ABSTRACT
The iprA gene (formerly known as yaiV or STM0374) is located in a two-gene operon in the Salmonella enterica serovar Typhimurium genome and is associated with altered expression during spaceflight and rotating-wall-vessel culture conditions that increase virulence. However, iprA is uncharacterized in the literature. In this report, we present the first targeted characterization of this gene, which revealed that iprA is highly conserved across Enterobacteriaceae. We found that S. Typhimurium, Escherichia coli, and Enterobacter cloacae ΔiprA mutant strains display a multi-log-fold increase in oxidative stress resistance that is complemented using a plasmid-borne wild-type (WT) copy of the S. Typhimurium iprA gene. This observation was also associated with increased catalase activity, increased S. Typhimurium survival in macrophages, and partial dependence on the katE gene and full dependence on the rpoS gene. Our results indicate that IprA protein activity is sensitive to deletion of the N- and C-terminal 10 amino acids, while a region that includes amino acids 56 to 80 is dispensable for activity. RNA sequencing (RNA-Seq) analysis revealed several genes altered in expression in the S. Typhimurium ΔiprA mutant strain compared to the WT, including those involved in fimbria formation, spvABCD-mediated virulence, ethanolamine utilization, the phosphotransferase system (PTS) transport, and flagellin phase switching from FlgB to FliC (likely a stochastic event) and several genes of hypothetical or putative function.
IMPORTANCE Overall, this work reveals that the conserved iprA gene measurably influences bacterial biology and highlights the pool of currently uncharacterized genes that are conserved across bacterial genomes. These genes represent potentially useful targets for bacterial engineering, vaccine design, and other possible applications.
INTRODUCTION
Many large-scale genomic studies, virulence factor identification assays, and whole-genome expression analyses have revealed bacterial genes that are uncharacterized but are associated with gene expression changes in different environments that can affect virulence and stress survival (see references 1–8 and several others). These studies reveal a pool of genes that are not understood or characterized but whose importance is highlighted due to how they were identified and, in some cases, to high conservation across bacterial genomes. The iprA gene (previously known as yaiV or STM0374) is located in a two-gene operon in the Salmonella enterica serovar Typhimurium genome and has been observed to display altered expression during spaceflight and under rotating-wall-vessel (RWV) culture conditions, which increase virulence (6, 7). In these studies, microarray analysis revealed that S. Typhimurium iprA was upregulated in cultures inoculated and grown during spaceflight (compared to identically grown ground controls) and in cultures grown under low-fluid-shear RWV conditions (compared to RWV controls in which low-fluid-shear conditions were disrupted) (6, 7). Interestingly, both spaceflight and RWV growth conditions increase the virulence of S. Typhimurium assayed using murine infection models (6, 9, 10). However, to our knowledge, the iprA gene is uncharacterized in the literature beyond these observations. Overall, little is known about the seven genes in the 8.9-kb locus between hemB and ddlA in the S. Typhimurium genome, which include yaiU, iprA, ampH, sbmA, yaiW, yaiY, and yaiZ (11). The ampH gene has been shown to be decreased in expression in the presence of butyrate (12). The sbmA and yaiW genes are cotranscribed and encode membrane proteins that affect sensitivity to antimicrobial peptides (13). The functional roles of the yaiU, iprA, yaiY, and yaiZ genes were hitherto unknown. In this report, we present the first targeted systematic characterization of the iprA gene, which reveals that iprA is highly conserved and found across Enterobacteriaceae genomes. Our results indicate a role for iprA in bacterial oxidative stress resistance across genera, where the deletion of iprA is associated with increased resistance to hydrogen peroxide, increased catalase activity, and increased S. Typhimurium survival in macrophage cells. We found the increased resistance to hydrogen peroxide to be partially dependent on the katE gene and fully dependent on the rpoS gene. We also found that deletion of S. Typhimurium iprA results in alteration of S. Typhimurium gene expression, including that of fimbrial genes, the ethanolamine utilization operon, plasmid-borne spvABCD genes, phosphotransferase system (PTS) transport genes, phage genes, and many putative or hypothetical genes. Thus, iprA is a previously unrecognized target gene for possible use in bacterial engineering, vaccine design, and other approaches where iprA-regulated phenotypes or associated gene pathways have a need to be manipulated for different applications. Based on this work, we introduce the name iprA (inhibitor of hydrogen peroxide resistance) for this gene. In addition, the results highlight undiscovered gene functions that reside in the pool of currently uncharacterized genes that are conserved across genera in bacterial genomes. We also discuss the role that iprA might play as a gene whose deletion results in increased resistance to oxidative stress.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The strains and plasmids used in this study are listed in Table 1. The construction of all deletion mutant strains utilized standard recombineering (14, 15), with PCR products obtained using the DNA primers listed in Table S1 in the supplemental material. The template plasmid pKD3, pKD4, or pJW102 was used as indicated in Table S1 to amplify the chloramphenicol resistance (Cmr), kanamycin resistance (Kmr), or spectinomycin resistance (Spr) marker for insertion, respectively, and the marker was subsequently removed using flippase (FLP) recombinase expressed from plasmid pCP20 (14, 15). Deletion endpoints were internal to the indicated gene open reading frames (ORFs) using the homology in the primers displayed in Table S1. Molecular verification of all mutations was performed via PCR, with primers amplifying specific products indicative of each deletion. Deletion mutations were transferred between S. Typhimurium strains using standard P22 HT/int phage transduction, as described previously (16). The transfer of deletion mutations between Escherichia coli strains was performed using purified donor chromosomal DNA transformed into recipient strains containing plasmid pKD46-RecA, as described previously (17). Construction of the S. Typhimurium ΔN10 and ΔC10 mutant strains was performed via genome recombineering that resulted in the in-frame removal of the very N-terminal and C-terminal 10 amino acids of the IprA protein, respectively. These mutations were verified via sequencing. The S. Typhimurium iprA wild-type (WT) allele and the Δ56-80 allele (which encodes a deletion of amino acids 56 to 80) were obtained via gene synthesis (Genewiz, Piscataway, NJ) and subcloned into the plasmid vector pBAD18 (18) at the XmaI site, using standard cloning procedures. These gene clones were verified via sequencing. Strains were grown using LB (Lennox) medium supplemented with the following antibiotics when necessary: ampicillin, 200 μg/ml; chloramphenicol, 10 μg/ml; kanamycin, 50 μg/ml; and spectinomycin, 125 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Plasmid or strain | Species | Reference or source |
|---|---|---|
| Strains | ||
| χ3339 | S. Typhimurium | 37 |
| χ3339 ΔU-Z | S. Typhimurium | This study |
| χ3339 ΔH-Z | S. Typhimurium | This study |
| χ3339 ΔU-V | S. Typhimurium | This study |
| χ3339 ΔU | S. Typhimurium | This study |
| χ3339 ΔiprA | S. Typhimurium | This study |
| χ3339 ΔkatN | S. Typhimurium | This study |
| χ3339 ΔkatG | S. Typhimurium | This study |
| χ3339 ΔkatE | S. Typhimurium | This study |
| χ3339 ΔoxyR | S. Typhimurium | This study |
| χ3339 ΔrpoS | S. Typhimurium | 49 |
| χ3339 Δdps | S. Typhimurium | This study |
| χ3339 ΔahpC | S. Typhimurium | This study |
| χ3339 ΔtsaA | S. Typhimurium | This study |
| χ3339 ΔkatN katG katE | S. Typhimurium | This study |
| χ3339 ΔiprA ΔkatN | S. Typhimurium | This study |
| χ3339 ΔiprA ΔkatG | S. Typhimurium | This study |
| χ3339 ΔiprA ΔkatE | S. Typhimurium | This study |
| χ3339 ΔiprA ΔkatN katG katE | S. Typhimurium | This study |
| χ3339 ΔiprA ΔrpoS | S. Typhimurium | This study |
| χ3339 ΔiprA Δdps | S. Typhimurium | This study |
| χ3339 ΔiprA ΔahpC | S. Typhimurium | This study |
| χ3339 ΔiprA ΔtsaA | S. Typhimurium | This study |
| χ3339 ΔiprA ΔoxyR | S. Typhimurium | This study |
| χ3339 ΔN10 | S. Typhimurium | This study |
| χ3339 ΔC10 | S. Typhimurium | This study |
| ATCC 29629 | S. Typhimurium | American Type Culture Collection |
| ATCC 29629 ΔiprA | S. Typhimurium | This study |
| NTCT74 | S. Typhimurium | 50 |
| NTCT74 ΔiprA | S. Typhimurium | This study |
| MG1655 | E. coli | 51 |
| MG1655 ΔiprA | E. coli | This study |
| JA221 | E. coli | 52 |
| JA221 ΔiprA | E. coli | This study |
| TOP10 | E. coli | Invitrogen |
| TOP10 ΔiprA | E. coli | This study |
| ATCC 23355 | Enterobacter cloacae | American Type Culture Collection |
| ATCC 23355 ΔiprA | E. cloacae | This study |
| Plasmids | ||
| pBAD18 | 18 | |
| pBAD18 + iprA | This study | |
| pBAD18 + iprA Δ56-80 | This study | |
| pKD3 | 14 | |
| pKD4 | 14 | |
| pCP20 | 14 | |
| pJW102 | 15 | |
| pKD46-RecA | Nature Technology Corp., Lincoln, NE |
Sequence analysis.
The IprA homologs from the indicated Gram-negative species were identified using BLAST search analysis with S. Typhimurium IprA (GenBank accession no. AAL19328) as the query (19). Homologs were aligned using CLUSTAL W analysis, as previously described (20, 21). Synteny analysis was performed using standard gene visualization tools via the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
Oxidative stress, catalase, macrophage survival, and Caenorhabditis elegans assays.
Oxidative stress assays were performed by adding hydrogen peroxide (Sigma, St. Louis, MO) to aliquots of cultures that had been grown for 4 h after inoculation from an overnight culture into fresh broth, as described previously (22–25). Cultures for the assays were used at an optical density at 600 nm (OD600) of 0.5 to 0.6, and graphs of the CFU amounts used in a typical experiment serve to show that samples were at equivalent CFU at the time of the stress assay (see Fig. S1 in the supplemental material). Survival was routinely assayed over 2 h using a hydrogen peroxide concentration of 70 mM; however, lower levels were tested for the ΔkatE, ΔkatG, ΔkatN, ΔrpoS, and Δdps background mutant strains due to hypersensitivity. Those levels were as follows: ΔkatE mutant, 27 mM: ΔkatG mutant, 35 mM; ΔkatN mutant, 35 mM; ΔkatE ΔkatG ΔkatN mutant, 17.5 mM; ΔrpoS mutant, 9 mM; and Δdps mutant, 17.5 mM. The oxidative stress data presented represent the mean and standard deviation of the results from at least four independent experiments, each plated in triplicate. Growth curve assays in LB broth culture were performed as described previously in the presence of 5 mM H2O2 to test inhibitory effects in this context (26). Bovine liver catalase protein (Sigma) was supplemented to solid medium at 140 μg/ml in the experiment shown in Fig. 7 (27). Catalase activity assays were performed as described previously, using at least six independent experimental samples for each strain shown (28). The invasion of Int407 intestinal epithelial cells and survival in J774 macrophages were determined using a 2-h postinfection time point, as described previously, using at least six independent cultures per sample, each plated in triplicate (22, 25, 29). Assays of C. elegans survival were performed using standard protocols, as described previously (30–32).
FIG 7.
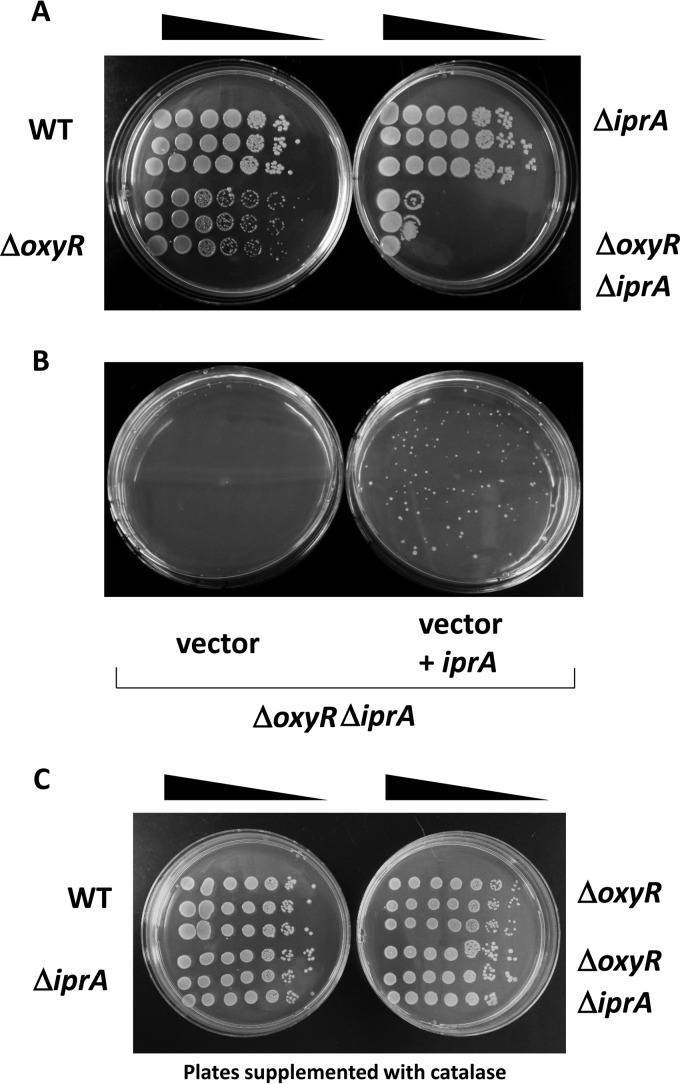
An S. Typhimurium ΔoxyR ΔiprA mutant strain displays a growth defect on solid medium. (A) S. Typhimurium χ3339 WT, ΔoxyR mutant, ΔiprA mutant, and ΔoxyR ΔiprA mutant strains were grown in liquid medium to equivalent OD600 values, serially diluted, and plated on solid medium for colony growth. (B) Strain χ3339 ΔoxyR ΔiprA containing either vector or vector plus iprA was diluted and plated for single-colony growth on solid medium. (C) The solid medium was supplemented with catalase protein (140 μg per ml of medium), and the indicated strains were grown and plated as described for panel A.
RNA-Seq and RT-qPCR analysis.
Total RNA was harvested from log- and stationary-phase cultures using the Qiagen RNAprotect and RNeasy reagents (Qiagen, Venlo, the Netherlands). RNA samples were treated with DNase (Ambion DNA-Free kit; Thermo Fisher Scientific, Waltham, MA) and submitted for RNA-sequencing (RNA-Seq) analysis using an Illumina HiSeq 2000 (Eurofins MWG Operon, Inc., Huntsville, AL) and the GeneSifter software (GeneSifter; Geospiza, Inc., Seattle, WA). Quality control statistics for the samples generated via FastQC are provided in Table S2 in the supplemental material. In GeneSifter, the following analysis settings were used: normalization to mapped reads, statistics using Fisher's exact test, quality cutoff level of 10, and a change in expression cutoff of ≥2-fold or ≤0.5-fold. The full RNA-Seq data set is provided in the supplemental material. Reverse transcription-quantitative PCR (RT-qPCR) analysis using primers for target genes indicated in Table S3 in the supplemental material was performed using the lpxC gene for normalization, as described previously (10, 24, 33).
Flagellin phase-switching analysis.
Confirmation of flagellin phase switching was performed at both the protein and DNA level, as described previously (34–36). The primers used to assay genomic DNA for flagellin phase are indicated in Table S3 in the supplemental material.
RESULTS
S. Typhimurium iprA gene encodes a conserved protein.
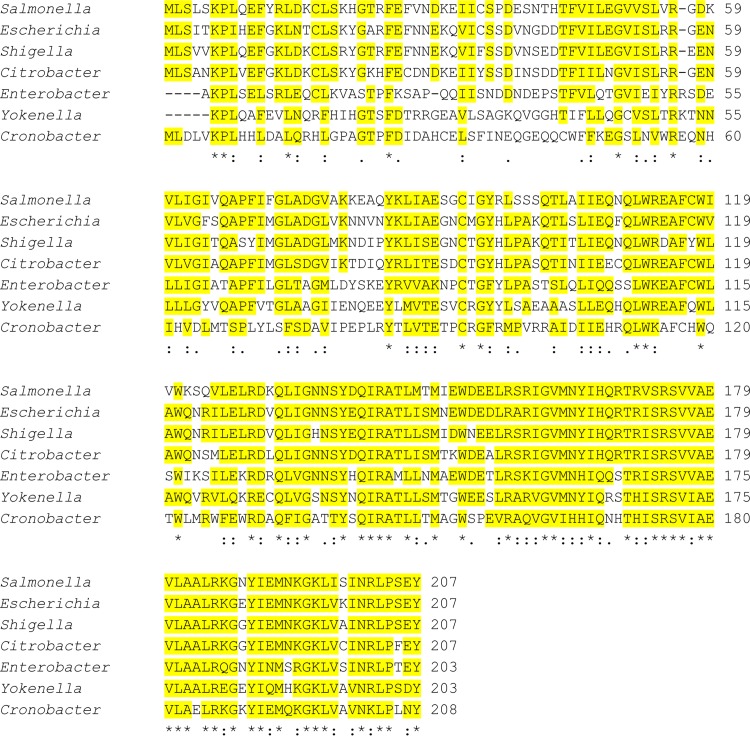
We used the S. Typhimurium IprA protein sequence (GenBank accession no. AAL19328) as a query in a BLAST search for homologs in the protein database. The results of this search indicated that the IprA protein sequence is highly conserved among Enterobacteriaceae genomes (E values ranging between 10−110 and 10−50), including the genera Escherichia, Shigella, Citrobacter, Enterobacter, Yokenella, Cronobacter, and Klebsiella (Fig. 1). Interestingly, the iprA gene is absent from other genera of Enterobacteriaceae, including Proteus, Serratia, Yersinia, and Erwinia, and the gene is also absent from other more distantly related genera, such as Pseudomonas, Acinetobacter, Xanthomonas, and Rhizobium (data not shown). In iprA-containing genomes, synteny analysis indicated the conservation of neighboring genes across genera (see Fig. S2 in the supplemental material). These results indicate that the iprA gene is conserved in Enterobacteriaceae across multiple genera and suggest evolutionary selection for the function of this gene in prokaryotic biology.
FIG 1.
Alignment of the S. Typhimurium IprA protein with homologs present in other Enterobacteriaceae genera. Yellow highlighting indicates amino acid identity or strong similarity (in at least four of the proteins). The E values for the aligned proteins range between 10−110 and 10−50. The accession numbers for sequences analyzed here are as follows: NP_459369 (Salmonella enterica serovar Typhimurium LT2), NP_414909 (Escherichia coli MG1655), NP_706193 (Shigella flexneri 2a strain 301), WP_044699331 (Citrobacter freundii), KPS97271 (Enterobacter cloacae), WP_006818715 (Yokenella regensburgei), and WP_004386860 (Cronobacter sakazakii). Asterisks indicate amino acids that are identical across all proteins; double dots indicate amino acids highly conserved across all proteins. A single dot indicates some high amino acid conservation coupled with divergence at this position.
Our sequence analysis revealed a predicted catabolite activator protein (CAP) effector binding domain in IprA that spans amino acid residues 23 to 116 and aligns with the consensus CAP effector binding domain sequence at an E value of 3.52 × 10−8 (see Fig. S3 in the supplemental material). The effector domain acts to bind a molecule that triggers conformational change to influence DNA binding of the protein (such as the cyclic AMP [cAMP] molecule does to the CAP/cAMP receptor protein [CRP]). Our analysis also found that the IprA protein contains a predicted winged helix-turn-helix (wHTH) DNA-binding domain that spans amino acids 138 to 205, which aligns with the consensus sequence at an E value of 1.9 × 10−29 (see Fig. S4 in the supplemental material). The identification of these predicted domains suggests that IprA could be a DNA-binding protein whose binding activity is influenced or regulated by the binding of an effector molecule via the predicted CAP effector domain.
Phenotypic analysis of S. Typhimurium ΔiprA mutant strains.
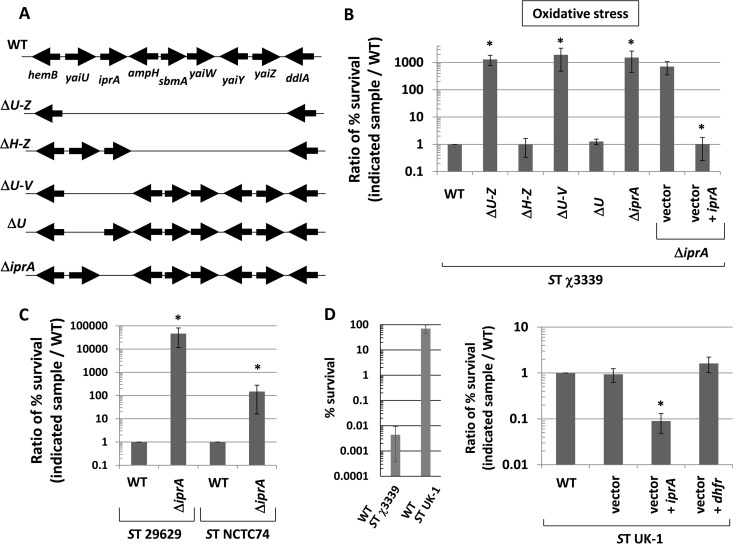
To determine the role of iprA and other nearby genes in bacterial biology, we constructed a series of deletions in the S. Typhimurium genome that removed different combinations of the genes between hemB and ddlA, which included yaiU, iprA, yaiW, yaiY, and yaiZ (Fig. 2A). We compared the survival of these mutant strains to that of the isogenic wild-type strain under various environmental stresses (including acid, oxidative, thermal, and osmotic) in the virulent S. Typhimurium background χ3339 (37). We found that all mutants containing a deletion of the iprA gene displayed enhanced survival of oxidative stress in this background at a level approximately 1,000-fold greater than that of the wild type (Fig. 2B). In addition, we set up growth curve assays with WT and ΔiprA mutant bacteria in the presence of inhibitory oxidative stress levels and found that the deletion of iprA resulted in enhanced survival under these conditions as well (see Fig. S5 in the supplemental material). The results of the acid, thermal, and osmotic stress assays were either inconclusive or displayed equivalent resistance levels in the mutant and wild-type strains (data not shown). The enhanced oxidative stress resistance phenotype of the S. Typhimurium ΔiprA mutant was fully complemented to the wild-type phenotype using a plasmid-borne copy of the S. Typhimurium iprA gene (Fig. 2B).
FIG 2.
Role of the iprA gene in oxidative stress resistance in S. Typhimurium (ST). (A) Gene map between hemB and ddlA in the S. Typhimurium genome showing different deletion mutations constructed in this region. (B) Oxidative stress resistance assays performed on S. Typhimurium strain χ3339 derivatives containing different mutations shown in panel A. The percent survival of bacteria after the addition of stress was obtained based on the initial CFU per milliliter before stress, and a ratio of the percent survival for each strain compared to the WT strain survival was calculated and plotted. (C) S. Typhimurium strains 29629 and NCTC74 containing ΔiprA mutations were compared to the corresponding WT strains in oxidative stress assays. (D) The S. Typhimurium strain UK-1 displays inherently high oxidative stress resistance in this study (as shown in the graph of oxidative stress assay results on the left). UK-1 strains containing plasmid vector, vector plus iprA, or vector plus the dihydrofolate reductase gene (dhfr) were tested for oxidative stress resistance. Data in each panel are shown as the mean plus standard deviation, and observed differences from the WT were found to be significant (as indicated by an asterisk) at a P value of <0.05 using a t test to compare the WT and the indicated mutant sample. For panels B and D, a t test comparison between the vector and the vector plus iprA was used.
To determine if we could observe the ΔiprA mutant phenotype in other S. Typhimurium strains, we transferred the ΔiprA mutation to S. Typhimurium strains 29629 and NTCT74 and compared the corresponding mutant and WT strains in the oxidative stress assay (Fig. 2C). The 29629 and NTCT74 ΔiprA mutant strains displayed approximately 40,000- and 100-fold enhanced oxidative stress survival, respectively, compared to the cognate WT strain, indicating that this phenomenon is not strain specific. The S. Typhimurium universal killer strain UK-1 displays an inherently high resistance to oxidative stress in our hands compared to χ3339 (Fig. 2D), and we reasoned that the overexpression of iprA in this strain would be predicted to decrease this resistance. When we expressed plasmid-borne iprA in S. Typhimurium UK-1, the oxidative stress resistance decreased as predicted, and this was not observed in control strains containing plasmid vector alone or expressing a heterologous protein (dihydrofolate reductase [DHFR]) from the same vector (Fig. 2D).
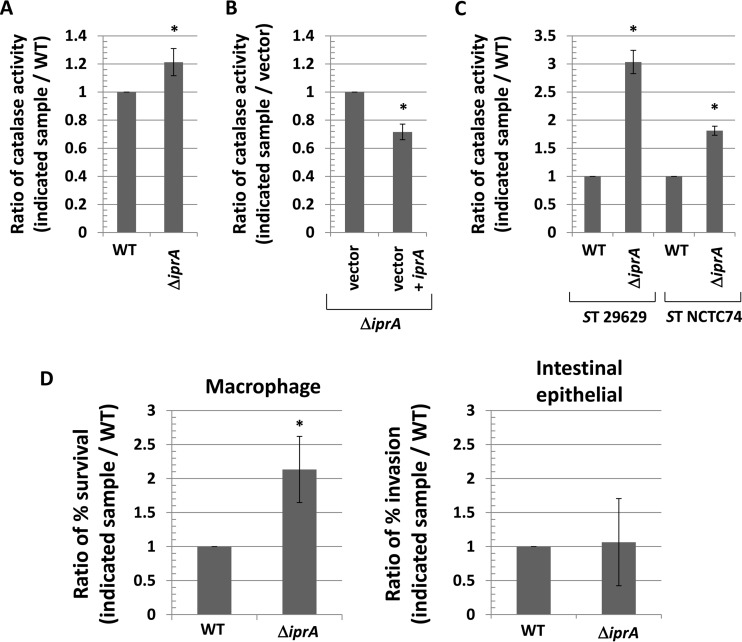
To determine if the increased resistance to hydrogen peroxide-induced oxidative stress in the S. Typhimurium χ3339 ΔiprA mutant strain corresponded with an increase in catalase activity, we measured the catalase activity in the WT and ΔiprA χ3339 strains (Fig. 3A). We found that χ3339 ΔiprA displayed approximately 20% more catalase activity than the WT strain, and this activity was correspondingly reversed by a WT copy of the iprA gene provided on a plasmid (Fig. 3B). We also measured the catalase activity in the S. Typhimurium 29629 and NTCT74 WT and ΔiprA mutant strains and found approximately 300% and 75% increases in catalase activity in the 29629 ΔiprA and NTCT74 ΔiprA mutant strains compared to the cognate WT, respectively (Fig. 3C). Thus, the S. Typhimurium ΔiprA mutation results in both an increase in oxidative stress resistance and an increase in catalase activity.
FIG 3.
Increased catalase activity and survival in macrophages associated with ΔiprA mutation in S. Typhimurium. (A) S. Typhimurium χ3339 WT and χ3339 ΔiprA mutant strains were measured for catalase activity, and a ratio of the activity in each sample to the WT value was calculated and plotted. (B) S. Typhimurium χ3339 ΔiprA mutant strains containing plasmid vector or vector plus iprA were measured for catalase activity, and a ratio of the activity in each sample compared to the vector strain activity was calculated and plotted. (C) S. Typhimurium strains 29629 and NTCT74 containing ΔiprA mutations were compared to each corresponding WT for catalase activity. (D) S. Typhimurium strain χ3339 WT and ΔiprA mutant strains were used to infect J774 macrophage cells and Int407 intestinal epithelial cells, and the percent survival (in macrophages) and percent invasion (for intestinal epithelial cells) were measured for each strain at 2 h postinfection. A ratio of these values for each sample to the values for the WT was calculated and plotted. Data in each panel are shown as the mean plus standard deviation, and observed differences from the WT were found to be significant (as indicated by an asterisk) at a P value of <0.05 using a t test to compare the WT and the indicated mutant sample or, for panel B, the vector and the vector plus iprA.
To test if the S. Typhimurium ΔiprA mutation alters survival in macrophages (where reactive oxygen species aid in killing phagocytosed bacteria), we infected macrophage cells with S. Typhimurium χ3339 WT and ΔiprA mutant strains and assayed survival at 2 h postinfection (Fig. 3D). We found that the ΔiprA mutant strain displayed approximately 2-fold greater survival than the WT in macrophages (Fig. 3D). This is consistent with the increased survival observed for S. Typhimurium ΔiprA mutant strains in the oxidative stress assays described above. We also compared the χ3339 WT and ΔiprA mutant strains for the invasion of intestinal epithelial cells (another key virulence attribute of S. Typhimurium) but found equivalent levels of invasion of these strains into these cells (Fig. 3D). We measured the survival of C. elegans worms infected with either the S. Typhimurium χ3339 WT or ΔiprA mutant strain (see Fig. S6 in the supplemental material), and we found no difference in the survival of the worms infected with these strains.
Escherichia coli and Enterobacter cloacae ΔiprA mutant strains.
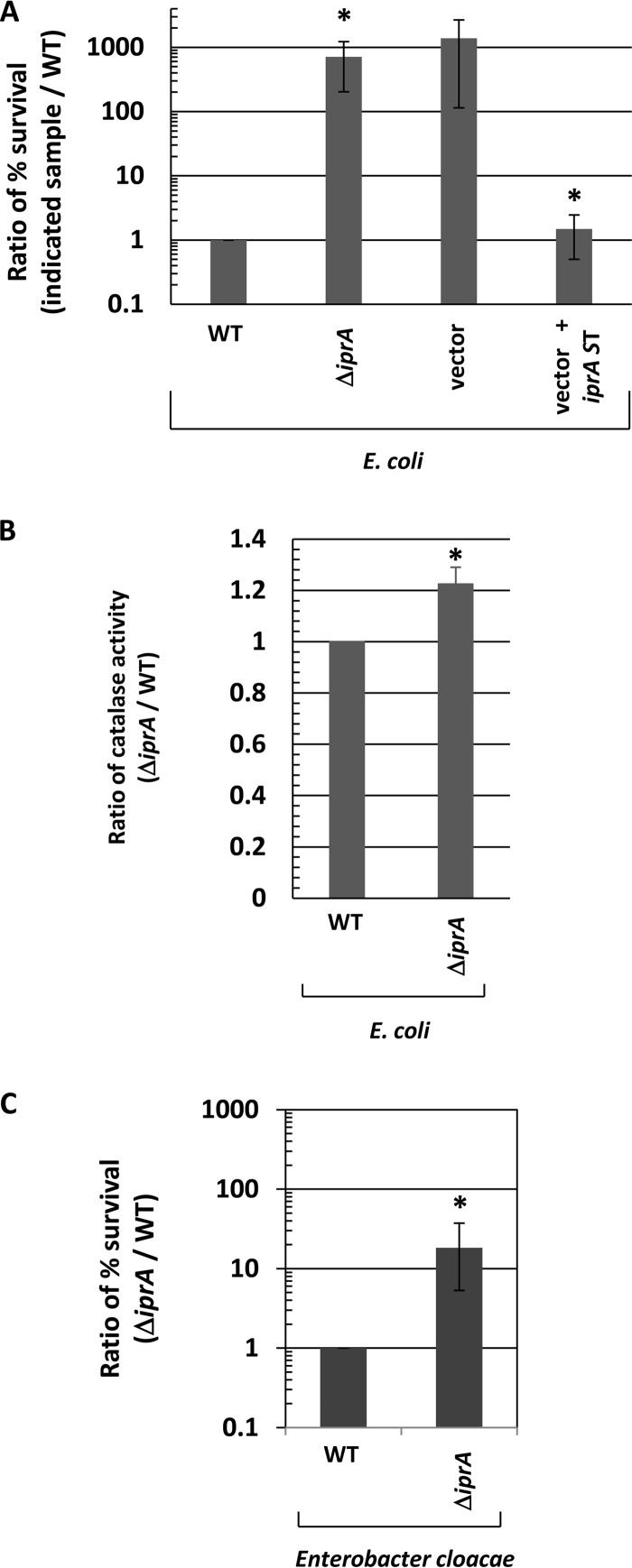
Given the strong conservation of the iprA gene across Enterobacteriaceae, we constructed a deletion mutation of the iprA gene in Escherichia coli, and we then compared the E. coli ΔiprA mutant strain to the corresponding WT strain for resistance to oxidative stress (Fig. 4A). We found that the ΔiprA mutation resulted in an approximately 700-fold increase in oxidative stress resistance compared to the WT in the E. coli TOP10 background (Fig. 4A). We then transferred the ΔiprA mutation to two other E. coli strain backgrounds, MG1655 and JA221. We found that the ΔiprA mutation resulted in approximately 80,000-fold and 20-fold increases in oxidative stress resistance compared to the WT in the MG1655 and JA221 backgrounds, respectively (see Fig. S7A in the supplemental material). We also measured the catalase activity of the above-mentioned E. coli ΔiprA mutant and WT strains and observed an increase in catalase activity for the ΔiprA mutant strains compared to the WT for each E. coli background (Fig. 4B; see also Fig. S7B). Since the homology between the S. Typhimurium and E. coli IprA proteins is high (71% identity, 86% similarity, E value = 10−108), we reasoned that the S. Typhimurium iprA gene could be used for complementation analysis in the E. coli ΔiprA mutant strain. We found that the S. Typhimurium iprA gene provided on a plasmid complemented the E. coli ΔiprA mutation and restored oxidative stress resistance to WT levels in these strains (Fig. 4A; see Fig. S7C). In addition, we also constructed a ΔiprA mutation in Enterobacter cloacae, another Gram-negative species that contains the iprA gene (as shown in Fig. 1). The E. cloacae ΔiprA mutant strain displayed approximately 20-fold increased resistance to oxidative stress compared to the corresponding WT strain (Fig. 4C). These results clearly demonstrate the conserved nature of the iprA gene and its function across Gram-negative genera.
FIG 4.
Characterization of E. coli and E. cloacae ΔiprA mutant strains. (A) A ΔiprA mutation was constructed in E. coli and transferred to E. coli strain TOP10. The percent survival of the ΔiprA mutant upon exposure to oxidative stress was measured and calculated for a ratio to the survival of the corresponding WT strain. TOP10 ΔiprA mutant strains containing plasmid vector and vector plus S. Typhimurium (ST) iprA were assayed for oxidative stress survival and compared to the corresponding WT strain. The S. Typhimurium iprA gene is the WT iprA gene cloned from S. Typhimurium. (B) Catalase activity was measured in the WT and E. coli TOP10 ΔiprA mutant strains and plotted as the ratio of the activity of each sample to that of the corresponding WT strain. (C) An ΔiprA mutation was constructed in E. cloacae, and the mutant was assayed for oxidative stress survival, as performed for the E. coli strains. Data in each panel are shown as the mean plus standard deviation, and the observed differences from the WT were found to be significant (as indicated by an asterisk) at a P value of <0.05 for all panels using a t test to compare the WT and the indicated mutant sample. For panel A, a t test comparison was also performed between vector and vector plus iprA.
Deletion of IprA protein regions.
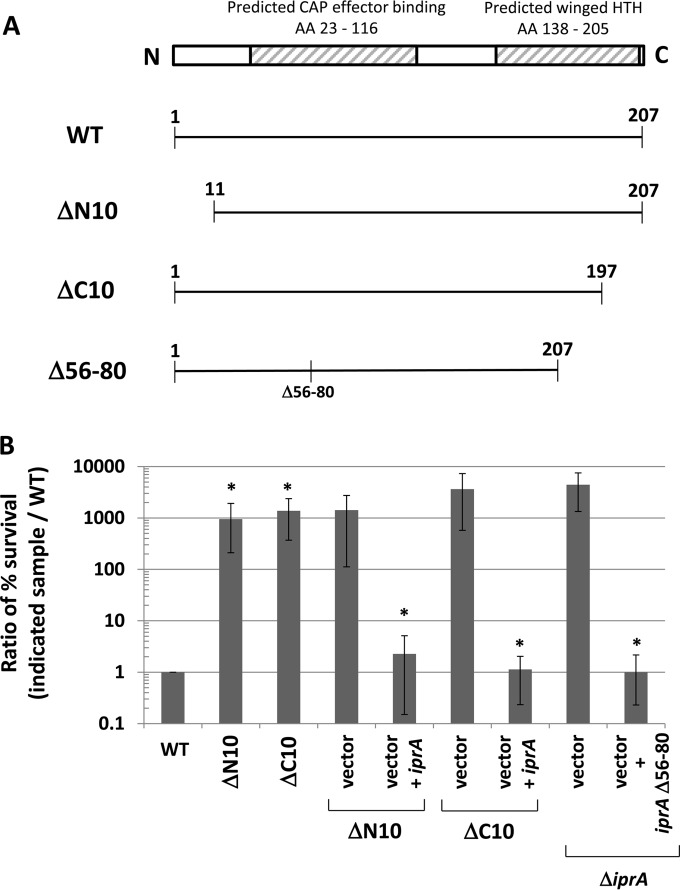
To determine which regions of the IprA protein are essential for its activity, we constructed in-frame deletion mutations in the iprA gene that removed peptide sequences from the corresponding IprA protein. We constructed two mutations, ΔN10 and ΔC10, which removed 10 amino acids from the very N and C termini of the S. Typhimurium IprA protein, respectively (Fig. 5A). Both mutations abolished iprA activity but could be complemented to the WT phenotype using a WT copy of the iprA gene provided on a plasmid (Fig. 5B). In addition, we constructed a mutation that removed amino acids 56 to 80 from the S. Typhimurium IprA protein and served to delete a significant portion from the middle of the predicted CAP effector binding domain that includes the predicted ligand binding site (Fig. 5A). This mutation did not alter iprA gene activity (Fig. 5B). These analyses demonstrate that the N and C termini of the IprA protein are critical to its activity and sensitive to removal and that the portion of the predicted CAP effector binding domain comprising amino acids 56 to 80 is dispensable.
FIG 5.
Deletion of IprA protein regions. (A) Diagram of WT IprA protein and the ΔN10, ΔC10, and Δ56-80 mutant derivatives constructed in S. Typhimurium for this study. The ΔN10 and ΔC10 alleles remove the first 10 amino acids (AA) from the N terminus and last 10 amino acids from the C terminus from S. Typhimurium IprA, respectively. The Δ56-80 allele contains a deletion of amino acids 56 to 80 located in the predicted CAP effector binding domain of the S. Typhimurium IprA protein. The ΔN10 and ΔC10 alleles were constructed in the chromosome, and the Δ56-80 allele was constructed in a plasmid. (B) S. Typhimurium strain χ3339 containing either the ΔN10 or ΔC10 mutation was tested for oxidative stress survival and compared to the isogenic WT strain, as described for previous experiments. The same mutant strains containing plasmid vector and vector plus iprA were also tested for oxidative stress survival and compared to the WT. The χ3339 ΔiprA mutant strain containing plasmid vector or vector plus the iprA Δ56-80 mutant was tested for oxidative stress survival in the same manner as the other strains. Data are shown as the mean plus standard deviation, and observed differences were found to be significant (as indicated by an asterisk) at a P value of <0.05 using a t test to compare either the WT and the indicated mutant or the vector and the vector plus iprA, as indicated.
Role of catalase genes, rpoS, and other genes in the ΔiprA mutant phenotype.
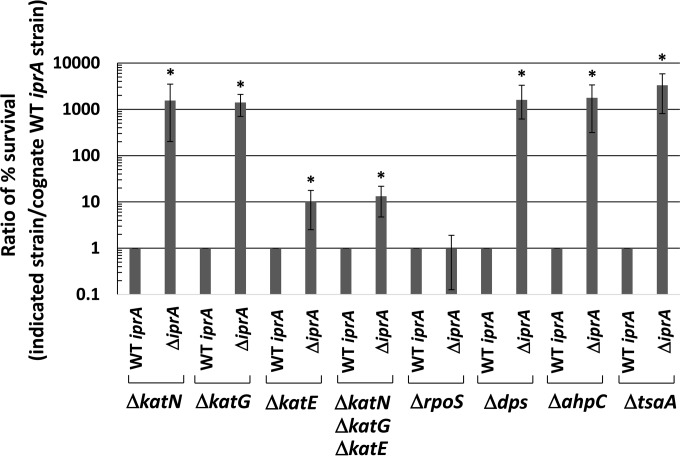
To learn what mechanistic pathways are involved in the increased oxidative stress resistance observed in the ΔiprA mutant strains, we constructed deletions in genes encoding catalase enzymes in S. Typhimurium: katN, katG, and katE (27). We constructed χ3339 double mutant strains that combined the ΔkatN, ΔkatG, or ΔkatE mutation with the ΔiprA mutation and compared these strains to the corresponding single-catalase-mutation strain. We specifically wanted to determine if any of the catalase mutations altered the increase in oxidative stress resistance observed in the presence of the ΔiprA mutation. The presence of the ΔiprA mutation increased oxidative stress resistance of the ΔkatN and ΔkatG mutant strains approximately 1,000-fold (Fig. 6). However, in the ΔkatE mutant background, the ΔiprA mutation increased oxidative resistance approximately 10-fold, indicating a role for the katE gene in this phenotype (Fig. 6). To see if the combined activity of the katN and katG genes could account for the remaining 10-fold resistance observed in the ΔkatE ΔiprA mutant strain, we constructed a strain containing mutations in all three catalase genes and then transferred the ΔiprA mutation to this background. The results show that the ΔiprA mutation increased resistance 10-fold in this background, indicating that the ΔiprA mutant phenotype works through the katE catalase for part of this phenotype and is likely independent of the katN and katG genes (Fig. 6). We then transferred the ΔiprA mutation into an isogenic S. Typhimurium ΔrpoS background mutant strain to test for a role of the rpoS gene in the ΔiprA mutant phenotype. The rpoS gene encodes a sigma factor involved in the expression of several stress resistance genes, including the katE gene (38, 39). Interestingly, we found that the ΔiprA mutation did not increase oxidative stress resistance in the ΔrpoS mutant background, indicating that the rpoS gene is essential for the full ΔiprA mutant phenotype (Fig. 6). This suggests that an additional RpoS-controlled factor plays a role in the ΔiprA oxidative stress resistance mutant phenotype. Therefore, we tested for a role of the dps gene in the ΔiprA mutant phenotype. The dps gene is regulated by RpoS and encodes a nonspecific DNA-binding protein that acts to protect bacterial cells against oxidative stress (40, 41). We constructed an S. Typhimurium Δdps mutant strain and transferred the ΔiprA mutation into this strain. However, we found that the ΔiprA mutation increased oxidative stress resistance 1,000-fold in the Δdps mutant strain, indicating that the dps gene is not involved in the ΔiprA mutant phenotype (Fig. 6).
FIG 6.
Role of catalase genes, rpoS, and other genes in the ΔiprA mutant phenotype. S. Typhimurium strain χ3339 containing the indicated mutations was tested for oxidative stress survival, and each strain was compared to the corresponding strain containing the WT iprA allele. Data are shown as the mean plus standard deviation, and observed differences from cognate strains containing WT iprA were found to be significant (as indicated by an asterisk) at a P value of <0.05 using a t test.
Two S. Typhimurium genes encoding separate alkyl hydroperoxide reductases, which can scavenge and degrade hydrogen peroxide, are ahpC and tsaA (27). To test the involvement of these genes in the ΔiprA mutant phenotype, we constructed ΔahpC and ΔtsaA mutations and tested them in combination with the ΔiprA mutation in oxidative stress assays (Fig. 6). We found that these mutations did not affect the ΔiprA mutant phenotype.
An S. Typhimurium ΔiprA ΔoxyR mutant displays a growth defect on solid medium.
During our efforts to combine mutations in stress-related genes with the ΔiprA mutation as described above, we constructed a deletion mutation in the S. Typhimurium oxyR gene, which regulates the expression of oxidative stress genes, including katG (42, 43). However, when we combined the ΔoxyR and ΔiprA mutations, we observed that this strain was severely growth defective on solid medium (Fig. 7A). This growth defect was reversed by providing a WT copy of the iprA gene in the ΔoxyR ΔiprA mutant background (Fig. 7B). Interestingly, the growth defect for the ΔoxyR ΔiprA mutant strain was not observed in liquid medium, as its growth curve in broth was equivalent to that of isogenic control strains (see Fig. S8 in the supplemental material). Thus, it appears that the loss of both the oxyR and iprA genes can result in a cellular effect that causes a defect for normal colony formation on solid medium. We found that the presence of catalase protein supplemented into the agar medium reversed this phenotype, indicating that enhanced oxidative stress as present in solid medium plays a role in this observation (Fig. 7C).
RNA-Seq analysis of S. Typhimurium ΔiprA mutant.
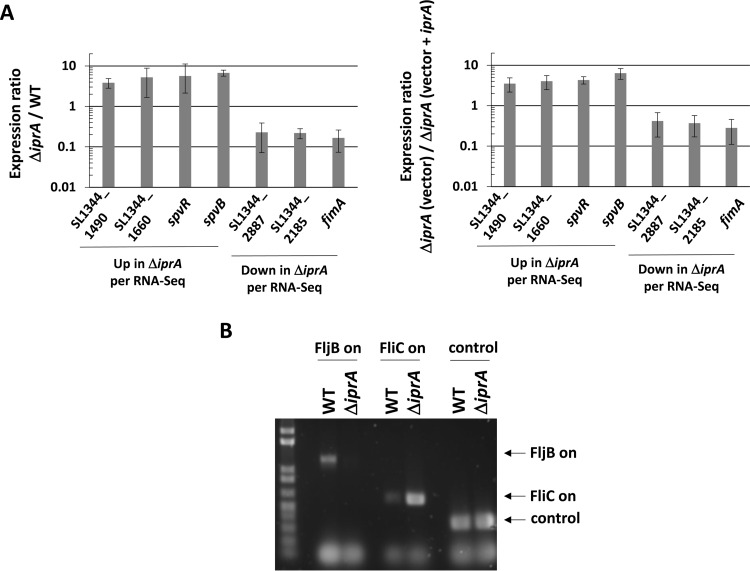
It is possible that any phenotypes observed in a ΔiprA mutant (including the increase in oxidative stress resistance) are the result of changes in gene expression due to loss of the iprA gene. To identify genes altered in expression in the S. Typhimurium ΔiprA mutant, we performed RNA-Seq analysis using total RNA obtained from S. Typhimurium χ3339 ΔiprA compared to the isogenic WT strain in both log and stationary phase. Quality control parameters from the analysis are available in Table S2 in the supplemental material. We identified 82 and 56 genes that were altered in expression in the ΔiprA mutant compared to the WT in log and stationary phases, respectively (Table 2; see also the supplemental material for full data set). Many of these hits were found to reside in the same operon or functional gene set, and several hits were observed in both phases of growth (Table 2). We used RT-qPCR to successfully confirm gene hits from the RNA-Seq analysis, and we used complementation via a plasmid copy of the iprA gene to restore WT gene expression phenotypes in the ΔiprA mutant strain (Fig. 8A).
TABLE 2.
Genes altered in expression in Salmonella Typhimurium χ3339 ΔiprA
| Growth phase and gene | Gene name and/or product | Fold differencea | Up- or downregulation | P value |
|---|---|---|---|---|
| Log phase | ||||
| Metabolism genes | ||||
| SL1344_0068 | carB; carbamoyl-PO4 synthase | 2.42 | Up | <1.0E−300 |
| SL1344_0114 | leuL; leu operon leader | 2.9 | Down | 0.0492303 |
| SL1344_0355 | Terminal oxidase subunit | 2.12 | Up | 2.38E−146 |
| SL1344_0456 | glnK; nitrogen regulatory protein | 2.9 | Down | 0.0492303 |
| SL1344_0548 | Bactoprenol glucose transferase | 2.74 | Down | 1.25E−09 |
| SL1344_0549 | Bactoprenol glucose transferase | 3.09 | Down | 7.95E−05 |
| SL1344_0743 | oadG2; oxaloacetate decarboxylase | 2.76 | Down | 0.0215319 |
| SL1344_1238 | argD; acetylornithine aminotransferase | 2.09 | Up | 2.29E−49 |
| SL1344_1488 | glgX; glycogen protein | 2.08 | Up | 8.93E−288 |
| SL1344_2017 | pduD; diol dehydratase | 2.01 | Up | 3.53E−27 |
| SL1344_2081 | cpsG; phosphomannomutase | 3.43 | Up | 1.19E−08 |
| SL1344_2900 | Decarboxylase | 2.48 | Up | 0.0105352 |
| SL1344_4390 | pyrI; aspartate carbamoyltransferase | 4.95 | Up | <1.0E−300 |
| SL1344_4391 | pyrB; aspartate carbamoyltransferase | 4.12 | Up | <1.0E−300 |
| PTS transport system genes | ||||
| SL1344_4466 | PTS transporter | 3.15 | Up | 1.31E−16 |
| SL1344_4467 | PTS transporter | 2.82 | Up | 5.13E−17 |
| SL1344_4468 | PTS transporter | 3.06 | Up | 5.59E−36 |
| SL1344_4469 | PTS transporter | 2.38 | Up | 3.84E−45 |
| SL1344_4470 | Putative sugar isomerase | 2.49 | Up | 2.50E−57 |
| SL1344_4471 | Putative sugar isomerase | 2.14 | Up | 5.10E−58 |
| Fimbrial genes | ||||
| SL1344_0536 | fimA; type 1 fimbrial protein | 2.24 | Down | 2.62E−253 |
| SL1344_0545 | fimW; fimbrial protein | 2.11 | Down | 3.63E−15 |
| SL1344_2128 | stcB; fimbrial chaperone | 2.8 | Down | 2.84E−05 |
| SL1344_2934 | Putative fimbrial subunit | 3.92 | Down | 0.000383 |
| Virulence genes | ||||
| SL1344_1026 | pipA; hypothetical protein | 2.33 | Down | 3.52E−18 |
| P1_0066 | spvR; plasmid virulence | 2.13 | Up | 2.01E−10 |
| P1_0067 | spvA; plasmid virulence | 2.31 | Up | 1.23E−261 |
| P1_0068 | spvB; plasmid virulence | 2.84 | Up | 7.31E−279 |
| P1_0069 | spvC; plasmid virulence | 3.6 | Up | 1.12E−63 |
| P1_0070 | spvD; plasmid virulence | 3.74 | Up | 2.48E−34 |
| Phage genes | ||||
| SL1344_0949 | Bacteriophage protein | 2.52 | Down | 0.0001826 |
| SL1344_1932 | Putative phage protein | 2.76 | Up | 0.0286893 |
| SL1344_1959 | Phage protein | 2.15 | Down | 1.99E−07 |
| SL1344_2673 | Putative DNA invertase | 5.55 | Down | 8.40E−05 |
| SL1344_2680 | Putative phage base protein | 3.32 | Down | 0.0369301 |
| SL1344_2682 | Putative phage tail protein | 2.23 | Down | 0.0201203 |
| SL1344_2683 | Phage lysis protein | 3.22 | Down | 0.0163595 |
| SL1344_2693 | Terminase subunit | 2.32 | Down | 0.0004644 |
| SL1344_2703 | Bacteriophage protein | 2.21 | Down | 0.0252955 |
| SL1344_4136 | Putative phage protein | 2.87 | Up | 4.28E−05 |
| SL1344_4141 | gtrA; phage glycosyltransferase | 2.17 | Down | 0.000599 |
| SL1344_4153 | Endolysin | 2.13 | Down | 0.002344 |
| SL1344_4154 | Bacteriophage protein | 2.51 | Down | 0.0062789 |
| Other genes | ||||
| SL1344_0044 | rpsT; 30S ribosomal protein S20 | 2.24 | Down | 3.15E−253 |
| SL1344_0490 | Secreted protein | 2.19 | Down | 1.49E−12 |
| SL1344_0705 | ABC transporter protein | 2.72 | Up | 0.0166391 |
| SL1344_1179 | envF; lipoprotein | 2.13 | Up | 0.0152697 |
| SL1344_1422 | ABC transporter protein | 2.05 | Up | 1.59E−29 |
| SL1344_1489 | Putative hydrolase | 2.16 | Up | 1.11E−02 |
| SL1344_1490 | Putative hydrolase | 2.15 | Up | 2.61E−256 |
| SL1344_1888 | fliC; flagellin 1 | 5.48 | Up | <1.0E−300 |
| SL1344_2755 | fljA; repressor of flagellin 2 | 143.1 | Down | <1.0E−300 |
| SL1344_2756 | flaG; flagellin 2 | 16.88 | Down | <1.0E−300 |
| SL1344_2757 | hin; DNA invertase | 6.08 | Down | <1.0E−300 |
| SL1344_2943 | ygcZ; glucarate transporter | 2.08 | Down | 1.14E−81 |
| SL1344_3183 | rpsU; 30S ribosomal protein S21 | 2.04 | Down | 3.55E−213 |
| SL1344_3807 | rnpA; RNase P component | 2.14 | Down | 3.43E−68 |
| SL1344_4052 | Inner membrane protein | 2.59 | Down | 0.0034985 |
| Genes encoding hypothetical/putative proteins | ||||
| SL1344_0291 | Hypothetical protein | 2.58 | Down | 0.0001506 |
| SL1344_0354 | Hypothetical protein | 2.39 | Up | 2.29E−42 |
| SL1344_0544 | Hypothetical protein | 2.56 | Down | 8.51E−05 |
| SL1344_0575 | Hypothetical protein | 4.55 | Up | 0.0470614 |
| SL1344_0736 | Hypothetical protein | 2.21 | Up | <1.0E−300 |
| SL1344_0815 | Hypothetical protein | 2.42 | Down | 0.0323411 |
| SL1344_1059 | Hypothetical protein | 2.57 | Up | <1.0E−300 |
| SL1344_1297 | Hypothetical protein | 2.29 | Down | 1.75E−05 |
| SL1344_1443 | Hypothetical protein | 2.47 | Up | 8.92E−65 |
| SL1344_1491 | Hypothetical protein | 2.04 | Up | 4.57E−44 |
| SL1344_1516 | Putative lipoprotein | 2.15 | Down | 0.0099574 |
| SL1344_1519 | Putative regulatory protein | 2.09 | Up | 5.01E−36 |
| SL1344_1659 | Hypothetical protein | 3.36 | Up | 1.11E−02 |
| SL1344_1660 | Hypothetical protein | 3.37 | Up | 1.83E−234 |
| SL1344_1661 | Hypothetical protein | 4.67 | Up | 4.52E−205 |
| SL1344_1830 | Pseudogene | 2.25 | Down | 0.0003631 |
| SL1344_2116 | Hypothetical protein | 2.62 | Down | 0.0050997 |
| SL1344_2185 | Hypothetical protein | 2.17 | Down | 9.98E−05 |
| SL1344_2343 | Putative DNA-binding protein | 4.53 | Down | 0.0043958 |
| SL1344_2345 | Putative lipoprotein | 2.67 | Down | 0.0002584 |
| SL1344_2887 | Hypothetical protein | 2.94 | Down | 3.65E−10 |
| SL1344_3653 | Hypothetical protein | 2.08 | Up | 3.17E−42 |
| SL1344_3910 | Hypothetical protein | 2.1 | Down | 0.000527 |
| SL1344_4257 | Hypothetical protein | 3.02 | Down | 5.05E−06 |
| Stationary phase | ||||
| Metabolism genes | ||||
| SL1344_0743 | oadG2; oxaloacetate decarboxylase | 3.05 | Down | 0.001348 |
| Ethanolamine utilization genes | ||||
| SL1344_2425 | eutJ; ethanolamine utilization | 2.46 | Up | 1.21E−18 |
| SL1344_2426 | eutE; aldehyde dehydrogenase | 2.24 | Up | 4.00E−35 |
| SL1344_2427 | eutN; ethanolamine utilization | 2.35 | Up | 9.54E−10 |
| SL1344_2429 | eutD; phosphate acyltransferase | 2.22 | Up | 3.40E−11 |
| SL1344_2430 | Cobalamin adenosyltransferase | 2.17 | Up | 1.27E−09 |
| SL1344_2431 | eutQ; ethanolamine utilization | 2.44 | Up | 7.97E−18 |
| SL1344_2432 | eutP; ethanolamine utilization | 3.7 | Up | 1.02E−16 |
| SL1344_2433 | eutS; ethanolamine utilization | 2.13 | Up | 0.0076109 |
| Fimbrial genes | ||||
| SL1344_0199 | stfE; fimbrial subunit StfE | 2.12 | Down | 0.0395983 |
| SL1344_0334 | stbB; fimbrial chaperone | 5.08 | Down | 0.0214963 |
| SL1344_0335 | stbA; fimbrial protein | 3.54 | Up | 0.010675 |
| SL1344_0536 | fimA; type 1 fimbrial protein | 4.77 | Down | <1.0E−300 |
| SL1344_0537 | fimI; pilin protein | 3.84 | Down | 3.81E−46 |
| SL1344_0538 | fimC; fimbrial chaperone | 3.6 | Down | 3.04E−33 |
| SL1344_0539 | fimD; usher protein | 2.64 | Down | 3.44E−36 |
| SL1344_0540 | fimH; fimbrial protein | 2.56 | Down | 6.68E−20 |
| SL1344_0542 | fimZ; transcription regulator | 3.59 | Down | 4.10E−10 |
| SL1344_0545 | fimW; fimbrial protein | 2.18 | Down | 2.66E−07 |
| SL1344_2934 | Putative fimbrial subunit | 7.11 | Down | 0.002188 |
| SL1344_3008 | stdA; fimbrial protein | 3.37 | Down | 1.39E−13 |
| Phage genes | ||||
| SL1344_1963 | Bacteriophage protein | 2.2 | Down | 6.97E−05 |
| SL1344_2213 | Bacteriophage holin | 2.58 | Up | 0.0242426 |
| SL1344_4140 | gtrB; phage glycosyltransferase | 2.31 | Down | 6.40E−06 |
| Other genes | ||||
| SL1344_0044 | rpsT; 30S ribosomal protein S20 | 2.14 | Down | 6.00E−177 |
| SL1344_0275 | sciN; lipoprotein | 8.86 | Up | 0.0004054 |
| SL1344_0278 | sciQ; putative membrane protein | 2.67 | Up | 0.0290844 |
| SL1344_0490 | Secreted protein | 3.87 | Down | 1.28E−07 |
| SL1344_0577 | fepE; ferric enterobactin | 2.28 | Down | 2.55E−16 |
| SL1344_0583 | entC; isochorismate synthase | 2.12 | Down | 1.62E−05 |
| SL1344_0705 | ABC transporter protein | 2.59 | Down | 0.0060809 |
| SL1344_1285 | Ferredoxin-like protein | 2.23 | Up | 0.009504 |
| SL1344_2755 | fljA; repressor of flagellin 2 | 18.72 | Down | 3.31E−119 |
| SL1344_2756 | flaG; flagellin 2 | 17.92 | Down | <1.0E−300 |
| SL1344_1888 | fliC; flagellin 1 | 3.62 | Up | <1.0E−300 |
| SL1344_3144 | Membrane transport protein | 2.16 | Down | 0.0002762 |
| SL1344_4052 | Inner membrane protein | 3.22 | Down | 0.0088688 |
| SL1344_4468 | PTS transporter | 2.14 | Down | 9.37E−05 |
| Genes encoding hypothetical/putative proteins | ||||
| SL1344_0011 | Hypothetical protein | 3.92 | Down | 0.0004655 |
| SL1344_0017 | Hypothetical protein | 2.71 | Down | 0.0334128 |
| SL1344_0032 | Hypothetical protein | 7.11 | Down | 0.002188 |
| SL1344_0100 | Hypothetical protein | 2.31 | Down | 0.0192471 |
| SL1344_0544 | Hypothetical protein | 4.32 | Down | 0.0040759 |
| SL1344_0637 | Putative hydrolase | 13.78 | Up | 0.0009813 |
| SL1344_0638 | Putative hydrolase | 2.45 | Up | 1.19E−05 |
| SL1344_0702 | Putative glycosyltransferase | 5.08 | Down | 0.0214963 |
| SL1344_0706 | Putative glycosyltransferase | 16.76 | Down | 1.57E−08 |
| SL1344_0785 | Putative inner membrane protein | 6.6 | Down | 2.60E−07 |
| SL1344_0814 | Hypothetical protein | 3.05 | Down | 0.0004317 |
| SL1344_0815 | Hypothetical protein | 3.2 | Up | 0.0491571 |
| SL1344_1516 | Putative lipoprotein | 2.27 | Down | 0.0131079 |
| SL1344_2185 | Hypothetical protein | 3.16 | Down | 6.42E−06 |
| SL1344_2343 | Putative DNA-binding protein | 3.05 | Down | 0.0139479 |
| SL1344_2379 | Hypothetical protein | 2.12 | Down | 1.93E−20 |
| SL1344_2738 | Putative hexulose-6-phosphate synthase | 2.31 | Down | 2.46E−11 |
| SL1344_4402 | Hypothetical protein | 3.81 | Down | 2.06E−07 |
Fold difference is the fold amount that gene expression is up- or downregulated in the ΔiprA mutant compared to the WT.
FIG 8.
Confirmation of RNA-Seq results. (A) RT-qPCR analysis was performed using total RNA harvested from S. Typhimurium χ3339 WT and ΔiprA mutant strains (graph on left) and from χ3339 ΔiprA containing either vector or vector plus iprA (graph on right). The qPCR was performed using primers hybridizing to the indicated genes identified from the RNA-Seq results shown in Table 2. The qPCR product levels were normalized to the level of the lpxC gene, and a ratio of each gene level for the ΔiprA mutant to the WT (left) or the ΔiprA mutant (vector) to the ΔiprA mutant (vector plus iprA) (right) was calculated to give a fold difference in expression between the corresponding samples. Differences in expression between the samples for each gene were significant at a P value of <0.05, using a t test to compare WT and ΔiprA mutant samples. (B) DNA analysis of flagellin phase switch in S. Typhimurium χ3339 ΔiprA compared to the WT. PCR products obtained from chromosomal DNA were analyzed to confirm the flagellin phase switch indicated by the RNA-Seq data. Primers that amplify PCR products indicative of either the FljB on (flagellin type 2) or FliC on (flagellin type 1) orientation of the fljA-fljB-hin locus were used to amplify the corresponding DNA fragments from the indicated strains, and the products were run on an agarose gel and stained for fluorescence. In addition, a control product not affected by phase switch was also amplified and run on the gel. The results of the DNA analysis confirm the switch to FliC on in the ΔiprA mutant strain.
Genes involved in type I fimbria formation, including fimA, fimW, fimI, fimC, fimD, fimH, fimZ, and fimW, were found to be decreased in expression in the ΔiprA mutant in either log or stationary phase, or both (Table 2). We also found other fimbrial genes (stcB, SL1344_2934, stfE, stbA, stbB, and stdA) to be altered in expression as well in the ΔiprA mutant strain (Table 2). The PTS transport system (found in genes SL1344_4466 through SL1344_4471) is increased in expression in the ΔiprA mutant in log phase (with a single gene hit from this system, SL1344_4468, being decreased in expression in stationary phase) (Table 2). In log phase, the plasmid-borne spvR and spvABCD genes involved in S. Typhimurium virulence were found to be increased in expression in the ΔiprA mutant (Table 2). In stationary phase, the large ethanolamine utilization gene operon (genes SL1344_2425 to SL1344_2433) was increased in expression in the ΔiprA mutant strain (Table 2). Many genes associated with phage were altered in expression in the ΔiprA mutant (SL1344_0949, SL1344_1932, SL1344_1959 to SL1344_1963, SL1344_2213, SL1344_2680, SL1344_2681 to SL1344_2683, SL1344_1703, SL1344_4136 to SL1344_4140, SL1344_4141 to SL1344_4153, and SL1344_4154) (Table 2). A large number of genes (48 [34% of the total hits]) encoding hypothetical or putative proteins were altered in expression in the ΔiprA mutant, including operons SL1344_1488 to SL1344_1491, SL1344_1659 to SL1344_1661, and SL1344_0637 and SL1344_0638 (Table 2).
Among the strongly altered genes (those with >5-fold change in expression) in both log and stationary phase were fliC, fljA, fljB (aka flaG), and hin, involved in the S. Typhimurium flagellin phase switch (between flagellin type 2 and flagellin type 1). The WT background strain χ3339 expresses flagellin type 2 (FljB/FlaG), and the RNA-Seq data indicated a phase switch to flagellin type 1 (FliC) in the ΔiprA mutant (Table 2). This phase switch was confirmed using protein gel analysis (data not shown) and chromosomal DNA PCR analysis (Fig. 8B), indicating correspondence to RNA-Seq data and that the phase switch occurred via the “textbook” mechanism of Hin-mediated DNA inversion at the fljAB promoter and not via some other uncharacterized mechanism associated with the ΔiprA mutation. However, when we attempted to complement this phenotype (back to the flagellin type 2 phenotype) using a plasmid-borne copy of the WT iprA gene, the complementation did not occur (data not shown). We then screened a panel of χ3339 background strains in our lab containing a range of different mutations, and we found a mixture of flagellin phase types across these strains, indicating that the phase switch appeared to occur randomly (data not shown). Thus, the flagellin phase switch observed in the ΔiprA mutant is likely a manifestation of stochastic events, although it is worth noting that the specific signals that regulate the timing or frequency of phase switch mechanisms are currently not well characterized (44).
We did not observe changes in the expression of previously characterized oxidative stress genes in the ΔiprA mutant within the cutoff values of our RNA-Seq analysis. However, the expression of the katE gene was increased 1.92-fold (just below the cutoff of 2-fold) in the ΔiprA mutant in log phase, with a statistically significant P value (data not shown). This is consistent with the phenotype of increased oxidative stress resistance in the S. Typhimurium ΔiprA mutant and its partial dependence on the katE gene, and it suggests that an increase in katE gene expression might play a role in the ΔiprA mutant phenotype (although the different aspects of this phenomenon are discussed below). The other genes analyzed in Fig. 6 (katG, katN, rpoS, dps, ahpC, and tsaA) did not display an expression change of >1.5-fold difference in the ΔiprA mutant compared to the WT (data not shown).
DISCUSSION
Our work has found that the protein encoded by the bacterial iprA gene is highly conserved, and that deletion of the iprA gene results in a dramatic increase in resistance to oxidative stress. How does the IprA protein function in relation to its role in oxidative stress resistance? A useful approach for framing such a discussion is to ask whether IprA is working at the transcriptional level (controlling transcription of relevant genes), at the posttranscriptional level (the IprA protein has some sort of enzymatic or protein interaction activity related to oxidative stress resistance), or at both levels. To address this question (and since the iprA gene was previously uncharacterized), we performed RNA-Seq analysis comparing the S. Typhimurium ΔiprA mutant and WT strains. We did not observe changes in the expression of previously characterized oxidative stress resistance genes beyond the 2-fold cutoff level of the analysis, but we did measure an increase in katE gene expression at a 1.92-fold greater level in the ΔiprA mutant strain than in the WT. This is consistent with the role of katE observed in the ΔiprA mutant phenotype but is likely not the whole story, since the effect of the katE gene was partial, and the rpoS gene appears to control other functions involved in the ΔiprA mutant phenotype beyond katE. Thus, the activity of IprA in relation to oxidative stress resistance appears to involve both KatE and another RpoS-controlled factor. IprA activity might involve protein-protein interactions between IprA and these factors that act at the posttranscriptional level to lower oxidative stress resistance. It is also possible that IprA acts at both the transcriptional level (i.e., is involved in transcriptional control pathways) and the posttranscriptional level (altering protein activity). Moreover, it is possible that some of the genes identified as altered in expression in the ΔiprA mutant are involved in oxidative stress resistance but have not been previously characterized as such. Another possibility is that the IprA protein is a target for reactive oxygen species in the cell, such that the interaction of the oxygen species with IprA is lethally toxic to the cells, and the removal of IprA results in increased survival in oxidative stress (see below for further discussion).
In our RNA-Seq analysis, we found that the deletion of S. Typhimurium iprA resulted in the altered expression of 82 and 56 genes in log and stationary phase, respectively, and these results were verified using RT-qPCR and complementation analysis. This indicates that IprA is involved in regulation pathways that control gene expression. We currently do not know if IprA participates in gene regulation via binding to DNA directly, via interactions with RNA, or via interactions with proteins that serve to regulate downstream gene expression or signaling pathways. However, the IprA protein contains a predicted winged helix-turn-helix (wHTH) DNA-binding domain in the C terminus, and deletion of the C-terminal 10 amino acids abolished WT IprA activity. Thus, IprA could use the wHTH to bind DNA as part of its action, and future work will be targeted at testing this activity. Based on deletion analysis, the predicted CAP effector binding domain of IprA does not appear to be involved with the role of IprA in oxidative stress resistance.
The genes altered in expression in the S. Typhimurium ΔiprA mutant are distributed across the S. Typhimurium genome and belong to varied functional groups, including virulence, fimbria production, PTS transport, ethanolamine utilization, various phage proteins, and many genes encoding hypothetical or putative proteins. We currently do not know if or how these genes potentially participate in the role of IprA in oxidative stress resistance. It is likely that IprA is involved in other functions beyond oxidative stress resistance, and further study based on the gene hits identified here may allow other IprA functions to be discovered. In regard to a possible link between IprA and the S. Typhimurium response to rotating-wall-vessel conditions, we found that the deletion of iprA had no effect on S. Typhimurium phenotypes observed in this environment (data not shown).
We found that the deletion of both oxyR and iprA resulted in an S. Typhimurium strain that displayed a severe growth defect on solid media. This is notable since both the oxyR and iprA genes participate in oxidative stress resistance, with OxyR controlling oxidative stress resistance genes and IprA serving to downregulate resistance to oxidative stress. It could be that the removal of both genes in the same strain causes genes/proteins associated with OxyR and IprA to act in an abnormal fashion, such that their additive or synergistic effects cause a growth defect. The effects of OxyR and IprA may act to balance genes/proteins involved in oxidative stress resistance, and when both OxyR and IprA are absent, this balance is abolished, and defective gene expression and/or protein activity cause growth problems. We found that supplementation of the agar medium with catalase protein served to reverse the phenotype, which means that the ΔoxyR ΔiprA mutant strain is hypersensitive to enhanced oxidative stress on solid medium. This is an interesting observation since the ΔiprA mutant causes increased oxidative stress resistance in broth, while the ΔiprA mutation in the presence of the ΔoxyR mutation causes decreased resistance on plates (suggesting additive effects or interaction of the two resistance schemes under certain conditions). Further study is needed to understand the nature of this phenomenon, but this observation serves as another piece of evidence of the involvement of IprA in the oxidative stress resistance program in bacteria.
Why would evolution select for a bacterial gene that serves to dramatically decrease resistance to oxidative stress resistance? This suggests that insufficient negative regulation of oxidative stress resistance is detrimental to fitness, possibly due to the use of excess metabolic energy in the expression of these functions. In addition, it is established that certain oxidative stress resistance mechanisms can be potentially mutagenic (45–48). Therefore, the evolution of regulation schemes to inhibit these mechanisms could have served to keep resulting mutations “in check,” which would likely be beneficial in the long term. Another possibility is that for some reason, the IprA protein itself is a target for oxidative stress, such that when hit with oxidative damage, it is highly harmful or toxic to the cell (and deletion of the gene removes this target). Future work will be directed at further understanding the selective benefits of the strong downregulation of oxidative stress resistance involving IprA.
Finally, this work serves to highlight the pool of currently uncharacterized genes that are conserved across bacterial genomes and that play as-of-yet-undiscovered roles in bacterial biology. These genes represent an “untapped pool” of potentially useful genes that might play a role in bacterial engineering, vaccine strain design, and other applications. Many of these untapped genes are highly conserved across different bacteria, which strongly suggests evolutionary selection for important functions. In addition, learning the functions of these uncharacterized yet highly conserved genes will allow greater understanding of bacterial growth, survival, and regulation across genera, including those containing pathogenic species.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Department of Biology, the Graduate School of Arts and Sciences, and the Dennis M. Cook Endowed Gregor Mendel Chair in Genetics (held by D.W.), Villanova University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00144-16.
REFERENCES
- 1.Handfield M, Levesque RC. 1999. Strategies for isolation of in vivo expressed genes from bacteria. FEMS Microbiol Rev 23:69–91. doi: 10.1111/j.1574-6976.1999.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 2.Kroger C, Colgan A, Srikumar S, Handler K, Sivasankaran SK, Hammarlof DL, Canals R, Grissom JE, Conway T, Hokamp K, Hinton JC. 2013. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe 14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Lévi-Meyrueis C, Monteil V, Sismeiro O, Dillies MA, Monot M, Jagla B, Coppee JY, Dupuy B, Norel F. 2014. Expanding the RpoS/σS-network by RNA sequencing and identification of σS-controlled small RNAs in Salmonella. PLoS One 9:e96918. doi: 10.1371/journal.pone.0096918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucchini S, Liu H, Jin Q, Hinton JC, Yu J. 2005. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun 73:88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh A, Charles RC, Sharmeen N, Rollins SM, Harris JB, Bhuiyan MS, Arifuzzaman M, Khanam F, Bukka A, Kalsy A, Porwollik S, Leung DT, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. 2011. In vivo expression of Salmonella enterica serotype Typhi genes in the blood of patients with typhoid fever in Bangladesh. PLoS Negl Trop Dis 5:e1419. doi: 10.1371/journal.pntd.0001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JW, Ott CM, Honer zu Bentrup K, Ramamurthy R, Quick L, Porwollik S, Cheng P, McClelland M, Tsaprailis G, Radabaugh T, Hunt A, Fernandez D, Richter E, Shah M, Kilcoyne M, Joshi L, Nelman-Gonzalez M, Hing S, Parra M, Dumars P, Norwood K, Bober R, Devich J, Ruggles A, Goulart C, Rupert M, Stodieck L, Stafford P, Catella L, Schurr MJ, Buchanan K, Morici L, McCracken J, Allen P, Baker-Coleman C, Hammond T, Vogel J, Nelson R, Pierson DL, Stefanyshyn-Piper HM, Nickerson CA. 2007. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci U S A 104:16299–16304. doi: 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JW, Ramamurthy R, Porwollik S, McClelland M, Hammond T, Allen P, Ott CM, Pierson DL, Nickerson CA. 2002. Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc Natl Acad Sci U S A 99:13807–13812. doi: 10.1073/pnas.212387899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon H, McDermott JE, Porwollik S, McClelland M, Heffron F. 2009. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog 5:e1000306. doi: 10.1371/journal.ppat.1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickerson CA, Ott CM, Mister SJ, Morrow BJ, Burns-Keliher L, Pierson DL. 2000. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun 68:3147–3152. doi: 10.1128/IAI.68.6.3147-3152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JW, Ott CM, Quick L, Davis R, Honer zu Bentrup K, Crabbe A, Richter E, Sarker S, Barrila J, Porwollik S, Cheng P, McClelland M, Tsaprailis G, Radabaugh T, Hunt A, Shah M, Nelman-Gonzalez M, Hing S, Parra M, Dumars P, Norwood K, Bober R, Devich J, Ruggles A, CdeBaca A, Narayan S, Benjamin J, Goulart C, Rupert M, Catella L, Schurr MJ, Buchanan K, Morici L, McCracken J, Porter MD, Pierson DL, Smith SM, Mergeay M, Leys N, Stefanyshyn-Piper HM, Gorie D, Nickerson CA. 2008. Media ion composition controls regulatory and virulence response of Salmonella in spaceflight. PLoS One 3:e3923. doi: 10.1371/journal.pone.0003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 12.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, Hinton JC, Van Immerseel F. 2006. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol 72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold MF, Caro-Hernandez P, Tan K, Runti G, Wehmeier S, Scocchi M, Doerrler WT, Walker GC, Ferguson GP. 2014. Enteric YaiW is a surface-exposed outer membrane lipoprotein that affects sensitivity to an antimicrobial peptide. J Bacteriol 196:436–444. doi: 10.1128/JB.01179-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quick LN, Shah A, Wilson JW. 2010. A series of vectors with alternative antibiotic resistance markers for use in lambda Red recombination. J Microbiol Biotechnol 20:666–669. doi: 10.4014/jmb.0909.09045. [DOI] [PubMed] [Google Scholar]
- 16.Maloy S. 1990. Experimental techniques in bacterial genetics. Jones and Bartlett Publishers, Boston, MA. [Google Scholar]
- 17.Williams JA, Luke J, Hodgson C. 2009. Strain engineering by genome mass transfer: efficient chromosomal trait transfer method utilizing donor genomic DNA and recipient recombineering hosts. Mol Biotechnol 43:41–51. doi: 10.1007/s12033-009-9177-5. [DOI] [PubMed] [Google Scholar]
- 18.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic Local Alignment Search Tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JW, Nickerson CA. 2006. A new experimental approach for studying bacterial genomic island evolution identifies island genes with bacterial host-specific expression patterns. BMC Evol Biol 6:2. doi: 10.1186/1471-2148-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennings ME, Quick LN, Soni A, Davis RR, Crosby K, Ott CM, Nickerson CA, Wilson JW. 2011. Characterization of the Salmonella enterica serovar Typhimurium ydcI gene, which encodes a conserved DNA binding protein required for full acid stress resistance. J Bacteriol 193:2208–2217. doi: 10.1128/JB.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon L, Shah A, Hannagan S, Wilson JW. 2014. Bacterial genus-specific tolerance for YdcI expression. Curr Microbiol 69:640–648. doi: 10.1007/s00284-014-0631-7. [DOI] [PubMed] [Google Scholar]
- 24.Soni A, O'Sullivan L, Quick LN, Ott CM, Nickerson CA, Wilson JW. 2014. Conservation of the low-shear modeled microgravity response in Enterobacteriaceae and analysis of the trp genes in this response. Open Microbiol J 8:51–58. doi: 10.2174/1874285801408010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson JW, Ott CM, Ramamurthy R, Porwollik S, McClelland M, Pierson DL, Nickerson CA. 2002. Low-shear modeled microgravity alters the Salmonella enterica serovar Typhimurium stress response in an RpoS-independent manner. Appl Environ Microbiol 68:5408–5416. doi: 10.1128/AEM.68.11.5408-5416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogomolnaya LM, Andrews KD, Talamantes M, Maple A, Ragoza Y, Vazquez-Torres A, Andrews-Polymenis H. 2013. The ABC-type efflux pump MacAB protects Salmonella enterica serovar Typhimurium from oxidative stress. mBio 4(6):e00630-13. doi: 10.1128/mBio.00630-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebrard M, Viala JP, Meresse S, Barras F, Aussel L. 2009. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol 191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwase T, Tajima A, Sugimoto S, Okuda K, Hironaka I, Kamata Y, Takada K, Mizunoe Y. 2013. A simple assay for measuring catalase activity: a visual approach. Sci Rep 3:3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson JW, Nickerson CA. 2006. Cloning of a functional Salmonella SPI-1 type III secretion system and development of a method to create mutations and epitope fusions in the cloned genes. J Biotechnol 122:147–160. doi: 10.1016/j.jbiotec.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Aballay A, Yorgey P, Ausubel FM. 2000. Salmonella Typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol 10:1539–1542. doi: 10.1016/S0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- 31.Marsh EK, van den Berg MC, May RC. 2011. A two-gene balance regulates Salmonella Typhimurium tolerance in the nematode Caenorhabditis elegans. PLoS One 6:e16839. doi: 10.1371/journal.pone.0016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu SN, Anriany Y, Grim CJ, Kim S, Chang Z, Joseph SW, Cinar HN. 2013. Identification of virulence properties in Salmonella Typhimurium DT104 using Caenorhabditis elegans. PLoS One 8:e76673. doi: 10.1371/journal.pone.0076673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hommais F, Zghidi-Abouzid O, Oger-Desfeux C, Pineau-Chapelle E, Van Gijsegem F, Nasser W, Reverchon S. 2011. lpxC and yafS are the most suitable internal controls to normalize real time RT-qPCR expression in the phytopathogenic bacteria Dickeya dadantii. PLoS One 6:e20269. doi: 10.1371/journal.pone.0020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eom JS, Kim JS, Jang JI, Kim HG, Bang IS, Park YK. 2012. Effect of iacP mutation on flagellar phase variation in Salmonella enterica serovar Typhimurium strain UK-1. J Bacteriol 194:4332–4341. doi: 10.1128/JB.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karavolos MH, Bulmer DM, Winzer K, Wilson M, Mastroeni P, Williams P, Khan CM. 2008. LuxS affects flagellar phase variation independently of quorum sensing in Salmonella enterica serovar Typhimurium. J Bacteriol 190:769–771. doi: 10.1128/JB.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutsukake K, Nakashima H, Tominaga A, Abo T. 2006. Two DNA invertases contribute to flagellar phase variation in Salmonella enterica serovar Typhimurium strain LT2. J Bacteriol 188:950–957. doi: 10.1128/JB.188.3.950-957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulig PA, Curtiss R III. 1987. Plasmid-associated virulence of Salmonella Typhimurium. Infect Immun 55:2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schellhorn HE. 2014. Elucidating the function of the RpoS regulon. Future Microbiol 9:497–507. doi: 10.2217/fmb.14.9. [DOI] [PubMed] [Google Scholar]
- 40.Calhoun LN, Kwon YM. 2011. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. J Appl Microbiol 110:375–386. doi: 10.1111/j.1365-2672.2010.04890.x. [DOI] [PubMed] [Google Scholar]
- 41.Halsey TA, Vazquez-Torres A, Gravdahl DJ, Fang FC, Libby SJ. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect Immun 72:1155–1158. doi: 10.1128/IAI.72.2.1155-1158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storz G, Tartaglia LA, Ames BN. 1990. The OxyR regulon. Antonie Van Leeuwenhoek 58:157–161. doi: 10.1007/BF00548927. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia LA, Storz G, Ames BN. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol 210:709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 44.Casadesús J, Low DA. 2013. Programmed heterogeneity: epigenetic mechanisms in bacteria. J Biol Chem 288:13929–13935. doi: 10.1074/jbc.R113.472274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konola JT, Sargent KE, Gow JB. 2000. Efficient repair of hydrogen peroxide-induced DNA damage by Escherichia coli requires SOS induction of RecA and RuvA proteins. Mutat Res 459:187–194. doi: 10.1016/S0921-8777(99)00073-7. [DOI] [PubMed] [Google Scholar]
- 46.Merrikh H, Ferrazzoli AE, Bougdour A, Olivier-Mason A, Lovett ST. 2009. A DNA damage response in Escherichia coli involving the alternative sigma factor, RpoS. Proc Natl Acad Sci U S A 106:611–616. doi: 10.1073/pnas.0803665106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prieto AI, Ramos-Morales F, Casadesús J. 2006. Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics 174:575–584. doi: 10.1534/genetics.106.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson AR, Soliven KC, Castor ME, Barnes PD, Libby SJ, Fang FC. 2009. The base excision repair system of Salmonella enterica serovar Typhimurium counteracts DNA damage by host nitric oxide. PLoS Pathog 5:e1000451. doi: 10.1371/journal.ppat.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nickerson CA, Curtiss R III. 1997. Role of sigma factor RpoS in initial stages of Salmonella Typhimurium infection. Infect Immun 65:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terabayashi Y, Juan A, Tamotsu H, Ashimine N, Nakano K, Shimoji M, Shiroma A, Teruya K, Satou K, Hirano T. 2014. First complete genome sequence of Salmonella enterica subsp. enterica serovar Typhimurium strain ATCC 13311 (NCTC 74), a reference strain of multidrug resistance, as achieved by use of PacBio single-molecule real-time technology. Genome Announc 2(5):e00986–14. doi: 10.1128/genomeA.00986-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 52.Lacadena J, Martinez del Pozo A, Mancheno JM, Gasset M, Onaderra M, Gavilanes JG. 1995. Escherichia coli JA221 can suppress the UAG stop signal. Lett Appl Microbiol 21:96–98. doi: 10.1111/j.1472-765X.1995.tb01015.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.