) or systolic blood pressure >20% of baseline values.
Continuous monitoring of HR, non-invasive blood pressure (NIBP), SpO2 and EtCO2 was done in the intra-operative period. The total amount of supplemental analgesia with fentanyl intra-operatively was recorded and compared between the groups.
Post-operatively, baseline visual analogue scale (VAS) score was noted in the recovery room (0–10). The nurses were explained and trained to assess pain scores and administer analgesia. Rescue analgesia was provided using IV bolus of 3 mg morphine, when patients had VAS score ≥4. A repeat bolus of 3 mg morphine was considered after 15 min if patients continued to have VAS score ≥4. The time to first analgesic dose requirement from the time the block was given and total amount of morphine administered in all the groups were recorded and compared for first 24 h in the post-operative period. In case any patient did not request for analgesia (VAS ≥4) in the study period, they were followed-up till the time they requested for analgesia and the time to first analgesic requirement was noted.
HR, NIBP, SpO2, respiratory rate and VAS was recorded every 30 min for 6 h and every 6 h for 24 h after surgery. Side effects in the form of nausea, vomiting, respiratory depression, urinary retention and pruritus were noted and treated.
The data from the study were analysed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. Quantitative parameters were compared using one-way ANOVA and Kruskal–Wallis test and qualitative data were analysed using Chi-square test.
RESULTS
The demographic parameters were comparable among the groups. Duration of surgery and intra-operative fentanyl consumption were also comparable. [Table 1] There was no difference in the intra-operative haemodynamic parameters between the groups.
Table 1.
Comparison of demographic variables, duration of surgery and intraoperative fentanyl requirements
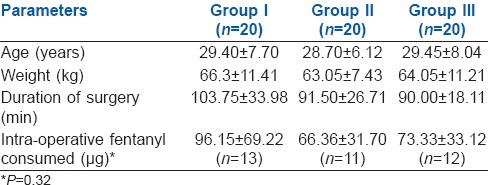
The time to first analgesic requirement was significantly prolonged in Group III (789 ± 436 min) compared to Group I (466 ± 290 min) and Group II (519 ± 274 min), (P = 0.02 and 0.05 respectively) [Table 2].
Table 2.
Time to first analgesic requirement

The total dose of morphine consumed over 24 h was 10.58 ± 4.51 mg, 8.53 ± 3.35 mg and 7.50 ± 4.38 mg, respectively, in Groups I, II and III and was statistically similar among the groups. However, significantly less number of patients in Group III (1/20) received morphine in the first 6 h post-operatively, compared to Group I (8/20) and Group II (6/20) with a P = 0.008 and 0.03, respectively. One patient each in Group I and II did not receive morphine, whereas four patients in Group III did not receive morphine in the 24 h period post-operatively. These values were comparable between the groups [Table 3].
Table 3.
Number of patients who received morphine and its total dose in 24 h
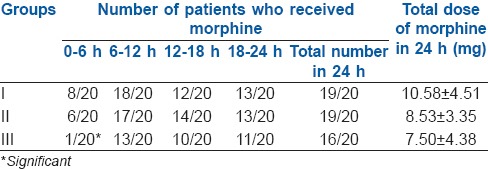
The post-operative VAS scores and the post-operative haemodynamic parameters were comparable among the groups. No side effects were noted.
DISCUSSION
ACL tears are common despite the awareness of coaches, trainers and athletes, as well as training programmes attempting to prevent these injuries. With >100,000 ACL tears per year projected in the United States alone, ligament reconstruction surgeries will contribute to a significant percentage of orthopaedic surgeries.[6] Nerve blocks for knee surgery have been shown to enhance functional recovery not only by improving post-operative analgesia but also by inhibiting inflammation of knee joint.[7] Femoral nerve block and saphenous nerve block have been proven to provide satisfactory analgesia following knee surgeries.[8,9] Although the primary innervation of the knee is by the femoral nerve, it also receives contributions from the obturator nerve, lateral cutaneous nerve and sciatic nerve. Hence, we used 3-in-1 block that blocks the femoral nerve, lateral cutaneous nerve and obturator nerve in our study.
A volume of at least 20 ml would be required to block all three nerves in a 3-in-1 block. In a study on paravascular lumbar plexus block to evaluate block extension after femoral nerve stimulation and injection, the volume of anaesthetic between 20 and 40 ml did not seem to influence the extent of the block.[10] Thus, we used a volume of 30 ml for the present study.
The block was given before the start of the surgery, assuming it will provide pre-emptive analgesia. As all the patients received general anaesthesia before the block, the success of block could not be monitored clinically. However, we monitored the requirement of fentanyl at the time of incision. It was noticed that none of the patients required fentanyl at the time of incision. This is attributed to the effect of the 3-in-1 block. Patients in all the groups showed the similar requirement of fentanyl boluses during the surgery. This can be explained by the fact that although all skin ports for arthroscopy and the skin incision for graft removal were within the supply territory of the femoral nerve, there was stimulation of the sciatic component due to continuous irrigation of saline into the joint capsule, and the stretch of the capsule, especially in the posterior part which is innervated by sciatic nerve. Furthermore, the graft used in our study was from the hamstring, supplied by the sciatic nerve.
The time to first analgesic requirement in the post-operative period showed a statistically significant difference between Group III, as compared to other groups, Group I and Group II. The duration of analgesia attained in our study with the 3-in-1 block alone is in concordance with another study that evaluated the effect of single injection femoral nerve block in ACL reconstruction and found the mean analgesic duration to be 670 ± 280 min in the group that received the block.[11] However, the duration of analgesia that was attained in our study with Mg as an adjuvant in the block was longer than the duration mentioned in that study, highlighting the role of Mg in prolonging the block.
It is known that prolonged analgesia and the time to and incidence of successful discharge are favourably related.[12] Since arthroscopic surgeries are increasingly being performed on an outpatient basis, the improved time to first analgesic requirement facilitates discharge. Pain is one of the most common symptoms requiring hospital admission after outpatient surgery.[13,14] In a retrospective study that involved 1200 patients who underwent complex outpatient knee procedures, it was found that the use of femoral or femoral-sciatic nerve block was associated with a 2.5-fold reduction in unplanned admission, compared to patients who received no block.[15] Adequate post-operative analgesia greatly facilitates early rehabilitation.[16]
The addition of Mg to the 3-in-1 block significantly prolonged the time to first analgesic requirement in our study. This is in accordance with a study, where Mg (2 ml of 10%) added as an adjuvant to 0.5% bupivacaine in interscalene block prolonged the mean duration of analgesia significantly when compared to 0.5% bupivacaine in the control group. Much like our study where total morphine consumption was statistically similar in the control groups, they also found that total post-operative fentanyl consumption did not differ statistically among the groups.[17] Similarly, in another study, the addition of 150 mg of Mg to 30 ml of 0.5% ropivacaine in supraclavicular brachial plexus block significantly increased the sensory and motor block duration and time to first analgesic requirement, compared to the control group where 0.5% ropivacaine was used. However, in that study the rescue analgesic used was diclofenac, and there was a significantly lesser need for post-operative rescue analgesic use in the group with Mg as an adjuvant.[18]
Mg has been used in IV, intrathecal, epidural/caudal routes to enhance analgesia.[19,20,21] The addition of Mg as an adjuvant in perioperative analgesia is due to the antagonist properties of Mg for the NMDA receptor and its inhibitory properties for calcium channels. Calcium channel blockers have shown antinociceptive effects in animals and morphine potentiation in patients with chronic pain.[22]
The dose of Mg (150 mg) used in our study was based on a previous study, where 150 mg Mg added to 2% prilocaine caused a pronounced prolongation of sensory and motor block.[23] This dose has been safely used, and no significant side effects have been reported.[18] Though the safety of Mg has been established in central neuraxial blocks, the literature on the safe dose of Mg that can be used as an adjuvant in peripheral nerve blocks is still sparse. Thus, we used Mg in doses that have been safely used in various studies.
In this study, we studied patients receiving 150 mg Mg IV and the 3-in-1 block with bupivacaine (group 2). This group was added to rule out the systemic absorption of Mg as a reason for the prolongation of analgesic time. IV Mg has also been reported to reduce analgesic requirements, but the dose needed to cause analgesia is much more than what was used in the present study.[24]
Three in one block in arthroscopic ACL reconstruction is known to reduce the post-operative opioid consumption.[6] Although total dose of morphine received in the first 24 h was less in Group III, it was not statistically significant. However, significantly less number of patients from Group III received morphine in the first 6 h post-operatively. The differential effect of the block in modifying the requirements of post-operative morphine in our study cannot be quantified or commented on because there was no control group that did not receive the 3-in-1 block.
In this study, Group III patients had significant prolongation of time to first analgesic requirement, also less number of patients required analgesia in the first 6 post-operative hours. Although Group III patients (Mg as adjuvant) had slightly lesser total morphine requirement in the post-operative period than the other groups and number of patients requiring morphine beyond first 6 h were less than in other groups, these values failed to achieve any statistical significance, possibly because of small sample size used in the study.
CONCLUSION
Magnesium is a promising adjuvant to bupivacaine for peripheral nerve blocks as it prolongs time to first analgesic requirement and reduces the number of patients requiring morphine in the immediate post-operative period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mitra S, Kaushal H, Gupta RK. Evaluation of analgesic efficacy of intra-articular bupivacaine, bupivacaine plus fentanyl, and bupivacaine plus tramadol after arthroscopic knee surgery. Arthroscopy. 2011;27:1637–43. doi: 10.1016/j.arthro.2011.08.295. [DOI] [PubMed] [Google Scholar]
- 2.Meftah M, Wong AC, Nawabi DH, Yun RJ, Ranawat AS, Ranawat CS. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics. 2012;35:e660–4. doi: 10.3928/01477447-20120426-19. [DOI] [PubMed] [Google Scholar]
- 3.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–16. doi: 10.1097/AIA.0b013e31820e4a49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han JU. About uses of magnesium during perioperative period. Korean J Anesthesiol. 2012;62:509–11. doi: 10.4097/kjae.2012.62.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung YC, Chen CY, Lirk P, Wang CF, Cheng JK, Chen CC, et al. Magnesium sulfate diminishes the effects of amide local anesthetics in rat sciatic-nerve block. Reg Anesth Pain Med. 2007;32:288–95. doi: 10.1016/j.rapm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mall NA, Wright RW. Femoral nerve block use in anterior cruciate ligament reconstruction surgery. Arthroscopy. 2010;26:404–16. doi: 10.1016/j.arthro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Martin F, Martinez V, Mazoit JX, Bouhassira D, Cherif K, Gentili ME, et al. Antiinflammatory effect of peripheral nerve blocks after knee surgery: Clinical and biologic evaluation. Anesthesiology. 2008;109:484–90. doi: 10.1097/ALN.0b013e318182c2a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima E Souza R, Correa CH, Henriques MD, de Oliveira CB, Nunes TA, Gomez RS. Single-injection femoral nerve block with 0.25% ropivacaine or 0.25% bupivacaine for postoperative analgesia after total knee replacement or anterior cruciate ligament reconstruction. J Clin Anesth. 2008;20:521–7. doi: 10.1016/j.jclinane.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Jin SQ, Ding XB, Tong Y, Ren H, Chen ZX, Wang X, et al. Effect of saphenous nerve block for postoperative pain on knee surgery: A meta-analysis. Int J Clin Exp Med. 2015;8:368–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Seeberger MD, Urwyler A. Paravascular lumbar plexus block: Block extension after femoral nerve stimulation and injection of 20 vs 40 ml mepivacaine 10 mg/ml. Acta Anaesthesiol Scand. 1995;39:769–73. doi: 10.1111/j.1399-6576.1995.tb04168.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiskira O, Alexandropoulou A, Polyzois VD. Single injection femoral nerve block after anterior cruciate ligament reconstruction: Is it adequate pain management? Reg Anesth Pain Med. 2007;32:404. [Google Scholar]
- 12.Fetherston CM, Ward S. Relationships between post operative pain management and short term functional mobility in total knee arthroplasty patients with a femoral nerve catheter: A preliminary study. J Orthop Surg Res. 2011;6:7. doi: 10.1186/1749-799X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krywulak SA, Mohtadi NG, Russell ML, Sasyniuk TM. Patient satisfaction with inpatient versus outpatient reconstruction of the anterior cruciate ligament: A randomized clinical trial. Can J Surg. 2005;48:201–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Viñoles J, Ibáñez MV, Ayala G. Predicting recovery at home after ambulatory surgery. BMC Health Serv Res. 2011;11:269. doi: 10.1186/1472-6963-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams BA, Kentor ML, Vogt MT, Williams JP, Chelly JE, Valalik S, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: A review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206–13. doi: 10.1097/00000542-200305000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs DM, Green TP, Esler CN. The local infiltration of analgesia following total knee replacement: A review of current literature. J Bone Joint Surg Br. 2012;94:1154–9. doi: 10.1302/0301-620X.94B9.28611. [DOI] [PubMed] [Google Scholar]
- 17.Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59:21–7. doi: 10.1007/s12630-011-9604-5. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee K, Das A, Basunia SR, Dutta S, Mandal P, Mukherjee A. Evaluation of magnesium as an adjuvant in ropivacaine-induced supraclavicular brachial plexus block: A prospective, double-blinded randomized controlled study. J Res Pharm Pract. 2014;3:123–9. doi: 10.4103/2279-042X.145387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mebazaa MS, Ouerghi S, Frikha N, Moncer K, Mestiri T, James MF, et al. Is magnesium sulfate by the intrathecal route efficient and safe? Ann Fr Anesth Reanim. 2011;30:47–50. doi: 10.1016/j.annfar.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JT, Higgins N, Toledo P, Scavone BM, McCarthy RJ, Wong CA. The effect of intravenous magnesium therapy on the duration of intrathecal fentanyl labor analgesia. Int J Obstet Anesth. 2012;21:212–6. doi: 10.1016/j.ijoa.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Kiran S, Gupta R, Verma D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postoperative pain after inguinal surgery. Indian J Anaesth. 2011;55:31–5. doi: 10.4103/0019-5049.76605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herroeder S, Schönherr ME, De Hert SG, Hollmann MW. Magnesium – Essentials for anesthesiologists. Anesthesiology. 2011;114:971–93. doi: 10.1097/ALN.0b013e318210483d. [DOI] [PubMed] [Google Scholar]
- 23.Gunduz A, Bilir A, Gulec S. Magnesium added to prilocaine prolongs the duration of axillary plexus block. Reg Anesth Pain Med. 2006;31:233–6. doi: 10.1016/j.rapm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Lysakowski C, Dumont L, Czarnetzki C, Tramèr MR. Magnesium as an adjuvant to postoperative analgesia: A systematic review of randomized trials. Anesth Analg. 2007;104:1532–9. doi: 10.1213/01.ane.0000261250.59984.cd. [DOI] [PubMed] [Google Scholar]


