Abstract
Mycotic keratitis is a major cause of corneal blindness, especially in tropical and subtropical countries. The prognosis is markedly worse compared to bacterial keratitis. Delayed diagnosis and scarcity of effective antifungal agents are the major factors for poor outcome. Over the last decade, considerable progress has been made to rapidly diagnose cases with mycotic keratitis and increase the efficacy of treatment. This review article discusses the recent advances in diagnosis and management of mycotic keratitis with a brief discussion on rare and emerging organisms. A MEDLINE search was carried out for articles in English language, with the keywords, mycotic keratitis, fungal keratitis, emerging or atypical fungal pathogens in mycotic keratitis, investigations in mycotic keratitis, polymerase chain reaction in mycotic keratitis, confocal microscopy, treatment of mycotic keratitis, newer therapy for mycotic keratitis. All relevant articles were included in this review. Considering the limited studies available on newer diagnostic and therapeutic modalities in mycotic keratitis, case series as well as case reports were also included if felt important.
Keywords: Antimicrobial peptides, confocal microscopy, fungal keratitis, mycotic keratitis, polymerase chain reaction, posaconazole, voriconazole
Mycotic keratitis, commonly known as fungal keratitis, accounts for approximately 1–44% of all cases of microbial keratitis, depending upon the geographic location.[1,2] Overall, it is more common in tropical and subtropical areas. The genera that commonly cause infection of the cornea include Fusarium, Aspergillus, Curvularia, Bipolaris, and Candida.[1,2,3] Most of the currently available antifungal medications have limitations, such as poor bioavailability and limited ocular penetration, especially in cases with deep-seated lesions.[4,5,6] These factors, especially in cases of severe fungal keratitis, account for the slow resolution of fungal infections, with most cases finally requiring a therapeutic penetrating keratoplasty (PKP).[6] To overcome these limitations a number of newer antifungal agents and drug delivery techniques are being tried to improve outcomes following fungal infections. In this review, we will discuss the recent innovations, in the diagnosis and management of fungal keratitis. In addition, a brief update about emerging fungal organisms would be discussed.
Method of literature search
A MEDLINE search was carried out for articles in the English language, with the key words, mycotic keratitis, fungal keratitis, emerging or atypical fungal pathogens in mycotic keratitis, investigations in mycotic keratitis, polymerase chain reaction (PCR) in mycotic keratitis, confocal microscopy, treatment of mycotic keratitis, newer therapy for mycotic keratitis. All relevant articles were included in this review. Considering the limited studies available on newer diagnostic and therapeutic modalities in mycotic keratitis, case series as well as case reports were also included if felt important.
Epidemiology and Risk Factors
A review of literature over the past few decades suggests that the most common risk factor for fungal keratitis is trauma with vegetable material or objects contaminated with soil.[1,2] While this has not changed much in developing countries, the use of contact lenses (CLs), with the solutions used to soak or clean lenses being the primary culprit, has emerged as an important risk factor for the occurrence of fungal keratitis in developed countries.[6,7] In a large case series of cases with fungal keratitis reported from 10 tertiary eye care centers across the United States over a 7-year period, CL wear was the presumed risk factor in 37% of the cases compared to ocular trauma, which was the presumed risk factor in 25% of the cases.[1] Keay et al. reported similar results from 11 tertiary care centers across the United States. In addition, to CL wear and ocular trauma, ocular surface disease (OSD) was the third most common risk factor accounting for 29% of cases in their study. Overall, yeasts were the most commonly isolated organisms in the presence of OSD. One important finding to notice from this study is that 65% cases of OSD were following PKP.[7]
Fungal Pathogens
Common organisms implicated in mycotic keratitis, include species of Aspergillus, Fusarium, Candida, Curvularia, and Penicillium.[1,2,3,4,5,6] Most of these species are saprobes. They invade traumatized or immunologically compromised corneas. The rarely reported fungal pathogens include Fonsecaea pedrosoi,[8] Lasiodiplodia theobromae,[9] Cylindrocarpon species,[10] Scedosporium prolificans,[11] Metarhizium anisopliae,[12] Paecilomyces species,[13] and Pythium insidiosum.[14]
Host Immune Response in Fungal Infections
An understanding of host immune response to fungal organisms is important to understand the healing process as well as devising new treatment strategies. In contrast to systemic fungal infections, which occur primarily in immunocompromised individuals, patients with fungal keratitis are immunocompetent and hence the immune response differs.[15] Both innate and adaptive immunity play an important role in infected corneal tissue at early and late stages of fungal keratitis. The various factors that have been implicated to play a role in host response includes polymorphisms in genes associated Dectin-1/CARD9 and Toll-like receptors (TLRs) pathways,[16] upregulation of indoleamine 2,3-dioxygenase,[17] thymic stromal lymphopoietin production by human corneal epithelial cells,[18] expression of interleukins (IL-8, IL-6 and IL-1β).[19] A recent study by Karthikeyan et al. has brought a lot of insight into the understanding of human immune response in fungal infections of cornea.[15] The authors analyzed the gene expression by quantitative PCR in RNA extracted from patients with either Fusarium solani or Aspergillus flavus corneal ulcers. The samples were taken both in early (within 1 week of infection, n = 85) and late (posttransplant corneas 2 weeks after infection, n = 20) stage of the disease. Based on their findings the authors have proposed the probable sequence of host immune reactions that includes adhesion of conidiophores containing multiple conidia to cornea following some form of trauma to the eye; germination of conidia, within the cornea, resulting in shedding the hydrophobin layer of resting conidia, and exposure of cell wall β-glucan on the surface that binds to Dectin-1 on resident corneal macrophages; Dectin-1 activates neighboring cells to produce C-X-C motif ligand (CXCL) chemokines and upregulates intercellular adhesion molecule 1 (ICAM-1) expression on vascular endothelial cells in the peripheral vessels; elevated ICAM-1 and CXC chemokines mediate recruitment of neutrophils to the corneal stroma; activation of Dectin-1 and TLR4 on neutrophils by cell surface β-glucan and mannosyl residues on hyphae in the cornea resulting in production of reactive oxygen species (ROS) and fungal killing.[15]
It is possible that targeting these receptors and proteins during corneal infection will help minimize host-mediated tissue damage while effectively killing fungal hyphae during corneal infection.
Clinical Features and Laboratory Diagnosis
A fungal corneal ulcer classically presents as a dry, raised lesion with crenate or feathery borders, presence of satellite lesions and a hypopyon. Conventional methods for the diagnosis of fungal keratitis include staining of tissue scrapings with Gram-stain, 10% potassium hydroxide (KOH) wet mount, lactophenol cotton blue, Giemsa, or calcofluor white.[1,2,3] KOH is one of the most commonly performed direct microscopy procedures for detection of fungi since it is a rapid and an inexpensive procedure. It has a sensitivity of 61–94% and specificity of 91–97% for detecting fungus.[2,3,4] Sabouraud dextrose agar is a very commonly used culture medium for isolating fungi.[2] Over the last decade a number of newer methods have been devised for detection of fungi. These methods are described below.
Polymerase chain reaction
PCR has emerged as a sensitive and specific test for the diagnosis of fungal keratitis.[20,21,22] Various studies have compared PCR with conventional diagnostic methods in cases with suspected fungal keratitis. PCR has the highest positive detection rate overall especially in cases with culture or smear-negative results [Table 1].[21,22,23,24,25]
Table 1.
Utilization of polymerase chain reaction in diagnosis of mycotic keratitis
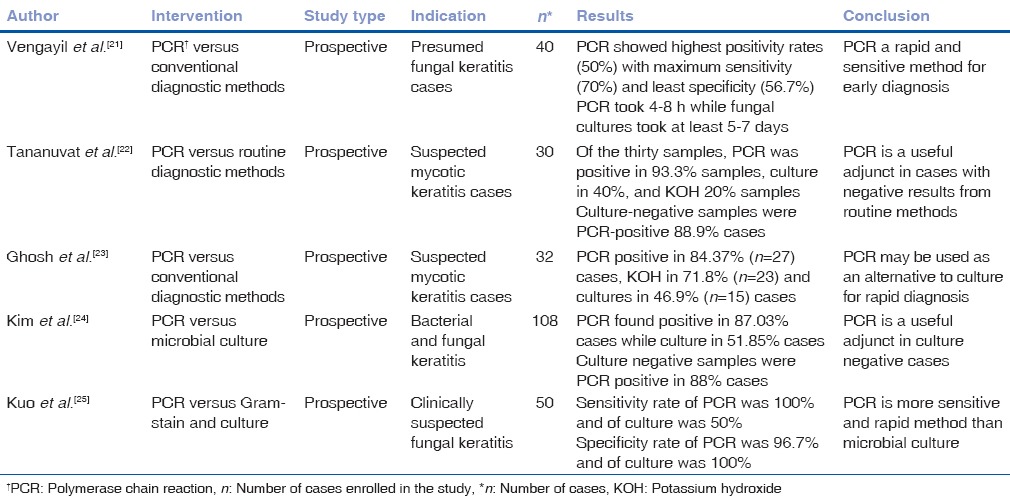
The advantage of PCR-based tests is that only a small clinical sample is needed for diagnosis and it is rapid. PCR assay takes 4–8 h, whereas positive fungal cultures require on an average of 2–7 days.[5] The major limitation of PCR is that it is expensive and therefore not readily available. Moreover, in a few countries like the United States, it has not been validated for corneal scraping specimens. In addition, artifactual amplification of nonpathogenic organisms, extraction of artifacts and amplification of nonpathogenic DNA can lead to overdiagnosis.[20,21] Thus, use of PCR as a stand-alone method for routine diagnosis of mycotic keratitis is not recommended. However, it can be useful in certain situations such as; to detect fungal DNA in corneal scrape material, thereby allowing antifungal therapy to be started at an early stage of the keratitis; to detect and then identify the fungal strain in the corneal material; and for rapid and accurate identification of fungal strains isolated from keratitis.
Genotyping
DNA sequence-based methods are used for rapid species identification of an organism. Recent reports suggest that filamentous fungi harbor unique species-specific in vitro antifungal agent susceptibility profiles as well as clinical characteristics.[26,27,28,29] Thus genotyping may help in explaining the variable presentation and response to treatment of the same genus among different patients. Oechsler et al. found a greater need for therapeutic PKP with Fusarium Solani isolates when compared with Fusarium oxysporum and Fusarium dimerum isolates. It has been demonstrated that the ability to form biofilm by Fusarium Solani makes it more resistant to antifungal agents than their planktonic counterparts without a biofilm.[30] In another study, Fusarium Solani isolates were found to have significantly higher voriconazole (VCZ) minimum inhibitory concentration 90 percentile (MIC90) values, while the corneal ulcers from which they were isolated showed a significantly longer time to cure, a worse follow-up visual acuity and an increased need for urgent surgical management, when compared to Fusarium nonsolani isolates and the corneal ulcers from which isolated.[31] Thus genotyping may yield important prognostic and therapeutic information that could improve the management of fungal ocular infections. At present genotyping is performed only in selective cases and by few laboratories especially in countries like India. However, considering the advantages it offers, its use might increase in the future.
Confocal microscopy
In vivo confocal microscopy (IVCM) uses a series of pinhole apertures to create optical sections of the cornea. It generates images from the cornea with a resolution of 1 μm, which is enough to yield instant imaging of organisms that are larger than a few micrometer such as Acanthamoeba cysts and fungal hyphae.[32] Aspergillus hyphae are 5–10 μm in diameter with septations, and branch dichotomously (at an angle of 45°) while hyphae of Fusarium species typically branch at an angle of 90°.[32,33] The hyper-reflective elements seen on IVCM must be differentiated from the basal corneal epithelial nerves, which have a more regular branching pattern. Stromal nerves, on the other hand, are much larger in diameter (25–50 μm). Both Aspergillus and Fusarium species hyphae are 200–400 μm long. In addition, yeasts such as Candida albicans have oval budding bodies that may develop pseudohyphae. They are 10–40 μm in length and 5–10 μm in width.[32,33]
The reported sensitivity of IVCM is between 80% and 94%.[32,33] There are now several studies of IVCM in fungal keratitis that compared the sensitivity of cultures to IVCM and found IVCM to be superior or at par with standard diagnostic procedures.[33,34,35,36,37,38,39] Brasnu et al. could diagnose all cases of suspected fungal keratitis (five out of five) using IVCM with sensitivity equal to the direct microscopy and culture.[37] Das et al. in a retrospective review found that IVCM had 83% (n = 5/6) sensitivity in diagnosing cases of deep fungal keratitis on the first day of presentation.[39] All these cases had undergone therapeutic keratoplasty and the subsequent histopathology of the corneal button revealed filamentous fungi in 83% (5/6) while, microbiology revealed filamentous fungi in 66% (4/6) of the cases.[39] Kanavi et al. studied 133 cases of infectious keratitis and found that IVCM has a sensitivity of 94% and it could identify fungal keratitis in 20.3% (n = 27) of cases in contrast to 12.0% (n = 16) of the cases by smear and culture examination.[36] Similarly Takezawa et al. reported identification of hyphae in 100% of the suspected fungal keratitis (n = 6) in contrast to 83% (5/6) with both smear and culture methods.[35] The advantages of IVCM include noninvasive in vivo technique, early identification of fungal elements, monitoring and guidance of treatment, and determination of the depth of infection.[33,35,37,38,39] There are several limitation of IVCM. The technique remains extremely user-dependent as it requires a skilled operator. Although detection of fungal elements is much easier compared to bacterial keratitis, the viewer requires some degree of experience. Patient collaboration and motion artifacts can affect the scanning. In addition, dense corneal infiltrates or scarring could preclude proper tissue penetration and visualization.[37,38,39] Moreover, the earlier versions were generally limited to scans of the central cornea. Lastly, in cases of smaller organisms IVCM is not helpful.[32]
Antifungal susceptibility testing
Unlike bacterial keratitis, susceptibility testing is not that frequently used in fungal keratitis. Although, a number of studies have reported the sensitivity of antifungals these studies often suffers from the limitation of small sample sizes, nonuniformity of data reported on MIC or focus on one particular genus or species.[40,41,42,43,44] Recently, Lalitha et al. reported the MIC of fungal isolates to natamycin (NTM) and VCZ in isolates from a relatively large sample size.[42] The MIC median (MIC50) and MIC90 for NTM were equal to or higher than VCZ for all organisms, except Curvularia species. Compared to other organisms, Fusarium species isolates had the highest MICs to VCZ and A. flavus isolates had the highest MICs to NTM. The result of this study reinforces the previous finding of mycotic ulcer topical treatment trial (MUTT) that NTM is better than VCZ. It also explains the clinical observation of poor response of Fusarium species to VCZ.[42]
Over the last few years clinicians have realized the value of susceptibility testing and a larger number of clinicians are using susceptibility test in the management of fungal keratitis. However, there is no consensus or any guidelines on the role of susceptibility testing in guiding treatment decisions and currently, fungal keratitis treatment is largely empirical.
Smartphone-based digital imaging
Recently Agarwal et al. have reported on the use of smartphone-based digital imaging in diagnosis and follow-up of keratitis.[44] Tissue samples obtained by conventional corneal scraping were stained and imaged using a smartphone coupled with a compact pocket magnifier and integrated light-emitting diode assembly. Photographs of multiple sections of slides were viewed using smartphone screen and pinch-to-zoom function. At present, the role of this technology is ill-defined and further studies are needed to elucidate its definitive role in mycotic keratitis.[44]
Advances in Medical Management
The routinely used topical antifungals, and their concentrations, are listed in Table 2.[45,46]
Table 2.
Currently available antifungal agents for treatment of mycotic keratitis
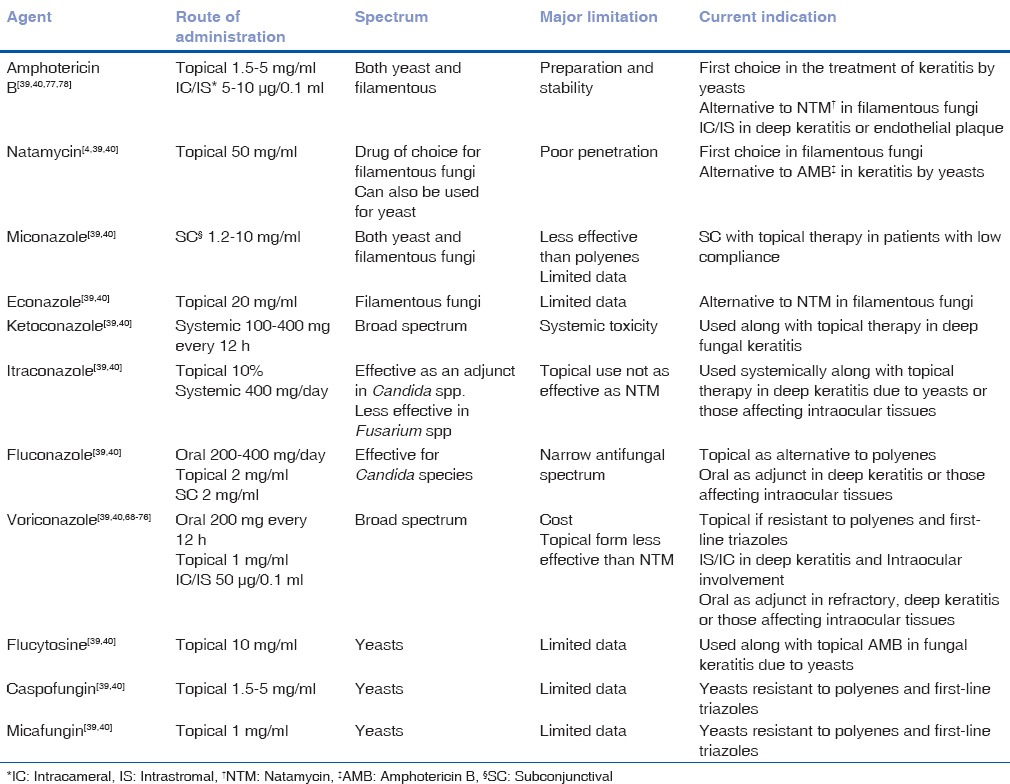
NTM is the treatment of choice for filamentous keratitis, especially that due to Fusarium species, as shown by the outcomes of the MUTT I.[47,48] The following section outlines the latest advances in medical management of fungal keratitis.
Posaconazole
Posaconazole is a new triazole, a synthetic structural analog of itraconazole.[48] The mechanism of action involves blocking of the fungal cell wall ergosterol synthesis.[49] In vitro and in vivo studies have shown that it has broad-spectrum activity against most Candida species, Cryptococcus neoformans, Aspergillus species, and zygomycetes, and endemic fungi (fungal pathogens in defined geographic locations around the world).[49,50,51]
Various published case reports have shown posaconazole to be an effective agent against Fusarium keratitis that was resistant to other antifungals [Table 3].[49,52,51,52,53] Posaconazole was used either systemically alone or in combination with topical posaconazole suspension in these studies. The dosage of oral posaconazole was 200 mg suspension four times daily or 400 mg twice a day in these studies.[49,52,51,52,53] The dosing schedule of topical formulation was 10 mg/0.1 ml and 4 mg/0.1 ml with hourly topical ocular application.[49,52,51,52,53] All cases had severe fungal keratitis with associated endophthalmitis and were resistant to routinely used antifungals including VCZ. Posaconazole use resulted in rapid resolution of infection in these cases without significant toxicity. Thus, it can be assumed that posaconazole can be used in cases of mycotic keratitis that are resistant to standard antifungal therapy. However, a few issues still need to be addressed. The use of topical posaconazole alone (without use of the oral preparation) needs to be investigated further. There is a difference in the reported concentration of the topical formulation. While Sponsel et al.[52] used 10 mg/0.1 ml, Altun et al.[51] used a concentration of 4 mg/0.1 ml. The safety and efficacy need further study, including study of a large number of cases.
Table 3.
Outcomes of newer modalities in the management of mycotic keratitis
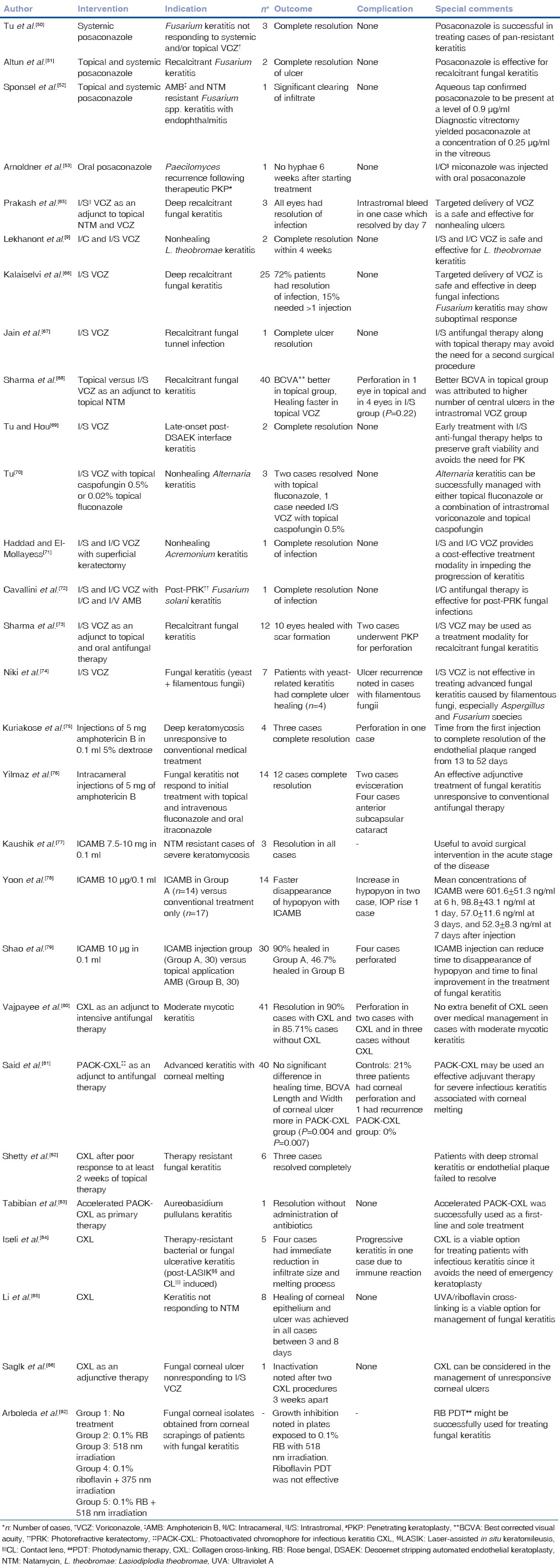
Echinocandins
Echinocandins are a group of newer antifungals, which act by inhibiting the synthesis of 1,3-β-d-glucan, leading to cell lysis due to increased permeability of the cell wall. Currently available echinocandins comprise caspofungin, micafungin and anidulafungin. Matsumoto et al. have reported successful use of topical 0.2% micafungin in cases of refractory fungal keratitis.[54] Topical caspofungin has been used in the cases of fungal keratitis refractory to VCZ.[55] There are limited data on the use of echinocandins to treat fungal keratitis in humans. There is a need for clinical studies, with adequate sample sizes, to validate the safety and efficacy of this group of antifungals.
Nano particles for sustained antifungal drug delivery
Cell-penetrating peptides (CPPs) are short peptide sequences that are able to transport molecules across the cell membrane. They are employed to enhance extracellular and intracellular internalization of various biomolecules including plasmid DNA, siRNA, oligonucleotide, peptide-nucleic acid, peptides, proteins and liposomes.[56] Jain et al., in an experimental in vitro study using cultured corneal epithelial cells, reported enhancing the penetrability of the antifungal drug, NTM, using such a CPP carrier, namely Tat-dimer (Tat2).[57] This led to an enhanced solubility of the drug in aqueous medium and increased cellular penetrability of NTM. When compared with unconjugated NTM, a 2-fold increase in antifungal activity against F. solani was noted following use of CPP-NTM complex. The formation of this CPP-NTM complex is clinically significant since it eliminates a major factor behind poor outcome in fungal keratitis, that is, poor bioavailability of antifungal agents in the corneal tissue. Thus future research on such nanoparticle-based therapy can be very useful in management of fungal keratitis.
Antimicrobial peptides
Antimicrobial peptides (AMPs) have significant potential for use as antimicrobial agents for ocular or other infections.[58] Nature provides us with numerous examples of the use of peptides and proteins with antimicrobial properties. These are also present in eye, either in tears or synthesized by conjunctival and corneal cells. Example of such peptides include the small peptides α and β defensins and LL-37, α 37-amino-acid peptide derived from the human cationic antimicrobial protein (CAP) 18, and proteins, such as lysozyme, lactoferrin, lactoferricin B, and mucins.[58,59] These natural antimicrobial agents act by several different mechanisms of action such as forming pores in bacterial membranes, resulting in cell death, preventing attachment, blocking entry, or both, chelating iron, and digestion of bacterial and possibly fungal cell walls by lysozyme.[58,59] In vitro studies have shown AMPs Pc-C and Pc-E reduced binding of Aspergillus fumigatus to cells, CAP37 inhibits infection, and also kills the pathogen, in cases of Candida infection, and the cecropin analog Shiva-11 exhibits antimicrobial activity against C. albicans.[58,59,60] Wu et al. evaluated the application of synthetic β-sheet forming peptide (IKIK) 2-NH2 and (IRIK) 2-NH2 for in vivo fungal keratitis treatment in comparison with amphotericin B (AMB).[61] It was found that topical solutions of the designed peptides were safe, and as effective as the clinically-used AMB. Compared to the costly and unstable AMB, these peptides are water-soluble, less expensive and stable. The authors concluded that the synthetic β-sheet forming peptides are promising candidates for the treatment of fungal keratitis.[61]
Theoretically, the use of the cationic peptides as antimicrobial agents has several distinct advantages: ability to effect killing of a broad spectrum of microorganisms including multidrug-resistant fungi, a low risk of development of resistance, synergy with conventional antibiotics, and amenability to synthesis.[58,59,60] However, the major limitation is that only experimental studies have been performed, and evidence in human eyes is lacking. Moreover, the possible use of the defensins in the eye has been known for more than two decades, but factors such as destruction of these peptides by hydrolytic enzymes in the tears or enzymes secreted by microbes have slowed down research in this field. Thus, future studies, including human subjects, and methods to overcome the above said limitations are needed to establish the role of AMPs in fungal keratitis.
Advances in Surgical Management
Recent advances have been made to ensure targeted drug delivery at the site of infection in the form of intrastromal injections, collagen cross-linking (CXL) and rose bengal (RB) aided photodynamic therapy (PDT).
Intrastromal voriconazole
VCZ is a triazole antifungal agent, structurally related to fluconazole but with a fluoropyrimidine group in place of triazole moiety.[62] Similar to other triazole agents, it inhibits the enzyme 14-alpha-lanosterol demethylase leading to lower levels of ergosterol, which is an essential component of fungal cell wall.[62] This inhibition is far more selective for fungal enzyme systems compared to the mammalian ones. VCZ has a broad-spectrum of action against fungal species, including Candida, Fusarium and Aspergillus species.[62,63,64]
Various routes of administration of VCZ include oral, topical, intacameral, and intrastromal delivery. Various studies have established the efficacy of topical, as well as systemic, VCZ. Targeted drug delivery of VCZ has been studied for the management of fungal keratitis not responding to standard topical therapy. Such a method of drug delivery overcomes a major limitation of topical antifungal therapy, which is poor bioavailability of drugs in cases of deep-seated fungal corneal ulcer. It provides a depot of drug, close to the ulcerated area, at a dose of 50 μg/0.1 ml in 5 divided doses, from where the drug is slowly released into the infected tissue.[65] Various studies in the literature have found targeted therapy with VCZ as an effective approach for deep-seated recalcitrant fungal corneal infections, not responding to conventional treatment modalities [Table 3].[65,66,67,68,69,70,71,72,73,74,75,76,77] Intrastromal VCZ has also been shown effective in managing secondary lamellar interface infection for late-onset infectious keratitis after Descemet stripping automated endothelial keratoplasty,[69] Alternaria keratitis,[70] recalcitrant Acremonium fungal keratitis,[71] and postphotorefractive keratectomy fungal keratitis.[72] However, a few issues must be kept in mind. First, performing any intervention through a normal cornea in the presence of keratitis may lead to new foci of infection. There is definitely a risk of inadvardent anterior chamber entry while performing the procedure in a hazy cornea. Moreover, a few studies have reported conflicting results. The study done by Sharma et al. found no benefit of intrastromal injections over topical VCZ in recalcitrant fungal keratitis cases not responding to topical NTM therapy for 2 weeks in cases of moderate fungal keratitis.[73] Similarly, Niki et al. found intrastromal VCZ to be successful in treating keratitis due to yeast only, but not keratitis due to filamentous fungi, which is in complete contrast to the experimental study results.[74] Thus, the role of intrastromal VCZ needs further research; however, at this point, it may be considered an alternative in selected recalcitrant cases of fungal keratitis.
Intracameral amphotericin B
AMB is a first-line treatment for keratitis caused by Candida species in many countries, and is used for the management of fungal keratitis in regions where NTM is not available.[45] AMB is also active against Aspergillus species but less effective against Fusarium species. Intracameral AMB is another approach that is being utilized for targeted drug delivery. It is indicated when medical treatment with topical and systemic antifungal has failed, especially in cases with deep mycosis, endothelial plaque and presence of hypopyon and/or inflammation of the anterior chamber.[75,76] The concentration injected, as described in literature, ranges between 5 and 10 μg/0.1 ml. The results of different studies and the reported complications are outlined in Table 3.[75,76,77,78,79]
Corneal collagen cross-linking (riboflavin with ultraviolet-A irradiation)
Corneal CXL has been found successful in halting the progression of keratoconus. Over the last few years there has been much interest in the role of CXL in infectious keratitis. Multiple studies have been published with conflicting results on the efficacy of CXL in infectious keratitis [Table 3].[80,81,82,83,84,85,86] Recently, to distinguish the use of CXL for the treatment of infectious keratitis from CXL for keratoconus, the term photoactivated chromophore for infectious keratitis (PACK)-CXL was created at the ninth cross-linking congress in Dublin, Ireland, in 2013.[81]
CXL may act in cases of mycotic keratitis by a direct antifungal effect and by halting the ongoing melting, thus helping to avoid emergency keratoplasty.[85,86,87] PACK-CXL has shown anti-fungal activity against pathogens such as C. albicans, Fusarium species, and A. fumigatus.[87] The result of various clinical studies are outlined in Table 3; unfortunately, the results are a bit contradictory. Vajpayee et al. found that PACK-CXL adds no extra advantage to the standard antimycotic regimen.[80] Similarly, a randomized controlled trial evaluating the efficacy of CXL as an adjuvant to appropriate antifungal therapy in nonresolving deep stromal fungal keratitis had to be stopped before full enrolment because of a high rate of perforation among the patients in the CXL group (four out of seven cases perforated in the CXL group compared to none out of six in the non-CXL group).[88] Said et al.[81] found that although PACK-CXL did not shorten the time to corneal healing, it prevented corneal melting. While Iseli et al.,[84] Saglk et al.[86] and Li et al.[85] found PACK-CXL to be useful in mycotic keratitis, Shetty et al.[82] reported good results in the management of superficial microbial keratitis and poor response in patients with deep stromal keratitis or endothelial plaque. Abbouda et al. reported halting of corneal melting with PACK-CXL in one case while the other developed perforation.[89]
The safety of CXL is of concern because the ultraviolet (UV)-A could damage intraocular structures. Recently, a detailed analysis of the expected damage compared with acceptable damage thresholds was published by Spoerl et al.[90] During standard CXL of a cornea with a 400-μm thickness, the irradiances of the UV light reaching the iris, lens, and retina are orders of magnitude smaller than the damage thresholds, and the only cell populations at risk are the microbes, the corneal endothelium, and the keratocytes.[81,90] Post-CXL complications, such as transient limbitis and a transient increase in the size of the hypopyon in the first 24 h followed by subsequent regression, has been reported.[81] Moreover, CXL itself can be complicated by infectious keratitis.
Rose bengal photodynamic therapy
PDT has been used for numerous applications such as choroidal neovascularization in age-related macular degeneration, corneal neovascularization, for tumor treatment, Acanthamoeba keratitis, and to prevent lenticular epithelial cell proliferation.[91] PDT involves the activation of photosensitizers using light of varying wavelengths. The photosensitizer is excited by the light and reacts with oxygen-generating ROS, which, in turn, react with various intracellular components to cause cell death.[92] Recently, in an experimental study, Arboleda et al.[92] have demonstrated RB PDT to be successful in infectious keratitis. However, there are no clinical studies to date to justify PDT with RB for treatment of fungal keratitis.
Management Guidelines
Management of fungal keratitis largely involves a decision on which antifungal to use and the route of administration. Current selection of antifungals is based on animal experiments, clinical experience, and published sensitivity data [Fig. 1]. In vitro sensitivity testing of a particular isolate is extremely useful and should be performed whenever its availability is not a concern. In most cases, clinical appearance of the keratitis is sufficient enough to determine whether it is responding to medical treatment or whether surgery is indicated.
Figure 1.
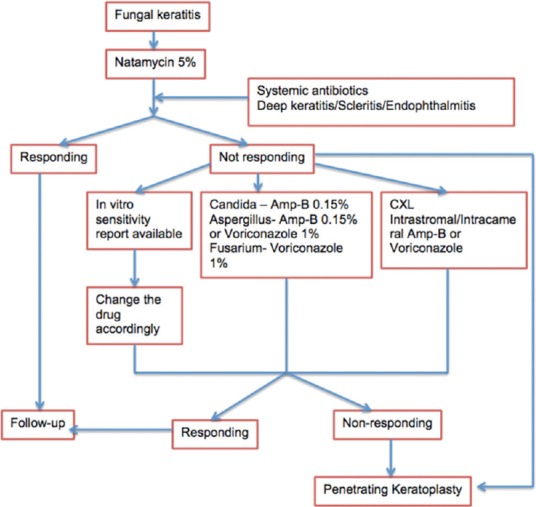
Flowchart for management of fungal keratitis
Clinically, commercially available NTM 5% suspension is the initial drug of choice for fungal keratitis. If worsening of the keratitis is observed on topical NTM or no improvement is seen after 2 weeks of therapy, topical AMB 0.15% can be substituted in cases of Candida spp. keratitis and Apergillus keratitis. A topical azole such as VCZ 1% can be substituted or added in cases Fusarium spp. and Apergillus keratitis. The clinician must determine the length of treatment for each case based on clinical response and experience. Treatment with a systemic antifungal agent is recommended in cases of severe deep keratitis, scleritis, and endophthalmitis. Systemic antifungals are also used after PKP for fungal keratitis. Several clinical and experimental studies have reported favorable results in the treatment of fungal keratitis with systemic ketoconazole, itraconazole, miconazole, fluconazole, VCZ, and posaconazole. The authors prefer ketoconazole for its broad spectrum activity and VCZ when cost of therapy is not a concern. The advantage of VCZ is that it has a favorable side-effect profile.
The corneal epithelium serves as a barrier to the penetration of most topical antifungal agents. Thus, debridement of the corneal epithelium can be helpful especially in cases of deep-seated keratitis. Although clinical trials have not shown any advantage of debridement, few clinicians still follow this.[47,48]
Keratoplasty is primarily indicated for medical treatment failure. However, under certain situations such as; advanced keratitis, severe corneal thinning, impending perforations, keratitis threatening to involve limbus the decision to perform keratoplasty must be taken early.
Treatment of atypical forms, or rarely reported fungus is difficult. The difficulty is primarily due to a delayed diagnosis or lack of evidence on susceptibility to routinely used antifungals agents. VCZ is the preferred drug in most such cases of fungal keratitis.[8,9,10,11,12,13] P. insidiosum keratitis is a vision-threatening keratitis that can lead to loss of eye in approximately 80% of the cases.[14] Few years back, it was considered to be a rare disease, however, a recent study by Sharma et al. clearly proved that it is not that uncommon, and the problem lies with the identification.[14] It is a fungus-like microbe that morphologically exhibits features of branching, sparsely septate or aseptate filaments and unlike fungi lacks the characteristic ergosterol in the cytoplasmic membrane.[93,94,95] Ocular trauma and CL use are often the predisposing factors. Reticular pattern of subepithelial and superficial infiltration or tentacle-like or dot-like corneal infiltrates are reported to be characteristic, but there is a variability in the reported studies so far.[93,94,95] Demonstration of zoospores, as proposed by Sharma et al. appears to be the is simplest way to diagnose these cases early.[14] Confirmation is done by DNA sequencing of the internal transcribed spacer region of the ribosomal DNA. Treatment is extremely difficult as the organism is not sensitive to any of the available antifungals. Wide surgical excision is the best way to treat such cases. Permpalung et al.[96] and Thanathanee et al.[95] have reported a lower enucleation rate (45%) with the use of immunotherapy and a combination of oral terbinafine and itraconazole. However, these authors remained unsure of the efficacy of the vaccine and attributed the lack of recurrence in their cases to early keratoplasty with a wide surgical excision.
Conclusion
Management of fungal keratitis remains a challenge to cornea specialists. Emerging fungal pathogens and resistance to existing antifungal drugs have further added to the reasons for poor prognosis in fungal keratitis. Newer investigative tools, such as PCR and IVCM, can help in reducing the time gap between clinical suspicion and microbiological diagnosis. Newer antifungal agents and newer methods of targeted drug delivery system can be helpful in treating recalcitrant cases. Nanoparticles and AMPs have shown promise in experimental studies and offer hope for improving prognosis in cases of fungal keratitis in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117:2263–7. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 2.Garg P. Fungal, mycobacterial, and nocardia infections and the eye: An update. Eye (Lond) 2012;26:245–51. doi: 10.1038/eye.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu S, Zhao GQ, Lin J, Wang X, Hu LT, Du ZD, et al. Natamycin in the treatment of fungal keratitis: A systematic review and Meta-analysis. Int J Ophthalmol. 2015;8:597–602. doi: 10.3980/j.issn.2222-3959.2015.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr Fungal Infect Rep. 2013;7:209–18. doi: 10.1007/s12281-013-0150-110.1007/s12281-013-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang HY, Chodosh J. Diagnostic and therapeutic considerations in fungal keratitis. Int Ophthalmol Clin. 2011;51:33–42. doi: 10.1097/IIO.0b013e31822d64dc. [DOI] [PubMed] [Google Scholar]
- 7.Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001-2007: A multicenter study. Ophthalmology. 2011;118:920–6. doi: 10.1016/j.ophtha.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangwan J, Lathwal S, Juyal D, Sharma N. Fonsecaea pedrosoi: A rare etiology in fungal keratitis. J Clin Diagn Res. 2013;7:2272–3. doi: 10.7860/JCDR/2013/6627.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lekhanont K, Nonpassopon M, Nimvorapun N, Santanirand P. Treatment With Intrastromal and Intracameral Voriconazole in 2 Eyes With Lasiodiplodia theobromae Keratitis: Medicine (Baltimore) Case Reports. 2015;94:e541. doi: 10.1097/MD.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaujoux T, Borsali E, Gavrilov JC, Touzeau O, Goldschmidt P, Despiau MC, et al. Fungal keratitis caused by Cylindrocarpon lichenicola. J Fr Ophtalmol. 2012;35:356.e1–5. doi: 10.1016/j.jfo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Jhanji V, Yohendran J, Constantinou M, Sheorey H, Vajpayee RB. Scedosporium scleritis or keratitis or both: Case series. Eye Contact Lens. 2009;35:312–5. doi: 10.1097/ICL.0b013e3181be722e. [DOI] [PubMed] [Google Scholar]
- 12.Motley WW, Melson AT, Mortensen JE. Pediatric Metarrhizium anisopliae keratitis. J AAPOS. 2011;15:101–3. doi: 10.1016/j.jaapos.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Yuan X, Wilhelmus KR, Matoba AY, Alexandrakis G, Miller D, Huang AJ. Pathogenesis and outcome of Paecilomyces keratitis. Am J Ophthalmol. 2009;147:691–6.e3. doi: 10.1016/j.ajo.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Balne PK, Motukupally SR, Das S, Garg P, Sahu SK, et al. Pythium insidiosum keratitis: Clinical profile and role of DNA sequencing and zoospore formation in diagnosis. Cornea. 2015;34:438–42. doi: 10.1097/ICO.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 15.Karthikeyan RS, Leal SM, Jr, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, et al. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or Fusarium. J Infect Dis. 2011;204:942–50. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leal SM, Jr, Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis – Insights from human disease and animal models. Cytokine. 2012;58:107–11. doi: 10.1016/j.cyto.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang N, Zhao G, Lin J, Hu L, Che C, Li C, et al. Indoleamine 2,3-dioxygenase is involved in the inflammation response of corneal epithelial cells to Aspergillus fumigatus infections. PLoS One. 2015;10:e0137423. doi: 10.1371/journal.pone.0137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Wang L, Wu X. Aspergillus fumigatus promotes T helper type 2 responses through thymic stromal lymphopoietin production by human corneal epithelial cells. Clin Experiment Ophthalmol. 2016 Jan 13; doi: 10.1111/ceo.12706. Doi: 10.1111/ceo12706. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Santacruz C, Linares M, Garfias Y, Loustaunau LM, Pavon L, Perez-Tapia SM, et al. Expression of IL-8, IL-6 and IL-1ß in tears as a main characteristic of the immune response in human microbial keratitis. Int J Mol Sci. 2015;16:4850–64. doi: 10.3390/ijms16034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas PA, Teresa PA, Theodore J, Geraldine P. PCR for the molecular diagnosis of mycotic keratitis. Expert Rev Mol Diagn. 2012;12:703–18. doi: 10.1586/erm.12.65. [DOI] [PubMed] [Google Scholar]
- 21.Vengayil S, Panda A, Satpathy G, Nayak N, Ghose S, Patanaik D, et al. Polymerase chain reaction-guided diagnosis of mycotic keratitis: A prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50:152–6. doi: 10.1167/iovs.07-1283. [DOI] [PubMed] [Google Scholar]
- 22.Tananuvat N, Salakthuantee K, Vanittanakom N, Pongpom M, Ausayakhun S. Prospective comparison between conventional microbial work-up vs PCR in the diagnosis of fungal keratitis. Eye (Lond) 2012;26:1337–43. doi: 10.1038/eye.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A, Basu S, Datta H, Chattopadhyay D. Evaluation of polymerase chain reaction-based ribosomal DNA sequencing technique for the diagnosis of mycotic keratitis. Am J Ophthalmol. 2007;144:396–403. doi: 10.1016/j.ajo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Chidambaram JD, Srinivasan M, Lalitha P, Wee D, Lietman TM, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol. 2008;146:714–23. doi: 10.1016/j.ajo.2008.06.009. 723.e1. [DOI] [PubMed] [Google Scholar]
- 25.Kuo MT, Chang HC, Cheng CK, Chien CC, Fang PC, Chang TC. A highly sensitive method for molecular diagnosis of fungal keratitis: A dot hybridization assay. Ophthalmology. 2012;119:2434–42. doi: 10.1016/j.ophtha.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Homa M, Shobana CS, Singh YR, Manikandan P, Selvam KP, Kredics L, et al. Fusarium keratitis in South India: Causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses. 2013;56:501–11. doi: 10.1111/myc.12062. [DOI] [PubMed] [Google Scholar]
- 27.Azor M, Gené J, Cano J, Manikandan P, Venkatapathy N, Guarro J. Less-frequent Fusarium species of clinical interest: Correlation between morphological and molecular identification and antifungal susceptibility. J Clin Microbiol. 2009;47:1463–8. doi: 10.1128/JCM.02467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Xiao M, Kong F, Chen S, Dou HT, Sorrell T, et al. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridization, and an in vitro antifungal susceptibility study. J Clin Microbiol. 2011;49:1890–8. doi: 10.1128/JCM.02415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzón A, Mellado E, Rodríguez-Tudela JL. Antifungal susceptibility profile of clinical Fusarium spp.isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–9. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- 30.Oechsler RA, Yamanaka TM, Bispo PJ, Sartori J, Yu MC, Melo AS, et al. Fusarium keratitis in Brazil: Genotyping, in vitro susceptibilities, and clinical outcomes. Clin Ophthalmol. 2013;7:1693–701. doi: 10.2147/OPTH.S40063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oechsler RA, Feilmeier MR, Miller D, Shi W, Hofling-Lima AL, Alfonso EC. Fusarium keratitis: Genotyping, in vitro susceptibility and clinical outcomes. Cornea. 2013;32:667–73. doi: 10.1097/ICO.0b013e318277ac74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar RL, Cruzat A, Hamrah P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin Ophthalmol. 2010;25:166–70. doi: 10.3109/08820538.2010.518516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labbé A, Khammari C, Dupas B, Gabison E, Brasnu E, Labetoulle M, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf. 2009;7:41–52. doi: 10.1016/s1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- 34.Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and Acanthamoeba keratitis. Ophthalmology. 2011;118:29–35. doi: 10.1016/j.ophtha.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Takezawa Y, Shiraishi A, Noda E, Hara Y, Yamaguchi M, Uno T, et al. Effectiveness of in vivo confocal microscopy in detecting filamentous fungi during clinical course of fungal keratitis. Cornea. 2010;29:1346–52. doi: 10.1097/ICO.0b013e3181cd3c84. [DOI] [PubMed] [Google Scholar]
- 36.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007;26:782–6. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]
- 37.Brasnu E, Bourcier T, Dupas B, Degorge S, Rodallec T, Laroche L, et al. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007;91:588–91. doi: 10.1136/bjo.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Li S, Liu M, Jin H, Xie L. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2008;246:581–6. doi: 10.1007/s00417-007-0719-x. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Samant M, Garg P, Vaddavalli PK, Vemuganti GK. Role of confocal microscopy in deep fungal keratitis. Cornea. 2009;28:11–3. doi: 10.1097/ICO.0b013e318181cff7. [DOI] [PubMed] [Google Scholar]
- 40.Lalitha P, Shapiro BL, Srinivasan M, Prajna NV, Acharya NR, Fothergill AW, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–93. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro BL, Lalitha P, Loh AR, Fothergill AW, Prajna NV, Srinivasan M, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94:384–5. doi: 10.1136/bjo.2009.158675. [DOI] [PubMed] [Google Scholar]
- 42.Lalitha P, Sun CQ, Prajna NV, Karpagam R, Geetha M, O'Brien KS, et al. In vitro susceptibility of filamentous fungal isolates from a corneal ulcer clinical trial. Am J Ophthalmol. 2014;157:318–26. doi: 10.1016/j.ajo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31:662–7. doi: 10.1097/ICO.0b013e31823f8ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal T, Bandivadekar P, Satpathy G, Sharma N, Titiyal JS. Detection of fungal hyphae using smartphone and pocket magnifier: Going cellular. Cornea. 2015;34:355–7. doi: 10.1097/ICO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 45.Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16:730–97. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller GG, Kara-José N, de Castro RS. Antifungals in eye infections: Drugs and routes of administration. Rev Bras Oftalmol. 2013;72:132–41. [Google Scholar]
- 47.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: A randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–9. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prajna NV, Mascarenhas J, Krishnan T, Reddy PR, Prajna L, Srinivasan M, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128:672–8. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: A broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5:775–85. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 50.Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592) Am J Ophthalmol. 2007;143:222–227. doi: 10.1016/j.ajo.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 51.Altun A, Kurna SA, Sengor T, Altun G, Olcaysu OO, Aki SF, et al. Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep Ophthalmol Med 2014. 2014 doi: 10.1155/2014/701653. 701653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sponsel WE, Graybill JR, Nevarez HL, Dang D. Ocular and systemic posaconazole (SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br J Ophthalmol. 2002;86:829–30. doi: 10.1136/bjo.86.7.829-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnoldner MA, Kheirkhah A, Jakobiec FA, Durand ML, Hamrah P. Successful treatment of Paecilomyces lilacinus keratitis with oral posaconazole. Cornea. 2014;33:747–9. doi: 10.1097/ICO.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto Y, Dogru M, Goto E, Fujishima H, Tsubota K. Successful topical application of a new antifungal agent, micafungin, in the treatment of refractory fungal corneal ulcers: Report of three cases and literature review. Cornea. 2005;24:748–53. doi: 10.1097/01.ico.0000154390.28254.54. [DOI] [PubMed] [Google Scholar]
- 55.Hurtado-Sarrió M, Duch-Samper A, Cisneros-Lanuza A, Díaz-Llopis M, Peman-Garcíia J, Vazquez-Polo A. Successful topical application of caspofungin in the treatment of fungal keratitis refractory to voriconazole. Arch Ophthalmol. 2010;128:941–2. doi: 10.1001/archophthalmol.2010.110. [DOI] [PubMed] [Google Scholar]
- 56.De Coupade C, Fittipaldi A, Chagnas V, Michel M, Carlier S, Tasciotti E, et al. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem J. 2005;390(Pt 2):407–18. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain A, Shah SG, Chugh A. Cell penetrating peptides as efficient nanocarriers for delivery of antifungal compound, natamycin for the treatment of fungal keratitis. Pharm Res. 2015;32:1920–30. doi: 10.1007/s11095-014-1586-x. [DOI] [PubMed] [Google Scholar]
- 58.Brandt CR. Peptide therapeutics for treating ocular surface infections. J Ocul Pharmacol Ther. 2014;30:691–9. doi: 10.1089/jop.2014.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mannis MJ. The use of antimicrobial peptides in ophthalmology: An experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc. 2002;100:243–71. [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira HA, Tsyshevskaya-Hoover I, Hinsley H, Logan S, Nguyen M, Nguyen TT, et al. Candidacidal activity of synthetic peptides based on the antimicrobial domain of the neutrophil-derived protein, CAP37. Med Mycol. 2010;48:263–72. doi: 10.1080/13693780903081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H, Ong ZY, Liu S, Li Y, Wiradharma N, Yang YY, et al. Synthetic ß-sheet forming peptide amphiphiles for treatment of fungal keratitis. Biomaterials. 2015;43:44–9. doi: 10.1016/j.biomaterials.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 62.Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–5. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 63.Diekema DJ, Messer SA, Hollis RJ, Jones RN, Pfaller MA. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J Clin Microbiol. 2003;41:3623–6. doi: 10.1128/JCM.41.8.3623-3626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jhanji V, Sharma N, Mannan R, Titiyal JS, Vajpayee RB. Management of tunnel fungal infection with voriconazole. J Cataract Refract Surg. 2007;33:915–7. doi: 10.1016/j.jcrs.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56–59. doi: 10.1016/j.ajo.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 66.Kalaiselvi G, Narayana S, Krishnan T, Sengupta S. Intrastromal voriconazole for deep recalcitrant fungal keratitis: A case series. Br J Ophthalmol. 2015;99:195–8. doi: 10.1136/bjophthalmol-2014-305412. [DOI] [PubMed] [Google Scholar]
- 67.Jain V, Borse N, Shome D, Natarajan S. Recalcitrant fungal tunnel infection treated with intrastromal injection of voriconazole. Int Ophthalmol. 2010;30:723–5. doi: 10.1007/s10792-010-9354-3. [DOI] [PubMed] [Google Scholar]
- 68.Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: Case series. Br J Ophthalmol. 2011;95:1735–7. doi: 10.1136/bjo.2010.192815. [DOI] [PubMed] [Google Scholar]
- 69.Tu EY, Hou J. Intrastromal antifungal injection with secondary lamellar interface infusion for late-onset infectious keratitis after DSAEK. Cornea. 2014;33:990–3. doi: 10.1097/ICO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 70.Tu EY. Alternaria keratitis: Clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofungin. Cornea. 2009;28:116–9. doi: 10.1097/ICO.0b013e31818225f8. [DOI] [PubMed] [Google Scholar]
- 71.Haddad RS, El-Mollayess GM. Combination of intracameral and intrastromal voriconazole in the treatment of recalcitrant Acremonium fungal keratitis. Middle East Afr J Ophthalmol. 2012;19:265–8. doi: 10.4103/0974-9233.95271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavallini GM, Ducange P, Volante V, Benatti C. Successful treatment of Fusarium keratitis after photo refractive keratectomy. Indian J Ophthalmol. 2013;61:669–71. doi: 10.4103/0301-4738.120213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma N, Chacko J, Velpandian T, Titiyal JS, Sinha R, Satpathy G, et al. Comparative evaluation of topical versus intrastromal voriconazole as an adjunct to natamycin in recalcitrant fungal keratitis. Ophthalmology. 2013;120:677–81. doi: 10.1016/j.ophtha.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Niki M, Eguchi H, Hayashi Y, Miyamoto T, Hotta F, Mitamura Y. Ineffectiveness of intrastromal voriconazole for filamentous fungal keratitis. Clin Ophthalmol. 2014;8:1075–9. doi: 10.2147/OPTH.S63516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuriakose T, Kothari M, Paul P, Jacob P, Thomas R. Intracameral amphotericin B injection in the management of deep keratomycosis. Cornea. 2002;21:653–6. doi: 10.1097/00003226-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Yilmaz S, Ture M, Maden A. Efficacy of intracameral amphotericin B injection in the management of refractory keratomycosis and endophthalmitis. Cornea. 2007;26:398–402. doi: 10.1097/ICO.0b013e318030767e. [DOI] [PubMed] [Google Scholar]
- 77.Kaushik S, Ram J, Brar GS, Jain AK, Chakraborti A, Gupta A. Intracameral amphotericin B: Initial experience in severe keratomycosis. Cornea. 2001;20:715–9. doi: 10.1097/00003226-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Yoon KC, Jeong IY, Im SK, Chae HJ, Yang SY. Therapeutic effect of intracameral amphotericin B injection in the treatment of fungal keratitis. Cornea. 2007;26:814–8. doi: 10.1097/ICO.0b013e31806c791e. [DOI] [PubMed] [Google Scholar]
- 79.Shao Y, Yu Y, Pei CG, Tan YH, Zhou Q, Yi JL, et al. Therapeutic efficacy of intracameral amphotericin B injection for 60 patients with keratomycosis. Int J Ophthalmol. 2010;3:257–60. doi: 10.3980/j.issn.2222-3959.2010.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vajpayee RB, Shafi SN, Maharana PK, Sharma N, Jhanji V. Evaluation of corneal collagen cross-linking as an additional therapy in mycotic keratitis. Clin Experiment Ophthalmol. 2015;43:103–7. doi: 10.1111/ceo.12399. [DOI] [PubMed] [Google Scholar]
- 81.Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121:1377–82. doi: 10.1016/j.ophtha.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Shetty R, Nagaraja H, Jayadev C, Shivanna Y, Kugar T. Collagen crosslinking in the management of advanced non-resolving microbial keratitis. Br J Ophthalmol. 2014;98:1033–5. doi: 10.1136/bjophthalmol-2014-304944. [DOI] [PubMed] [Google Scholar]
- 83.Tabibian D, Richoz O, Riat A, Schrenzel J, Hafezi F. Accelerated photoactivated chromophore for keratitis-corneal collagen cross-linking as a first-line and sole treatment in early fungal keratitis. J Refract Surg. 2014;30:855–7. doi: 10.3928/1081597X-20141113-06. [DOI] [PubMed] [Google Scholar]
- 84.Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27:590–4. doi: 10.1097/ICO.0b013e318169d698. [DOI] [PubMed] [Google Scholar]
- 85.Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultravoilet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97:669–71. doi: 10.1136/bjophthalmol-2012-302518. [DOI] [PubMed] [Google Scholar]
- 86.Saglk A, Uçakhan OO, Kanpolat A. Ultraviolet A and riboflavin therapy as an adjunct in corneal ulcer refractory to medical treatment. Eye Contact Lens. 2013;39:413–5. doi: 10.1097/ICL.0b013e3182960fdf. [DOI] [PubMed] [Google Scholar]
- 87.Sauer A, Letscher-Bru V, Speeg-Schatz C, Touboul D, Colin J, Candolfi E, et al. In vitro efficacy of antifungal treatment using riboflavin/UV-A (365 nm) combination and amphotericin B. Invest Ophthalmol Vis Sci. 2010;51:3950–3. doi: 10.1167/iovs.09-4013. [DOI] [PubMed] [Google Scholar]
- 88.Uddaraju M, Mascarenhas J, Das MR, Radhakrishnan N, Keenan JD, Prajna L, et al. Corneal cross-linking as an adjuvant therapy in the management of recalcitrant deep stromal fungal keratitis: A randomized trial. Am J Ophthalmol. 2015;160:131–4.e5. doi: 10.1016/j.ajo.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 89.Abbouda A, Estrada AV, Rodriguez AE, Alió JL. Anterior segment optical coherence tomography in evaluation of severe fungal keratitis infections treated by corneal crosslinking. Eur J Ophthalmol. 2014;24:320–4. doi: 10.5301/ejo.5000424. [DOI] [PubMed] [Google Scholar]
- 90.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–9. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 91.Szentmáry N, Goebels S, Bischoff M, Seitz B. Photodynamic therapy for infectious keratitis. Ophthalmologe. 2012;109:165–70. doi: 10.1007/s00347-011-2511-x. [DOI] [PubMed] [Google Scholar]
- 92.Arboleda A, Miller D, Cabot F, Taneja M, Aguilar MC, Alawa K, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158:64–70.e2. doi: 10.1016/j.ajo.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kosrirukvongs P, Chaiprasert A, Uiprasertkul M, Chongcharoen M, Banyong R, Krajaejun T, et al. Evaluation of nested pcr technique for detection of Pythium insidiosum in pathological specimens from patients with suspected fungal keratitis. Southeast Asian J Trop Med Public Health. 2014;45:167–73. [PubMed] [Google Scholar]
- 94.Thanathanee O, Enkvetchakul O, Rangsin R, Waraasawapati S, Samerpitak K, Suwan-apichon O. Outbreak of Pythium keratitis during rainy season: A case series. Cornea. 2013;32:199–204. doi: 10.1097/ICO.0b013e3182535841. [DOI] [PubMed] [Google Scholar]
- 95.Tanhehco TY, Stacy RC, Mendoza L, Durand ML, Jakobiec FA, Colby KA. Pythium insidiosum keratitis in Israel. Eye Contact Lens. 2011;37:96–8. doi: 10.1097/ICL.0b013e3182043114. [DOI] [PubMed] [Google Scholar]
- 96.Permpalung N, Worasilchai N, Plongla R, Upala S, Sanguankeo A, Paitoonpong L, et al. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: A retrospective study of 18 patients. J Antimicrob Chemother. 2015;70:1885–92. doi: 10.1093/jac/dkv008. [DOI] [PubMed] [Google Scholar]


