ABSTRACT
Neisseria gonorrhoeae causes the human-specific disease gonorrhea and is transmitted from person to person primarily via sexual contact. During transmission, N. gonorrhoeae is often exposed to seminal fluid and must adapt to this change in environment. Previous work demonstrated that seminal fluid facilitates N. gonorrhoeae motility and alters epithelial cell interactions. In this study, exposure to seminal fluid was found to decrease surface adherence of gonococci in a manner that was independent of Opa adhesin proteins or type IV pilus retraction. Semen was also shown to cause dispersal of bacteria that had previously established surface adherence. Although surface adherence decreased, interbacterial interactions were increased by seminal plasma both in long-term static culture and on a cell-to-cell basis over shorter time periods. The result of increased bacterium-bacterium interactions resulted in the formation of microcolonies, an important step in the N. gonorrhoeae infectious process. Seminal fluid also facilitated increased bacterial aggregation in the form of shear-resistant three-dimensional biofilms. These results emphasize the importance of the gonococcal response to the influx of seminal fluid within the genital niche. Further characterization of the N. gonorrhoeae response to semen will advance our understanding of the mechanisms behind the establishment of infection in naive hosts and the process of transmission.
IMPORTANCE N. gonorrhoeae is the causative agent of the globally prevalent sexually transmitted infection gonorrhea. An understudied aspect of this human-adapted pathogen is the change in bacterial physiology that occurs during sexual transmission. N. gonorrhoeae encounters semen when transmitted from host to host, and it is known that, when N. gonorrhoeae is exposed to seminal fluid, alterations in bacterial motility and type IV pilus arrangement occur. This work extends our previous observations on this modulation of gonococcal physiology by seminal fluid and demonstrates that seminal plasma decreases surface adherence, promotes interbacterial interactions, and enhances biofilm formation.
INTRODUCTION
The study of bacterial physiology during pathogen transmission is limited in practical terms by the degree to which experimental conditions can approximate the transmission environment, since very few animal transmission models exist. Investigations of this type are further complicated in cases where the bacterium is capable of infecting multiple host species, as each environment likely triggers unique physiological responses from the bacterium. Neisseria gonorrhoeae is a human-specific pathogen that is transmitted through sexual contact and has no known environmental reservoir. Infection with N. gonorrhoeae most frequently results in either urethritis in males or cervicitis in females, indicating a restricted niche even within the already narrow human environment. One of the major elements of sexual transmission is the presence of seminal fluid. Despite this, there have been a limited number of studies investigating the physiological role that seminal fluid has on N. gonorrhoeae. Early studies investigating N. gonorrhoeae and semen indicated that gonococci were capable of attaching to sperm cells (1), in part through interactions via the bacterial type IV pilus (T4P) (2) and lipooligosaccharide (3). More recently, the semen-derived enhancer of viral infection, which is a cationic amyloid fibril found in semen (4), was shown to bind N. gonorrhoeae and increase cellular phagocytosis of the bacterium (5). In our own studies, twitching motility of N. gonorrhoeae was shown to be facilitated by the presence of seminal plasma (SP), with bacterial motility exhibiting a more random directional pattern in the presence of seminal fluid compared to control conditions (6). This same study also demonstrated that exposure to semen resulted in the disruption of T4P bundles into individual fibers, providing initial evidence that SP exposure modulates the physical state of this critical gonococcal virulence factor.
One of the early steps in transmission of a pathogen to an uninfected host is attachment and adherence to host surfaces. In the case of N. gonorrhoeae, bacteria first colonize the mucosal surfaces of either the urethral or vaginal epithelium. In immortalized cell lines and primary cell culture, it has been well established that early adherent gonococci actively aggregate to form microcolonies on the surface of epithelial cells (7, 8). Furthermore, bacteria in microcolonies elicit rearrangement of host cell cytoskeletal components (9, 10) and modulate cell signaling (11, 12), indicating that these cell surface clusters of gonococci influence host interactions. Our recent work has also demonstrated that exposure to SP enhances formation of microcolonies on cultured epithelial cells (6). Several of the above studies have contributed to the conclusion that two of the major bacterial factors required for formation of microcolonies are T4P and the pilus retraction motor PilT. However, less is known about the cellular and environmental factors that influence this microcolony formation and pilus retraction. The host membrane phospholipid phosphatidylinositol-3,4,5-trisphosphate has thus far been the only cellular factor that has been shown to stimulate microcolony formation (12). Therefore, additional investigation into the environmental factors that modulate aggregation and microcolony formation is necessary to better understand this critical aspect of the N. gonorrhoeae-host interaction.
The multicellular nature of microcolonies and the intimate bacterium-bacterium associations that result, suggests that these structures may be a form of gonococcal biofilm or may represent the initial stages of a larger biofilm structure. Bacterial biofilms have been implicated in the pathogenesis of a number of different organisms, and it was previously demonstrated that N. gonorrhoeae is capable of forming biofilm structures both on abiotic surfaces and on the surface of epithelial cells (13). While biofilms lack bacterially derived exopolysaccharide, the extracellular matrix of gonococcal biofilms has been shown to contain DNA (14). The potential sources for this extracellular DNA include that liberated from autolysis (15), DNA associated with membrane blebbing (16, 17), and single-stranded DNA secreted via the type IV secretion system (18), in addition to any potential host DNA sources. Furthermore, the thermostable nuclease Nuc has been shown to be important for reorganization and dispersal of gonococcal biofilms (14). Given the status of N. gonorrhoeae as an exclusively human pathogen, the role of the human genital environment on the establishment and dispersal of biofilms remains an important area of study. Recently, it was shown that the biologically relevant polyamine spermine was capable of inhibiting the first steps of biofilm formation (19) and that the availability of oxygen was critical for establishing and maintaining biofilms (20). However, the role of host biological fluids on modulating N. gonorrhoeae biofilms has yet to be tested.
Over 300,000 cases of gonorrhea were reported in the United States in the most recent Centers for Disease Control Summary of Notifiable Diseases (21), and an estimated 100 million cases occur annually worldwide (22). Investigations into the physiology and host interactions of N. gonorrhoeae are becoming increasingly important given the high level of observed drug resistance for this organism and the limited number of antibiotics that remain viable treatment options (23). The objective of this study was to further characterize the response of N. gonorrhoeae to SP. Specifically, we determined the influence that SP has on bacterial adherence and biofilm formation. Based on the results described here, we propose that seminal plasma plays an important role in the transmission of N. gonorrhoeae by promoting interbacterial interactions and the formation of biofilms.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Routine culture of N. gonorrhoeae was conducted using gonococcal medium base (GCB) (Difco) at 37°C in 5% CO2 or in GCB liquid broth containing Kellogg supplements (24). GW is a fully defined liquid medium (25) that was used to test the response of N. gonorrhoeae strains to SP and other compounds, when indicated. Pooled human semen was purchased from both Lee Biosolutions and Innovative Research. No differences in experimental outcome were observed between the two sources. SP was prepared from whole semen immediately prior to use by centrifugation to remove spermatozoa and other insoluble material. Sterile SP was obtained by passing cleared fluid through a 0.22-μm syringe filter.
N. gonorrhoeae strains used in this study are derivatives of the FA1090 clinical isolate harboring the 1-81-S2 pilE variant sequence (26). This strain was later modified to have reduced spontaneous T4P antigenic variation through the introduction of a mutation in the guanine quartet-forming region upstream of pilE (27). All previously described N. gonorrhoeae strains are listed in Table 1. Strains lacking Opa (Opaless), which have null mutations in all 11 opa genes, and harboring the established nonfunctional pilT::erm allele (28) were generated by spot transformation (29) using purified genomic DNA from existing strains. Erythromycin-resistant transformants were confirmed to carry the pilT::erm allele by PCR. Genomic DNA from a single isolate from each transformation was then used to backcross the pilT::erm mutation into the Opaless parent. Backcrossed isolates containing the pilE 1-81-S2 sequence, as determined by DNA sequencing, were selected for further use.
TABLE 1.
N. gonorrhoeae strains used in this study
| Strain name or genotype | Description | Source or reference |
|---|---|---|
| FA1090NV | FA1090 1-81-S2 with a nonvariant pilE G4 mutation (pilENV) and a pilC1 phase-locked allele | 6 |
| pilT::erm | FA1090 harboring pilENV and pilT::erm alleles | 6 |
| Opaless | pilENV strain containing loss-of-function mutations in all 11 opa alleles | 39 |
| Opaless, OpaD+ | Opaless strain harboring a nonvariable copy of the opaD gene | 39 |
| Opaless, ΔpilE | Opaless strain harboring pilE deletion allele | 39 |
| Opaless, pilT::erm | Opaless strain harboring the pilT insertion allele | This study |
| Opaless, OpaD+ pilT::erm | Opaless, OpaD+ strain harboring the pilT insertion allele | This study |
Microtiter adherence assays.
The ability of SP to inhibit N. gonorrhoeae adherence was assessed in polystyrene 96-well plates. Bacteria from GCB plate growth were used to create a dense suspension in GW medium. All suspensions were normalized to an optical density at 550 nm of 1.5 prior to addition of either SP or spermine (Sigma-Aldrich) at the indicated concentrations. The addition of phosphate-buffered saline (PBS) to GW suspensions was used as a negative control. Aliquots (100 μl) of each suspension were then added to replicate wells and incubated statically at 37°C for 24 h. The amount of bacteria adhering to individual wells was quantitated based on a previously described method (30). Briefly, planktonic bacteria and media were aspirated, and wells were washed once with PBS. A 0.1% solution of crystal violet was added to each well and allowed to stand for 10 min at room temperature. Excess crystal violet was decanted, and wells were washed three times with PBS before plates were allowed to dry overnight. Dried stain was then solubilized in 125 μl of 30% acetic acid for 10 min and quantitated by measuring absorbance at 550 nm. Stereo microscope images of bacterial aggregate formation were captured immediately prior to staining of cultures with crystal violet.
The ability of SP to disrupt adherence was tested by first establishing adherent gonococci in microtiter wells, as described above. After 24 h, the supernatant was aspirated, and a solution of 1:20 SP or PBS in GW was added to wells and allowed to stand at 37°C for 1 h. The amount of adherent bacteria remaining after treatment was then quantitated via crystal violet staining, as described above.
Quantitation of bacterial microcolony formation.
The formation of N. gonorrhoeae microcolonies was measured by quantitative analysis of bacterial aggregates on glass slides. The N. gonorrhoeae FA1090NV strain was grown on GCB agar and suspended in GW medium to a density of ∼2 × 108 CFU/ml. Bacterial suspensions were mixed with a 1:20 volume of SP or GW as a negative control. A 100-μl aliquot of each suspension was then transferred to a glass coverslip and allowed to stand at 37°C for 1 h. The remaining liquid was then aspirated, and the coverslips were immediately mounted and imaged on a Nikon 90i microscope using differential interference contrast (DIC) with a 40× objective lens. A minimum of 10 fields was captured for each experiment, and aggregation was quantitated using NIS Elements v.4. Briefly, the detect-DIC-objects function was used to generate regions of interest (ROI) encompassing individual bacteria or bacterial aggregates of any size. The area (μm2) of each ROI was calculated using the software, and the distribution of object areas was compared between SP-treated and untreated bacteria.
Biofilm growth in continuous-flow chambers.
N. gonorrhoeae FA1090NV was grown in continuous-flow chambers using GW medium diluted 1:5 with PBS (Becton-Dickinson), as previously described (17). Prior to filter sterilization, a 1:20 dilution of human semen was added to the medium. Biofilms were formed in a 47-mm circular chamber on a 25-mm glass coverslip, which was sealed using a rubber gasket and screws that fasten the top and bottom portions together. Chambers were inoculated with ∼3 × 108 CFU and incubated under static conditions at 37°C with 5% CO2 for 1 h to allow attachment to the glass surface. A flow rate of 60 μl/min was applied to biofilm chambers and allowed to proceed at 37°C for 24 h. Following incubation, the biofilm effluent was cultured to assess purity, and biofilm formation was imaged with a Nikon CI confocal microscope using a live/dead stain (Life Technologies). The stacked images were analyzed using Imaris-8 3D image processing (Oxford Instruments) for surface-rendered images and statistical analysis (Mann-Whitney comparison) of mass and height of the biofilms. Biofilm experiments were performed in duplicate in two independent experiments.
RESULTS
SP inhibits surface adherence of N. gonorrhoeae.
Our previous work demonstrated that N. gonorrhoeae treated with SP had altered epithelial cell adherence characteristics (6). To further investigate the influence of SP on N. gonorrhoeae adherence and to determine the role of gonococcal adhesins in this phenotype, we sought to differentiate surface adherence from bacterium-bacterium adherence using a cell-free microtiter-based assay (31). In this assay, dense bacterial suspensions were incubated statically in a defined medium in the presence or absence of SP, and surface adherence was quantitated by crystal violet staining. N. gonorrhoeae cultures treated with a 1:20 dilution of SP exhibited a complete loss of surface adherence compared to untreated cultures (Fig. 1A). Suspensions from both the untreated and SP-treated conditions contained approximately equivalent numbers of viable bacteria following the incubation period (data not shown). Further dilution of SP resulted in a similar loss of adherence until a concentration of ∼0.3% was reached, at which point bacteria regained partial surface adherence compared to the untreated control. These results indicate a potent inhibition of gonococcal surface adherence even in the presence of dilute concentrations of SP. It is interesting to note that a 1:320 dilution of SP is also the point at which a loss of semen-enhanced gonococcal motility was observed, indicating that this may represent the minimal concentration for the N. gonorrhoeae semen response (6). Similar observations regarding the loss of surface adherence in the presence of spermine have previously been made by Goytia et al. (19). Since spermine is a polyamine that is naturally found in the male urogenital tract, and is abundantly found in SP, we sought to compare the inhibitory activity of spermine and SP at physiologically relevant concentrations (32). The highest tested concentration of spermine exhibited a significant decrease in bacterial adherence compared to the negative control (Fig. 1A), indicating that spermine can contribute to the loss of gonococcal surface adherence, as previously demonstrated (19). However, since the naturally occurring levels of spermine present in the 1:20 dilution of SP (complete loss of adherence) are much lower than the highest concentration of purified spermine tested (1.25 mg/ml), it is unlikely that the surface adherence-inhibiting activity of SP is due solely to the presence of spermine.
FIG 1.
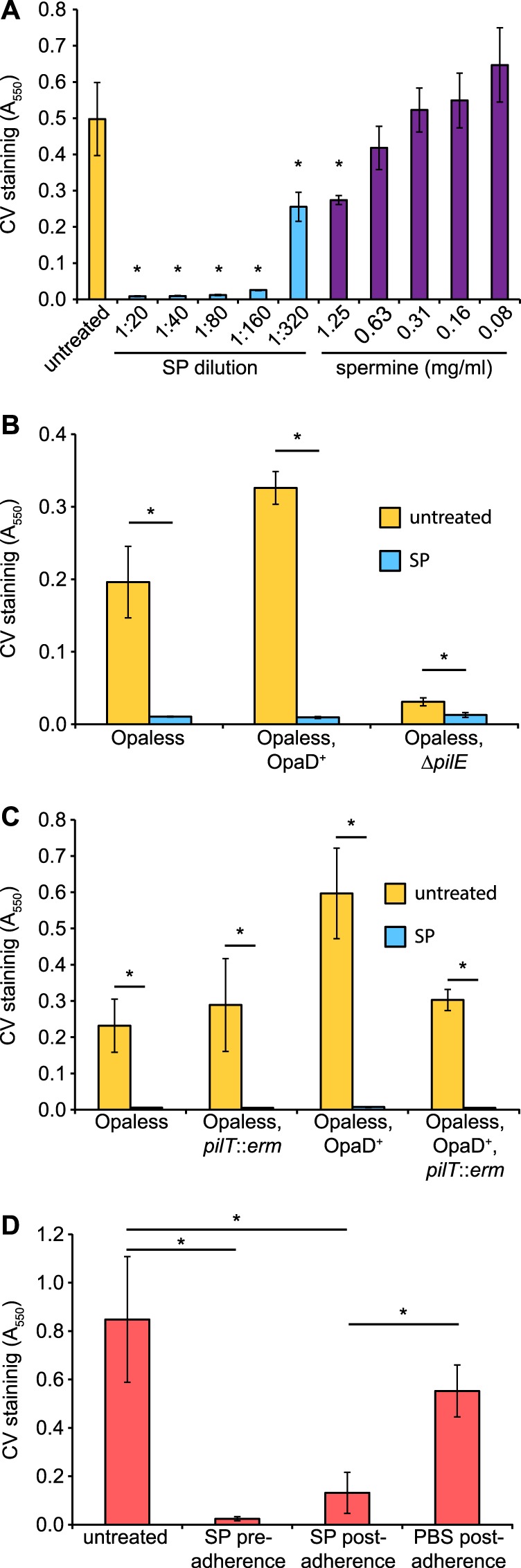
SP inhibits surface adherence of N. gonorrhoeae. (A) N. gonorrhoeae strain FA1090NV was grown statically for 24 h in 96-well plates containing SP or spermine at the indicated concentrations. Bars indicate the mean (±standard deviation) level of adherent bacteria as determined by crystal violet (CV) staining. Conditions resulting in significantly lower staining than the untreated sample are indicated with asterisks. The requirement for the T4P major pilin PilE (B) and the pilus retraction ATPase PilT (C) in SP-mediated adherence inhibition was tested using N. gonorrhoeae strains either lacking all Opa adhesins (Opaless) or constitutively expressing the opaD allele (Opaless, OpaD+). (D) To test the ability of SP to disrupt established adherence, bacteria were incubated in 96-well plates for 24 h in the absence of SP. Following removal of planktonic bacteria, wells were treated for 1 h with either SP or PBS (postadherence). The level of CV staining was compared to untreated bacteria and bacteria that had been exposed to SP for the duration of the experiment (preadherence). An SP concentration of 1:20 was used for all experiments unless indicated otherwise. Asterisks indicate statistically significant differences as determined by Student's t test (P < 0.01; n = 4).
The Opa family of proteins is a group of major N. gonorrhoeae adhesins that interact principally with carcinoembryonic antigen cell adhesion molecule family proteins on the surface of host cells (33). The Opa proteins are also known to facilitate gonococcal aggregation (34); as such, the influence of Opa proteins on SP-mediated loss of adherence was tested. An N. gonorrhoeae strain lacking Opa proteins (Opaless) was demonstrated to have reduced overall adherence compared to an isogenic strain that constitutively expresses OpaD (Opaless, OpaD+) (Fig. 1B). Importantly, both strains exhibited a complete loss of surface adherence when cultures were treated with SP, indicating that SP-mediated adherence inhibition is independent of the Opa adhesins. In contrast, an Opaless ΔpilE mutant strain exhibited no adherence under any of the conditions tested, demonstrating that the T4P acts as the primary adhesin under these conditions. Since it was not possible to test whether pili were required for SP-mediated loss of adherence using the ΔpilE mutant strain, due to the complete lack of adherence, a nonfunctional pilT mutant was instead used to assess the requirement for pilus retraction. Interestingly, loss of pilus retraction did not alter the effect of SP on adherence, as evidenced by the complete absence of adherent gonococci in SP-treated cultures, regardless of the opa or pilT genotype of the tested bacteria (Fig. 1C). These results demonstrate that neither surface adherence nor SP-mediated adherence inhibition requires pilus retraction, thereby genetically differentiating the effects of SP on adherence from that of SP-meditated twitching motility (6).
In the case of a preexisting N. gonorrhoeae infection, bacteria are likely already surface associated when they are exposed to seminal fluid during transmission. To determine whether bacteria that have already established surface adherence would also be effected by exposure to SP, bacteria were incubated in microtiter wells for 24 h followed by a 1 h exposure to 1:20 SP or PBS. This short treatment with SP resulted in a significant loss of bacterial surface adherence, similar to the levels observed when SP is present for the duration of the 24-hour incubation (Fig. 1D). This is in contrast to the control treatment, in which surface-adherent bacteria were exposed to PBS instead of SP, and which showed no significant (P > 0.01) reduction in surface adherence compared to the untreated cultures. These results demonstrated that SP can disrupt established gonococcal surface adherence, potentially via the disruption of pilus-surface interactions.
SP treatment promotes bacterial aggregation.
While performing the surface adherence assays described above, it was noted that SP-treated bacteria formed tightly associated aggregates at the bottom the microtiter wells during static incubation. N. gonorrhoeae aggregates have been observed previously in vitro and were shown to require T4P and pilus retraction for formation (35). Since we had previously determined that SP mediates microcolony formation on the surface of epithelial cells, we sought to further our understanding of the Opa and T4P requirements for SP-mediated aggregate formation using a cell-free system. Although untreated Opaless bacteria exhibited a moderate amount of aggregation, exposure to SP clearly resulted in a more dense association of bacteria (Fig. 2). This phenotype was even more pronounced in bacteria constitutively expressing the opaD allele. Untreated OpaD+ bacteria were uniformly distributed in microtiter wells, in marked contrast to the dense aggregates that formed in the presence of SP, similar to those observed with the strain devoid of Opa proteins. Together, these results demonstrate that SP-mediated aggregation does not occur through interactions with the Opa proteins. Both the Opaless and Opaless, OpaD+ strains retained functional T4P. Interestingly, piliated bacteria that were incapable of pilus retraction (pilT::erm) exhibited no evidence of bacterial aggregation in either untreated medium or SP-containing medium. However, bacteria that completely lacked T4P (ΔpilE) demonstrated levels of SP-mediated aggregation that were similar to those of the piliated parent strain. These results suggest that pili are not required for bacterial aggregation facilitated by exposure to semen; however, the presence of nonfunctional pili clearly inhibits aggregate formation.
FIG 2.
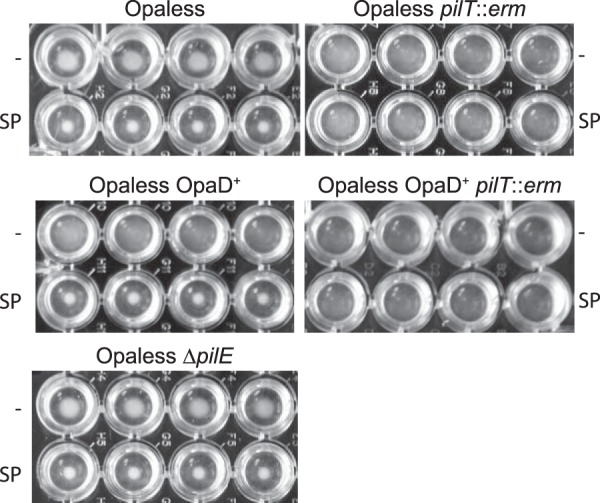
SP facilitates bacterial aggregation. N. gonorrhoeae strains were grown statically in 96-well plates for 24 h in the presence (SP) or absence (−) of 1:20 SP. Images of replicate (n = 4) wells are shown from a top-down perspective. Aggregative bacteria were observed as a tightly associated cell mass at the bottom of the wells.
To assess the effect of SP on N. gonorrhoeae aggregation quantitatively, a live-cell imaging approach using DIC microscopy was performed. Bacterial suspensions were incubated on glass coverslips in the presence or absence of SP and imaged using DIC microscopy. ROI were generated around DIC objects consisting of individual bacteria and any multicellular bacterial aggregates that were present, followed by the quantitation of all object areas within a field. The result of this analysis demonstrated that, when N. gonorrhoeae was treated with SP, there was a significantly greater portion of large bacterial aggregates within the analyzed fields than within the fields containing untreated bacteria (Fig. 3A). Although the median ROI area was similar between the two conditions due to a substantial majority of nonassociated diplococci, the average abundance of microcolonies with areas ≥10 μm2 per field was 5.8 for SP-treated gonococci but only 0.1 for untreated bacteria. The SP-induced aggregates consisted of large numbers of bacteria forming a dense three-dimensional arrangement on the surface of the slide. This aggregation phenomenon was in contrast to the untreated bacteria, which consisted largely of isolated diplococci or smaller bacterium-bacterium associations between a few cells (Fig. 3B). These results are consistent with the observations made using the microtiter-based assay and clearly demonstrate that exposure to SP promotes bacterium-bacterium interactions.
FIG 3.
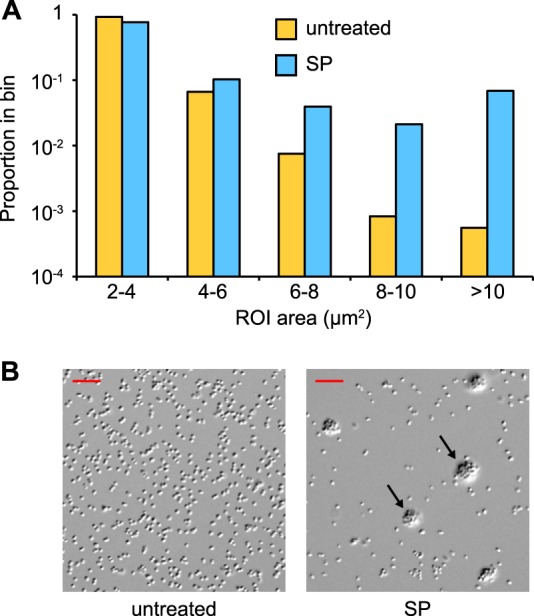
Exposure to SP stimulates formation of microcolonies. Suspensions of FA1090NV were incubated on glass coverslips for 1 h in the presence (SP) or absence (untreated) of 1:20 SP and imaged using DIC microscopy. (A) The distribution of ROI areas from 20 fields. The median ROI areas for untreated and SP-treated bacteria were 2.47 μm2 (n = 3,596) and 2.70 μm2 (n = 1,693), respectively. The distributions were significantly different by the Mann-Whitney rank sum test (U = 2,393,191; P < 0.0001). ROI areas of <2 μm2 were excluded from the analysis. (B) Representative DIC images from both conditions; arrows indicate microcolonies. Scale bars are 10 μm.
SP treatment enhances biofilm formation.
The ability of SP to promote N. gonorrhoeae aggregation and microcolonies suggests that these small bacterial communities may have the potential to develop into more complex and shear-resistant biofilm structures. Biofilm-like growth has previously been observed on excised human tissues from infected individuals, suggesting a biological role for the formation of this type of bacterial community during infection (17). The ability of SP to influence biofilm formation in this study was determined using a continuous-flow chamber on glass slides, using the FA1090NV strain to avoid spontaneous loss of pilus expression during the experiment. Bacteria that were exposed to SP during flow-chamber incubation exhibited significantly greater live biofilm volume than did the bacteria that were untreated (Fig. 4A). The effect of SP treatment on biofilm characteristics could easily be observed in z-series reconstructions of the biofilm surface, with SP-treated bacteria forming large three-dimensional aggregates compared to those formed in the absence of SP (Fig. 4B). Furthermore, SP treatment did not result in an increased proportion of nonviable bacteria compared to untreated bacteria in this experiment (Fig. 4A). This result is important, given the established role of extracellular nucleic acid in N. gonorrhoeae biofilm formation (14) and the potential for an increase in extracellular bacterial DNA with the loss of viability. The FA1090 parent strain also lacks the gonococcal genetic island, which encodes DNA secretion functions that are independent of bacterial lysis (36). The ability of SP to promote N. gonorrhoeae biofilms is likely to play a role in maintaining colonization of host tissues during sexual transmission.
FIG 4.
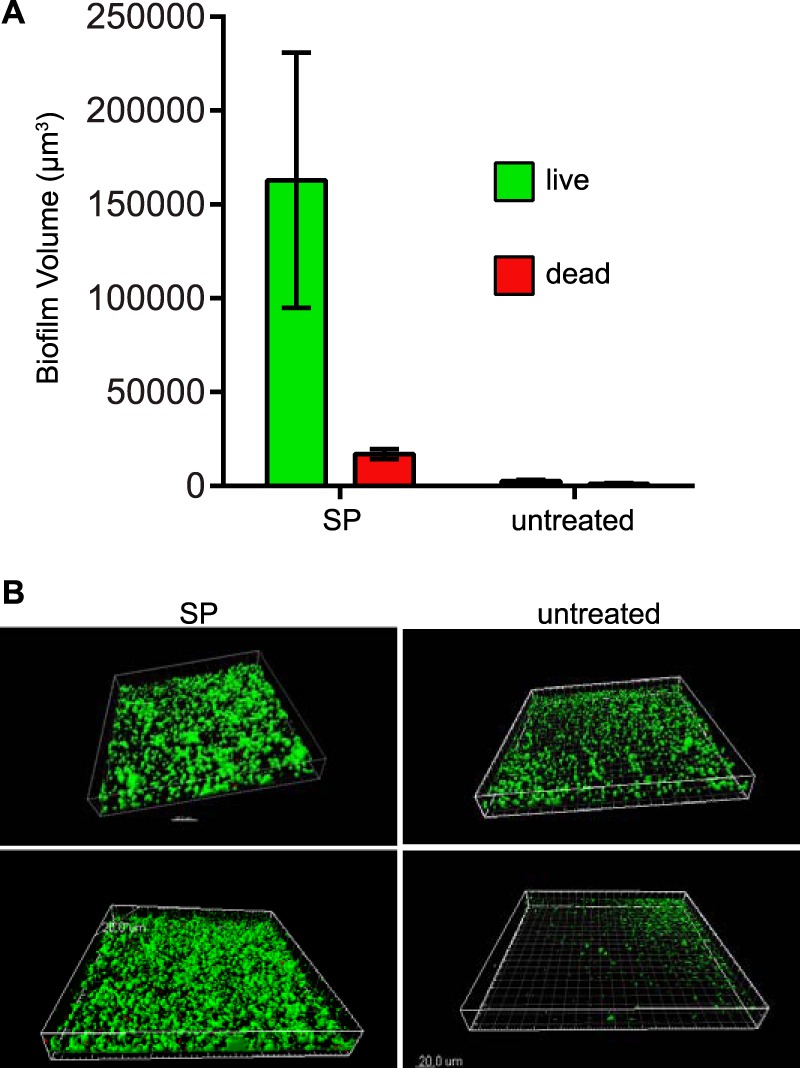
SP enhances biofilm formation of N. gonorrhoeae. (A) N. gonorrhoeae FA1090NV was grown in biofilm flow chambers for 24 h at a flow rate of 60 μl/min in the presence (SP) or absence (untreated) of a 1:20 dilution of SP. Bars represent mean ± standard deviation total fluorescent biofilm volumes. Viability of bacteria in biofilms was determined using a live/dead stain, with green and red fluorescence indicating viable and nonviable bacteria, respectively. The viable biofilm volume was significantly different between the SP-treated and untreated conditions, as assessed by the Mann-Whitney test (P < 0.02). (B) Representative three-dimensional reconstructions from confocal images of biofilm growth in SP-containing and untreated medium. Scale bar represents 20 μm.
DISCUSSION
Although N. gonorrhoeae is capable of colonizing body sites, such as the eye or nasopharynx, these types of infections are rare compared to the overwhelming majority of infections that are localized to the urogenital tract or rectum. N. gonorrhoeae is also not known to naturally infect nonhuman animals or exist in any environmental reservoir. Therefore, it is certain that N. gonorrhoeae has evolved specific mechanisms to cope with the introduction of seminal fluid into the genital environment, an event that represents a dramatic physiological change. In this study, we have extended our initial observations on the physiology of N. gonorrhoeae in the presence of seminal fluid. In addition to facilitating bacterial twitching motility and epithelial cell microcolony formation, exposure to SP has now also been demonstrated to inhibit gonococcal surface adherence, promote bacterium-bacterium interactions, and enhance biofilm formation. Each of these SP-dependent phenotypes have potential roles in facilitating the establishment and maintenance of colonization during sexual transmission of N. gonorrhoeae, the period in which the urogenital tract of both sexes is likely exposed to this host secretion.
Important consequences of gonococcal SP exposure are the decreased ability of bacteria to establish surface adherence and the disruption of already adherent N. gonorrhoeae. SP-mediated loss of adherence in vivo has the potential to contribute to disease transmission by helping established bacterial infections detach from host cell surfaces. For example, bacteria that are colonized within the male urethra may be more readily liberated by exposure to semen, thus enhancing transmission to a sexual partner. The microtiter-based assay used to assess surface adherence in this work was based on a method that was previously developed and popularized as a simple and high-throughput method of quantitating static bacterial biofilm formation (31). It would therefore seem contradictory that SP reduces surface adherence in the microtiter-based system yet promotes biofilm formation in a flow chamber apparatus. However, it is worth noting that the microtiter-based method is best suited to quantitate only the initial stages of biofilm development, specifically surface adherence under static conditions. The formation of a robust gonococcal biomass in the flow chamber would still be possible after the initial surface adherence, even if only a small portion of the initial population was able to adhere to the flow chamber surface, given the ability of SP to also promote bacterium-bacterium interactions. This idea is supported by the ability of SP to cause gonococcal aggregation both in microtiter wells (Fig. 2) and on the surface of glass slides (Fig. 3). An alternative possibility is that the ability of SP to disrupt surface adherence depends on the nature of the surface, since the static experiments were carried out in polystyrene plates, whereas the biofilm assays were conducted in a glass flow chamber. Regardless, the results described here using abiotic surfaces as a substrate will guide further work to investigate SP modulation of biofilm formation on host epithelial cells.
The role of T4P in responding to the presence of seminal fluid was first established when we observed that SP facilitated twitching motility of N. gonorrhoeae, a process that is dependent on the PilT-mediated retraction of pili (6). In this study, SP disrupted adherence of both the pilT mutant and parent strains (Fig. 1C), despite the requirement for pili in overall adherence under these conditions (Fig. 1B). This indicates that retraction of pili, and therefore twitching motility, is not the cause of adherence inhibition in the presence of SP. Interestingly, these results contrast with what was observed for bacterial aggregate formation under the same conditions (Fig. 2). SP was capable of stimulating similar levels of bacterial aggregation in both the presence and the absence of pili. However, when T4P were present, bacterial aggregation was dependent on PilT function and the ability to retract pili. One potential explanation for these results is that SP-mediated aggregation requires intimate contact between the cell bodies of multiple bacteria, a state that can be achieved either in the complete absence of pili or when proximal bacteria are capable of drawing closer together through pilus attachment and retraction. The presence of nonfunctional pili may then interfere with the ability of gonococci to achieve close cell-to-cell contact, in effect by holding neighboring bacteria at a distance. Indeed, lack of PilT function has previously been demonstrated to alter aggregation of bacteria (35) and microcolony morphology (37). The presence or absence of Opa proteins did not alter SP-mediated loss of surface adherence (Fig. 1) or SP-mediated bacterial aggregation (Fig. 2), indicating that this major class of gonococcal adhesins is unlikely to facilitate interactions with SP components. It remains possible that other gonococcal surface structures, such as lipooligosaccharide and porin proteins, bind SP components. Identifying any potential interactions between SP and N. gonorrhoeae surface structures will be a goal of future work, along with characterizing the SP response of different gonococcal isolates.
Seminal fluid is a mixture of multiple secretions that includes high concentrations of soluble proteins, small molecules, monosaccharides, and metal ions (38). Seminal fluid also contains high concentrations of polyamines, such as spermine (32), which has previously been implicated in inhibiting N. gonorrhoeae biofilm formation (19). Although similar microtiter-based adherence assays were used both in the previous study and here, spermine exhibited only a modest effect on gonococcal adherence in our hands. Several possible explanations exist for this apparent discrepancy, including the use of different N. gonorrhoeae strains. The presence of spermine in seminal fluid probably contributes to the lack of surface adherence under these conditions, but it is not sufficient, since SP was much more potent than spermine when tested at physiologically relevant concentrations (Fig. 1A). Furthermore, spermine alone was not capable of disrupting established adherence (19), while treatment with SP at a 1:20 dilution resulted in the near-complete loss of adherent bacteria (Fig. 1D). The implication of these findings, together with our previous work that demonstrated that three different SP glycoproteins could facilitate twitching motility (6), is that multiple SP components are involved in the T4P-associated phenotypes observed when N. gonorrhoeae is exposed to SP. Since it is clear that the modulating activity of SP toward gonococci is a not a simple binary interaction, further experiments were conducted to identify nonprotein molecules that could mimic the effects of SP. However, a series of model biomolecules, including the monosaccharides sialic acid, N-acetylglucosamine, fucose, fructose, and galactose; the polycations spermidine, poly-l-lysine, and poly-l-arginine; the polyanions poly-dl-aspartic acid and DNA; and leucine-proline dipeptide, all failed to replicate the effect of SP in a transwell motility assay (6) (data not shown).
The ability of N. gonorrhoeae to adapt the human genital environment is a critical component of the success of this prevalent sexually transmitted infection. Part of the role of seminal fluid during gonococcal transmission is undoubtedly to serve as a vehicle for bacterial transfer. However, this work further demonstrates that exposure of N. gonorrhoeae to seminal fluid alters gonococcal physiology in a manner that also has the potential to influence the process of transmission. The lack of an appropriate animal model to study sexual transmission of N. gonorrhoeae necessitates the use of in vitro assays, such as those described in the current work, to mimic relevant in vivo conditions. Further investigation into the biology of gonococcal-semen interactions is warranted, as a complete understanding of N. gonorrhoeae biology during the transmission state is a critical step toward limiting the spread of this pathogen.
ACKNOWLEDGMENT
We thank Adrienne Chen for the critical reading of this report.
REFERENCES
- 1.Howard TL. 1971. Bacterial hitch-hikers. J Urol 106:94. [DOI] [PubMed] [Google Scholar]
- 2.James-Holmquest AN, Swanson J, Buchanan TM, Wende RD, Williams RP. 1974. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun 9:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey HA, Porat N, Campbell CA, Jennings M, Gibson BW, Phillips NJ, Apicella MA, Blake MS. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol Microbiol 36:1059–1070. doi: 10.1046/j.1365-2958.2000.01938.x. [DOI] [PubMed] [Google Scholar]
- 4.Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Giménez-Gallego G, Sánchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel J-C, Margolis L, Keppler OT, Forssmann W-G, Kirchhoff F. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Easterhoff D, Ontiveros F, Brooks LR, Kim Y, Ross B, Silva JN, Olsen JS, Feng C, Hardy DJ, Dunman PM, Dewhurst S. 2013. Semen-derived enhancer of viral infection (SEVI) binds bacteria, enhances bacterial phagocytosis by macrophages, and can protect against vaginal infection by a sexually transmitted bacterial pathogen. Antimicrob Agents Chemother 57:2443–2450. doi: 10.1128/AAC.02464-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson MT, Dewenter L, Maier B, Seifert HS. 2014. Seminal plasma initiates a Neisseria gonorrhoeae transmission state. mBio 5:e01004-01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draper DL, Donegan EA, James JF, Sweet RL, Brooks GF. 1980. In vitro modeling of acute salpingitis caused by Neisseria gonorrhoeae. Am J Obstet Gynecol 138:996–1002. doi: 10.1016/0002-9378(80)91095-9. [DOI] [PubMed] [Google Scholar]
- 8.Higashi DL, Lee SW, Snyder A, Weyand NJ, Bakke A, So M. 2007. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect Immun 75:4743–4753. doi: 10.1128/IAI.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards JL, Shao JQ, Ault KA, Apicella MA. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun 68:5354–5363. doi: 10.1128/IAI.68.9.5354-5363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merz AJ, Enns CA, So M. 1999. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol Microbiol 32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich M, Bartfeld S, Munke R, Lange C, Ogilvie LA, Friedrich A, Meyer TF. 2011. Activation of NF-κB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell Microbiol 13:1168–1182. doi: 10.1111/j.1462-5822.2011.01607.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Higashi DL, Snyder A, Merz AJ, Potter L, So M. 2005. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell Microbiol 7:1271–1284. doi: 10.1111/j.1462-5822.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, Apicella MA. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect Immun 73:1964–1970. doi: 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steichen CT, Cho C, Shao JQ, Apicella MA. 2011. The Neisseria gonorrhoeae biofilm matrix contains DNA, and an endogenous nuclease controls its incorporation. Infect Immun 79:1504–1511. doi: 10.1128/IAI.01162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmros T, Burman LG, Bloom GD. 1976. Autolysis of Neisseria gonorrhoeae. J Bacteriol 126:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorward DW, Garon CF, Judd RC. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol 171:2499–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steichen CT, Shao JQ, Ketterer MR, Apicella MA. 2008. Gonococcal cervicitis: a role for biofilm in pathogenesis. J Infect Dis 198:1856–1861. doi: 10.1086/593336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweig M, Schork S, Koerdt A, Siewering K, Sternberg C, Thormann K, Albers SV, Molin S, van der Does C. 2014. Secreted single-stranded DNA is involved in the initial phase of biofilm formation by Neisseria gonorrhoeae. Environ Microbiol 16:1040–1052. doi: 10.1111/1462-2920.12291. [DOI] [PubMed] [Google Scholar]
- 19.Goytia M, Dhulipala VL, Shafer WM. 2013. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 343:64–69. doi: 10.1111/1574-6968.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewenter L, Volkmann TE, Maier B. 2015. Oxygen governs gonococcal microcolony stability by enhancing the interaction force between type IV pili. Integr Biol (Camb) 7:1161–1170. doi: 10.1039/C5IB00018A. [DOI] [PubMed] [Google Scholar]
- 21.Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, Schley AW, Anderson WJ, Grigoryan A, Aranas AE, Wodajo MS, Abellera JP, Centers for Disease Control and Prevention (CDC) . 2014. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep 61:1–121. [PubMed] [Google Scholar]
- 22.World Health Organization. 2012. Global incidence and prevalence of selected curable sexually transmitted infection, 2008. WHO, Geneva, Switzerland. [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 24.Kellogg DS, Peacock WL, Deacon WE, Brown L, Pirkle CI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wade JJ, Graver MA. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol Lett 273:35–37. doi: 10.1111/j.1574-6968.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 26.Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest 93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325:764–767. doi: 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. 2003. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect Immun 71:6279–6291. doi: 10.1128/IAI.71.11.6279-6291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell-Adams B, Wainwright LA, Seifert HS. 1996. The size and position of heterologous insertions in a silent locus differentially affect pilin recombination in Neisseria gonorrhoeae. Mol Microbiol 22:509–522. doi: 10.1046/j.1365-2958.1996.00128.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp 24:pii=2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 32.Tabor H, Tabor CW. 1964. Spermidine, spermine, and related amines. Pharmacol Rev 16:245–300. [PubMed] [Google Scholar]
- 33.Sadarangani M, Pollard AJ, Gray-Owen SD. 2011. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol Rev 35:498–514. doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 34.Swanson J. 1978. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect Immun 19:320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merz AJ, So M, Sheetz MP. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 36.Dillard JP, Seifert HS. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol 41:263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 37.Higashi DL, Zhang GH, Biais N, Myers LR, Weyand NJ, Elliott DA, So M. 2009. Influence of type IV pilus retraction on the architecture of the Neisseria gonorrhoeae-infected cell cortex. Microbiology 155:4084–4092. doi: 10.1099/mic.0.032656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen DH, Katz DF. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl 26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 39.Ball LM, Criss AK. 2013. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J Bacteriol 195:2982–2990. doi: 10.1128/JB.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


