Abstract
Purpose:
To study the efficacy of intravitreal interferon alpha-2b for endotoxin-induced uveitis.
Materials and Methods:
A total of 36 rabbits were randomly allocated to one of the three groups: (1) received interferon plus balanced-salt solution; (2) received lipopolysaccharide (LPS) plus interferon; and (3) received LPS plus balanced-salt solution. Intraocular inflammation was evaluated by slit-lamp biomicroscopy (standardization of uveitis nomenclature grading), binocular indirect ophthalmoscopy (BIO) score, and histopathology.
Results:
Group 2 showed significantly lower mean (±standard deviation) anterior chamber reaction than Group 3 (3.1 ± 0.9 vs. 3.8 ± 0.4) on day 1 postinjection, lower vitreous cells on days 1 through 7 (day 1: 3.1 ± 0.9 vs. 3.8 ± 0.4; day 3: 2.1 ± 1.6 vs. 3.8 ± 0.4; day 7: 1.9 ± 1.3 vs. 3.6 ± 0.7), and lower BIO score on days 1–7 (day 1: 3.3 ± 1.2 vs. 4.4 ± 0.7; day 3: 3.0 ± 1.4 vs. 4.3 ± 0.9; day 7: 2.4 ± 1.4 vs. 3.7 ± 1.2). The protein content of anterior and vitreous aspirates was lower in Group 2 than 3 (1618.5 ± 411.4 vs. 2567.3 ± 330.8 and 2157.0 ± 283.3 vs. 3204.6 ± 259.5, respectively).
Conclusion:
Intravitreal interferon alpha-2b was effective in controlling endotoxin-induced uveitis.
Keywords: Endotoxin, interferon alpha-2b, intravitreal, lipopolysaccharide, uveitis
One of the important causes of visual impairment worldwide is uveitis.[1] A variety of disorders such as Behcet's disease, Vogt-Koyanagi-Harada's disease, sympathetic ophthalmia, sarcoidosis, and white dot syndromes, are classified as noninfectious uveitis. These types of uveitis are often caused by an imbalance in the ocular immune system.[2]
Some types of medications have been used in the treatment of noninfectious uveitis. Corticosteroids are the main-stay of treatment, but they are not always effective, and long-term use is associated with significant ocular or systemic side effects.[3] Immunomodulatory agents have been successfully applied as adjunctive or steroid-sparing agents in cases with associated systemic disorders or in severe recalcitrant isolated uveitis.[4] Suppression of the immune system and life-threatening infections are possible complications of these drugs. Research should be continued to find novel effective and safe therapeutic medications for treatment of ocular inflammation.
Some immunomodulatory agents, with proven systemic efficacy against autoimmune disorders, have been used intravitreally. The intravitreal injection may enhance ocular efficacy and minimize systemic toxicity. Intravitreal infliximab, for instance, showed promising effects in treating experimental or clinical uveitis.[5,6,7] If proven as an effective and safe therapy, other immunomodulatory agents also have the potential to be used intravitreally in the treatment of uveitis.
Interferons, a class of cytokines, were originally described in 1957 as natural antiviral substances that are produced by most cells in response to viral infection. They can interfere with viral replication, reduce cell proliferation, and alter immunity.[8]
Interferons can form a network of complex interactions with other cytokines, and connect innate and adaptive immunity. They seem to be involved in the induction of autoimmune disorders as well as their treatment.[9] Several animal and human studies revealed that interferons have rather immunomodulatory effects than immunosuppressive.[10] Overall, these agents have the potential to be used in the management of uveitis.
Interferons are classified into Type 1 (interferon α, interferon β) and Type 2 (interferon γ).[9,10] There are several reports of successful systemic interferon alpha-2b use in treating uveitis.[11,12,13] Because of lower applied doses, perfect penetration, assumed endurable efficacy, and minimal systemic side effects, intravitreal administration of these agents may be more promising in isolated uveitis. In this study, we aimed to evaluate the efficacy of intravitreal interferon alpha-2b in the management of experimental endotoxin-induced uveitis.
Materials and Methods
Thirty-six New Zealand white rabbits were included in this study. Only right eyes of the animals were used for the experiment. All rabbits were anesthetized and treated after receiving approval from the institutional review board at the Shiraz University of Medical Sciences.
Procedure
Before each procedure, general anesthesia was induced with an intramuscular injection of ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (10 mg/kg). The rabbits were randomly assigned to one of the three groups: The first group (n = 12) received intravitreal injection of 200,000 IU/0.1 mL interferon alpha-2b (PDferon®, Pooyeshdarou Pharmaceutical Co., Tehran, Iran), plus 0.1 mL balanced salt solution; the second group (n = 12) received 2 μg/0.1 mL salmonella typhimurium lipopolysaccharide (LPS) endotoxin (L6511; Sigma Chemical, St. Louis, MO, USA), plus 200,000 IU/0.1 mL interferon alpha-2b; and the third group (n = 12) received 2 μg/0.1 mL LPS, plus 0.1 mL balanced salt solution intravitreally. To mitigate postinjection increase in intraocular pressure, 0.1 mL of aqueous was aspirated from the anterior chamber of all rabbits before intravitreal injection. The rational for the dosage of interferon alpha-2b in this study was adopted from a previous safety study on rabbits, which reported intraocular toxicity for 2,000,000 IU of the drug, but not for 1,000,000 IU.[14] To leave a confident safety zone, we used a 1/10th of the toxic threshold.
Under the aseptic condition, intravitreal injections were performed through pars plana, 2.5 mm posterior to the limbus, using a 27-gauge needle. Topical ciprofloxacin and povidone-iodine were applied before and after injections. To confirm the healthiness of the studied eyes, the right eyes of all animals were examined by slit-lamp biomicroscopy and binocular indirect ophthalmoscope (BIO) before the injections. All injections were performed by a single investigator (Mehrdad Afarid) in a masked manner.
Evaluation of inflammation
On days 1, 3, and 7 after injection, the intensity of intraocular inflammation was evaluated using slit-lamp biomicroscopy and BIO by a single masked observer (Mehrdad Afarid). The Standardization of Uveitis Nomenclature Working Group grading scheme for anterior chamber cells was used for evaluating anterior chamber inflammation:[15] Vitreous inflammation was graded from 0 to 4+ based on the density of vitreal inflammatory cells.[16] Vitreous haze was evaluated using BIO and a 20 D condensing lens.[17]
On the 7th postinjection day, under general anesthesia, 0.1 mL of aqueous and 0.5 mL of vitreous samples were obtained from each eye, using a 27-gauge needle attached to a 1 mL tuberculin syringe, and 19-gauge needle attached to a 2 mL syringe, respectively. Caution was exerted to avoid injury to the lens, iris, and retina during sampling. Aqueous and vitreous cell count was evaluated using a hemocytometer slide under a microscope at ×100 magnification (IX71 Microscope, Olympus, Tokyo, Japan). Measurement of protein levels of the aspirate was performed using pierce kit (Pierce BCA Protein Assay kit, code 23227, USA), in which the bovine serum albumin was considered as the standard. Next, the right eyes of all animals were enucleated. The enucleated globes were processed for light microscopy after fixation in 10% buffered formalin for 2 days, hematoxylin and eosin staining method was used. Infiltrating cells in ten random, noncontiguous fields at ×200 magnification for each of the anterior (the iris-ciliary body) and posterior (the retina) segment fields counted by a single masked pathologist. A semi-logarithmic grading scale, adopted from Verma et al.,[18] was used to compare infiltrating cells among the three groups.
Statistical analysis
Statistical analysis was performed using SPSS version 17 software (SPSS Inc., Chicago, Illinois, USA). Measurements from the three groups were compared using analysis of variance ([ANOVA]; with Tukey honestly significant difference [HSD] for pairwise comparisons) or Kruskal–Wallis test (with Mann–Whitney U-test for pair-wise comparisons), when appropriate. All reported P values were two-sided and considered statistically significant if <0.05.
Results
Slit-lamp examination
Comparison of the anterior chamber and anterior vitreous cellular reaction according to slit-lamp examination grading among the three groups are presented in Tables 1 and 2, respectively. Group 1 showed a consistent lower cellular reaction (median = 0 in all occasions) in both anterior chamber and vitreous compared to other groups. On the 1st postinjection day, Group 2 showed significantly lower mean (±standard deviation) anterior chamber reaction compared to Group 3 (3.1 ± 0.9 vs. 3.8 ± 0.4; P = 0.023), whereas no significant difference was found on days 3 or 7. For vitreous cells, however, Group 2 showed lower values on days one through seven [Table 2].
Table 1.
Comparison of anterior chamber cellular reaction according to slit-lamp examination grading between the three groups

Table 2.
Comparison of vitreous humor cellular reaction according to slit-lamp examination grading between the three groups

Binocular indirect ophthalmoscopy score
Comparison of vitreous haze according to BIO examination grading between the three groups is presented in Table 3. The vitreous haze was significantly lower in Group 1 than Groups 2 or 3, and in Group 2 than Group 3. Table 4 presents a comparatively detailed scoring of individual rabbits in the LPS versus interferon/LPS groups.
Table 3.
Comparison of vitreous haze according to binocular indirect ophthalmoscope score between the three groups

Table 4.
Comparative detailed scoring of individual rabbits in the lipopolysaccharide versus interferon/lipopolysaccharide groups

Aqueous and vitreous aspirates
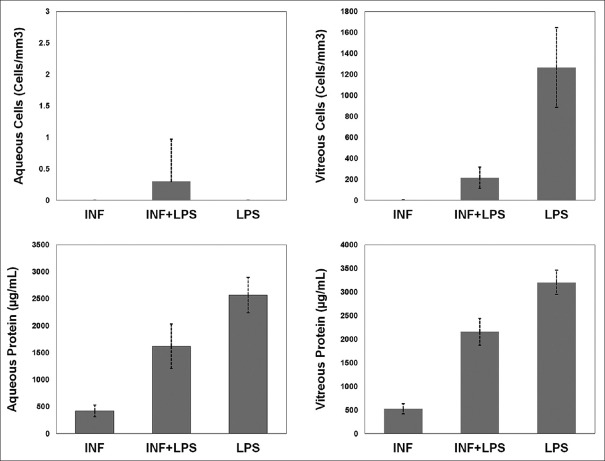
The number of inflammatory cells and the amounts of protein in aqueous and vitreous samples of different groups are depicted in Fig. 1. The mean of anterior chamber cells were not statistically different: 0 ± 0 cells/mm3 in Group 1, 0.3 ± 0.7 in Group 2, and 0 ± 0 in Group 3 (P = 0.084, Kruskal–Wallis test). The mean of vitreous cells were 1.2 ± 3.7 cells/mm3 in Group 1, 216.0 ± 102.2 in Group 2, and 1266.4 ± 379.9 in Group 3 (P < 0.001, Kruskal–Wallis test; pair-wise comparison [Mann–Whitney U-test]: Group 1 vs. 2, P < 0.001; Group 1 vs. 3, P < 0.001; Group 2 vs. 3, P < 0.001). The mean of anterior chamber protein was 419.7 ± 108.4 μg/mL, 1618.5 ± 411.4, and 2567.3 ± 330.8 in Groups 1, 2, and 3, respectively (P < 0.001, ANOVA test; pair-wise comparison [Tukey HSD test]: P < 0.001 for all pairs). The mean of vitreous humor protein was 525.3 ± 109.2 μg/mL, 2157.0 ± 283.3, and 3204.6 ± 259.5 in Groups 1, 2, and 3, respectively (P < 0.001, ANOVA test; pair-wise comparison [Tukey HSD test]: P < 0.001 for all pairs).
Figure 1.
Comparison of aqueous and vitreous aspirates cells and protein between the three groups. INF: Interferon, LPS: Lipopolysaccharide
Histopathologic examination
The mean histopathologic score in iris-ciliary body section was 0.3 ± 0.5 in Group 1, 2.8 ± 0.6 in Group 2, and 2.6 ± 0.5 in Group 3 (P < 0.001, Kruskal–Wallis test; pair-wise comparison [Mann–Whitney U-test]: Group 1 vs. 2, P < 0.001; Group 1 vs. 3, P < 0.001; Group 2 vs. 3, P = 0.527). The mean histopathologic score in retina section was 0 ± 0 in Group 1, 2.8 ± 0.4 in Group 2, and 3.0 ± 0.7 in Group 3 (P < 0.001, Kruskal–Wallis test; pair-wise comparison [Mann–Whitney U-test]: Group 1 vs. 2, P < 0.001; Group 1 vs. 3, P < 0.001; Group 2 vs. 3, P = 0.533). For both the iris-ciliary body and the retina sections, the score was significantly lower in Group 1 than Groups 2 or 3, and the score was not significantly different between Groups 2 and 3. Analysis of Group 1 histologic sections revealed that intravitreal injection of 200,000 IU/0.1 mL interferon alpha-2b was associated with no histopathologically evident toxic ocular effects. Fig. 2 demonstrates the light microscopy images of ciliary body and retina of a typical sample from each group.
Figure 2.

(a and b) Light microscopy images of ciliary body and retina of Group 1 animal show no inflammation (H and E, ×100); (c and d) ciliary body and retina of Group 2 animal show mild infiltration of inflammatory cells (H and E, ×40); (e and f) ciliary body and retina of Group 3 animal show marked inflammation (H and E, ×100)
Discussion
The results of this study showed that intravitreal administration of 200,000 IU/0.1 mL interferon alpha-2b was associated with significant decrease in intraocular inflammation, and particularly the vitreous inflammation, in an endotoxin-induced model of uveitis. Slit-lamp examination of anterior vitreous, vitreous haze evaluation by BIO score and cell and protein analysis of vitreous aspirates were all in favor of significant anti-inflammatory effect of interferon alpha-2b. However, for anterior chamber inflammation, the results were not as consistent. Slit-lamp examination showed a significant decrease in anterior chamber cellular reaction on postinjection day 1 in Group 2 compared to Group 3, but not on days 3 and 7. Anterior chamber aspirates were generally hypocellular and no significant difference was found between groups. In line with vitreous aspirates, the aqueous protein level was significantly lower in Group 2 than Group 3.
In the eye, interferons may exert their anti-inflammatory effects via changes in vitreous microenvironment or enhancing barrier function of diseased retinal capillaries.[10,19] However, the exact mechanism of their action in controlling exuberant immune reaction is yet to be elucidated. The observed greater effect of treatment on vitreous inflammation may be explained by the intravitreal rout that used to deliver both the immunogenic LPS and the interferon. Our histopathologic evaluation of iris-ciliary body and retinal sections revealed no difference between Groups 2 and 3 in the inflammatory cell infiltrates, suggesting that intravitreal interferon alpha-2b may have poor penetration into the retina. This issue has not been addressed in previous studies and could be the subject of future investigations.
In this study, we did not observe any clinical or histopathologic evidence for retinal toxicity after intravitreal injection of 200,000 IU/0.1 mL interferon alpha-2b. A previous experimental study suggested that intravitreal interferon alpha-2b up to 1,000,000 IU was safe according to histopathologic examination in rabbits.[14] In the only report of intravitreal interferon alpha-2b use in human, Kertes et al.[20] evaluated long-term effects and safety of a single injection of 100,000 IU of the drug in two cases of neovascular age-related macular degeneration. They did not report any clinical ocular or systemic adverse effect; however, in both treated eyes, they observed a marked generalized reduction in the amplitude of the bright-flash dark-adapted electroretinogram 1 month after injection that had returned to preinjection levels at 5 months after treatment. In addition, systemic therapy with interferon alpha-2b has been associated with ischemic retinopathy characterized by cotton wool spots and capillary dropouts in a subset of treated patients.[21,22,23] These features of retinopathy had typically occurred 1–3 months after the inception of therapy.[23] Together these reports raise concerns about the possibility of long-term toxicities of intravitreal interferon therapy that have not yet been addressed by the current literature.
This study has several limitations. First, we only used one type of experimental uveitis, and findings of this study should be corroborated by other experimental studies using other methods for inducing uveitis (such as human interphotoreceptor retinoid binding protein-derived peptide induced uveitis). In addition, we evaluated the short-term effects of one injection up to 1 week after treatment, and the long-term efficacy of the treatment and the role of multiple injections remain unknown. Furthermore, we did not have access to anterior chamber flare cell meter which would be a better method for evaluating anterior chamber inflammation. Finally, we did not obtain electroretinography as a safety measure, because we did not have access to the electroretinography animal set at the time of the study. However, safety was not our primary goal in this study.
Conclusion
A single intravitreal injection of 200,000 IU/0.1 mL interferon alpha-2b is effective in controlling experimental endotoxin-induced uveitis in short-term. Concerns about the long-term safety and efficacy should be addressed before progressing to clinical trials on humans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We appreciate Nader Tanideh and Mahjoub Vahedi (Laboratory Animal Research Center, Shiraz University of Medical Sciences), and Afsoon Hakimzadeh (Central Laboratory, Khalili Hospital, Shiraz University of Medical Sciences) for their kind cooperation in this study. We also thank Narges Rousta (Department of Biostatistics, Shiraz University of Medical Sciences) for her valuable help and advice in statistical analysis of data.
References
- 1.Pereira DV, Steckert AV, Mina F, Petronilho F, Roesler R, Schwartsmann G, et al. Effects of an antagonist of the gastrin-releasing peptide receptor in an animal model of uveitis. Invest Ophthalmol Vis Sci. 2009;50:5300–3. doi: 10.1167/iovs.09-3525. [DOI] [PubMed] [Google Scholar]
- 2.Schewitz-Bowers LP, Lee RWJ, Dick AD. Immune mechanisms of intraocular inflammation. Expert Rev Ophthalmol. 2010;5:43–58. [Google Scholar]
- 3.Rothova A. Corticosteroids in uveitis. Ophthalmol Clin North Am. 2002;15:389–94. doi: 10.1016/s0896-1549(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 4.Rao NA, Rajeev B. Treatment of uveitis with immunosuppressive agents. Indian J Ophthalmol. 1993;41:107–13. [PubMed] [Google Scholar]
- 5.Farvardin M, Afarid M, Mehryar M, Hosseini H. Intravitreal infliximab for the treatment of sight-threatening chronic noninfectious uveitis. Retina. 2010;30:1530–5. doi: 10.1097/IAE.0b013e3181d3758a. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini H, Safaei A, Khalili MR, Nowroozizadeh B, Eghtedari M, Farvardin M, et al. Intravitreal infliximab in experimental endotoxin-induced uveitis. Eur J Ophthalmol. 2009;19:818–23. doi: 10.1177/112067210901900521. [DOI] [PubMed] [Google Scholar]
- 7.Farvardin M, Afarid M, Shahrzad S. Long-term effects of intravitreal infliximab for treatment of sight-threatening chronic noninfectious uveitis. J Ocul Pharmacol Ther. 2012;28:628–31. doi: 10.1089/jop.2011.0199. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–67. [PubMed] [Google Scholar]
- 9.Mackensen F, Max R, Becker MD. Interferon therapy for ocular disease. Curr Opin Ophthalmol. 2006;17:567–73. doi: 10.1097/ICU.0b013e328010ab35. [DOI] [PubMed] [Google Scholar]
- 10.Mackensen F, Max R, Becker MD. Interferons and their potential in the treatment of ocular inflammation. Clin Ophthalmol. 2009;3:559–66. doi: 10.2147/opth.s3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler NJ, Suhler EB, Rosenbaum JT. Interferon alpha 2b in the treatment of uveitic cystoid macular edema. Ocul Immunol Inflamm. 2012;20:86–90. doi: 10.3109/09273948.2011.645989. [DOI] [PubMed] [Google Scholar]
- 12.Demiroglu H, Ozcebe OI, Barista I, Dündar S, Eldem B. Interferon alfa-2b, colchicine, and benzathine penicillin versus colchicine and benzathine penicillin in Behçet's disease: A randomised trial. Lancet. 2000;355:605–9. doi: 10.1016/S0140-6736(99)05131-4. [DOI] [PubMed] [Google Scholar]
- 13.Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol. 2007;144:55–61. doi: 10.1016/j.ajo.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Kertes PJ, Britton WA, Jr, Addison DJ, Munro SM, Marshall DH, Leonard BC. Toxicity of intravitreal interferon alpha-2b in the rabbit. Can J Ophthalmol. 1995;30:355–9. [PubMed] [Google Scholar]
- 15.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephen Foster C, Vitale A. Diagnosis and Treatment of Uveitis. 2nd ed. New Dehli, India: Jaypee Brothers Medical Publishers; 2013. [Google Scholar]
- 17.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 18.Verma MJ, Mukaida N, Vollmer-Conna U, Matsushima K, Lloyd A, Wakefield D. Endotoxin-induced uveitis is partially inhibited by anti-IL-8 antibody treatment. Invest Ophthalmol Vis Sci. 1999;40:2465–70. [PubMed] [Google Scholar]
- 19.Gillies MC, Su T. Interferon-alpha 2b enhances barrier function of bovine retinal microvascular endothelium in vitro . Microvasc Res. 1995;49:277–88. doi: 10.1006/mvre.1995.1024. [DOI] [PubMed] [Google Scholar]
- 20.Kertes PJ, Britton WA, Jr, Leonard BC. Intravitreal interferon alpha-2b for the treatment of neovascular age-related macular degeneration: A pilot study. Can J Ophthalmol. 1997;32:185–8. [PubMed] [Google Scholar]
- 21.Hayasaka S, Nagaki Y, Matsumoto M, Sato S. Interferon associated retinopathy. Br J Ophthalmol. 1998;82:323–5. doi: 10.1136/bjo.82.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves LL, Farias AQ, Gonçalves PL, D'Amico EA, Carrilho FJ. Branch retinal vein thrombosis and visual loss probably associated with pegylated interferon therapy of chronic hepatitis C. World J Gastroenterol. 2006;12:4602–3. doi: 10.3748/wjg.v12.i28.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain K, Lam WC, Waheeb S, Thai Q, Heathcote J. Retinopathy in chronic hepatitis C patients during interferon treatment with ribavirin. Br J Ophthalmol. 2001;85:1171–3. doi: 10.1136/bjo.85.10.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]