ABSTRACT
The tetrachloroethene (PCE)-respiring bacterium Sulfurospirillum multivorans produces a unique cobamide, namely, norpseudo-B12, which, in comparison to other cobamides, e.g., cobalamin and pseudo-B12, lacks the methyl group in the linker moiety of the nucleotide loop. In this study, the protein SMUL_1544 was shown to be responsible for the formation of the unusual linker moiety, which is most probably derived from ethanolamine-phosphate (EA-P) as the precursor. The product of the SMUL_1544 gene successfully complemented a Salmonella enterica ΔcobD mutant. The cobD gene encodes an l-threonine-O-3-phosphate (l-Thr-P) decarboxylase responsible for the synthesis of (R)-1-aminopropan-2-ol O-2-phosphate (AP-P), required specifically for cobamide biosynthesis. When SMUL_1544 was produced in the heterologous host lacking CobD, norpseudo-B12 was formed, which pointed toward the formation of EA-P rather than AP-P. Guided cobamide biosynthesis experiments with minimal medium supplemented with l-Thr-P supported cobamide biosynthesis in S. enterica producing SMUL_1544 or S. multivorans. Under these conditions, both microorganisms synthesized pseudo-B12. This observation indicated a flexibility in the SMUL_1544 substrate spectrum. From the formation of catalytically active PCE reductive dehalogenase (PceA) in S. multivorans cells producing pseudo-B12, a compatibility of the respiratory enzyme with the cofactor was deduced. This result might indicate a structural flexibility of PceA in cobamide binding. Feeding of l-[3-13C]serine to cultures of S. multivorans resulted in isotope labeling of the norpseudo-B12 linker moiety, which strongly supports the hypothesis of EA-P formation from l-serine-O-phosphate (l-Ser-P) in this organism.
IMPORTANCE The identification of the gene product SMUL_1544 as a putative l-Ser-P decarboxylase involved in norcobamide biosynthesis in S. multivorans adds a novel module to the assembly line of cobamides (complete corrinoids) in prokaryotes. Selected cobamide-containing enzymes (e.g., reductive dehalogenases) showed specificity for their cobamide cofactors. It has recently been proposed that the structure of the linker moiety of norpseudo-B12 and the mode of binding of the EA-P linker to the PceA enzyme reflect the high specificity of the enzyme for its cofactor. Data reported herein do not support this idea. In fact, norpseudo-B12 was functional in the cobamide-dependent methionine biosynthesis of S. enterica, raising questions about the role of norcobamides in nature.
INTRODUCTION
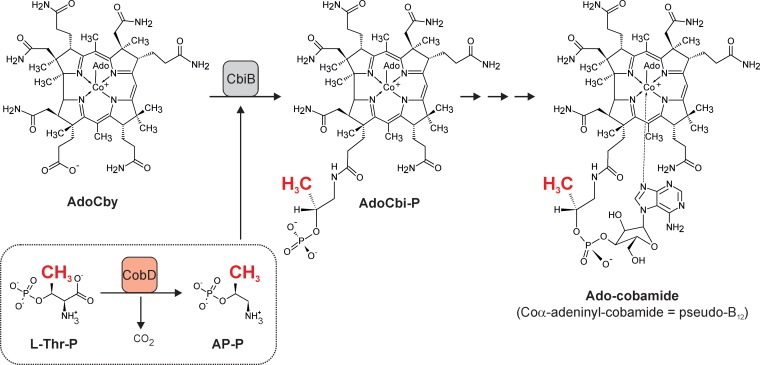
Cobamides, such as cobalamin (also known as Cbl or, in its cyano form, as vitamin B12), are complex natural products exclusively produced by prokaryotes (1). They act as essential cofactors of a defined set of enzymes that are either mutases, eliminases, methyltransferases, or reductive dehalogenases (2, 3). Cobamides share a complex core structure, the corrin ring, which is a unique contracted tetrapyrrole ring system that binds a central cobalt ion (4). When the cobalt ion is in its Co3+ oxidation state, it binds two additional ligands. In natural cobamides, different upper ligands, e.g., a 5′-deoxyadenosyl moiety, a methyl group, or a hydroxyl group, have been identified on the β-face of the corrin ring. Purines (e.g., adenine) or benzimidazoles serve as the lower ligand (on the α-face of the corrin ring) (5). Structural diversity among cobamides is limited to variations in the type of upper and lower ligands and, in one special case, a modification to the linker moiety of the nucleotide loop (6). The lower ligand is part of the nucleotide loop tethering the ligand base to the corrin ring. In almost all cobamides, a linker unit derived from (R)-1-aminopropan-2-ol O-2-phosphate (AP-P) connects the nucleotide to the D ring of the cobalt porphinoid. The assembly of the nucleotide loop is the final part of the multistep cobamide biosynthesis (1). In Salmonella enterica serovar Typhimurium strain LT2, two enzymes are responsible for the synthesis of AP-P from l-threonine (l-Thr). The S. enterica pduX gene encodes the l-Thr kinase (PduX, EC 2.7.1.177), involved in cobamide biosynthesis (7); PduX generates l-threonine-O-3-phosphate (l-Thr-P) from l-Thr at the expense of ATP. In the second step, l-Thr-P is decarboxylated by the l-threonine-O-3-phosphate decarboxylase (CobD, EC 4.1.1.81), yielding AP-P (Fig. 1) (8). Finally, the adenosylcobinamide-phosphate (AdoCbi-P) synthase (CbiB, EC 6.3.1.10) combines AP-P and adenosylcobyric acid (AdoCby) to yield adenosylcobinamide-phosphate (AdoCbi-P) (9).
FIG 1.
Schematic representation of the generation and incorporation of the cobamide linker moiety in S. enterica. The methyl group at position 176 that is absent in norcobamides but present in the cobamide structure is highlighted in red. l-Thr-P, l-threonine-O-3-phosphate; AP-P, (R)-1-aminopropan-2-ol O-2-phosphate; AdoCby, adenosylcobyric acid; AdoCbi-P, adenosylcobinamide-phosphate.
CobD homologues are ubiquitously present in the genomes of cobamide-producing prokaryotes (10). The CobD decarboxylase is a pyridoxal-5′-phosphate (PLP)-dependent enzyme and shows structural similarities to PLP-dependent aminotransferases (11). The cobD gene product was shown to be required for the synthesis of cobamides, such as pseudo-B12 (Coα-adeninyl-cobamide; Fig. 1), in S. enterica. The growth defect caused by the absence of CobD was corrected by the addition of 1-aminopropan-2-ol (AP) to the culture medium (8, 12). AP appeared to be phosphorylated by the cells and used for cobamide biosynthesis.
The occurrence of an alternative linker unit in cobamides has been reported only for norpseudo-B12 (Coα-adeninyl-176-norcobamide), produced by the Gram-negative epsilonproteobacterium Sulfurospirillum multivorans (6). In comparison to pseudo-B12 (Fig. 1), norpseudo-B12 lacks a methyl group at position 176 in the linker moiety. It was assumed that in this special case ethanolamine-O-phosphate (EA-P) rather than AP-P was used as the precursor. The unusual norpseudo-B12 is utilized as a cofactor in the tetrachloroethene (PCE) reductive dehalogenase (PceA) in S. multivorans (13). The PceA enzyme functions as the terminal reductase in the organohalide respiratory metabolism of this anaerobe (14). Recently, the three-dimensional crystal structure of the PceA enzyme was solved and the mode of norpseudo-B12 binding was reported (15). In the structure, the norcobamide cofactor is bound deeply inside PceA in the base-off conformation, which means that the adeninyl moiety does not form a coordination bond with the cobalt ion in the center of the corrin ring. Notably, the lower ligand base is not tightly enclosed by the protein environment, and in fact, it is accessible to solvent in a cavity of the PceA structure. In contrast, the adjoining linker moiety is completely packed by the protein. The backbone of a histidine residue (His357) in the PceA structure is located near the carbon atom at position 176 in the linker moiety (15). Based on this observation, we surmised that the PceA structure might favor the binding of norcobamides rather than AP-P-containing cobamides. The methyl group at position 176 in the AP-P linker moiety of cobamides may hinder binding to the site.
The availability of the S. multivorans genome sequence (16) provided new impetus to efforts aimed at understanding why this bacterium makes norpseudo-B12 rather than pseudo-B12. A single large gene cluster was identified in the organohalide respiration gene region, which encodes a complete set of cobamide biosynthesis proteins. The production of the respective gene products appeared to be triggered by the availability of PCE, which highlights the close interconnection between cobamide production and PCE respiration in this organism (17). The second gene (locus tag, SMUL_1544) of the cobamide biosynthesis gene cluster in S. multivorans showed similarity to the cobD gene of Salmonella enterica (22% sequence identity). In the study presented here, the role of the SMUL_1544 protein in norpseudo-B12 biosynthesis in S. multivorans was examined. Its putative function as a decarboxylase specifically required for norcobamide formation was investigated using an S. enterica ΔcobD mutant strain. To prove that the SMUL_1544 protein is specific for the synthesis of norcobamide, the S. enterica ΔcobD strain harboring a plasmid carrying the SMUL_1544 gene was cultivated in the presence of l-Thr-P. The cobamide synthesized by the tester strain was identified as pseudo-B12, providing valuable insights into the substrate specificity of the SMUL_1544 protein in vivo. Finally, the role of l-serine (l-Ser) as a putative precursor of the norpseudo-B12 linker moiety was investigated by isotopic labeling experiments.
MATERIALS AND METHODS
Cultivation of bacteria.
S. multivorans (DSMZ 12446, formerly Dehalospirillum multivorans) was grown anaerobically in a defined mineral medium (18) in the absence of yeast extract and vitamin B12. Pyruvate (40 mM) served as the electron donor, and, unless otherwise stated, PCE (nominal concentration, 10 mM) served as the terminal electron acceptor. PCE was added from a sterile stock solution (0.5 M) in hexadecane. Where indicated, l-Thr-P, l-Ser-P, l-Thr, l-Ser, or l-[3-13C]serine (all chemicals were purchased from Sigma-Aldrich Chemie GmbH, Munich, Germany) was added to the medium from sterile stock solutions to a final concentration of 1 mM. Growth was monitored photometrically by measuring the optical density (OD) at 578 nm. The maximal growth rate (μmax) was calculated on the basis of a logarithmic plot of the average results. Derivatives of S. enterica serovar Typhimurium strain LT2 were cultivated on no-carbon essential (NCE) minimal medium (19) containing glycerol (22 mM for growth studies or 55 mM for cobamide analysis) as the growth substrate under aerobic conditions. Trace minerals (20), MgSO4 (1 mM), and ampicillin (100 μg/ml) were added to the medium. For growth studies, 250 μM arabinose was added, and for cobamide analysis, 1.3 mM arabinose was added. Cultures prepared for cobamide analysis contained 1,2-propanediol (5 mM), cobyric acid (38 nM), and adenine (300 μM). Growth studies were performed at 37°C in duplicate in a 96-well microtiter plate with a computer-controlled BioTek ELx808-1 ultramicroplate reader 132 (BioTek Instruments, Inc.). Cell density analysis was performed by measuring the OD at 630 nm every 15 min for 48 h. Inoculation was done by adding 2 μl of an overnight culture grown in lysogenic broth (LB) to 198 μl of fresh medium.
Strain and plasmid construction.
The S. enterica mutant strains (Table 1) generated in this study are variants of S. enterica strain JE7088. The plasmid pBAD24+SMUL_1544 was constructed as follows. Using genomic DNA of S. multivorans as the template, a PCR fragment which covered the coding sequence of the SMUL_1544 gene was produced. The sequences of the oligonucleotides (forward primer, 5′-AGCGTCATGATTGATACAATGAATGCACG-3′; reverse primer, 5′-TCAGAAGCTTTCACTTACTATCCTTTAGTATGC-3′) contained NcoI and HindIII restriction sites. Plasmid pBAD24+SMUL_1544 was obtained after ligation of the cut PCR fragment into vector pBAD24 (21) cut with the same enzymes. Plasmid pBAD24+SMUL_1544 was stored in Escherichia coli DH5α grown on LB containing 100 μg/ml ampicillin. Finally, plasmid pBAD24+SMUL_1544 was transferred into S. enterica JE12941 via electroporation. Strain JE7088 and its derivatives cannot utilize arabinose as the carbon and energy source. Gene expression from the pBAD24 vector was induced with l-(+)-arabinose.
TABLE 1.
Bacterial strains used in this studya
| Strain | Genotype | Source or reference |
|---|---|---|
| JE7088 | ΔmetE2702 ara-9 | J. C. Escalante-Semerena strain collection |
| Derivatives of JE7088 | ||
| JE11685 | pBAD24b | J. C. Escalante-Semerena strain collection |
| JE12941 | ΔcobD1371 | J. C. Escalante-Semerena strain collection |
| JE14935 | ΔcobD1371/pBAD24 | J. C. Escalante-Semerena strain collection |
| JE20971 | ΔcobD1371/pBAD24+SMUL_1544 | This study |
All strains used in this study were derivatives of Salmonella enterica serovar Typhimurium strain LT2.
From reference 21.
Cobamide extraction and analysis.
S. enterica strains JE11685 and JE20971 were cultivated as described above. Cultures were grown in 2-liter Erlenmeyer flasks containing 1 liter of medium. After a 3-day incubation period at 37°C (shaking at 100 rpm), cells were harvested by centrifugation at 4°C using an Avanti J-25I centrifuge equipped with a JA25.50 rotor; cell pellets were stored at −20°C until they were used. Cobamide extraction was conducted in accordance with the method described elsewhere (22). For cobamide extraction, S. multivorans was grown on pyruvate- and fumarate-containing medium. The procedure for cobamide extraction and analysis via high-performance liquid chromatography (HPLC) is described elsewhere (23). High-resolution mass spectra were recorded using a Bruker Maxis high-resolution quantitative time of flight mass spectrometer equipped with an electrospray ionization source operated in the positive mode (Bruker Daltonik GmbH, Bremen, Germany). HPLC separations were achieved using a Dionex UltiMate 3000 instrument equipped with a Phenomenex Gemini C18 column (250 by 2 mm, 5 μm) operated at a flow rate of 400 μl/min starting with 3% aqueous acetonitrile for 5 min, followed by a linear gradient to 100% acetonitrile within 35 min, using 0.5% acetic acid as an additive. PceA activity measurement and immunological detection of the enzyme in crude cell extracts of S. multivorans cells were performed as previously described (23).
Isotopic labeling with l-[3-13C]serine and NMR spectroscopy.
S. multivorans was grown and subcultured in medium containing PCE as the electron acceptor in the presence of 1 mM l-Ser. The third culture contained 1 mM l-[3-13C]serine. After harvesting of the cells, cobamide extraction was performed as described above. Norpseudo-B12 (20 μg) was isolated by semipreparative HPLC using an Agilent HP-1100 HPLC instrument equipped with a Grom-Sil 120 ODS-4 HE column (250 by 8 mm; particle size, 5 μm) and UV detector at a λ of 360 nm and coupled to a Gilson 206 Abimed fraction collector. A flow rate of 2 ml/min was used, starting at 3% acetonitrile in 0.1% aqueous acetic acid for 3 min, followed by a linear increase to 100% acetonitrile with 0.1% acetic acid over 30 min. Nuclear magnetic resonance (NMR) spectra were recorded in D2O on a Bruker Avance III HD700 NMR spectrometer (Bruker BioSpin, Karlsruhe, Germany) at operating frequencies of 700 MHz for 1H and 176 MHz for 13C. A triple-resonance TCI 1.7-mm MicroCryoProbe was used to measure the spectra at 298 K.
RESULTS
Complementation of a Salmonella enterica ΔcobD strain.
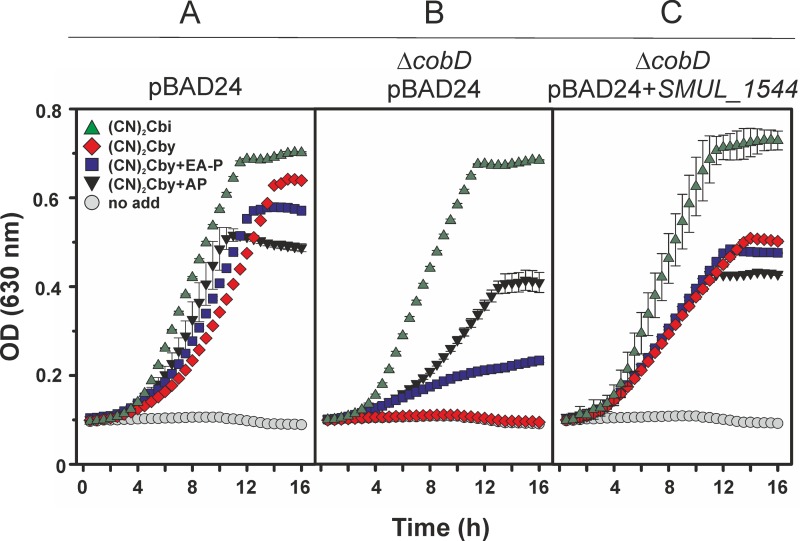
A characterization of the SMUL_1544 gene function in S. multivorans has not been possible due to the lack of the genetic tools needed to inactivate the genes of interest in this organism. Therefore, heterologous expression of the gene encoding SMUL_1544 in appropriate S. enterica mutant strains offers a viable option for the testing of enzyme function. S. enterica strain JE7088 (Table 1) lacks the gene encoding the cobamide-independent methionine synthase (MetE). Methionine biosynthesis in this strain requires a functional cobamide-dependent methionine synthase (MetH). Hence, growth of the strain is strictly dependent on the availability of methionine or cobamides. The latter can be provided either by de novo biosynthesis or exogenously. The wild-type allele of SMUL_1544 cloned under the control of the ParaBAD promoter was used in the experiments described below. Three derivatives of strain JE7088 were analyzed. Strain JE11685, which carried the empty cloning vector, served as a positive control. Strain JE14935, which harbored the empty cloning vector and a chromosomal deletion of the cobD gene, served as the negative control. Strain JE20971 harbored a cobD deletion and a plasmid containing the SMUL_1544 gene. The strains used either did not make CobD or produced S. enterica CobD or SMUL_1544. We monitored the growth of the above-described strains under aerobic conditions in minimal medium supplemented with either dicyano-cobinamide [(CN)2Cbi] or dicyano-cobyric acid [(CN2)Cby] (9, 24). As shown in Fig. 2, none of the mutant strains grew in the absence of these additives (circles). This effect was reversed in all cultures when 1 nM vitamin B12 (Coβ-cyano-Coα-5,6-dimethylbenzimidazolyl-cobamide) was added to the medium (data not shown). The addition of vitamin B12 to the medium had the same effect on the growth rate and yield for all the strains tested. A similar result but with a lower rate and a lower yield (reduction of about 50%) was obtained when 1 nM (CN)2Cbi was present in the medium (Fig. 2, green triangles). As expected, when (CN)2Cbi was used, the CobD function was not required, since (CN)2Cbi already contains the linker moiety. When 1 nM (CN2)Cby was added to the cultures, the presence of a functional CobD was necessary, as seen by the growth defect of strain JE14935, which lacks the cobD gene (Fig. 2B, diamonds). This defect was partially reversed by the addition of either 1 mM AP (Fig. 2B, black triangles) or 1 mM EA-P (Fig. 2B, squares). S. enterica was previously shown to be able to use exogenous AP and EA-P for cobamide biosynthesis (8, 9, 12). The correction of the growth defect by (CN2)Cby plus EA-P was not as efficient as that for (CN2)Cby plus AP, which suggested a preference of MetH for cobamides rather than norcobamides. A growth defect in the presence of (CN2)Cby was not detectable for strain JE11685, which had a functional cobD gene in its genome, and for strain JE20971, which produced SMUL_1544 exclusively from the plasmid (Fig. 2A and C, diamonds). The latter observation clearly shows that SMUL_1544 can compensate for the absence of the CobD function in S. enterica. The growth of strains JE11685 (in which cobD is encoded by the genome) and JE20971 (in which SMUL_1544 is carried by a plasmid) in the presence of (CN2)Cby and the addition of either AP or EA-P (Fig. 2A and C, black triangles and squares, respectively) did not result in the recovery of the growth rate and yield achieved with (CN2)Cbi supplementation (Fig. 2A and C, green triangles). The latter observation might be explained by the additional efforts that the cells have to make in taking up EA-P or AP. Phosphorylation of AP is required prior to its use in cobamide linker biosynthesis (9).
FIG 2.
Cobamide-dependent growth of S. enterica mutant strains. (A) Strain JE11685/pBAD24; (B) strain JE14935 ΔcobD/pBAD24; (C) strain JE20971 ΔcobD/pBAD24+SMUL_1544. Cultivation was conducted in the presence of either dicyanocobinamide [(CN)2Cbi; 1 nM], dicyanocobyric acid [(CN)2Cby; 1 nM], (CN)2Cby and 1-aminopropan-2-ol (AP; 1 mM), (CN)2Cby, or ethanolamine phosphate (EA-P; 1 mM) or without any amendment (no add, no addition).
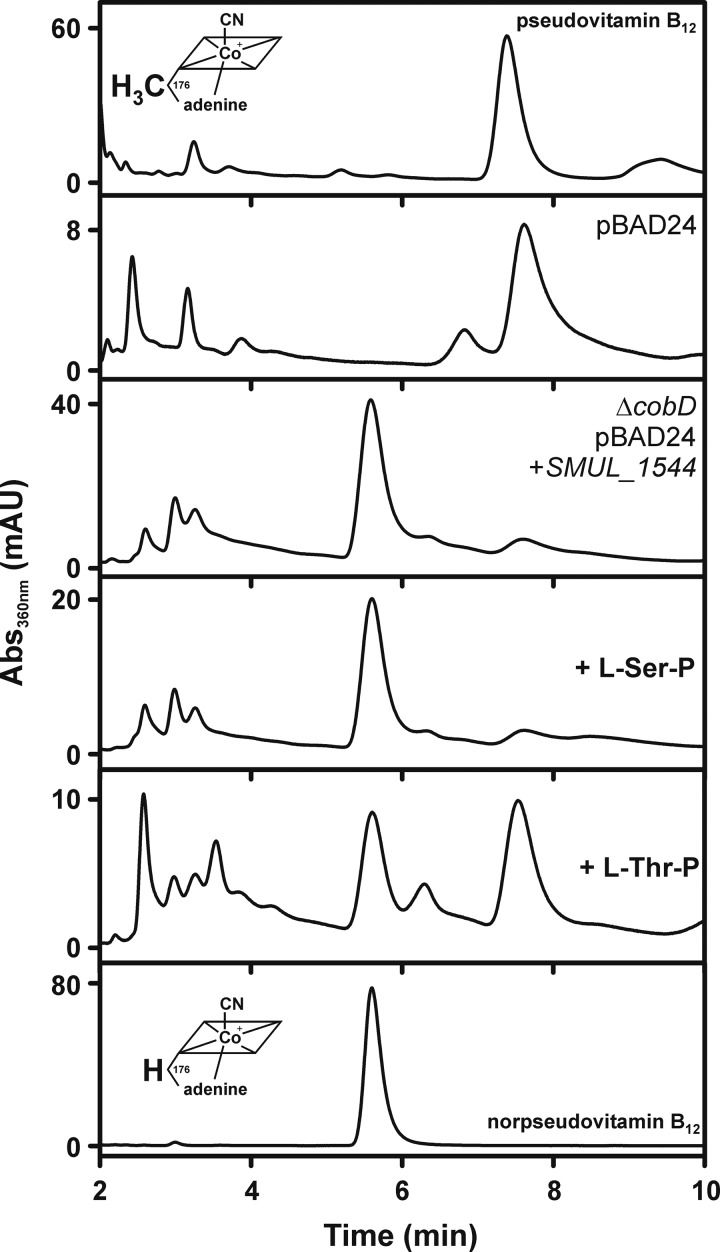
To identify the type of cobamide generated by the SMUL_1544-producing strain (JE20971), cobamide extraction and analysis were performed (Fig. 3). The cobamide extract was analyzed via HPLC, and the profile was compared to that for a cobamide extract from strain JE11685, which synthesized the native S. enterica CobD enzyme. Cells were grown aerobically on glycerol-containing medium supplemented with (CN)2Cby. Adenine (300 μM) was added to the medium in order to sustain cobamide production. From the comparison with the HPLC elution profile of the pseudovitamin B12 standard, it was concluded that strain JE11685 produced pseudo-B12 under these growth conditions. In contrast, the SMUL_1544-producing strain JE20971 appeared to form mainly norpseudo-B12. No change in the HPLC profile was observed when 1 mM l-Ser-P was added to the growth medium of JE20971. l-Ser-P is the putative substrate of SMUL_1544. Surprisingly, strain JE20971 produced both pseudo-B12 and norpseudo-B12 when 1 mM l-Thr-P was present in the growth medium. This observation suggested that SMUL_1544 might be able to use l-Ser-P and l-Thr-P as the substrates to yield EA-P and AP-P, respectively.
FIG 3.
HPLC analysis of cobamide extracts from S. enterica JE7088 mutant strains. Strain JE11685/pBAD24 and strain JE20971 ΔcobD/pBAD24+SMUL_1544 were analyzed. l-Ser-P or l-Thr-P was added to the culture of JE20971 at a concentration of 1 mM. Pseudo-B12 extracted from Propionibacterium acidipropionici and norpseudo-B12 extracted from S. multivorans served as standards. The suffix vitamin refers to the presence of a cyano group as the upper ligand in the extracted cobamides. mAU, milli-absorbance units.
Production of pseudo-B12 in S. multivorans.
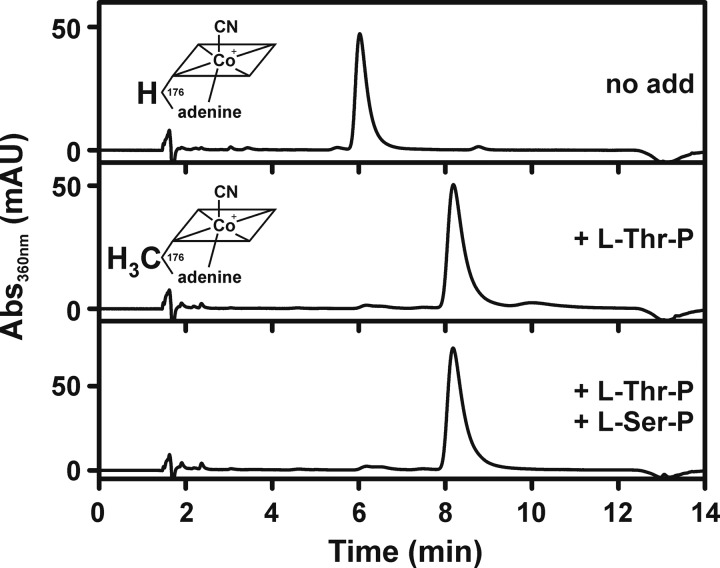
The evidence for the conversion of l-Thr-P by SMUL_1544 obtained by the previous experiment raised the question of pseudo-B12 formation in S. multivorans. Guided cobamide biosynthesis was applied to test the organism for this synthetic capability. Cobamides from cells grown in the presence of 1 mM l-Thr-P were purified and analyzed via HPLC (Fig. 4). A cobamide which, in comparison to the elution profile of norpseudo-B12, showed a peak at a higher retention time in the HPLC analysis was obtained. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, the cobamide was identified as pseudovitamin B12 on the basis of the single charged molecular ion signal at m/z 1,345.5489 [M+H]+ (calculated for C59H85CoN17O14P, m/z 1,345.5526; difference, −2.7 ppm) and the double charged molecular ion signal at m/z 673.2801 [M+2H]2+ (calculated for C59H86CoN17O14P, m/z 673.2799; difference, −0.3 ppm). Confirmation of the LC-MS/MS data was obtained with an authentic standard isolated from Propionibacterium acidipropionici. The addition of 1 mM l-Ser-P did not counteract the effect of l-Thr-P.
FIG 4.
HPLC analysis of cobamide extracts from S. multivorans. l-Ser-P or l-Thr-P was added to the medium to a final concentration of 1 mM.
The presence of the methyl group at position 176 in the linker moiety of pseudo-B12 might have a negative impact on the binding of the cofactor to the PceA enzyme in S. multivorans. Such an effect might be observable from a lower growth rate or a lower yield of the organism with PCE as the sole electron acceptor. For this reason, we analyzed the growth kinetics of the organism (Fig. 5A). Two subsequent precultures were grown under conditions identical to those of the growth experiment. The inoculum (10%, vol/vol) was transferred after 24 h when the culture without additives reached stationary phase. The growth rate and yield of the cultures with l-Thr-P (1 mM) were not drastically altered in comparison to those of the cultures without supplementation. However, growth started after an extended lag phase. For the culture without an amendment, a maximal growth rate of 0.059 h−1 was calculated. This value was similar to the value obtained for the culture treated with l-Thr (0.060 h−1). The maximal growth rate of the cells cultivated in the presence of l-Thr-P was 0.041 h−1. No effect was observed when l-Thr rather than l-Thr-P was in the medium. The effect of l-Thr-P was specific for the PCE-dependent growth of S. multivorans, since cells cultivated on fumarate rather than PCE as the electron acceptor were not affected by the presence of l-Thr-P (data not shown).
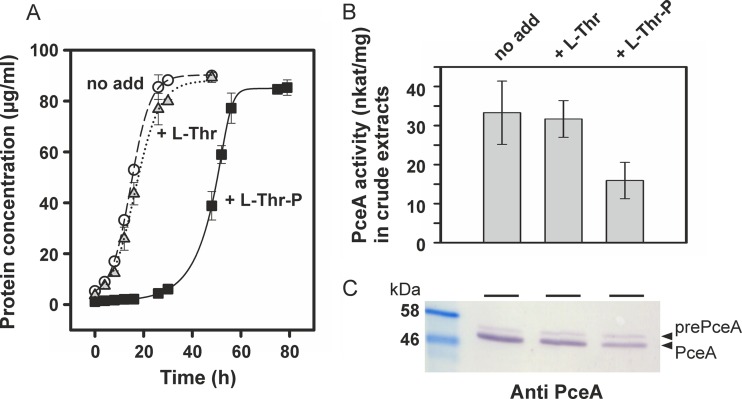
FIG 5.
PCE-dependent growth and PceA activity in S. multivorans. (A) Growth on pyruvate- and PCE-containing medium; (B) PceA enzyme activity in crude extracts; (C) immunological detection of PceA in crude extracts separated via SDS-PAGE. l-Thr or l-Thr-P was added to the medium at a final concentration of 1 mM.
The PceA enzyme activity was measured in crude extracts of PCE-grown cells cultivated either with or without l-Thr or l-Thr-P (Fig. 5B). For all conditions applied in this experiment, the cells were harvested in the late exponential growth phase (protein concentration, 70 to 80 μg/ml). The presence of l-Thr had no impact on the conversion of PCE by PceA in the photometric enzyme assay. In crude extracts obtained from cells grown in the presence of l-Thr-P, a 50% reduction in the PceA activity was measured. This result does not exactly reflect the impact of l-Thr-P on growth in the respective culture (reduction of μmax by about 30%) and might point toward a surplus of PceA in S. multivorans cells, the activity of which is not linked to energy conservation and growth. The proteins in the crude cell extracts were separated by SDS-PAGE and analyzed by Western blot analysis using polyclonal PceA antibodies (Fig. 5C). As it was shown before, two forms of the PceA enzyme were detected. The slower-migrating band represented the Tat signal peptide-bearing precursor (prePceA), and the faster-migrating band represented the mature form of the enzyme without the signal peptide (PceA). In cells grown in the absence or presence of l-Thr, the mature form of PceA was the dominant species. In cells cultivated in medium supplemented with l-Thr-P, the overall amount of PceA was slightly reduced, which suggested a decrease in the stability of the enzyme.
Isotopic labeling of the linker moiety norpseudo-B12.
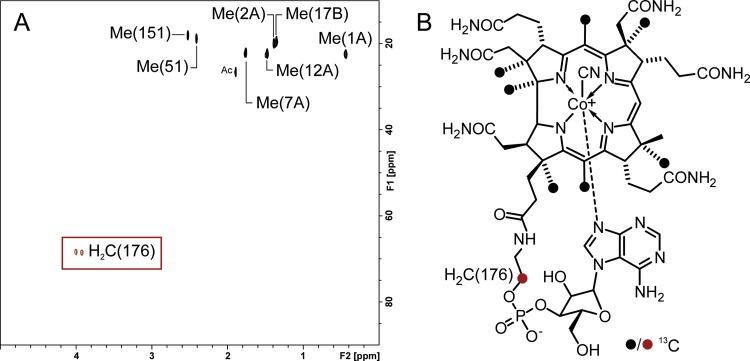
Cobamide linker biosynthesis in S. multivorans can be easily redirected by the addition of l-Thr-P to the growth medium, which points toward a biosynthetic pathway comparable to what was reported for S. enterica (Fig. 1). For a better understanding of the synthesis of EA-P, it is important to determine its metabolic origin. l-Ser-P is produced as an intermediate in l-Ser biosynthesis from 3-phosphoglycerate in S. multivorans. This was inferred from the analysis of the organism's genome. In analogy to the linker biosynthetic pathway in S. enterica, where l-Thr is phosphorylated by the specific kinase (PduX) and used as the linker precursor, l-Ser might be used in a similar manner by S. multivorans. A PduX-like protein was not encoded by the norcobamide biosynthesis gene cluster of S. multivorans, nor was a gene encoding a putative kinase identified among the 25 open reading frames containing most of the cobamide biosynthetic genes of this bacterium. To analyze the ability of S. multivorans to utilize l-Ser as a precursor for the linker moiety in norpseudo-B12, an isotopic labeling approach was conducted using l-[3-13C]serine. NMR analysis of a purified norpseudo-B12 sample from S. multivorans grown in the presence of 1 mM l-[3-13C]Ser revealed the 13C incorporation into position 176 in the linker moiety (Fig. 6), demonstrating that this unit was derived from l-Ser. These data reveal the possibility of the formation of EA-P via l-Ser-P from serine and underpin the previous observation that, besides l-Thr-P, l-Ser-P might also be a substrate of SMUL_1544. Hence, this result indicated the utilization of l-Ser-P as a precursor of the EA-P linker moiety in norpseudo-B12 and makes the use of other putative intracellular sources of EA-P (e.g., phospholipids) rather unlikely. Incorporation of 13C from l-[3-13C]Ser into seven methyl groups of the corrin ring was also observed. The same methyl units are known to be labeled by [S-13CH3]methionine ([S-13CH3]Met) (25), which is converted into S-adenosylmethionine (SAM), the methyl donor used by cobamide biosynthetic enzymes. The formation of Met from homocysteine is catalyzed by a cobamide-independent methionine synthase (MetE) in S. multivorans (16). Methyltetrahydrofolate serves as the donor of the methyl group transferred in this reaction (26). Tetrahydrofolate can be methylated at the expense of Ser in S. multivorans, which channels the 13C label of l-[3-13C]-Ser into Met, SAM, and, finally, the methyl groups of the corrin ring (27).
FIG 6.
Incorporation of l-[3-13C]Ser into the linker moiety in norpseudo-B12. (A) Partial heteronuclear single quantum coherence spectrum of norpseudo-B12 after feeding with l-[3-13C]serine shows peaks corresponding to seven SAM-derived methyl groups of the corrin ring as well as the methylene group at position 176 [H2C(176)] of the linker moiety (the peak assignments are according to previous work [6]). (B) Structure of norpseudo-B12. 13C-labeled positions are indicated by filled circles. Me, methyl group; Ac, acetic acid.
DISCUSSION
The CobD-mediated formation of AP-P as a precursor of the linker moiety is one of the last steps in the formation of cobamides. The absence of this enzyme arrests the cobamide-dependent growth of S. enterica. Herein we showed that expression of the gene SMUL_1544 from S. multivorans (Fig. 2) complemented this effect. From this result, it could be concluded that the gene product SMUL_1544 functions as an l-Thr-P decarboxylase. However, when SMUL_1544 was produced in S. enterica, this bacterium synthesized norpseudo-B12 rather than pseudo-B12 (Fig. 3). This finding pointed toward the formation of EA-P rather than AP-P and suggested that SMUL_1544 prefers l-Ser-P over l-Thr-P as the substrate. l-Thr-P is specifically formed for cobamide biosynthesis in S. enterica, and l-Ser-P is an intermediate of the common serine biosynthetic pathway from 3-phosphoglycerate. Hence, both compounds should be available in S. enterica cells. Excess l-Thr-P in the medium led to the production of pseudo-B12 by the SMUL_1544-producing strain (Fig. 3). l-Thr-P is taken up by the cells in an amount that appears to suppress the use of l-Ser-P by SMUL_1544. These observations led to the conclusion that SMUL_1544 is a decarboxylase that can use l-Ser-P or l-Thr-P as the substrate, but with a preference for l-Ser-P. Direct proof of the conversion of l-Ser-P and l-Thr-P by SMUL_1544 has to await the purification and characterization of the protein. The decarboxylation of l-Ser-P has not been investigated for S. enterica CobD so far but is also going to be assessed in future studies.
The hypothesis that SMUL_1544 can decarboxylate both l-Thr-P and l-Ser-P was tested in the PCE-respiring bacterium S. multivorans. l-Ser-P is expected to be available in S. multivorans, since it is an intermediate of the serine biosynthetic pathway. The organism lacks a specific l-Thr kinase (PduX), which makes the availability of intracellular l-Thr-P unlikely. The formation of pseudo-B12 was exclusively observed when the organism was cultivated in the presence of l-Thr-P (Fig. 4). This finding revealed the efficient uptake of the phosphorylated amino acid in this bacterium. In S. enterica and S. multivorans, the effect of l-Thr-P was not reversed by the addition of l-Ser-P at the same concentration. A rate of l-Ser-P uptake lower than the rate of l-Thr-P uptake might be the reason for this result. An excess of l-Thr-P in the cells could overcome the preferential use of l-Ser-P by SMUL_1544. The utilization of l-Ser-P by SMUL_1544 in its native host was deduced from the incorporation of exogenous 13C-labeled serine into the norpseudo-B12 cofactor produced by S. multivorans (Fig. 6). At present, it is unclear whether or not a serine kinase is specifically functioning in norpseudo-B12 biosynthesis in S. multivorans. Results from our 13C-NMR experiments (Fig. 6) suggest that this may be the case.
The identification of the norpseudo-B12 cofactor in S. multivorans more than 10 years ago raised the question of a specific need for this unusual type of cobamide in the organism. In the study presented here, it was shown that the formation of pseudo-B12 in S. multivorans does not hinder the maturation of the cobamide-containing PceA enzyme (Fig. 5). A negative effect on the maturation of the enzyme may be concluded from the decrease in PceA enzyme activity and the slight reduction in the amount of PceA in cells forming pseudo-B12. However, besides an extension in the lag phase of a pseudo-B12-producing culture growing with PCE, no severe effect on the growth rate or yield was detected. The almost exclusive production of pseudo-B12 concomitantly with the formation of active PceA enzyme makes the binding of this cofactor to the enzyme's precursor in the cytoplasm very likely. Hence, the PceA structure might adopt a conformation which allows the binding of either norpseudo-B12 or pseudo-B12. Such an acceptance of a structurally different cobamide cofactor by PceA has not been observed before, when the lower base of the nucleotide loop in norpseudo-B12 was exchanged from adenine to 5,6-dimethylbenzimidazole (23). Current work in our laboratory seeks to determine whether or not catalytically active PceA from cells cultivated in the presence of l-Thr-P contains pseudo-B12 or whether it selectively binds traces of norpseudo-B12.
The production of norcobamides has not yet been reported for B12-synthesizing prokaryotes different from S. multivorans. Furthermore, a gene product with high similarity to SMUL_1544 and with a defined role in cobamide biosynthesis has not been identified so far. The firmicute Ilyobacter polytropus, which harbors the cobamide biosynthesis genes with the highest similarity to those of S. multivorans, was shown to produce pseudo-B12 (16). The sequence identity of CobD from I. polytropus and SMUL_1544 is exceptionally low compared to that of the other cobamide biosynthesis genes. The low sequence similarity toward CobD homologues in general and the special function of SMUL_1544 in norcobamide biosynthesis make this enzyme occupy an exceptional position among the PLP-dependent decarboxylases.
ACKNOWLEDGMENTS
This work was financially supported by the German Research Foundation (DFG project SCHU2605/1-1) and the German Academic Exchange Service (DAAD). Work in the J. C. Escalante-Semerena group was supported by USPHS grant R37 GM040313 from the National Institutes of Health to J.C.E.-S.
We thank G. Diekert for helpful discussions and critical reading of the manuscript. Peggy Brand-Schön is acknowledged for skillful technical assistance, and Bernd Schneider (Max Planck Institute for Chemical Ecology, Jena, Germany) is acknowledged for NMR measurements.
REFERENCES
- 1.Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat Prod Rep 19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem 72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 3.Buckel W, Golding BT. 2008. B12-dependent enzyme reactions, chemistry of, p 1–9. In Wiley encyclopedia of chemical biology. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 4.Lenhert PG, Hodgkin DC. 1961. Structure of the 5,6-dimethyl-benzimidazolylcobamide coenzyme. Nature 192:937–938. doi: 10.1038/192937a0. [DOI] [PubMed] [Google Scholar]
- 5.Renz P. 1999. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids, p 557–576. In Banerjee R. (ed), Chemistry and biochemistry of B12. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 6.Kräutler B, Fieber W, Ostermann S, Fasching M, Ongania KH, Gruber K, Kratky C, Mikl C, Siebert A, Diekert G. 2003. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo-B12, a new type of a natural corrinoid. Helv Chim Acta 86:3698–3716. doi: 10.1002/hlca.200390313. [DOI] [Google Scholar]
- 7.Fan C, Bobik TA. 2008. The PduX enzyme of Salmonella enterica is an l-threonine kinase used for coenzyme B12 synthesis. J Biol Chem 283:11322–11329. doi: 10.1074/jbc.M800287200. [DOI] [PubMed] [Google Scholar]
- 8.Brushaber KR, O'Toole GA, Escalante-Semerena JC. 1998. CobD, a novel enzyme with l-threonine-O-3-phosphate decarboxylase activity, is responsible for the synthesis of (R)-1-amino-2-propanol O-2-phosphate, a proposed new intermediate in cobalamin biosynthesis in Salmonella typhimurium LT2. J Biol Chem 273:2684–2691. doi: 10.1074/jbc.273.5.2684. [DOI] [PubMed] [Google Scholar]
- 9.Zayas CL, Claas K, Escalante-Semerena JC. 2007. The CbiB protein of Salmonella enterica is an integral membrane protein involved in the last step of the de novo corrin ring biosynthetic pathway. J Bacteriol 189:7697–7708. doi: 10.1128/JB.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem 278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- 11.Cheong CG, Bauer CB, Brushaber KR, Escalante-Semerena JC, Rayment I. 2002. Three-dimensional structure of the l-threonine-O-3-phosphate decarboxylase (CobD) enzyme from Salmonella enterica. Biochemistry 41:4798–4808. doi: 10.1021/bi012111w. [DOI] [PubMed] [Google Scholar]
- 12.Grabau C, Roth JR. 1992. A Salmonella typhimurium cobalamin-deficient mutant blocked in 1-amino-2-propanol synthesis. J Bacteriol 174:2138–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann A, Wohlfarth G, Diekert G. 1996. Purification and characterization of tetrachloroethene reductive dehalogenase from Dehalospirillum multivorans. J Biol Chem 271:16515–16519. doi: 10.1074/jbc.271.28.16515. [DOI] [PubMed] [Google Scholar]
- 14.Miller E, Wohlfarth G, Diekert G. 1996. Studies on tetrachloroethene respiration in Dehalospirillum multivorans. Arch Microbiol 166:379–387. doi: 10.1007/BF01682983. [DOI] [PubMed] [Google Scholar]
- 15.Bommer M, Kunze C, Fesseler J, Schubert T, Diekert G, Dobbek H. 2014. Structural basis for organohalide respiration. Science 346:455–458. doi: 10.1126/science.1258118. [DOI] [PubMed] [Google Scholar]
- 16.Goris T, Schubert T, Gadkari J, Wubet T, Tarkka M, Buscot F, Adrian L, Diekert G. 2014. Insights into organohalide respiration and the versatile catabolism of Sulfurospirillum multivorans gained from comparative genomics and physiological studies. Environ Microbiol 16:3562–3580. doi: 10.1111/1462-2920.12589. [DOI] [PubMed] [Google Scholar]
- 17.Goris T, Schiffmann CL, Gadkari J, Schubert T, Seifert J, Jehmlich N, von Bergen M, Diekert G. 2015. Proteomics of the organohalide-respiring epsilonproteobacterium Sulfurospirillum multivorans adapted to tetrachloroethene and other energy substrates. Sci Rep 5:13794. doi: 10.1038/srep13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholz-Muramatsu H, Neumann A, Messmer M, Moore E, Diekert G. 1995. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol 163:48–56. doi: 10.1007/BF00262203. [DOI] [Google Scholar]
- 19.Berkowitz D, Hushon JM, Whitfield HJ Jr, Roth J, Ames BN. 1968. Procedure for identifying nonsense mutations. J Bacteriol 96:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atlas R. 1995. Handbook of media for environmental microbiology, p 6. CRC Press, Boca Raton, FL. [Google Scholar]
- 21.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CH, Escalante-Semerena JC. 2011. ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis. Mol Microbiol 81:952–967. doi: 10.1111/j.1365-2958.2011.07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller S, Ruetz M, Kunze C, Kräutler B, Diekert G, Schubert T. 2014. Exogenous 5,6-dimethylbenzimidazole caused production of a non-functional tetrachloroethene reductive dehalogenase in Sulfurospirillum multivorans. Environ Microbiol 16:3361–3369. doi: 10.1111/1462-2920.12268. [DOI] [PubMed] [Google Scholar]
- 24.Jeter RM, Olivera BM, Roth JR. 1984. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol 159:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott AI, Townsend CA, Okada K, Kajiwara M, Cushley RJ, Whitman PJ. 1974. Biosynthesis of corrins. II. Incorporation of 13C-labeled substrates into vitamin B12. J Am Chem Soc 96:8069–8080. [DOI] [PubMed] [Google Scholar]
- 26.González JC, Peariso K, Penner-Hahn JE, Matthews RG. 1996. Cobalamin-independent methionine synthase from Escherichia coli: a zinc metalloenzyme. Biochemistry 35:12228–12234. doi: 10.1021/bi9615452. [DOI] [PubMed] [Google Scholar]
- 27.Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, Howard MJ, Rigby SE, Warren MJ. 2013. Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc Natl Acad Sci U S A 110:14906–14911. doi: 10.1073/pnas.1308098110. [DOI] [PMC free article] [PubMed] [Google Scholar]