Abstract
Antibody-drug conjugates are highly complex entities that combine an antibody, a linker and a toxin. This complexity makes them demanding both technically and from a regulatory point of view, and difficult to deal with in their patent aspects. This article discusses different issues of patent protection and freedom to operate with regard to this promising new class of drugs.
Keywords: ADC, antibody-drug conjugates, antibody, freedom to operate, immunotoxin, Kadcyla, patent
Abbreviations
- FDA
Food and Drug Administration
- FTO
Freedom to Operate
- IP
Intellectual property
- ADC
Antibody-Drug Conjugates
- EPO
European Patent Office
- USPTO
United States Patent And Trademark Office
- CDR
Complementarity Determining Region
- HC/LC
Heavy Chain/Light Chain
- DAR
Drug-Antibody Ratio
- IPR
Inter Partes Review
- PTAB
Patent Trial and Appeal Board
Introduction
Antibody-drug conjugates (ADCs) are one of the most promising classes of new drugs, although the idea is not new.1 ADCs embody the oft-cited concept of “magic bullets,” which was described by Paul Ehrlich over 100 y ago. As of 2015, 3 ADCs have been approved by the US Food and Drug Administration (FDA), namely gemtuzumab ozogamacin (Mylotarg®), ado-trastuzumab emtansine (Kadcyla®) and brentuximab vedotin (Adcetris®). Their characteristics are shown in Table 1. More than 40 other ADCs are in clinical studies today.2
Table 1.
Characteristics of the 3 ADCs approved to date by the US Food and Drug Administration
| ADC name | Antibody target | Key IP right US/EP | Target also used in conventional mAb therapy? | Linker | Linker cleavable? | Toxin | Site specific conjugation? | Drug Antibody Ratio |
|---|---|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin** | CD33 | US5773001/ EP0689845B1* | no | hydrazone | yes | calicheamicin | no | Only 50% of the antibody is loaded at all (avg 4 – 6) |
| Trastuzumab emtansine | HER-2/neu | US8337856/ EP2283867B1 | yes | SMCC (maleimide) | no | DM1 (maytansinoid) | no | 0–8 (avg 3,5) |
| Brentuximab vedotin | CD30 | US7829531/ EP2353611B1 | no | maleimidocaproyl spacer, valine–citrulline linker, and PABC spacer | yes(cathepsin) | MMAE (auristatin) | no | 3–5 |
expired;
product voluntarily withdrawn from the market in 2010.
ADCs combine an antibody, a linker and a toxin (often called “payload” or, more martial, “warhead”), and, for that reason, they are technically demanding to develop and pose challenges in manufacturing.3 They can also raise regulatory issues.4 because ADCs can be considered prodrugs that release their active compound–the toxin–at the site of action.
While their complexity has been called an„invitation to innovation,”5 ADCs are difficult to deal with in their intellectual property (IP) aspects. Both Freedom to Operate (FTO), as well as the protection of ADCs and technologies to generate them, are affected by the complexity of the molecules, and thus those who want to develop and sell new ADCs or protect the compounds and technologies from which they are derived are faced with challenges when making business decisions. In addition, the numerous players active in this field have created a maze of third-party IP rights that is difficult to navigate. Further, because ADCs combine biotechnology and organic chemistry, IP counsels working in this area need a thorough technical background in both disciplines.
This article discusses some of these aspects in more detail, and discloses IP rights that stand exemplarily for a given technology or concept. It can, however, not replace a case-specific FTO analysis or novelty search. Some of the IP rights discussed herein may not have been granted yet, or they may have already expired or been revoked. The latter, which are marked with an asterisk in the respective tables, may thus constitute free prior art, and, as such, provide a valuable source of information for competitors.
Freedom to operate
The term “Freedom to Operate” refers to a determination that the commercialization of a product does not infringe third-party IP rights, in particular patents. Establishing FTO requires that all components of the respective technology, encompassing methods as well as compounds and intermediates, are analyzed with respect to whether they are the subject of valid and enforceable third-party IP rights.
Because IP rights have a territorial effect and a restricted lifetime only, an FTO analysis does not only focus on the technologies as such, but also considers where IP rights are in force and when they expire. In this context, the estimated time to market of the product that is to be commercialized should be weighed against the lifetime of a given IP right. Further considerations should focus on potential research exemptions as well as on questions of exhaustion, or process patents that might extend their protection on products obtained therewith. These non-ADC specific regulations are subject to large variations between different jurisdictions, and thus are outside the scope of this article.
If, in the course of an FTO analysis, IP rights that are likely to be infringed by the commercialization of a given product are detected, one should consider whether or not they are valid and enforceable. If not, respective countermeasures should be considered, like invalidity opinions, oppositions, nullity actions, or post grant review/inter partes review.
As an alternative, in-licensing of the respective IP rights could be a solution. This approach is frequently used in cases where the patent protected technology is an enabling technology, or refers only to a part of the molecule, like a linker. These technologies have often been developed by technology companies who use out-licensing as their business model. Large biopharmaceutical companies are generally less inclined to grant a license, in particular on a compound-related patent, because they seek exclusivity rather than royalties.
For ADCs, an FTO analysis encompasses all components, i.e., the antibody, the toxin and the linker. Numerous players have already staked their claims, and many patents and patent applications refer to a combination of 2 of the components, very often a combination of a toxin and a linker. The existing IP landscape thus appears more complicated than for naked antibodies, with overlapping patent estates assigned to different owners. Navigating this landscape can become a laborious challenge, and again requires a thorough understanding of the technical background, including biotechnology and organic chemistry, and the filing strategies used by competitors.
Further, inventors often believe that, once a patent has been granted on a given invention, FTO would be automatically warranted. This thinking relies on a misconception. The truth is that even if a patent has been awarded on a structurally improved second-generation antibody, it can still be the subject of earlier third-party patents protecting the starting antibody, if these are still in force. In the following sections, the methods and compounds that are relevant in an ADC FTO analysis will be briefly addressed.
Active IP strategies
ADCs do also offer new possibilities to obtain patent protection. ADCs can, in some way, be considered as an advancement of conventional therapeutic antibodies, and, according to a general principle, each bit of advancement can be made subject of a patent application.
According to a study performed at Tufts University in 2007, the estimated average costs of developing a new biologic was 1.2 billion USD.6 This figure has been adjusted upwards in 2014 to even 2.6 billion USD.7 Further, development times in biologics are slightly longer than those reported for small molecular drugs.8
Because of the higher complexity and the higher regulatory burden, it can be assumed that, generally, these figures will be even higher in ADCs. A meaningful patent strategy is thus indispensable to ensure that these investments can be recuperated over at least a given period of time. ADC-related embodiments that can become subject of patent protection will also be discussed in the following.
The antibody component
An antibody as such can be subject of third-party patents. This may encompass 4 categories: 1) patents that protect any kind of antibody binding a particular target, which at the filing date was novel (and specified by the applicant in a sufficient way as to enable skilled persons to make an antibody thereagainst); 2) patents that protect all antibodies against a given epitope of such target (if binding said epitope has unprecedented effect); 3) patents that protect all antibodies against a given target that have a particular functionality (e.g., minimum affinity, inhibition of a given effect); or 4) patents that protect a specific antibody (either defined by the respective expressor cell, or by a particular sequence, e.g., of the CDRs, the variable domains or the HC/LC sequences).
It needs to be added, in this context, that new targets for ADC therapy are hard to find,9 thus making patents of categories 1 and 2 less frequent nowadays. Further, it appears that the US Patent and Trademark Office (USPTO) and the European Patent Office (EPO) have become more critical toward patents of category 3 because a functional claim feature is quite often considered to be a mere desideratum only, putting into question the true possession of the entire invention by the applicant at the filing date, as well as the inventive character involved. Regarding category 4, it seems that the EPO applies a higher standard than the USPTO by requiring that the applicant disclose some kind of surprising effect of the new sequence-wise specified antibody over prior art antibodies addressing the same target.10
As a general rule, all therapeutic antibodies on the market are, or were, protected by such compound patents. The use of an approved antibody to generate an ADC is thus likely to fall under the scope of the respective naked antibody patent, provided it is still in force. The fact that the antibody is conjugated to a toxin does not per se change this situation.
In case the planned ADC comprises an existing antibody that is already on the market, or will enter the market, a thorough FTO analysis should be carried out in order to define when FTO can be established, and in which markets. The same applies in cases where the target of the planned ADC is the subject of third-party patents. Patents of such type are on the decline (simply because quite a few targets have already been described 10 y ago or earlier), but still exist and provide meaningful patent protection. Table 2 shows typical examples of different types of naked antibody compound patents, as granted by the EPO.
Table 2.
Examples of different types of naked antibody compound patents
| Category | Example IP right | Assignee | Target | Claim language |
|---|---|---|---|---|
| Antibody claimed by its target or target epitope | EP1587837B1 | Proscan RX | PSMA | An antigen comprising an immunogenic moiety or carrier and an epitope of the extracellular region of PSMA consisting of SEQ ID NO:8, wherein the N-terminal cysteine residue on SEQ ID NO:8 is optional. An isolated antibody or antigen binding fragment thereof, which binds to the antigen wherein the antibody and/or antigen binding fragment thereof also binds to PSMA. |
| Antibody claimed by functional properties | EP1347730B2 | Seattle Genetics | CD30 | An antibody that immunospecifically binds CD30 and exerts a cytostatic or cytotoxic effect on a Hodgkin's disease cell line in the absence of conjugation to a cytostatic or cytotoxic agent. |
| Antibody claimed by expressor cell | EP0660721B1* | Dana Farber | CD22 | A monoclonal antibody that: is produced by a hybridoma cell line selected from the group consisting of HB22–7 (ATCC No. HB 11347), HB22–22 (ATCC No. HB 11348) and HB22–23 (ATCC No. HB11349) […] |
| Antibody claimed by sequence | EP0590058B1* | Genentech | HER2 | A humanized Antibody which comprises a VL domain comprising the polypeptide sequence X and a VH domain comprising the polypeptide sequence Y. |
| Antibody claimed by sequence | EP1951757B1 | Xencor | CD30 | An anti-CD30 antibody, comprising a variable heavy chain sequence 1 and a variable light chain sequence 2 |
expired; the symbol […] indicates that claim language has been truncated.
As shown above, some ADC approaches use existing antibodies that have already proven useful either in the clinic or in preclinical research, and may have a substantial global market. For example, trastuzumab, which as a solo product, generated global sales of 6.5 bn USD in 2013.
As can be seen in Table 2, Claim 3 of EP0590058 (which has expired June 2012) protected Genentech's anti-HER2 antibody trastuzumab by its VL and VH sequence. ADCs comprising trastuzumab, such as Genentech's ado-trastuzumab emtansine, would therefore fall under claim 3 of EP0590058B1. Interestingly, claim 11 explicitly specified, as a preferred embodiment, an immunotoxin comprising trastuzumab plus a cytotoxin.
The use of an approved naked antibody for making an ADC has its merits. For example, it may appear useless to “reinvent the wheel,” i.e., to develop a new antibody when ones that bind relevant targets with high specificity and affinity are already on the market, and have proven sufficiently safe and efficient to be approved. However, not all therapeutic antibodies on the market are suitable as ADCs.
For ADCs, internalization of the antibody may be necessary, whereas, for naked antibodies that evoke antibody-dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity (e.g., anti-CD20 rituximab), quick internalization would be counterproductive. Further, antibodies that bind cytokines (e.g., anti-TNF adalimumab, anti-VEGF-A bevacizumab) instead of cell surface antigens are unlikely to be useful for ADC therapy because they would not deliver their toxin to a suitable target cell. An ideal ADC target should therefore: 1) reliably differentiate cancer cells from healthy cells; 2) occur in sufficient abundancy on the cell surface; 3) internalize the bound ADC with sufficient speed and efficacy.
Concerns have already been raised that all acceptable targets meeting these requirements have been discovered already, and that it is unlikely new ones will be found. 11 Regardless, in the event a novel and suitable target for ADC therapy is found, it is definitely worth seeking patent protection for antibodies against said target on the basis of the classical categories of antibody compound protection (see above). If the target is already known but has not yet been described as a target for ADC therapy, patent protection may focus on an ADC binding to said target. An example of the latter category is shown in Table 3.
Table 3.
Suitable patent category if target and antibody are known, but not in an ADC
| Embodiment | Target | Example IP right | Assignee | Claim language |
|---|---|---|---|---|
| Target and antibody thereagainst are known, but not in an ADC | cKIt | US20140271688 | Novartis | ADC of the formula Ab-(L-(D)n)n or a pharmaceutically acceptable salt thereof; wherein Ab is an antibody or antigen binding fragment thereof that specifically binds to an epitope of human cKIT; L is a linker; D is a drug moiety; m is an integer from 1 to 8; and n is an integer from 1 to 10. |
In addition thereto, further developments regarding the antibody concept as such can be made the subject of patent protection. This could involve, for example, the use of new antibody formats or protein binders derived from alternative scaffolds. Another approach is to modify antibodies in such way that they become active only at the tumor site, even if they target an antigen that is expressed both on healthy cells and tumor cells.12 This approach relies on specific environmental conditions at the tumor site (e.g., abundance of proteases) to activate the antibody, thus avoiding damage to healthy cells are bound. In such way, targets that are not druggable due to insufficient discrimination between cancer cells and healthy cells can be used. Still other approaches have identified targets that do not require internalization of the ADC. Accumulation of the ADC in the sub-endothelial extracellular matrix of tumors was found efficient at very low side effects.13 Table 4 shows some of these approaches where the antibody concept has been further developed, plus exemplary IP rights.
Table 4.
Patents protecting new antibody concepts that can be used in ADCs
| Technology | Embodiment | Example IP right | Assignee | Claim language |
|---|---|---|---|---|
| New antibody format | Diabody | EP2516462B1 | Avipep | An isolated protein comprising an immunoglobulin variable region comprising:(i) at least 2 cysteine residues positioned within framework region (FR) 2, wherein if at least 2 of the cysteine residues in FR2 are not conjugated to a compound then a disulfide bond is capable of forming between the cysteine residues in FR2; and/or(ii) at least 2 cysteine residues positioned within framework region (FR) 3, wherein if at least 2 of the cysteine residues in FR3 are not conjugated to a compound then a disulfide bond is capable of forming between the cysteine residues in FR3. |
| Conditionally active Biologics |
Antibodies are activated and/or inactivated at defined physiological conditions. | US8709755 | BioAtla | A method of preparing a conditionally active antibody, the method comprising the steps of:i. selecting a wild-type antibody against an antigen;ii. evolving the DNA which encodes the wild-type antibody using one or more evolutionary techniques to create mutant DNAs;iii. expressing the mutant DNAs to obtain at least one mutant antibody;iv. subjecting the at least one mutant antibody and the wild-type antibody to an assay under a normal physiological condition selected from the group consisting of temperature, pH, osmotic pressure, osmolality, oxidation and electrolyte concentration, and to an assay under an aberrant condition selected from the group consisting of temperature, pH, osmotic pressure, osmolality, oxidation and electrolyte concentration; andv. selecting the conditionally active antibody from the at least one mutant antibody […] |
| Cleavable masking peptides |
Masking peptides are released, e.g., by proteases secreted by the tumor | US20100189651 | CytomX | A modified antibody comprising: an antibody or antibody fragment (AB), capable of specifically binding its target, coupled to a masking moiety (MM), wherein the coupling of the MM reduces the ability of the AB to bind its target such that that the dissociation constant (Kd) of the AB when coupled to the MM toward the target is at least 100 times greater than the Kd of the AB when not coupled to the MM toward the target |
| Target which does not require toxin internalization | Extra domains A and B of fibronectin | US20150030536 | Philogen | A method of treating lung cancer or lymphoma in an individual, comprising administering to the individual a therapeutically effective amount of an antibody, or antigen-binding fragment thereof, which binds Extra Domain-A (ED-A) of fibronectin comprising a VH domain and a VL domain with given CDR sequences and wherein the antibody is conjugated to a molecule that has biocidal or cytotoxic activity or is conjugated to a radioisotope. |
the symbol […] indicates that claim language has been truncated.
The linker technology
In principle, a large variety of linker technologies exists to bind small molecules to proteins. A well-established technique is the maleimide-based conjugation of thiol-comprising molecules to proteins. This approach has often been used in first-generation ADCs. Other linkers frequently used rely on hydrazone, disulfide or amide bonds.
Early attempts to improve linker technology focused mainly on increasing linker stability, to avoid cleavage thereof when the ADC is still in the bloodstream. These demands were met, e.g., by linkers developed by Seattle Genetics or ImmunoGen. See Table 5 for some exemplary IP rights. However, there was, and still is, further potential for innovation and, accordingly, new IP, in the field of ADC linker technology for various reasons, including insufficient linker stability, insufficient site specificity of the conjugation or insufficient stoichiometry. These problems affect the efficacy and safety of an ADC and, as such, may pose challenges during regulatory agency reviews. New linker technologies therefore strive to: 1) increase linker stability (and thus avoid cleavage thereof in the extracellular space); 2) modulate cleavability after internalization (cleavage by plasma enzymes or medium conditions, e.g., pH, allows the drug to leak into neighboring cells; non-cleavable linkers release the toxin only after degradation of the antibody); 3) increase site specificity of the conjugation site to establish homogenicity of the ADC and avoid steric hindrance of the binding domains; or 4) increase stoichiometry (also called „drug-antibody ratio,” DAR) to yield sufficient efficacy of the ADC, e.g., by avoiding antibodies that carry no toxin, which would compete with the ADC for target binding and thus affect efficacy of the ADC. Table 6 shows some new approaches related to ADC linker technology, and exemplary IP rights.
Table 5.
Early ADC linker technology patents
| Technology | Example IP right | Assignee | Claim language |
|---|---|---|---|
| Maleimidocaproyl-val-cit-PAB (combination with auristatins) | US7745394 | Seattle Genetics | 1. A method for treating cancer comprising administering to a patient in need thereof an effective amount of an antibody-drug conjugate compound having formula Ic: Ab  Aa-Ww-Yy-D)p Ic Aa-Ww-Yy-D)p Icwherein Ab is an antibody which binds to one or more tumor-associated antigens D is a drug moiety selected of Formula DE: 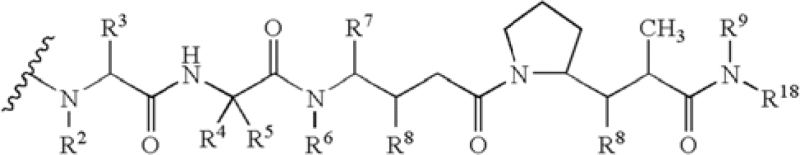 [ ] [ ] |
| Maytansinoids plus SMCC linker | US5208020* | ImmunoGen | A cytotoxic agent comprising one or more maytansinoids linked to a monoclonal antibody or fragment thereof via a disulfide bridge at the C-3, −14, −15, or −20 position of said maytansinoids and wherein said monoclonal antibody or fragment thereof is selective for tumor cell antigens. |
expired; the symbol […] indicates that claim language has been truncated.
Table 6.
Patents protecting new approaches related to ADC linker technology
| Technology | Example IP right | Assignee | Claim language |
|---|---|---|---|
| Non-natural amino acids | EP1968635B1 | Ambrx | A polypeptide, or salt thereof, containing at least one compound selected from the group consisting of: 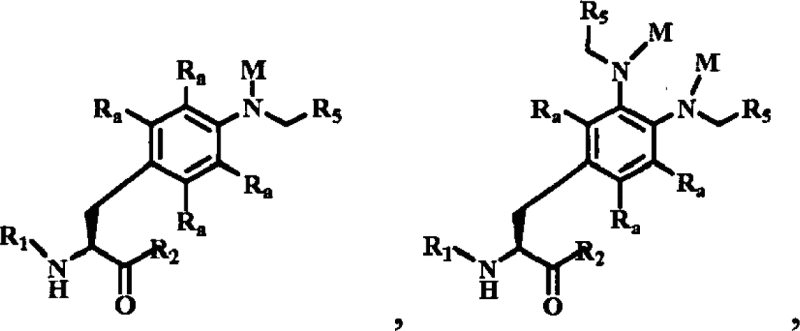 wherein each Ra is independently selected from the group consisting of […] wherein each Ra is independently selected from the group consisting of […] |
| Cysteine-engineered antibodies (THIOMAB) | US20070092940 | Genentech | A cysteine engineered antibody comprising one or more free cysteine amino acids having a thiol reactivity value in the range of 0.6 to 1.0, wherein the cysteine engineered antibody is prepared by a process comprising replacing one or more amino acid residues of a parent antibody by cysteine. |
| SMAC (sortase- mediated antibody conjugation) with N-terminal LPTXG tag/linker | WO2014140317 | NBE therapeutics | A method of producing an immunoligand/payload conjugate, which method encompasses conjugating a payload to an immunoligand by means of a sequence- specific transpeptidase, or a catalytic domain thereof. |
| SpaceLink Technology | EP1370298B1 | Syntarga | A compound of the formula V-(W)k-(X)l-A-Z wherein: V is a specifier which is removed by an enzyme, optionally after prior binding to a receptor; (W)k-(X)l-A is an elongated self-eliminating spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m, wherein Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclisation elimination spacer; Z is a therapeutic or diagnostic moiety; k and l are independently an integer from 0 (included) to 5 (included); m is an integer from 1 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), andk+1>0. |
| Microbial transglutaminase (MTGase) | WO2014202775 | Innate | A method for conjugating a hydrophobic, high molecular weight or charged organic compound to an antibody, comprising the steps of:a) providing an antibody or antibody fragment comprising an acceptor glutamine residue;b) reacting said antibody with a linking reagent comprising a primary amine and a moiety of interest (Z), wherein (Z) is a hydrophobic compound, a charged organic compound and/or organic compound having a molecular weight of at least 500, 700 or 800 g/mol, in the presence of a TGase, under conditions sufficient to obtain an antibody comprising an acceptor glutamine linked to said moiety of interest (Z), via the linking reagent […] |
| SMARTag | US20120183566 | Redwood | An isolated aldehyde-tagged immunoglobulin (Ig) polypeptide comprising an Ig constant region amino acid sequence comprising an amino acid sequence of a sulfatase motif, wherein the sulfatase motif is positioned within or adjacent a solvent-accessible loop region of the Ig polypeptide constant region, and wherein the sulfatase motif is not at the C-terminus of the Ig polypeptide chain. |
| Transglutaminase + “endosome escaping non cleavable” (EENC) linkers | US provisional 62/011,534, not published yet | Dophen | Not published yet |
the symbol […] indicates that claim language has been truncated.
The toxin
Before the toxin component of a projected ADC is selected, consideration should be given to whether the respective toxin is subject to third-party IP, and, if so, where, and until when, and whether or not it can be in-licensed. Today, 3 classes of toxins are typically used in ADCs, namely maytansinoids, auristatins, and calcheamicins, but others are under investigation. Table 7 shows some selected toxins and exemplary IP rights, some of which refer to the combination of a toxin and a given linker or antibody.
Table 7.
Patents protecting selected toxins
| Technology | Example IP right | Assignee | Claim language |
|---|---|---|---|
| Auristatins (MMAE, MMAF) | US6884869 | Seattle Genetics | A compound of the formula 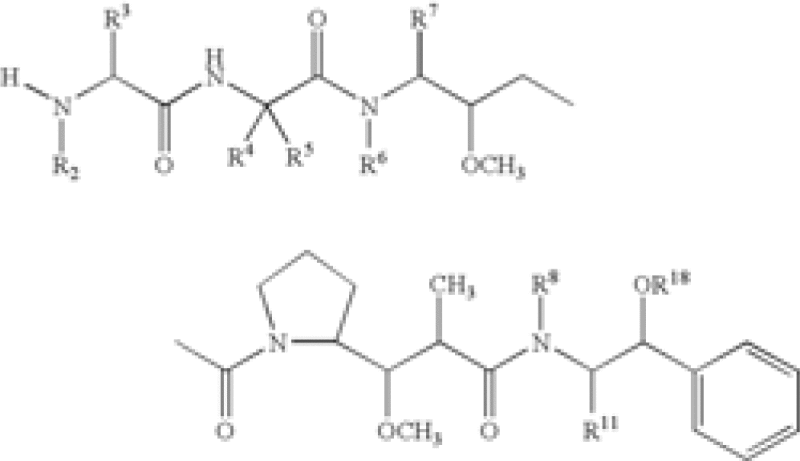 and pharmaceutically acceptable salts and solvates there of wherein […] |
| Maytansinoids plus SMCC linker | US5208020* | ImmunoGen | A cytotoxic agent comprising one or more maytansinoids linked to a monoclonal antibody or fragment thereof via a disulfide bridge at the C-3, −14, −15, or −20 position of said maytansinoids and wherein said monoclonal antibody or fragment thereof is selective for tumor cell antigens. |
| New anthracyclin derivatives | US8900589 | Genentech | An antibody-drug conjugate compound comprising an antibody covalently attached by a linker L and an optional spacer Z to one or more anthracycline derivative drug moieties D, the compound having the formula (D-L-(Z)m)p-Ab or a pharmaceutically acceptable salt thereof, wherein: Ab is an antibody; |
D is an anthracycline derivative selected from the structures A and B: 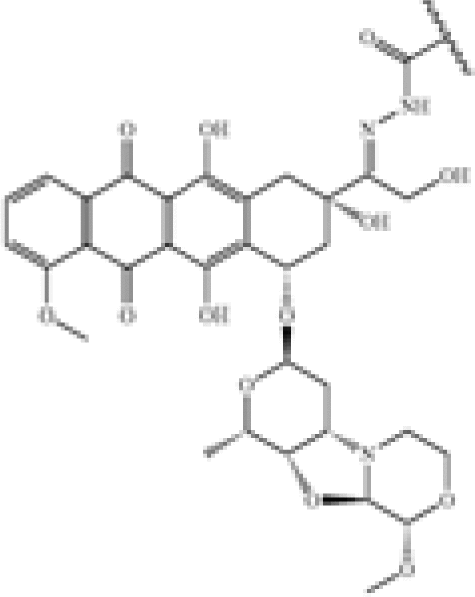 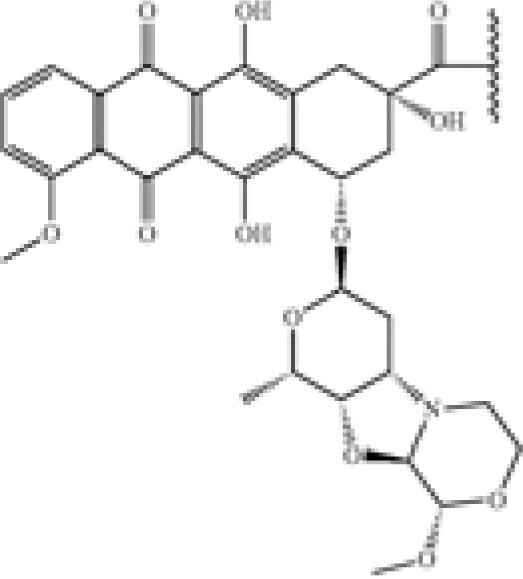 […] […] |
|||
| Calicheamicins | US5714586 | American Cyanamid Company | A method for preparing monomeric calicheamicin derivative/carrier conjugates with higher drug loading/yield and decreased aggregation having the formula, Pr(–X–S–S–W)m wherein: Pr is a proteinaceous carrier, X is a linker that comprises a product of any reactive group that can react with a proteinaceous carrier, W is the calicheamicin radical formed by removal of the naturally occurring methyl trisulfide group […] |
| Duocarmycins | EP2560645A2 | Syntarga | 1. A compound of formula (III): 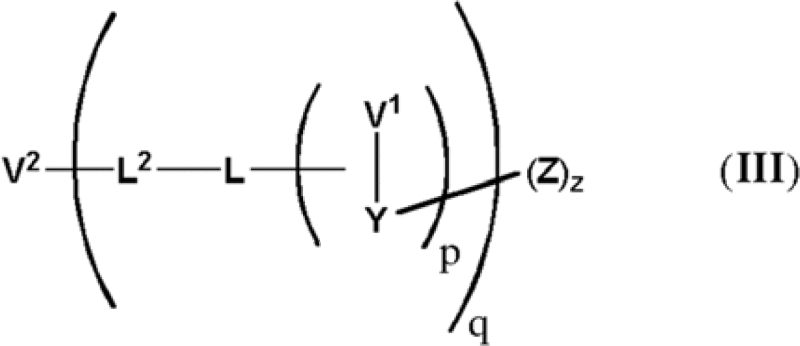 wherein […] each Z is independently a compound of formula (I), (II), (I'), or (IΓ):  […] |
| Novel taxanes | US7390898 | ImmunoGen | A compound represented by formula (I):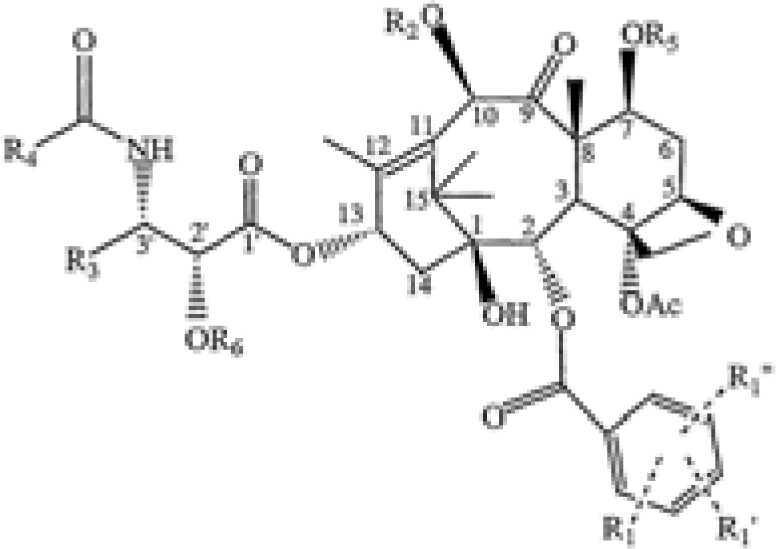 wherein […] |
| Pyrrolobenzodiazepine | EP2766048B1 | ADC Therapeutic & Spirogen Limited | A conjugate of formula ConjB::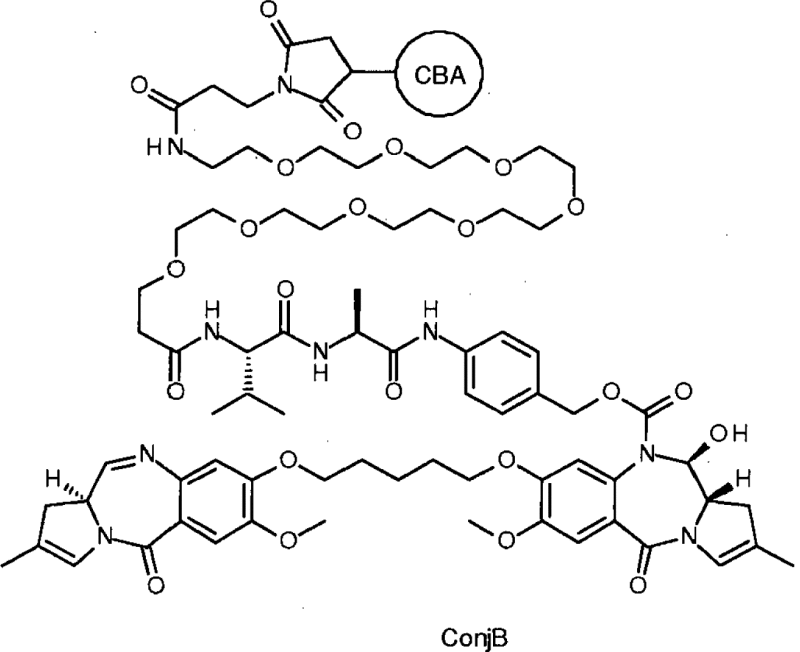 where CBA represents a cell binding agent. where CBA represents a cell binding agent. |
| α-amanitin | EP2436398B1 | Heidelberg Pharma | A conjugate comprising a target-binding moiety linked via a linker L to an amatoxin, wherein the linker L is connected to the amatoxin via(i) the γ C-atom of amatoxin amino acid 1, particularly via an amide linkage;(ii) an oxygen atom bound to the δ C-atom of amatoxin amino acid 3, particularly via an ester linkage, an ether linkage or a urethane linkage; or(iii) the 6′ C-atom of amatoxin amino acid 4, particularly via an oxygen atom bound to the 6′ C-atom of amatoxin amino acid 4; in each case wherein the linker L is connected to the target-binding moiety via a urea moiety. |
| CC-1065 (duocarmycin) | US5475092* US5585499* |
ImmunoGen | A cytotoxic agent comprising a cell binding agent linked via a disulfide bond to one or more analogs or derivatives of a cyclopropylbenzindole-containing cytotoxic drug, wherein said cell binding agent is a monoclonal antibody or an antigen-binding fragment of a monoclonal antibody having at least one binding site thereof, and wherein prior to linking said analogs or derivatives to said cell binding agent said analogs or derivatives are selected from the group consisting of analogs or derivatives formed from an A subunit of the formulae (A-3) or (A-4) and a B-C subunit of the formulae (F-3), (F-4), (F-5) or (F-6), said B-C subunit having a B moiety shown on the left-hand side of the formulae and a C moiety shown on the right-hand side of the formulae and wherein said A subunit is covalently linked to said B-C subunit via an amide bond from the secondary amino group of the pyrrole moiety of the A subunit to the C-2 carboxyl group of the B moiety of the B-C subunit, wherein the formulae (A-3) and (A-4) are as follows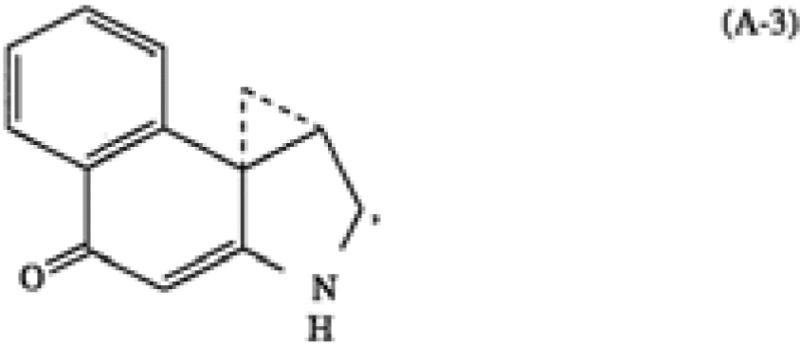 […] wherein the formulae (F-3) to (F-6) are as follows: 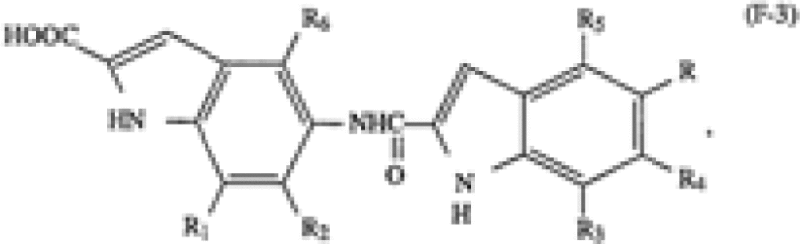 […] |
| De-immunized bouganin protein toxin | US8716234 | Merck | A method of inhibiting or destroying a lymphoma cancer cell comprising administering a cytotoxin to an animal having lymphoma, wherein said cytotoxin comprises a targeting moiety that binds to the lymphoma cancer cell and is linked to a modified bouganin protein, wherein said modified bouganin protein comprises AA sequence SEQ ID NO: 11, […] |
| Tubulysins | US2015141646 | Concortis | 1. A compound having the structure of Formula I: 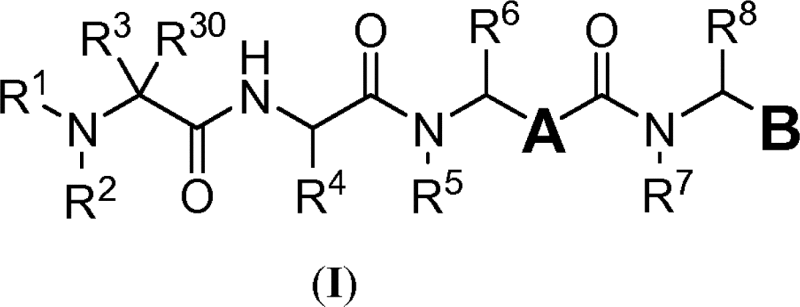 or a pharmaceutically acceptable salt thereof, wherein: A is a tubulin binding moiety; B is a functional moiety; […] |
| Benzodiazepine derivatives | US8765740 | ImmunoGen | 1. A compound represented by the following formula: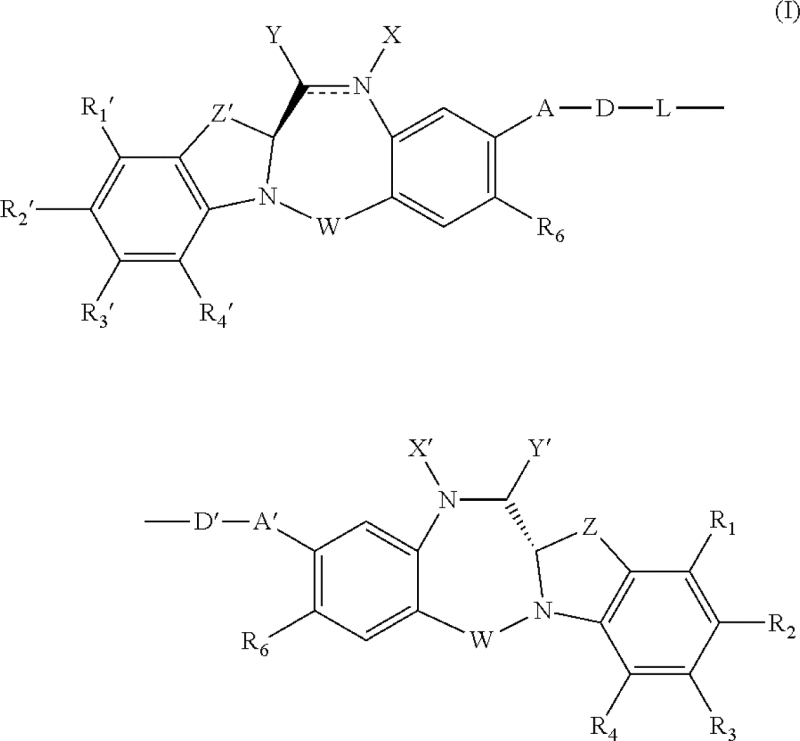 or a pharmaceutically acceptable salt thereof, wherein: the double line – between N and C represents a single bond or a double bond, […] |
| Pyrrolobenzodiazepine | US2013028919 | Seattle/Spirogen | A Conjugate having formula L-(LU-D)p or a pharmaceutically acceptable salt or solvate thereof; wherein L is a Ligand unit, LU is a Linker unit, p is 1 to 20; and D is a Drug unit comprising a PBD dimer having the following formula II 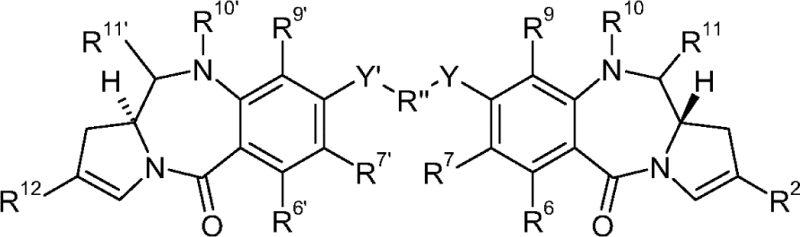 wherein: R2 is 2'X […] |
| Pyrrolobenzodiazepines | US20110256157 | Spirogen | A conjugate of formula (A)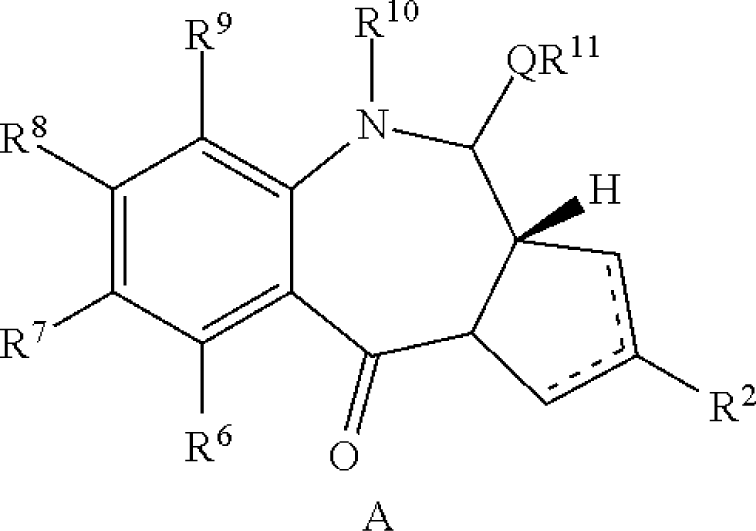 and salts and solvates thereof, wherein: the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; […] or the compound is a dimer with each monomer being of formula (A), or with one monomer being of formula (A) and the other being of formula (B): 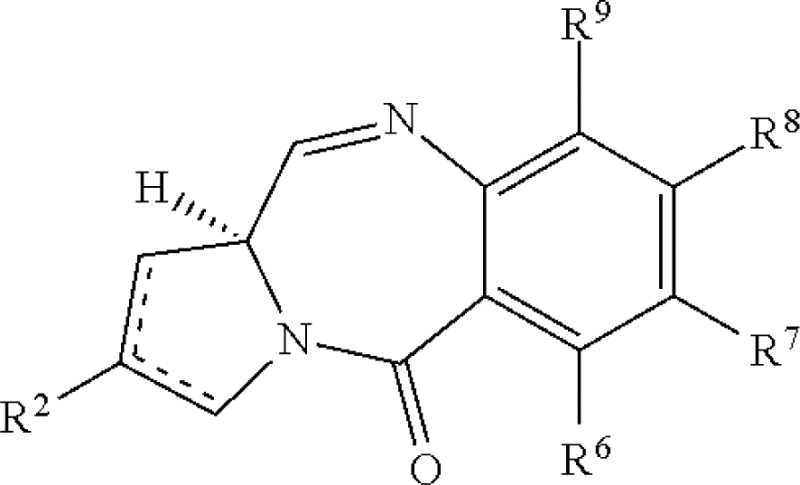 wherein […] |
expired; the symbol […] indicates that claim language has been truncated.
Interestingly, of 47 ADC candidates in clinical studies as of 2014,14 16 candidates use maytansinoids, while 22 candidates use auristatins. In most cases, a license, or a purchase of the respective toxin, from ImmunoGen, or Seattle Genetics, respectively, would be necessary, because these 2 companies are the major IP holders regarding these toxins, or toxin-linker combinations (see Table 7).
With respect to ado-trastuzumab emtansine, which carries ImmunoGen's maytansinoid DM1, the original deal forged between Genentech and ImmunoGen provided 2 mn USD upfront payments, plus 44 mn USD in milestone and royalty payments.15 This is quite modest in view of the commercial power of ado-trastuzumab emtansine, which was launched in February 2013, achieved sales of 91 mn USD in the first half of 2013, and may achieve sales of 2.55 bn USD in 2018 according to analysis by Fierce Pharma. It appears, however, that in more recent deals, ImmunoGen has negotiated more favorable terms. A deal signed with Novartis in 2010 provided an upfront payment of 45 mn USD, and milestone payments totaling ∼200 mn USD per target, plus royalties on the sales. ImmunoGen's other deals include, among others, Eli Lilly (2011, 20 mn USD upfront and approximately 200 mn USD per target in milestones), Takeda (2015, 440 mn USD upfront and milestones), or Sanofi (2006, 32 mn USD upfront and milestones per target),16 demonstrating that there is substantial value even in just the toxin technology used in ADCs.
The first experimental ADCs used cytotoxic payloads that were already established in conventional chemotherapy, e.g., methotrexate,17 vinblastine.18 However, ADCs have a distinct advantage over conventional chemotherapy because they can direct their toxic payload to the target tissue with high specificity. For this reason, this approach opens the possibility to reduce non-specific side effects common to chemotherapy, thus broadening the therapeutic window. This again allows the use of toxic payloads that otherwise would be too toxic for systemic administration, or have a too short half-life in the human plasma.
On the other hand, antibodies are relatively large molecules (the molecular weight of an IgG is ∼150 KDa), compared to which the toxin, which is commonly an organic molecule, is small (molecular weight usually in the range of 0.3 to 1 KDa). Therefore, the total amount of toxin that can be administered with ADC therapy is relatively small. Accordingly, an ADC carrying 4 toxin molecules comprises only 0.8–2.67% w/w toxin. Further, it has been reported that, despite the target specificity an ADC has due to its antibody component, only 1.56% of the administered dose of the toxin will reach the intracellular target.19 This in turn means that a toxin candidate must show high potency to be useful in ADC therapy.
As discussed already, 3 classes of toxins have been typically used in ADCs, namely maytansinoids, auristatins and calcheamicins. Other classes are in clinical or preclinical trials,20 including pyrrolobenzodiazepines and other benzodiazepine derivatives, duocarmycins, tubulysins, α-amanitin or bouganin protein toxin (see Table 7).
Most of these toxins are significantly more potent than toxins used for conventional chemotherapy. In any case, the discovery of a new toxin (either a derivative from an existing class or a whole new class) that can be used in ADCs can give rise to specific patent protection. The same applies for the transfer of an existing toxin into the ADC context. Typical patent categories that cover such types of innovations are shown in Table 8.
Table 8.
Claim categories for different types of toxin inventions
| Subject matter | Claim category | Claim language |
|---|---|---|
| New class of toxin | Compound protection | Compound according to the general structural formula X, with R1 – Rx being [.] |
| Class is known, but specific toxin is novel | Compound protection | Compound of class X, having the following structural formula Y |
| Class or specific toxin is known, but transfer to ADC is novel | (i) Use/process protection or (ii) purpose bound compound protection | (i) Use of toxin x for the manufacture of an ADC(ii) Toxin x for use in an ADC for the treatment of cancer |
the symbol […] indicates that claim language has been truncated.
Second medical use patents and other higher generation patents
Second medical use claims strive to protect the use of a given drug for a new indication discovered after the drug (and at least one medical indication) was already known. They usually specify the drug and its new use in a language such as “antibody X for the treatment of disease Y.”
If an ADC comprising a given antibody is used for the treatment of a disease that is the subject of a third-party patent claiming a second medical use of that naked antibody, it should be thoroughly checked as to whether or not said use for the ADC falls under the scope of said second medical use patent. The fact alone that the antibody has been structurally modified by conjugating a toxin thereto does not automatically mean that it would no longer fall under the scope of such patent, at least as long as the claim language does not exclude such modification explicitly. Similar logic applies to other higher generation patents, like dosage patents or formulation patents, in which the claims refer to a naked antibody. However, it appears unlikely that an ADC using a given naked antibody would use the same dosage or formulation.
Table 9 shows typical claim categories for second medical use antibody patents and other higher generation antibody patents. With ADCs, the same rules exist as with naked antibodies, and similar second generation claim categories can hence be generated. Table 10 shows some examples of higher generation ADC patents.
Table 9.
Claim categories for higher generation antibody patents
| Category | Example IP rights | Assignee | Target | Claim language |
|---|---|---|---|---|
| 2nd medical use | EP1734996B1 | University of California | α vbeta 5 integrin | Use of an antibody that specifically binds to α vbeta 5 integrin, wherein the antibody is a humanized form of the antibody produced by the hybridoma deposited as ATCC Deposit No. PTA-5817, for the manufacture of a medicament for treating pulmonary edema. |
| Dosage patent | EP1210115B1* | Genentech | HER2 | Use of the anti-ErbB2 antibody huMab 4D5 for treating a human patient diagnosed with a breast cancer characterized by overexpression of ErbB2, said method comprising the steps of administering to the patient an initial dose of 8mg/kg of the anti-ErbB2 antibody; and administering to the patient a plurality of subsequent doses of the antibody in an amount that is 6 mg/kg, wherein the doses are separated in time from each other by 3 weeks. |
| Formulation | EP2459167B1 | Roche | HER2 | A highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody for subcutaneous injection comprising:a. about 50 to 350 mg/ml anti-HER2 antibody;b. about 1 to 100 mM of a buffering agent providing a pH of 5.5 ± 2.0;c. about 1 to 500 mM of a stabilizer or a mixture of 2 or more stabilizers;d. about 0.01 to 0.08 % of a nonionic surfactant; ande. more than 150 to about 16′000 U/ml, about 2′000 U/ml, or about 12′000 U/ml, respectively of a hyaluronidase enzyme. |
EP Patent revoked in opposition, appeal pending. In UK finally revoked.
Table 10.
Higher generation ADC patents
| Category | Example IP rights | Assignee | Target | Claim language |
|---|---|---|---|---|
| 2nd medical use | US20110165155 | Genentech | HER2 | A method for the treatment of metastatic or unresectable locally advanced HER2 positive cancer in a patient comprising administering a therapeutically effective amount of trastuzumab-MCC-DM1 wherein the patient has been previously treated with at least 2 anti-HER2 agents. |
| Formulation | WO2014143765 | Abbvie | EGFR | A formulation comprising an anti-Epidermal Growth Factor Receptor (EGFR) antibody drug conjugate (ADC), a sugar, histidine, and a surfactant, wherein said formulation has a pH of about 5 - 7, and wherein said anti-EGFR ADC comprises an anti-EGFR antibody, or antigen-binding portion thereof, conjugated to an auristatin. |
Specific combination of antibody and toxin
The specific combination of an antibody and a toxin can be also patent eligible. A given tumor cell type characterized by a specific antigen can, for example, be particularly susceptible to a given toxin conjugated to an antibody against said target. In such case, patent claims providing the broadest protection would merely recite the antibody target and the toxin class.
If such a broad concept is already anticipated, or does not meet the non-obviousness/inventive step criterion, fallback positions for the antibody component could focus on a target epitope, a specific binding behavior or a structurally defined antibody. For the toxin component, fallback positions lie in a more restricted definition of the specific toxin. Even though narrow on paper, such restricted claims can still provide meaningful patent protection when backed by a respective marketing authorization.
So far, the legal framework for ADC biosimilar products is far from clear, and the US and European Union regulatory agencies have only recently begun evaluations of biosimilar antibodies. However, experience with regulations for generics and biosimilars teaches that, once established, respective approval pathways do not allow any chemical modifications of the toxin or sequence modifications of the antibody. Therefore, although the scope of such claims could theoretically be bypassed by even minor modification of either the toxin or the antibody, such strategy would necessitate an entirely new approval, because the product would no longer be a biosimilar. Table 11 shows examples of patents claiming specific antibody/toxin combinations.
Table 11.
Examples of patents claiming specific antibody/toxin combinations
| Category | Example IP right | Assignee | Claim language |
|---|---|---|---|
| Combination of target and toxin class | US8337856 | ImmunoGen | An immunoconjugate comprising an anti-ErbB2 antibody conjugated to a maytansinoid, wherein the antibody is huMAb4D5–8 |
| Combination of specific antibody and toxin | US8153768 | Wyeth Holdings Corporation | A composition comprising a drug conjugate, wherein said drug conjugate comprises calicheamicin derivatives and an anti-CD22 antibody and has the formula: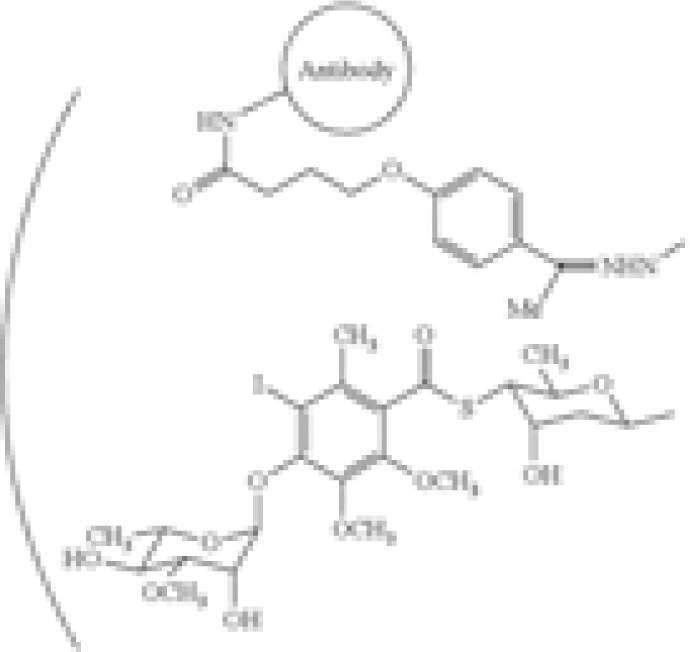 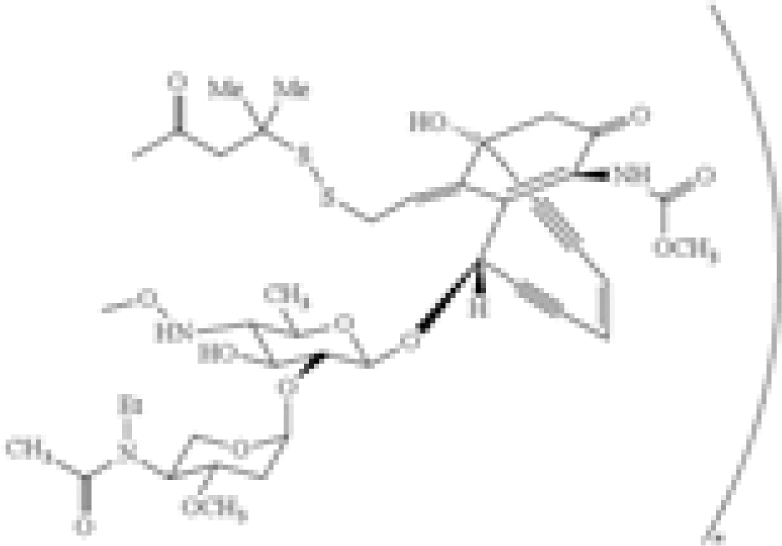 [b]wherein the antibody comprises SEQ ID NO. […] [b]wherein the antibody comprises SEQ ID NO. […] |
the symbol […] indicates that claim language has been truncated.
Non-obviousness / inventive step
Patent claims relating to ADCs or their components must pass the test on non-obviousness, as codified on 35 USC. § 103, or its European counterpart, inventive step, as codified in Art. 56 of the European Patent Convention. Both tests decline patentability to an alleged invention, even if it is novel, in case it was obvious for a person of skill in the art in view of existing prior art. In the US, different tests have been applied in the past.
In the decision KSR v. Teleflex,21 the US. Supreme Court set forth that the true test of nonobviousness is the so called “Graham analysis,” thus denouncing a different type of test that was used by the lower instance courts, which the Supreme Court deemed too liberal. The Supreme Court declared that the bar on patents claiming obvious subject matter should not be confined within a test „too constrained to serve its purpose.“ In the Graham analysis, the courts examine the scope and content of the prior art, the level of ordinary skill in the art; the differences between the claimed invention and the prior art; and objective evidence of non-obviousness. Examples for the latter are, e.g., commercial success, long-felt but unsolved needs, and failure of others.
The EPO applies the so-called “problem solution approach.” In this test, the closest prior art is defined first. Second, the difference between the claimed invention and the prior art is determined, and its technical effect is established. Accordingly, it is stipulated that it would be the objective technical object of the alleged invention to obtain such effect started from the closest prior art. Eventually, it is considered whether it was obvious for the person of skill to solve this objective technical problem.
Despite all attempts to make the aforementioned tests as reproducible as possible, large uncertainties still exist. The practical implementations differ not only between the US and European Union or other jurisdictions, but also between different technical disciplines, and even between different examination divisions in the same jurisdiction.
Table 12 gives an overview of the European patents protecting gemtuzumab ozogamicin, ado-trastuzumab emtansine and brentuximab vedotin (see also Table 1), and the reasons why the respective examiner found the claimed subject matter inventive over the prior art. It can be seen that in each case, the respective examiner's motif to allow the patent was different.
Table 12.
European patents protecting gemtuzumab ozogamicin, ado-trastuzumab emtansine and brentuximab vedotin
| EP Patent | Claimed subject matter | Closest prior art | Why inventive? |
|---|---|---|---|
| EP0689845B1* | ADC with calicheamicin and hydrazine linker | EP0392384 discloses Calicheamicin succinimidyl derivatives conjugated to an antibody | Examiner accepted that “nothing in the prior art suggests that the use of the current linker system to bind calicheamicin to an antibody would yield conjugates having high immunoaffinity to the target, low toxicity and high antitumour activity.” (EP office action of May 11, 2001) |
| EP2283867B1 | Trastuzumab maytansinoid conjugate for treatment of cancer over-expressing ErbB2 | WO0069460 discloses trastuzumab, plus generally mentions combination therof with maytansine |
Examiner accepted applicant's arguments of Nov 18, 2013, that (i) the specific selection of trastuzumab and maytansine would be novel over WO'460. In support of inventive step, applicant argued (ii) against Chari et al (1992), which discloses a murine anti-cancer ADC comprising a maytansinoid and an anti ErbB2 antibody as not being the closest prior art, and (ii) that it was surprising that trastuzumab retained its cytostatic activity in an ADC, and would not be degraded to a mere targeting device, thus leading to an ADC where the antibody and the toxin act in concert. Applicant had also argued that at the priority date, there was uncertainty regarding the therapeutic potential of immunoconjugates. |
| EP2353611B1 | ADC with Pentapeptide linker plus auristatin | WO02088172 discloses pentapeptide linkers, and ADCs using them, but not auristatin | WO0208817 was the only prior art document, but although pre-filed, published after the priority date, and did thus not affect inventive step |
expired
A look into the board of appeal decisions database of the EPO does not provide better guidance either. In June 2015, only 2 decisions existed that relate to patentability issues of ADC patents, one of which is referring to sufficiency of disclosure, while the other one refers to inventive step. Table 13 shows these 2 decisions. Again, no true guidance can be derived from these decisions. Thus, unlike in naked antibody patent claims that specify the antibody by a particular sequence, and where a clear guidance as to how inventive step is to be assessed exists,22 no such guidance has so far been established with respect to inventive step questions of patent claims for ADCs.
Table 13.
Abstract of the 2 EPO Appeal decisions that relate to ADC patents
| Decision | Date | Patent/ application | Claimed subject matter | Outcome |
|---|---|---|---|---|
| T 0619/01 | August 2004 | EP0634938A1* | conjugate of SMPT linked-humanized M195 antibody and 2-iminothiolane modified gelonin or recombinant gelonin for the treatment of leukemia | Claims found unallowable for insufficient disclosure, because the term “term “recombinant gelonin” was not properly defined. The only vague definition (JM105 E. coli expressing optimized gelonin”) would leave the burden of finding out how to arrive at recombinant gelonin entirely upon the skilled reader. |
| T 1014/01 | Nov 2003 | EP0439095B1** | Antibody-based fusion protein comprising an Ig portion capable of directing the fusion protein to a tumor, an IL2 molecule capable of promoting lymphocyte proliferation, and a modified IgG1 hinge region with the 2 Cys residues replaced by Pro and Serine to permit greater flexibility in the fused molecule. | Claim 1 (3rd request) was found inventive because prior art suggested that the hinge feature would avoid the reactive side groups in the AA sequence, not for increasing the flexibility. Further, prior art would contains no pointer to replace Cys with Pro, while prejudice against the use of Pro (“helix-killer”) would exist. 2nd request, which recited, as an alternative, also a natural hinge region, was not deemed inventive. |
rejected;
expired
It needs to be recalled, in this context, that combining a particular toxin with superior potency and an antibody with excellent target affinity does not necessarily yield a clinically effective ADC. The binding process, or the linker as such, can affect either the toxin, or the antibody, or both. Likewise, the toxin may not be adequate for the target bound by the antibody. As can be seen in Table 12, Genentech has successfully used this line of argumentation when defending their patent application EP2283867, protecting ado-trastuzumab emtansine, over their own prior art, which disclosed trastuzumab, plus generally mentioned the combination thereof with maytansinoids.
For these reasons, the mere provision of a functional ADC comprising a known toxin and antibody can already meet the inventive step criterion, at least in the recent past. However, with technical progress, the likelihood that a prior art benchmark exists already (e.g., an ADC using, for example, the same antibody, or at least an antibody against the same target), increases. The patentee may in such case be required to provide further arguments, or restrict the claim scope, to meet the non-obviousness/inventive step criterion.
This criterion is thus a moving target, where, with technical progress of the respective discipline, requirements are changed accordingly. Predictions of the requirements the offices will set with respect to a given ADC patent application are thus educated guesses that take into account the applicable state of the art, not only with respect to the target, but also with respect to the toxin and the linker technology.
One objective evidence for inventive step commonly accepted by EPO examiners is if the novel embodiment has surprising properties. In the ADC world, and in particular regarding specific antibody-toxin combinations, this would mean that a specific characteristic of a claimed ADC would be unprecedented even in view of the prior art benchmark. Such characteristic would not necessarily mean efficacy or potency. It could also be reduced side effects or, at least in the US and European Union, even non-clinical advantages, e.g., shelf life, ease of manufacture.
The problem of trade secrets
The term “trade secret” relates to a product, process or know how that provides a commercial advantage to its owner or creator, who, however, prefers to not make it the subject of a patent application. In some cases, creators or owners of such embodiments see it as a problem that the content of a patent application is published after 18 months, while it is not yet clear at that time whether a patent will be awarded on the respective subject matter or not. Further, if eventually granted, a patent expires roughly after 20 years, after which the content of the patent becomes public domain. As a consequence, an invention may not be a subject of a patent application, but rather it may be kept a trade secret. While this strategy does not work with products that are to be sold (because putting them to the market without patent protection makes such products public domain), it may have its merits with respect to technologies, in particular methods, that are practiced in-house, at least in cases where a marketed product does not carry any traceable marks that enable competitors to reverse-engineer said technology.
A trade secret, however, is not an exclusive right. If the secret is disclosed, unintentionally, intentionally or even in bad faith, its content becomes public domain immediately, and can be practiced by competitors. Therefore, in a strategy that relies on trade secrets, measures must be taken that ensure these secrets cannot be disclosed, e.g., by former employees, nor be discovered by reverse-engineering. In cases when the owner of a trade secret collaborates with third parties to whom said trade secret needs to be disclosed, non-disclosure agreements should be signed. In case such agreement is violated by the third party, the latter will be liable for damages; however, the disclosure as such involving the loss of the trade secret cannot be undone.
For ADCs, the supply chain can be very long. While an innovator can be responsible for a series of innovative technologies covering the entire supply chain of an ADC (encompassing, antibodies, linker technology, toxins, methods of production and so forth), reality shows that, at least when commercialization starts, different steps of the supply chain are taken over by contract manufacturers.23 In such an environment, where different steps are outsourced to different partners, a trade secret strategy bears particular risks, which may result in the loss of a given trade secret and, accordingly, availability of the respective technology to competitors. Therefore, ADC innovators should use trade secrets with utmost care, and consider relying on patent applications to protect their intellectual property.
Immunotoxins and radiolabelled antibodies
Although not specifically discussed herein, most of the principles set forth above do also apply to immunotoxins and radiolabelled antibodies. Immunotoxins are fusion proteins that consist of a targeting protein, ideally an antibody, fused to a toxic protein. Immunotoxins offer advantages over ADCs in manufacture (because they can be produced by recombinant protein expression in one step) as well as to site specificity and stoichiometry of the conjugation. Disadvantages may lie in an increased immunogenicity. So far only one immunotoxin has received approval in the US, namely denileukin diftitox (Ontak®), which consists of an interleukin-2 protein fused to a diphtheria toxin.
Radiolabelled antibodies are antibodies that carry a radioactive isotope. These conjugates do not have to be internalized into the cells to become effective, and can thus also be used to attack cellular targets that do not involve internalization, just like the CD20 receptor. Two radiolabelled antibodies have been approved, Genentech's 90Y ibritumomab tiuxetan (Zevalin®; Key IP right: EP1112084B1) and 131I tositumomab (Bexxar®; Key IP right: US6090365; marketing discontinued in 2014), which both include an anti-CD20 antibody component. Both have been the subject of litigation between Genentech and GlaxoSmithKline.24
Case study 1
Because of their commercial potential, ADC patents will inevitably become the subject of IP litigation. One very recent dispute is evolving between Phigenix, based in Atlanta, Georgia, and Genentech of South San Francisco. Phigenix, who on their website claim that they “will leverage licensed patented technology to establish a strong first-mover advantage in Personalized Medicine and forge a lasting leadership position in the rapidly evolving cancer diagnostic and therapeutics industry,” has in 2014 filed requests for inter partes review (IPR) against Genentech's US Patent 7575748 (IPR2014–00842) and ImmunoGen's US patent 8337856 (IPR2014–00676), which both protect Genentech's ADC ado-trastuzumab emtansine. Further, Phigenix sued Genentech on Jan 31, 2014 for patent infringement of their own US patent 8080534 in the Georgia Northern District Court (1:14-cv-00287). Table 14 shows the respective patents and selected claim language. As discussed above, Genentech has acquired a license from ImmunoGen for use of the SMCC linker and the DM1 toxin conjugated to trastuzumab. A conjugate of said toxin-linker combination with an antibody was protected, among others, by ImmunoGen's patent US5208020 (now expired; see Table 5).
Table 14.
Patents that play a role in the Phigenix-Genentech dispute
| Novel embodiment | Assignee | Case No | Claim language |
|---|---|---|---|
| US7575748 | Genentech | IPR2014–00842 | 1. A method for the treatment of a tumor in a mammal, comprising the steps of (i) identifying said tumor as being characterized by overexpression of an ErbB2 receptor and as being a tumor that does not respond, or responds poorly, to treatment with an anti-ErbB antibody, and (ii) intravenously administering to the mammal a therapeutically effective amount of a conjugate of a humanized antibody huMab 4D5–8 covalently linked via a thioether linking group with a maytansinoid DM1 having the structure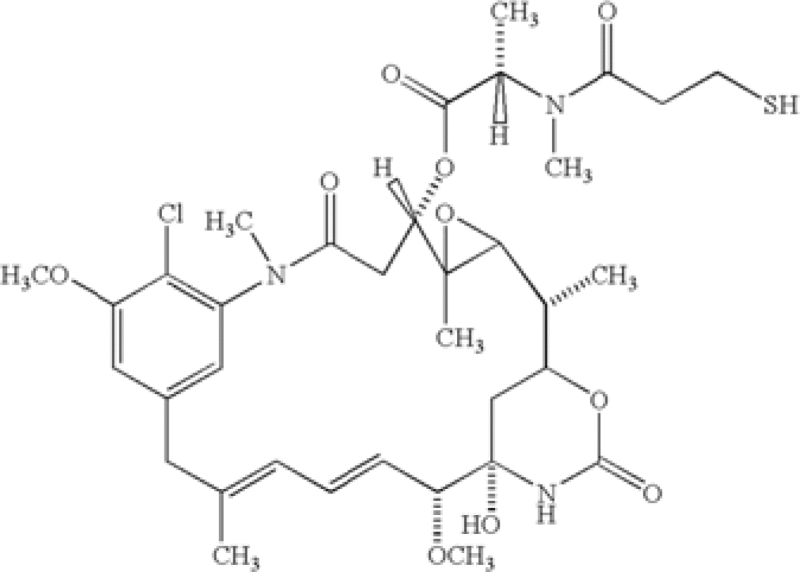 at a dose of between about 0.2 mg/kg and about 10 mg/kg (antibody-maytansinoid conjugate weight/body weight) and at a frequency of dosing selected from the group of dosing frequencies consisting of bolus, less than about 1 time per week, one time per week, 2 times per week, more than 2 times per week, and continuous infusion, whereby said tumor characterized by overexpression of an ErbB2 receptor and that does not respond, or responds poorly, to treatment with an anti-ErbB antibody, is treated. |
| US8337856 | ImmunoGen | IPR2014–00676 | 1. An immunoconjugate comprising an anti-ErbB2 antibody conjugated to a maytansinoid, wherein the antibody is huMAb4D5–8 |
| US8080534 | Phigenix | 1:14-cv-00287 | 1. A method for treating a breast condition in a subject, comprising administering to a breast tissue of the subject, a composition that (1) inhibits PAX2 expression or PAX2 activity, (2) expresses DEFB1 or (3) inhibits PAX2 expression or PAX2 activity and expresses DEFB1. 15. The method of claim 1, further comprising the step of: administering to the subject an effective amount of an anti-HER-2 agent. 16. The method of claim 15, wherein the anti-HER-2 agent is trastuzumab. 19. A method for treating a breast condition in a subject, comprising:(a) determining the PAX2-to-DEFB1 expression ratio in a diseased breast tissue from said subject;(b) determining the ER/PR status of said diseased breast tissue from said subject; and(c) based on the results of (a) and (b), administering to a breast tissue of said subject, a first composition that (1) inhibits PAX2 expression or PAX2 activity, (2) expresses DEFB1 or (3) inhibits PAX2 expression or PAX2 activity and expresses DEFB1. |
On December 9, 2014, the Patent Trial and Appeal Board (PTAB) of the USPTO denied institution of IPR2014–00842 against US7575748, on the grounds that Phigenix did not establish a reasonable likelihood of prevailing with respect to any challenged claim. Phigenix' attacks were based on alleged obviousness in view of the trastuzumab (Herceptin®) 1998 label and several prior art documents. According to the Board, which applied the “broadest reasonable construction in light of the specification of the patent,” Phigenix failed to explain adequately how, nor provided sufficient evidence indicating that, the teaching in the trastuzumab label that certain patients failed to respond to the product would have motivated an ordinary artisan to treat such patients using a trastuzumab (huMab 4D5–8) conjugate.
In contrast thereto, IPR2014–00676 against US8337856 was instituted on October 29, 2014. The PTAB found that Phigenix has demonstrated that there is a reasonable likelihood that it would prevail on the ground that claims 1–8 of the patent would have been obvious over some of the prior art documents in view of the trastuzumab label. This, however, is not a final determination on the patentability of the challenged claims. IPR proceedings were thus instituted. The case is ongoing as of mid-2015.
Regarding the litigation at the Georgia Northern District Court, Phigenix asserted that Genentech would infringe their patent US8080534 via certain acts, “directly and/or indirectly, of making, using, selling, or offering for sale the drug ado-trastuzumab emtansine under the trade name Kadcyla®, and inducing healthcare professionals to prescribe and administer Kadcyla®,” among others by distribution of the respective prescribing information.
The infringement contentions were served to Genentech's attorneys in September 2014, but are so far not public yet. As of mid-2015, Phigenix has not publicly disclosed which part of ado-trastuzumab emtansine they believe to inhibit PAX2 expression or PAX2 activity and/or express DEFB1, as set forth in claim 1 of Phigenix's patent. However, dependent claim 15 stipulates that the therapy can also comprise administration of an anti-Her-2 agent. This suggests that Phigenix considers the DM1 toxin as the part of ado-trastuzumab emtansine that inhibits PAX2 expression or PAX2 activity and/or expresses DEFB1, as set forth in claim 1, while the trastuzumab part would be defined in said dependent claim 15.
Furthermore, claim 19 claims administering the composition to a patient after determining the PAX2-DEFB1 expression ratio and the ER/PR status in a breast cancer tissue isolated from the patient. However, the prescribing information,25 of ado-trastuzumab emtansine does not mention either PAX2 or DEFB1. Therefore, it remains unclear so far what exactly the infringement contentions refer to. Upon motion of Genentech, the infringement action was transferred to the Northern District of California Court on March 17, 2015 (3:2015cv01238), where it is still pending.
This case impressively demonstrates how even originators of a naked antibody can run into patent litigation when marketing an ADC, even if said ADC comprises their established antibody, and even if the respective toxin and linker technology have been in-licensed. Further, this case shows that even having obtained a lege artis FTO opinion – an exercise Genentech has undoubtedly gone through before marketing ado-trastuzumab emtansine – does not provide a guarantee against IP attacks from unforeseen corners. This again demonstrates the complexity of the IP landscape in the ADC field, and the residual risk players must deal with even with a proper FTO opinion in their hands.
Phigenix also filed an opposition against ImmunoGen's EP counterpart of US patent 8337856, EP2283867 (see Tables 1 and 12) on February 19, 2015. In the opposition, Phigenix alleges that the patent claims would lack novelty over WO0069460, and lack inventive step over, among others, a journal article authored by Chari et al.,26 and the trastuzumab label, in combination with other documents disclosing maytansinoid toxins. While Phigenix thus largely relies on prior art that has already been considered by the office (see Table 12), their main line of argumentation is that the selection of trastuzumab and a maytansinoid would not be a specific selection that would provide novelty over WO0069460. Further, they argue that Chari et al. would indeed be the closest prior art, as it relates to a functional anti-cancer ADC comprising a maytansinoid and a murine anti-ErbB2 antibody, thus rebutting Genentech's arguments according to which it was surprising that trastuzumab retained its cytostatic activity in an ADC, and would not be degraded to a mere targeting device.
A first decision in this case cannot be expected prior to mid-2016. Interestingly, on February 2015, Genentech received the allowance for another EP application of the same family, EP2283866, with an almost identical claim scope. The opposition term of this patent will be open until November 2015.
Case study 2
HER2 seems to be an attractive target for ADC therapy, not only because it has proven safe and efficacious in antibody therapy, but also because it meets the other requirements set forth above, including rapid internalization.27 Unsurprisingly, Genentech is not the only company that has an anti-HER2 ADC in their portfolio. In some way, ado-trastuzumab emtansine can be considered a first-generation ADC, while second-generation anti-HER2 ADCs are already under development.
Based in Nijmegen, The Netherlands, Synthon has developed SYD985, which comprises trastuzumab conjugated to a cleavable linker and duocarmycin payload. According to Synthon, SYD985 is also active against tumors that exhibit low expression of HER2, and does thus allow extension of the target population of cancer patients who may respond to this treatment to include FISH (fluorescence in situ hybridization)-negative/ immunohistochemistry (IHC)-HER2 1+ and 2+ patients.28 According to the FDA label, ado-trastuzumab emtansine is, however, only indicated for the treatment of patients with HER2-positive breast cancer in which the tumors show HER2 overexpression defined as 3+ IHC. While trastuzumab is beginning to come off patent (EP0590058 has expired June 15, 2012), the linker technology and the duocarmycin used in SYD985 are being pursued in pending application EP2560645 (see Table 12), assigned to Syntarga, which is a subsidiary of Synthon. Although SYD985 addresses a similar market as ado-trastuzumab emtansine, and clinical trials are currently under way,29 it has, so far, not been subject of a patent litigation by third parties. It appears that SYD985 would at least not fall under the scope of US8337856 protecting ado-trastuzumab emtansine (see Table 15) because SYD985 does not comprise a maytansinoid.
Table 15.
Details of different anti-HER2 ADCs in development
| ADC name | Antibody | Linker | Toxin | Key IP right | Company | Status | Claim language |
|---|---|---|---|---|---|---|---|
| Trastuzumab emtansine | Trastuzumab | SMCC | DM1 | US8337856 | Genentech | Approved in US and EU | An immunoconjugate comprising an anti-ErbB2 antibody conjugated to a maytansinoid, wherein the antibody is huMAb4D5–8. |
| MM-302 | scFv anti-HER2 | PEG-DSPE | Liposome-encapsulateddoxorubicin | US2014023698 | Merrimack | Phase 2/3 | A method of treating a human cancer patient by administration of anthracycline - comprising anti-HER2 immunoliposomes, the method comprising determining a first dosage, such a dosage indicating a dose magnitude and frequency of dosing, for a patient diagnosed with a cancer characterized by expression of HER2 receptor, the first dosage being for a liposomal anthracycline chemotherapeutic agent that does not comprise an immunoliposome, which dosage is determined to provide to the patient a safe and effective amount of the liposomal formulation, and administering anthracycline-comprising anti-HER2 immunoliposomes, a plurality of which immunoliposomes is each bearing a plurality of anti-HER2 antibody molecules on its surface and each containing the anthracycline chemotherapeutic agent, wherein the anthracycline-comprising anti-HER2 immunoliposomes are administered to the patient at the first dosage. |
| SYD985 | Trastuzumab | SpaceLink | Duocarmycin | EP2560645A2 | Syntarga | Phase 1 | A compound of formula (III):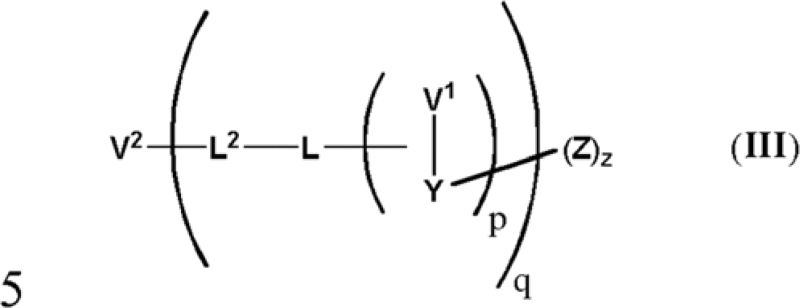 or a pharmaceutically acceptable salt, hydrate, or solvate thereof, wherein […] |
| ARX788 | Undisclosed | Undisclosed | Amberstatin AS269 | US2015141624 | AmbrX | Preclinical* |
 wherein […] |
| Not available | Trastuzumab | Transglutaminase + EENC linker | MMAE, hybrid ADCs with different toxins | Not available | Dophen | R&D | Not available |
| Not available | Trastuzumab | SMAC with N-terminal LPTXG tag/linker | (i) DM1 (mertansine), (ii) maytansine, (iii) MMAE/MMAF (iv) α-amanitin (v) undisclosed new toxin, respectively |
(i) – (iv) WO2014140317 (v) not disclosed |
NBE Therapeutics | R&D | 1. A method of producing an immunoligand/payload conjugate, which method encompasses conjugating a payload to an immunoligand by means of a sequence- specific transpeptidase, or a catalytic domain thereof. 6. The method according to any of the aforementioned claims, wherein the sequence-specific transpepeptidase is at least one selected from the group consisting of• a sortase enzyme, or one or more fragments or derivatives thereof• a split-intein, or one or more fragments or derivatives thereof |
| Not available | Trastuzumab | N-Hydroxysuccinimide ester | Tubulysin | Not available | VUMC Amsterdam | R&D | Not available |
Phase 1 study (NCT02512237) scheduled to start in September 2015; the symbol […] indicates that claim language has been truncated.
The same circumstances apply to the anti-HER2 ADC ARX788 developed by Ambrx. ARX788 comprises an anti-HER2 antibody coupled site specifically to a toxin called Amberstatin (AS269) by means of Ambrx' technology using non-natural amino acids (EP1968635B1, see Table 6). Like Synthon, Ambrx claims that ARX788 serves a broader spectrum of HER2+ cancer patients than ado-trastuzumab emtansine. Similarly, it appears that ARX788 has so far not been the subject of a patent litigation by third parties. ARX788 is covered by Ambrx's international patent application WO2013192360. The linker, toxin and antibody are not chemically specified, but according to a report published in 2012,30 it appears that the antibody could be a modified trastuzumab that carries a p-acetylphenylalanine (pAcPhe) residue instead of a naturally occurring amino acid reside, to which an alkoxy-amine derivatized auristatin F (MMAF) is conjugated by oxime ligation.
Another anti-HER2-toxin construct in clinical development is MM-302 developed by Merrimack Pharmaceuticals Inc. MM-302 is not a conjugate in strictu sensu because it consists of a liposome 75–110 nm in diameter that encapsulates doxorubicin. The lipid membrane is composed of phosphatidylcholine, cholesterol, and PEGylated phosphatidylethanolamine (1 PEG molecule for 200 phospholipid molecules), wherein one PEG molecule for each 1780 phospholipid molecules bears at its end an anti-HER2 scFv antibody fragment called F5.31
Exact targeting is critical in this ADC because cardiomyocytes, which are affected by the highly cardiotoxic doxorubicin, must be avoided. The fact that doxorubicn is encapsulated by liposomes further protects the cardiomyocytes. As with SYD985 and ARX788, it appears that MM-302, which is covered by international patent application WO2012078695, has so far not been subject of a patent litigation by third parties.
Dophen Biomed of Sacramento, USA, has recently presented results32 on an anti-HER2 ADC consisting of trastuzumab and MMAE, conjugated to one another by Dophen's transglutaminase using Dophen's endosome escaping non-cleavable” (EENC) linkers (see Table 6). The company claims that this ADC has 100% stability and higher potency than a comparable ADC with a cleavable linker. Further, Dophen uses this technology to generate anti-HER2 ADC comprising trastuzumab and 2 different toxins of undisclosed nature. Quite apparently, at least one of these toxins is a tubulysin because of a collaboration with Austrian tubulysin specialist Tubepharma.33 It has been reported that such a hybrid ADC has better potency than the respective homogeneous ADCs of the same toxins, and also better potency than a 1:1 mixture of the 2 homogeneous ADCs. No patent information is available for Dophen's technologies.
NBE Therapeutics of Basel, Switzerland, has created different anti-HER2 ADCs with trastuzumab using their sortase-mediated antibody conjugation technology (SMAC) with N-terminal LPTXG tag/linker (see Table 6, WO2014140317) to couple trastuzumab to DM1 (mertansine), maytansine, MMAE, MMAF and α-amanitin, and thus create ADCs with site-specifically conjugated toxins of homogeneous DAR. The first 2 have been reported to be equally potent as ado-trastuzumab emtansine. Another ADC comprises an undisclosed new toxin, which has demonstrated significantly better potency in cells expressing only low amounts of HER2 than ado-trastuzumab emtansine.34
Still another anti-HER2 ADC has been developed by researchers of the VU University Medical Center Amsterdam, who used a moderately toxic tubulysin analog (TUB-OMOM). Tubulysins have a narrow therapeutic window and are thus interesting for ADC use. For this purpose, tubulysin-NHS-esters were coupled to trastuzumab, while for control purposes, 131I-tubulysin and 89Zr-trastuzumab were used. The conjugation reaction was 45–55% efficient, resulting in ADCs with 96–98% radiochemical purity and an average DAR of between 2 and 4. The researchers report a potency comparable to that of ado-trastuzumab emtansine, while the availability of synthetic tubulysins that are more potent than TUB-OMOM offers additional options to make more potent ADCs.35 No patent information is available for this approach. Table 15 shows some details of the anti-HER2 ADCs discussed above.
Summary
Regarding IP aspects, ADCs pose severe challenges, but also tremendous possibilities. ADCs comprise a field of their own, in which only part of the rules can be translated from antibody IP. Further, because many players are active in this field, the third-party IP situation is complicated, with many overlapping patent estates. A meaningful IP strategy for protecting ADC inventions and establishing FTO requires specific expertise in this challenging IP discipline, as well as a thorough technical understanding of both biotechnology and organic chemistry.
Disclosure of Potential Conflicts of Interest
The author is involved in the prosecution of some of the patent applications mentioned herein.
The information provided herein reflect the personal views and considerations of the author. They do not represent legal counsel and should not be attributed to Michalski · Hüttermann and Partner Patent Attorneys or to any of its clients. Patent numbers and patent lifetimes have been verified with utmost care, but no liability is taken for their correctness.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Beck A, Reichert JM. Antibody-drug conjugates: present and future. MAbs 2014; 6(1):15-17; PMID:24423577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostami S, Qazi I, Sikorski R. The Clinical Landscape of Antibody-drug Conjugates. ADC Review 2014; August 1, 2014. [Google Scholar]
- 3.Rohrer T. Consideration for the safe and effective manufacturing of antibody drug conjugates. Chemistry Today 2012; 30(5):76-79. [Google Scholar]
- 4.Wu WJ. Regulatory Perspective on Challenges in ADC Development (Keynote presentation) PepTalk 2015; January 19–23;2015, San Diego, CA. [Google Scholar]
- 5.Lash A. Antibody-drug conjugates: the next generation of moving parts. START-UP 2001; 27 Dec 2011. [Google Scholar]
- 6.DiMasi JA, Grabowski HG. The cost of biopharmaceutical R&D: is biotech different? Manag Decis Econ 2007; 28:469-479. [Google Scholar]
- 7.Mullard A. New drugs cost US$2.6 billion to develop. Nature Reviews Drug Discovery 2014; 13(12):877-877 [Google Scholar]
- 8.Grabowski H, Cockburn I, Long G. The market for follow-on biologics: how will it evolve? Health Affairs (Project Hope) 2006; 25(5):1291-301; PMID:16966725 [DOI] [PubMed] [Google Scholar]
- 9.Lash A. Antibody-drug conjugates: the next generation of moving parts. START-UP 2001; 27 Dec 2011. [Google Scholar]
- 10.Stewart M, Kent L, Smith A, Bassinder E. The special inventive step standard for antibodies. epi information 2011; 2/2011:72-76. [Google Scholar]
- 11.Bander NH. Antibody-drug conjugate target selection: critical factors. Methods Mol Biol 2013; 1045:29-40; PMID:23913139 [DOI] [PubMed] [Google Scholar]
- 12.Erster O, Thomas JM, Hamzah J, Jabaiah AM, Getz JA, Schoep TD, Daugherty PS. Site-specific targeting of antibody activity in vivo mediated by disease-associated proteases. J Control Release 2012; 161(3):804-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.List T, Neri D. Biodistribution studies with tumor-targeting bispecific antibodies reveal selective accumulation at the tumor site. mAbs 2012; 4(6):775-83; PMID:23032949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostami S, Qazi I, Sikorski R. The clinical landscape of antibody-drug conjugates. ADCReview 2014; August 1, 2014. [Google Scholar]
- 15.Lash A. Antibody-drug conjugates: the next generation of moving parts. START-UP 2001; 27 Dec 2011. [Google Scholar]
- 16.ImmunoGen press releases of October 11, 2013 (http://investor.immunogen.com/releasedetail.cfm?ReleaseID=796395); December 20, 2011 (http://investor.immunogen.com/releasedetail.cfm?ReleaseID=634459); March 23, 2015 (http://investor.immunogen.com/releasedetail.cfm?ReleaseID=902766) and December 26, 2006 (http://www.biospace.com/News/immunogen-inc-announces-that-sanofi-aventis-has/41104). [Google Scholar]
- 17.Endo N, Takeda Y, Umemoto N, Kishida K, Watanabe K, Saito M, Hara T. Nature of linkage and mode of action of methotrexate conjugated with antitumor antibodies: implications for future preparation of conjugates. Cancer Res 1988; 48(12):3330-5; Retrieved from http://cancerres.aacrjournals.org/content/48/12/3330.short; PMID:3259466 [PubMed] [Google Scholar]
- 18.Starling JJ, Maciak RS, Hinson NA, Nichols CL, Briggs SL, Laguzza BC. In vivo efficacy of monoclonal antibody-drug conjugates of three different subisotypes which bind the human tumor-associated antigen defined by the KS1/4 monoclonal antibody. Cancer Immunol Immunother 1989; 28(3):171-8; Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2784353; PMID:2784353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res 2011; 17(20):6389-97; PMID:22003066; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1417 [DOI] [PubMed] [Google Scholar]
- 20.Rostami S, Qazi I, Sikorski R. The Clinical Landscape of Antibody-drug Conjugates. ADCReview 2014. August 1; 2014. [Google Scholar]
- 21.Decision KSR Int'l Co. v. Teleflex, Inc., 550 US 398, US Supreme Court 2007. [Google Scholar]
- 22.Stewart M, Kent L, Smith A, Bassinder E. The special inventive step standard for antibodies. epi information 2011; 2/2011; 72-76. [Google Scholar]
- 23.Wooge C, Swamy J. Next-Gen ADCs: collaborating to improve supply chains for development and technology transfer with novel technology platforms. Contract Pharma 2014; Nov/Dec 2014; 16(9):50. [Google Scholar]
- 24.Storz U. Rituximab: how approval history is reflected by a corresponding patent filing strategy. MAbs 2014; 6(4):820-37; PMID:24866199; http://dx.doi.org/ 10.4161/mabs.29105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescribing information for Kadcyla® (Ado-trastuzumab emtasine); http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf [Google Scholar]
- 26.Chari RV, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, Goldmacher VS. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res 1992; 52(1):127-31; Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1727373; PMID:1727373 [PubMed] [Google Scholar]
- 27.Minami T, Kijima T, Kohmo S, Arase H, Otani Y, Nagatomo I, Kumanogoh A. Overcoming chemoresistance of small-cell lung cancer through stepwise HER2-targeted antibody-dependent cell-mediated cytotoxicity and VEGF-targeted antiangiogenesis. Sci Rep 2013; 3:2669; PMID:24036898; http://dx.doi.org/ 10.1038/srep02669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synthon press release of April 02, 2014; http://www.synthon.com/en/Corporate/News/PressReleases/Synthons-Anti-HER2-ADC-Frontrunner-SYD985-Outperforms-Only-Available-HER2-targeting-ADC.aspx [Google Scholar]
- 29. https://clinicaltrials.gov/ct2/show/NCT02277717 [Google Scholar]
- 30.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Schultz PG. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci USA 2012; 109(40):16101-6; PMID:22988081; http://dx.doi.org/ 10.1073/pnas.1211023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. http://worldwide.espacenet.com/publicationDetails/originalDocument?CC=WO&NR=2014089127A1&KC=A1&FT=D&ND=&date=20140612&DB=&&locale=en_EP International Patent Application WO2014089127A1, assigned to Merrimack. 12 June 2014; Available at. [Google Scholar]
- 32.Hu S. Homogeneous ADCs of Herceptin-MMAE with Non-cleavable Linker is more Potent than that with a Cleavable Linker. Oral presentation at ICA 7th Annual Internatl Congress on Antibodies; Nanjing, CN; April 27, 2015 [Google Scholar]
- 33.Allen L, Mi Y, Abbas M, Richter W, Hu S. Homogeneous ADCs bearing two different payloads show strong synergy in tumor killing. Poster presented at World ADC Summit; 2014; San Diego, CA Available at http://www.eposters.net/redirect/?ID=2456&UID=0&Link=http://www.eposters.net/pdfs/homogeneous-adcs-bearing-two-different-payloads-show-strong-synergy-in-tumor-killing.pdf [Google Scholar]
- 34.Grawunder U. Sortase-mediated generation of site-specifically conjugated next-generation ADCs for treatment of cancer. Oral Presentation at PEGS conference, Boston,USA, on May 06 2015; 2015. [Google Scholar]
- 35.Cohen R, Vugts DJ, Visser GWM, Stigter-van Walsum M, Bolijn M, Spiga M, van Dongen GAMS. Development of novel ADCs: conjugation of tubulysin analogues to trastuzumab monitored by dual radiolabeling. Cancer Res 2014; 74(20):5700-10; PMID:25145670; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


