Abstract
Background:
Vitiligo is a progressive depigmenting disorder characterized by the loss of functional melanocytes from the epidermis. The etiopathogenesis of vitiligo is still unclear. Heat shock proteins (HSPs) are prime candidates to connect stress to the skin. HSPs were found to be implicated in autoimmune diseases such as rheumatoid arthritis and other skin disorders as psoriasis.
Aim and Objectives:
The aim of this study was to map the level of HSP-70 in vitiligo lesions to declare its role in the pathogenesis and activity of vitiligo.
Materials and Methods:
The study included thirty patients with vitiligo and 30 age- and sex-matched healthy controls. Vitiligo patients were divided as regards to the disease activity into highly active, moderately active, and inactive vitiligo groups. Skin biopsies were taken from the lesional and nonlesional skin of patients and from the normal skin of the controls. HSP-70 messenger RNA (mRNA) expression was estimated using quantitative real-time polymerase chain reaction.
Results:
Our analysis revealed a significantly higher expression of HSP-70 mRNA in lesional skin biopsies from vitiligo patients compared to nonlesional skin biopsies from vitiligo patients (P < 0.001) and compared to skin biopsies from healthy controls (P < 0.001). The level of HSP-70 was not found to be correlated with age, sex, or disease duration. The expression of HSP-70 was correlated with the disease activity and patients with active vitiligo showed higher mean HSP-70 level compared to those with inactive disease.
Conclusions:
HSP-70 plays a role in the pathogenesis of vitiligo and may enhance the immune response in active disease.
Keywords: Heat shock protein-70, messenger RNA, real-time polymerase chain reaction, vitiligo
Introduction
What was known?
Vitiligo is a depigmenting disease with possible autoimmune etiology
Stress is involved in induction of vitiligo
Heat shock proteins (HSPs) are prime candidates to connect stress to the skin
HSPs were found to be implicated in autoimmune diseases.
The heat shock protein-70 (HSP-70) family is composed of at least 17 highly related genes on chromosomes 1, 5, 6, 9, 11, 14, and 21 in humans, encoding constitutively expressed and inducible proteins.[1] The common denominator is expression induced by elevated temperatures (heat shock) of proteins with an approximate molecular weight of 70 kDa (66–78 kDa).[2] Family members serve as chaperones, guiding intracellular proteins to respective organelle targets.[3] In this function, HSP-70 facilitates folding, binding and translocation of proteins.[4]
Vitiligo is a common pigmentary disorder characterized by well-demarcated depigmented patches or macules of different shapes and sizes and caused by the destruction of functional melanocytes in the involved epidermis.[5]
Genetic studies support the involvement of abnormalities affecting immune function in vitiligo.[6] T-cell infiltrates are observed in the perilesional skin of patients with active vitiligo.[7] T cells isolated from vitiligo skin are cytotoxic toward melanocytes.[8] Thus, vitiligo is primarily a T cell-mediated disease; although, humoral responses may also contribute to disease development.[9]
Intrinsic abnormalities that were found in vitiligo melanocytes, including dilated endoplasmic reticulum (ER) profiles, mitochondrial abnormalities, and abnormal melanosome compartmentalization possibly rendering the cells increasingly sensitive to stress.[10,11] Patients consider stress a precipitating factor for their disease,[12] known stressors, including bleaching phenols, ultraviolet irradiation, and mechanical injury, invoke a Koebner phenomenon in about half of patients.[13] In terms of emotional stress, obsession and phobia have been correlated with autoimmune markers in vitiligo.[14]
In this respect, HSPs are prime candidates to connect stress to the skin with the autoimmune response to follow. In particular, HSPs 60, 70, and 90 have been implicated in immune cell activation.[15] Stress proteins support immune reactivity by activating dendritic cells to more efficiently phagocytize, process, and present antigens.[16]
The aim of this study was to declare whether HSP-70 secretion could contribute to the pathogenesis and the activity of vitiligo.
Materials and Methods
Selection of the study subjects
The study included 30 patients with vitiligo referred to the dermatology clinic at the Beni-Suef University Hospitals, Beni Suef, Egypt and thirty age- and gender-matched healthy volunteers who were donating blood.
Subjects (patients and controls) with malignancies, autoimmune diseases other than vitiligo and those receiving phototherapy, oral steroids, or topical treatments in the past 6 months were excluded from the study.[17] Subjects more than 60-year-old were also excluded from the study.[18]
The complete clinical examination was done of the skin and mucosa for the exclusion of any associated disease. Patients’ information was collected by one dermatologist, including age, sex, type of vitiligo (generalized, localized or universal),[19] and affected body surface area according to the rule of nines.[20]
Patients were classified into three groups as regards to vitiligo activity; highly active vitiligo group (i.e., appearance of new lesions or progression of older lesion within the past3 months), moderately active group (i.e., appearance of new lesions or progression of older lesion within the past >3 but <6 months), and inactive group (i.e., spontaneous improvement of existing lesions and or no appearance of new lesions or progression of older lesion within the past 6 months).
Four millimeters punch biopsies were taken from lesional skin and nonlesional skin of every patient and the normal skin biopsies from the controls. All the skin biopsies were stored at − 80°C until real-time polymerase chain reaction (RT-PCR) was performed for the detection of HSP-70 messenger RNA (mRNA) expression.
Patients consent
The purpose of this study was explained for each patient. A written informed consent was taken from each patient. Local research ethics approval was taken before starting data collection. With respect to patients’ confidentiality, they were represented in the study by code numbers. All personal data were concealed. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institution's Human Research Committee.
Detection of heat shock protein-70 gene expression in tissue using real-time polymerase chain reaction
RNA extraction
Total RNA was isolated from skin tissue homogenates using RNeasy Purification Reagent (Qiagen, Valencia, CA, USA) according to manufacturer's instruction. The purity (A260/A280 ratio) and the concentration of RNA were obtained using spectrophotometry (GeneQuant 1300, Uppsala, Sweden). RNA quality was confirmed by gel electrophoresis.
Complementary DNA synthesis
First-strand complementary DNA was synthesized from 4 μg of total RNA using an Oligo (dT) 12-18 primer and Superscript™ II RNase Reverse Transcriptase, This mixture was incubated at 42°C for 1 h, the kit was supplied by SuperScript Choice System (Life Technologies, Breda, The Netherlands).
Real-time quantitative polymerase chain reaction
RT-PCR amplification was carried out using 10 μL amplification mixtures containing Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), equivalent to 8 ng of reverse-transcribed RNA and 300 nM primers, the HSP-70 primer sequence was as follows: 5’-AGCGT AACAC CACCA TTCC-3’ (forward) and 5’-TGGCT CCCAC CCTAT CTC-3’ (reverse).
Reactions were run on an ABI PRISM 7900 HT detection system (Applied Biosystems, Foster City, CA, USA) PCR reactions consisting of 95°C for 10 min (one cycle), 94°C for 15 s, and 60°C for 1 min (forty cycles), data were analyzed with the ABI Prism sequence detection system software and quantified using the v1.7 Sequence Detection Software from PE Biosystems (Foster City, CA, USA). Relative expression of studied genes was calculated using the comparative threshold cycle method. All values were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes.[21] The GAPDH sequence was: Forward primer 5’-AGC CAC ATC GCT GAG ACA C-3’, reverse primer 5’- GCC CAA TAC GACCAA ATCC-3’.
Statistical analysis
Comparison of quantitative variables between the study groups was carried out using the Mann–Whitney U-test for independent samples. Comparison of sex distribution between the study groups was carried out using the Chi-square test. The correlation between various variables was assessed using the Spearman's rank correlation equation for nonnormal variables. The statistical significance level was set at (P ≤ 0.05). All statistical calculations were carried out using computer programs Microsoft Excel 2007 (Microsoft Corporation, Redmond, Washington, USA) and Statistical Package for the Social Science (SPSS Inc., Chicago, Illinois, USA) version 15 for Microsoft Windows.
Results
Patients with vitiligo were (17 women, 13 men; mean ± standard deviation [SD] age 28.9 ± 13.18 years), and healthy controls were (18 women, 12 men; mean ± SD age 33.67 ± 9.13). The disease duration ranged from 1 to 120 months (mean value = 35.5, median value = 30 months).
All the patients and controls expressed HSP-70 mRNA in their skin biopsies. The mean level of HSP-70 mRNA was significantly higher in the lesional biopsies from vitiligo patient group than in the control group (mean ± SD: 0.72 ± 0.28 vs. 0.025 ± 0.028; P < 0·001); and in nonlesional biopsies than the normal control biopsies (mean ± SD: 0.15 ± 0.05 vs. 0.025 ± 0.028; P < 0·001) [Figure 1].
Figure 1.
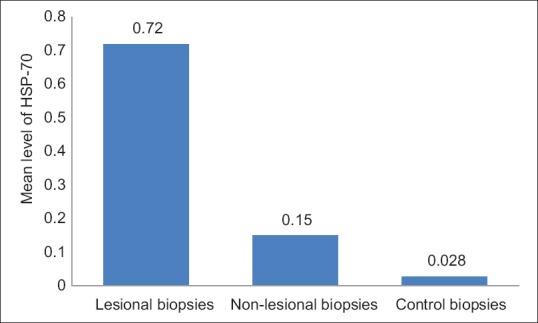
The mean level of heat shock protein-70 in lesional skin biopsies was higher than the nonlesional and control skin biopsies (P < 0.001)
There was no significant correlation between HSP-70 mRNA expression (in both lesional and nonlesional skin biopsies) and the age, family history, disease duration, or vitiligo type (P > 0.05 in all).
Relation of mean level of heat shock protein-70 to disease activity
The highly active vitiligo group (n = 13) showed significantly higher mean level of HSP-seventy compared to those with moderately active (n = 8) and compared to those with inactive vitiligo (n = 9) groups in the lesional skin biopsies (mean ± SD: 0.92 ± 0.16 vs. 0.85 ± 0.06; P < 0.01, 0.92 ± 0.16 vs. 0.52 ± 0.21; P < 0.01, respectively) and we got the same results when we compared the mean level of HSP-70 in the nonlesional skin biopsies obtained from normal skin from the vitiligo patients (mean ± SD: 0.21 ± 0.05 vs. 0.18 ± 0.05; P < 0.01, 0.21 ± 0.05 vs. 0.14 ± 0.05; P < 0.01, respectively) [Figure 2].
Figure 2.
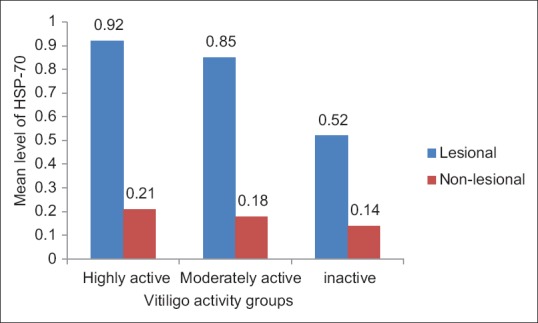
Patients were divided as regards to the disease activity into highly active, moderately active and inactive groups. The level of heat shock protein-70 was higher in the highly active groups compared to the moderately active and the inactive groups (P < 0.01)
Discussion
Cells under stress halt mainstream protein synthesis in favor of HSPs and/or glucose-regulated protein synthesis.[22] In the ER, stress can activate the unfolded protein response, upregulating HSP.[23] Among the HSPs, inducible HSP-70 stands out as it can be secreted by live cells.[24]
As said before, intracellular HSP-70 functions as chaperone molecules protecting cellular proteins from premature degradation by supporting proper protein folding. In normal, nonstressed cells HSP-70 is expressed at very low levels.[25]
The unfolded protein response has been implicated in many diseases including vitiligo.[26] This finding is congruent with dilated ER profiles reported for vitiligo melanocytes.[27]
On the other hand, extracellular located HSP-70 mediates the immunological response to proteins and peptides derived from the cells under stress; HSP-70 is also implicated in other autoimmune diseases as well, including rheumatoid arthritis, and other skin disorders such as psoriasis.[28]
All of these findings prompted us to investigate the role of HSP-70 in the pathogenesis of vitiligo.
In the current study, we found that HSP-70 expression in lesional and nonlesional biopsies from patients with vitiligo was significantly increased compared to the normal skin from the healthy controls. These results are inconsistence with earlier observations of Le Poole and Luiten,[29] who observed a differential expression of HSP-70 in nonlesional and vitiligo skin from three vitiligo patient.
Similarly, our results are inconsistence with Abdou et al.,[30] who used immunohistochemistry to detect intense and diffuse expression of HSP-70 in the nuclei and cytoplasm of keratinocytes from the epidermis of vitiliginous lesions with more prominent nuclear in the active lesions.
Furthermore, these results are in agreement with Denman et al.[31] and Mosenson et al.[32] who induced depigmentation in mouse models when they injected these mouse models with animal and human derived HSP-70 vaccine.
As a consequence, we propose that HSP-70 could contribute to the development of vitiligo through enhancing the unfolded protein response within the cell and promoting autophagy to avert cellular apoptosis.[33]
HSP-70 could also play a role in the immunological mechanism leading to vitiligo through activation of lymphocytes and macrophages, activation and maturation of dendritic cells as an antigen presenting cells and initiating the release of cytokines, including tumor necrosis factor-α, interleukin (IL)-1β, IL-12, and IL-6 which are apoptosis mediators and melanocytes proliferation and melanogenesis inhibitor leading to vitiligo.[25]
Conclusion
Our study showed that the expression of HSP-70 was related to the disease activity which may provide a link between stress as an activity inducer in vitiligo and the immunological mechanism that is involved in the pathogenesis of vitiligo.
Although, there have been previous in vitro studies that reported secretion of HSP-70 by melanocyte cell models in response to stress induced by chemicals as 4-tertiary butyl phenol[34] and monobenzyl ether of hydroquinone,[35] to the best of our knowledge, this is the first study to correlate the vitiligo disease activity with the HSP-70 expression in skin biopsies from vitiligo patients.
Our study was limited by the small number of patients that is why further large scale of patients are recommended.
The fact that depigmentation could be induced by HSP-70 vaccine urges further studies aiming at inhibiting the release of these stress-induced molecules to control the disease activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
HSP-70 play a role in the pathogenesis of vitiligo
Expression of HSP-70 was related to the vitiligo disease activity.
References
- 1.Brocchieri L, Conway de Macario E, Macario AJ. HSP70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–8. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriechbaumer V, von Löffelholz O, Abell BM. Chaperone receptors: Guiding proteins to intracellular compartments. Protoplasma. 2012;249:21–30. doi: 10.1007/s00709-011-0270-9. [DOI] [PubMed] [Google Scholar]
- 4.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 5.AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25:749–57. doi: 10.1111/j.1468-3083.2010.03876.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–97. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–28. [PMC free article] [PubMed] [Google Scholar]
- 8.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–32. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 9.Kemp EH, Emhemad S, Akhtar S, Watson PF, Gawkrodger DJ, Weetman AP. Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35–40. doi: 10.1111/j.1600-0625.2010.01181.x. [DOI] [PubMed] [Google Scholar]
- 10.Boissy RE, Liu YY, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97:395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- 11.Le Poole IC, Yang F, Brown TL, Cornelius J, Babcock GF, Das PK, et al. Altered gene expression in melanocytes exposed to 4-tertiary butyl phenol (4-TBP): Upregulation of the A2b adenosine receptor 1. J Invest Dermatol. 1999;113:725–31. doi: 10.1046/j.1523-1747.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- 12.Cedercreutz K, Denman CJ, Klarquist J, et al. Vitiligo eitiology and treatment: Parameters derived from a patient survey. J Dermatol Nurses Assoc. 2010;2:265–72. [Google Scholar]
- 13.van Geel N, Speeckaert R, De Wolf J, Bracke S, Chevolet I, Brochez L, et al. Clinical significance of koebner phenomenon in vitiligo. Br J Dermatol. 2012;167:1017–24. doi: 10.1111/j.1365-2133.2012.11158.x. [DOI] [PubMed] [Google Scholar]
- 14.Moretti S, Arunachalam M, Colucci R, Pallanti S, Kline JA, Berti S, et al. Autoimmune markers in vitiligo patients appear correlated with obsession and phobia. J Eur Acad Dermatol Venereol. 2012;26:861–7. doi: 10.1111/j.1468-3083.2011.04171.x. [DOI] [PubMed] [Google Scholar]
- 15.Multhoff G. Heat shock proteins in immunity. Handb Exp Pharmacol. 2006;172:279–304. doi: 10.1007/3-540-29717-0_12. [DOI] [PubMed] [Google Scholar]
- 16.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: Agents of antigen cross-presentation. Expert Rev Vaccines. 2008;7:1019–30. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- 17.El-Din AG, Saleh H, Fattah NA, Abdel Hamid M, et al. Evaluation of heat shock protein-70 in psoriasis. JASMR. 2008;3:103–10. [Google Scholar]
- 18.Njemini R, Bautmans I, Onyema OO, Van Puyvelde K, Demanet C, Mets T. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 2011;12:24. doi: 10.1186/1471-2172-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortonne J. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. Spain: Elsevier; 2008. pp. 913–20. [Google Scholar]
- 20.Kanthraj GR, Srinivas CR, Shenoi SD, Deshmukh RP, Suresh B. Comparison of computer-aided design and rule of nines methods in the evaluation of the extent of body involvement in cutaneous lesions. Arch Dermatol. 1997;133:922–3. [PubMed] [Google Scholar]
- 21.Kenneth KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: The recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21:500–11. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–84. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- 25.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–10. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 26.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–9. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan C, Lin F, Zhou M, Hong W, Fu L, Xu W, et al. The role of VIT1/FBXO11 in the regulation of apoptosis and tyrosinase export from endoplasmic reticulum in cultured melanocytes. Int J Mol Med. 2010;26:57–65. doi: 10.3892/ijmm_00000435. [DOI] [PubMed] [Google Scholar]
- 28.Millar DG, Ohashi PS. HSP70 family members, danger signals and autoimmunity. In: Asea AA, De Maio A, editors. Heat Shock Proteins: Potent Mediators of Inflammation and Immunity. Dordrecht: Springer; 2007. pp. 189–211. [Google Scholar]
- 29.Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. 2008;10:227–43. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- 30.Abdou AG, Maraee AH, Reyad W. Immunohistochemical expression of heat shock protein 70 in vitiligo. Ann Diagn Pathol. 2013;17:245–9. doi: 10.1016/j.anndiagpath.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patiño JA, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–8. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25:88–98. doi: 10.1111/j.1755-148X.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286–97. [PubMed] [Google Scholar]
- 34.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: Relevance to vitiligo. J Invest Dermatol. 2005;124:798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosenson JA, Flood K, Klarquist J, Eby JM, Koshoffer A, Boissy RE, et al. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell Melanoma Res. 2014;27:209–20. doi: 10.1111/pcmr.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]


