Abstract
Many therapeutic monoclonal antibodies (mAbs) are clinically administered through intravenous infusion after mixing with a diluent, e.g., saline, 5% dextrose. Such a clinical setting increases the likelihood of interactions among mAb molecules, diluent, and plasma components, which may adversely affect product safety and efficacy. Avastin® (bevacizumab) and Herceptin® (trastuzumab), but not Remicade® (infliximab), were shown to undergo rapid aggregation upon dilution into 5% dextrose when mixed with human plasma in vitro; however, the biochemical pathways leading to the aggregation were not clearly defined. Here, we show that dextrose-mediated aggregation of Avastin or Herceptin in plasma involves isoelectric precipitation of complement proteins. Using mass spectrometry, we found that dextrose-induced insoluble aggregates were composed of mAb itself and multiple abundant plasma proteins, namely complement proteins C3, C4, factor H, fibronectin, and apolipoprotein. These plasma proteins, which are characterized by an isoelectronic point of 5.5–6.7, lost solubility at the resulting pH in the mixture with formulated Avastin (pH 6.2) and Herceptin (pH 6.0). Notably, switching formulation buffers for Avastin (pH 6.2) and Remicade (pH 7.2) reversed their aggregation profiles. Avastin formed little, if any, insoluble aggregates in dextrose-plasma upon raising the buffer pH to 7.2 or above. Furthermore, dextrose induced pH-dependent precipitation of plasma proteins, with massive insoluble aggregates being detected at pH 6.5–6.8. These data show that isoelectric precipitation of complement proteins is a prerequisite of dextrose-induced aggregation of mAb in human plasma. This finding highlights the importance of assessing the compatibility of a therapeutic mAb with diluent and human plasma during product development.
Keywords: aggregation, complement proteins, compatibility, diluent, therapeutic proteins, isoelectric point, monoclonal antibodies, plasma, product formulation
Abbreviations
- API
active pharmaceutical ingredient
- FDA
US. Food and Drug Administration
- IV
intravenous
- LC-MS
Liquid chromatography–mass spectrometry
- mAb
monoclonal antibody
- MFI
micro-flow imaging
- MS/MS
tandem mass spectrometry
- pI
isoelectric points
Introduction
The formation of particulate matters in injectable pharmaceuticals can adversely affect patient safety and product efficacy; 1-4 for instances, immunogenicity,4 pulmonary emboli,5 immune system dysfunction,6 organ dysfunction,7,8 and even death.5,9-12 Therefore, the levels of particulates in pharmaceuticals for injection must be adequately assessed during formulation, storage, and clinical administration. The typical quality control strategy involves release and stability testing for subvisible particulates sized ≥10 microns and ≥25 microns following the US. Pharmacopoeial Convention general chapter <788> Particulate Matter in Injections. For therapeutic proteins, it is also recommended to assess smaller subvisible particles in the range of 2–10 microns according to the recently published US. Food and Drug Administration (FDA) Guidance on Immunogenicity Assessment for Therapeutic Protein Products. Many therapeutic proteins, including monoclonal antibodies (mAbs), are clinically administered via intravenous (IV) infusion after mixing with a diluent (e.g., 5% dextrose, 0.9% NaCl). Such a clinical procedure increases the likelihood of drug-matrix interactions, which may facilitate product aggregation. Arvinte et al.13 recently showed that the therapeutic mAbs Avastin® (bevacizumab) and Herceptin® (trastuzumab) formed aggregates when mixed with 5% dextrose and human plasma in vitro. The contributing factors were suggested to be related to the exposure of hydrophobic residues or inappropriate handling of the mAb product at the clinic.14,15 However, the precise mechanisms by which a therapeutic mAb forms insoluble aggregates in dextrose and plasma were not clearly defined.
In this study, we made a similar observation that Avastin and Herceptin, but not Remicade® (infliximab) rapidly formed insoluble aggregates after mixing with 5% dextrose and human plasma in vitro. Notably, dextrose-mediated protein aggregation was only observed for those products that are formulated in acidic pH buffer (pH 6.0–6.2). The insoluble aggregates were found to contain several abundant plasma proteins, namely complement C3, C4, and factor H, whose isoelectric points (pI values) are close to the acidic pH of Avastin formulation (pH 6.2). Our studies reveal that those abundant plasma proteins (pI ˜5.5–6.7) undergo isoelectric precipitation when mixed with 5% dextrose in an acidic buffer (pH 6.0–6.2), which in turn leads to co-precipitation of mAb molecules through interactions with the complement proteins.
Results
Characterization of protein aggregates formed when mixing therapeutic mAbs with dextrose and human plasma in vitro
Several therapeutic mAbs were shown to form insoluble aggregates when mixed with dextrose and human plasma in vitro;13 however, the biochemical pathways leading to the aggregation were not clearly defined. We sought to identify factors in diluent and product formulations that contribute to aggregation of therapeutic mAbs in plasma. To this end, mAb-containing protein aggregates were generated following the procedures previously described.13 Briefly, aliquots (10–25 µl) of mAb solution were diluted into 300 µl of 5% dextrose or 0.9% saline followed by addition of human plasma (10 µl), resulting in a mixture with a typical volume ratio at mAb (1) : diluent (30) : plasma (1). The mixture was incubated at 25°C for 30 min and then centrifuged at 21,000 g for 3 min at 25°C. The particulate morphology and counts in the mixtures of Avastin (3 µL), 5% dextrose (1 mL), and plasma (3 µL) were determined using micro-flow imaging (MFI) analysis. The particles are amorphous and tend to be translucent especially for the smaller particles (Fig. 1A). After 30 min incubation, the mixture was found to contain 478,196 ± 63,195 particles/mL, predominantly (>90%) in the size range of 1–2 µm. The particulate counts dropped to 25,719 ± 2,328 particles/mL in the supernatant post-centrifugation (Fig. 1B), which is comparable with the basal level of particulates (13,940 ± 3,781 particles/mL) detected in the mixture of Avastin with saline and plasma. This data shows that the centrifugation procedure was effective in isolating at least 95% of insoluble particulates present in the mixture, and therefore such procedures were followed in this study to assess protein aggregates formed under different conditions. The insoluble aggregates were collected by centrifugation and resolved onto SDS-PAGE (Fig. 2A). Aliquots of individual mAb and human plasma alone were included as controls. As expected,13 all 3 mAbs tested formed little (if any) insoluble aggregates when mixed with saline (0.9% sodium chloride) and human plasma (lanes 5–7). By contrast, Avastin and Herceptin, but not Remicade, formed substantial insoluble aggregates when mixed with dextrose and human plasma (lane 8–10).
Figure 1.
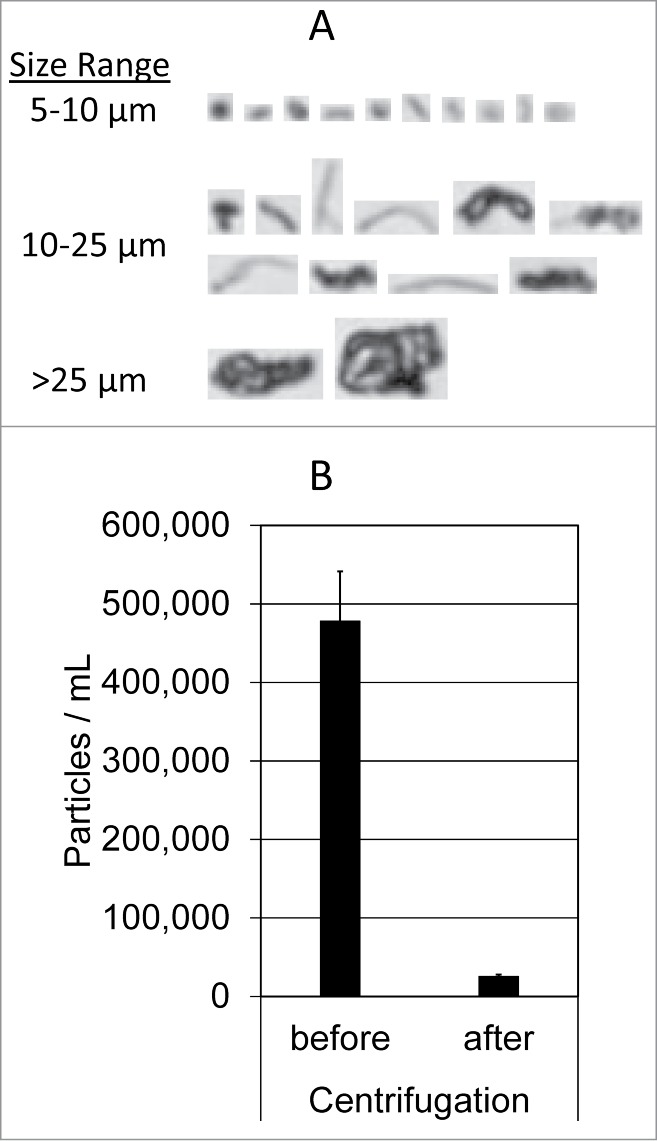
MFI analysis of insoluble aggregates. (A) Representative MFI images of particles (>5 µm) formed in the mixture of Avastin, 5% dextrose, and human plasma. (B) Particle counts in the sizes of 1–70 µm were determined using MFI for the resulting mixtures before and after centrifugation at 21,000 g for 3 minutes. Shown are representatives of 3 independent experiments.
Figure 2.
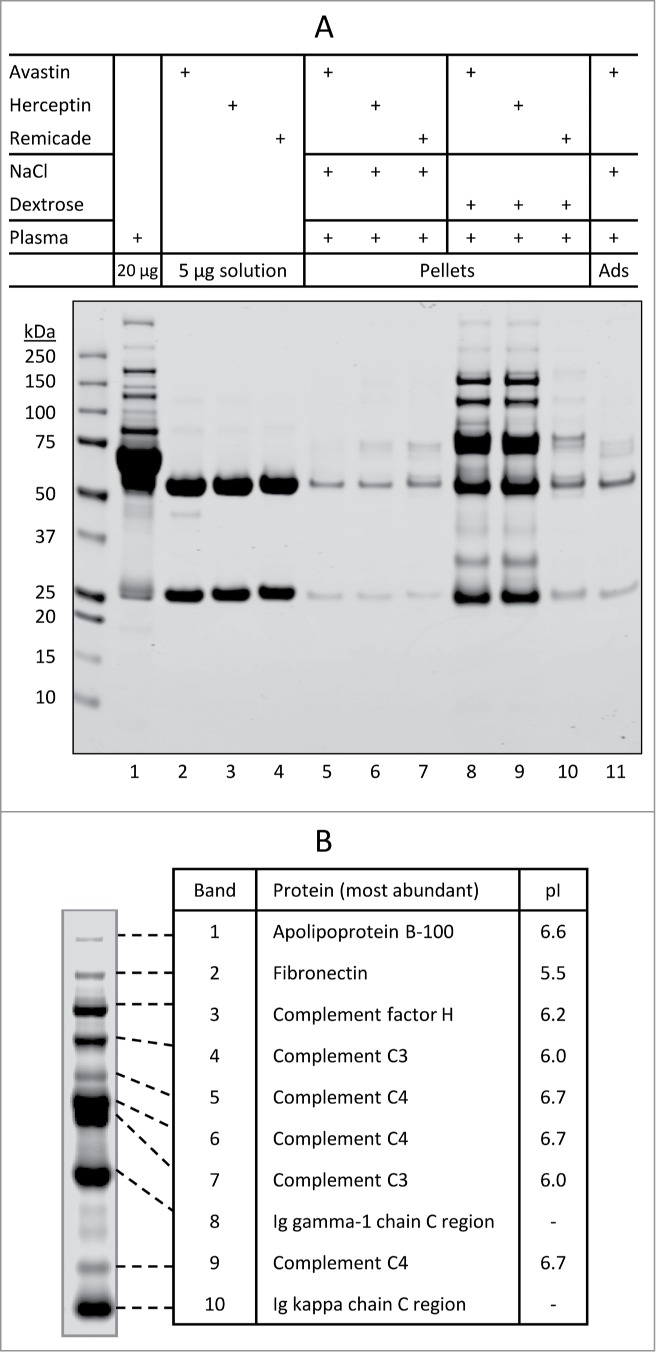
Characterization of insoluble protein aggregates formed when therapeutic mAbs are mixed with dextrose and human plasma. (A) SDS-PAGE analysis of individual protein samples (lanes 1–4), insoluble aggregate pellets collected using centrifugation (lanes 5–10), and basal protein adsorption (Ads) to the microtube surface (lane 11). Samples were prepared as described in Materials and Methods. Shown are representatives of 2 independent experiments. (B) The most abundant proteins that are identified by in-gel digestion and LC-MS/MS from samples in the corresponding bands of the SDS-PAGE gel. Note: pI values taken from the UniProtKB/Swiss-Prot database.
To characterize dextrose-mediated protein aggregates, visible protein bands (Fig. 2A; lane 8) were excised from the SDS-PAGE gel and analyzed by in-gel digestion and liquid chromatography-tandem mass spectrometry (LC-MS/MS). A number of proteins were identified (Table 1); the most abundant ones, including apolipoprotein B-100, fibronectin, complement factor H, and complement protein C3 and C4, are highlighted in Fig. 2B. These proteins are known to be abundant in human plasma, and are characterized by a pI value falling within the range of 5.5 to 6.7. In addition, bands 8 and 10 were identified as IgG1-related species that likely correspond to the heavy chain and light chain of Avastin IgG1 mAb. These data demonstrate for the first time that dextrose-induced Avastin aggregation in plasma involves mAb itself and complement proteins from human plasma.
Table 1.
Protein candidates identified in the insoluble aggregates formed in the mixture of dextrose-Avastin-plasma
| Band # | Distinct Peptides | Molecular Weight (kDa)* | Database Accession # | Protein Name |
|---|---|---|---|---|
| 1 | 4 | 517 | P04114 | Apolipoprotein B-100 |
| 2 | 24 | 266 | P02751 | Fibronectin |
| 3 | 29 | 144 | P08603 | Complement factor H |
| 5 | 189 | P01024 | Complement C3 | |
| 4 | 190 | P01031 | Complement C5 | |
| 4 | 38 | 189 | P01024 | Complement C3 |
| 12 | 190 | P01031 | Complement C5 | |
| 3 | 42 | P01860 | Ig gamma-3 chain C region | |
| 3 | 107 | P19823 | Inter-alpha-trypsin inhibitor heavy chain H2 | |
| 5 | 16 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B |
| 7 | 55 | P05155 | Plasma protease C1 inhibitor | |
| 8 | 82 | P00736 | Complement C1r subcomponent | |
| 9 | 189 | P01024 | Complement C3 | |
| 3 | 66 | Q08380 | Galectin-3-binding protein | |
| 4 | 93 | P00747 | Plasminogen | |
| 3 | 78 | P09871 | Complement C1s subcomponent | |
| 6 | 16 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B |
| 14 | 69 | P04003 | C4b-binding protein alpha chain | |
| 22 | 189 | P01024 | Complement C3 | |
| 8 | 50 | P01871 | Ig mu chain C region | |
| 9 | 72 | P00734 | Prothrombin | |
| 4 | 96 | P02671 | Fibrinogen alpha chain | |
| 4 | 190 | P01031 | Complement C5 | |
| 3 | 12 | P01834 | Ig kappa chain C region | |
| 7 | 35 | 189 | P01024 | Complement C3 |
| 16 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B | |
| 12 | 69 | P04003 | C4b-binding protein alpha chain | |
| 8 | 190 | P01031 | Complement C5 | |
| 4 | 96 | P02671 | Fibrinogen alpha chain | |
| 5 | 37 | P01857 | Ig gamma-1 chain C region | |
| 3 | 65 | P02748 | Complement component C9 | |
| 6 | 77 | P07225 | Vitamin K-dependent protein S | |
| 8 | 10 | 37 | P01857 | Ig gamma-1 chain C region |
| 3 | 42 | P01860 | Ig gamma-3 chain C region | |
| 3 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B | |
| 9 | 7 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B |
| 4 | 27 | P02746 | Complement C1q subcomponent subunit B | |
| 3 | 36 | P02649 | Apolipoprotein E | |
| 10 | 6 | 12 | P01834 | Ig kappa chain C region |
| 4 | 11 | P0CG05 | Ig lambda-2 chain C regions | |
| 5 | 194 | P0C0L4 or P0C0L5 | Complement C4-A or C4-B | |
| 7 | 31 | P02647 | Apolipoprotein A-1 | |
| 3 | 26 | P02743 | Serum amyloid P-component |
Protein molecular weight and the corresponding Database Accession numbers obtained from UniProtKB/Swiss-Prot database.
Biochemical pathways triggering aggregation of therapeutic mAbs in plasma
The presence of complement proteins in dextrose-induced Avastin aggregates suggested that these proteins might be the trigger of aggregation. To test this possibility, we assessed the aggregation potential of Avastin in plasma samples with immune-depletion of specific complement proteins, including C1q, C2, C3, C4, factor B, and factor D. These complement proteins are known to be essential in complement activation pathways in which they trigger a cascade of protein complex formation.16 As shown in Fig. 3A, depletion of a single complement protein from human serum had little or no effect on dextrose-mediated aggregation of Avastin in terms of the amount of insoluble aggregates or major protein components. We also compared serum samples freshly collected from wild-type mouse and C3-knocked out mouse. Under similar conditions as in Fig. 2A, Avastin displayed a near identical aggregation pattern when exposed to C3-deficient mouse serum, wild-type mouse serum, and whole human plasma (Fig. 3B). In addition, heat inactivation of the complement system, which was achieved by incubation of human plasma at 56°C for 60 minutes,17 had little or no effect on Avastin aggregation (data not shown). Collectively, dextrose-induced aggregation of Avastin in plasma was not affected by removal of individual complement protein or by heat inactivation of the complement system.
Figure 3.
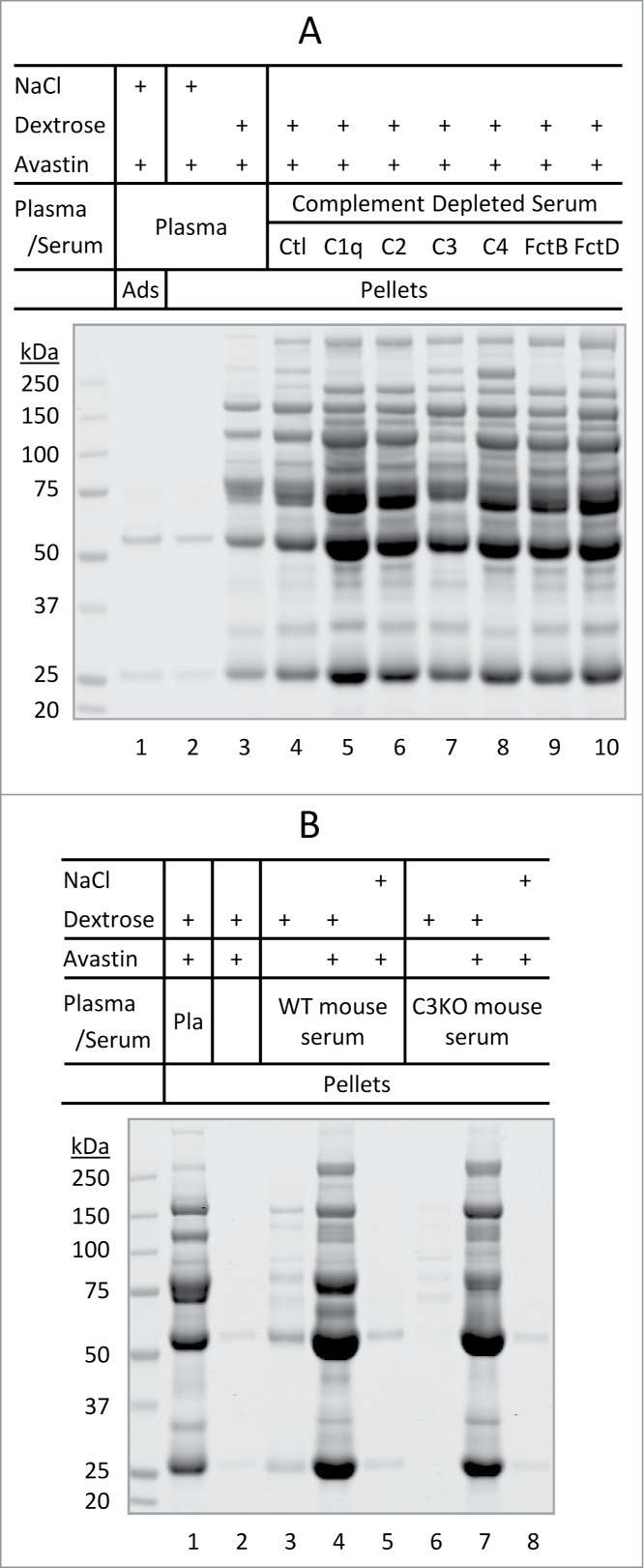
Dextrose-mediated aggregation of Avastin in immunodepleted serum. (A) Dextrose-mediated aggregation of Avastin in modified human serum. Lanes 1–3, protein adsorption/aggregation in human plasma. Lanes 4–10, protein aggregation in complete human serum (Ctl) or immunodepleted human serum void of one of the following complement proteins: C1q, C2, C3, C4, factor B (FctB), or factor D (FctD). (B) Dextrose-mediated aggregation of Avastin in human plasma (Pla) or in fresh mouse serum from wild-type (WT) or C3 knocked out (C3KO) mouse.
Next, we asked if product formulation plays a role in the observed aggregation phenomena. To this end, we switched formulation buffers for Avastin and Remicade according to the approved formulation formula (Table 2). This was achieved through sequential dilution (1:800) and concentration procedures (see detail in Materials and Methods). The resulting reformulated samples were tested for aggregation. Strikingly, when Remicade (pH 7.2) was placed into Avastin formulation (pH 6.2) and then mixed with dextrose and plasma as in Fig 2A, massive insoluble protein aggregates were detected (Fig. 4A; lane 3). The Remicade-containing aggregates displayed a similar band pattern on SDS-PAGE as observed for Avastin (lane 5). By contrast, Avastin lost the ability to form aggregates when exchanged into Remicade formulation (lane 6). These data indicate that product formulation is a critical determination of mAb aggregation when diluting with dextrose and plasma.
Table 2.
Comparison of the approved product formulations
| Product | Avastin* | Herceptin** | Remicade** |
|---|---|---|---|
| Aggregation in product-dextrose-plasma mixture | yes | yes | no |
| Active pharmaceutical ingredient (Concentration in mg/mL) | Bevacizumab (25) | Trastuzumab (21) | Infliximab (10) |
| Formulation (mg/mL) | |||
| α,α-trehalose dihydrate | 60 | 20 | |
| sucrose | 50 | ||
| NaH2PO4•H2O | 5.8 | 0.22 | |
| Na2HPO4 | 1.2 | ||
| Na2HPO4•2H2O | 0.61 | ||
| L-histidine HCl | 0.495 | ||
| L-histidine | 0.32 | ||
| polysorbate 20 | 0.4 | 0.09 | |
| polysorbate 80 | 0.05 | ||
| pH | 6.2 | 6.0 | 7.2 |
Obtained from the FDA approved label for liquid formulated Avastin.
Calculated from the formula information on the FDA approved labels for lyophilized Herceptin and Remicade after reconstitution with the specified volumes of water.
Figure 4.
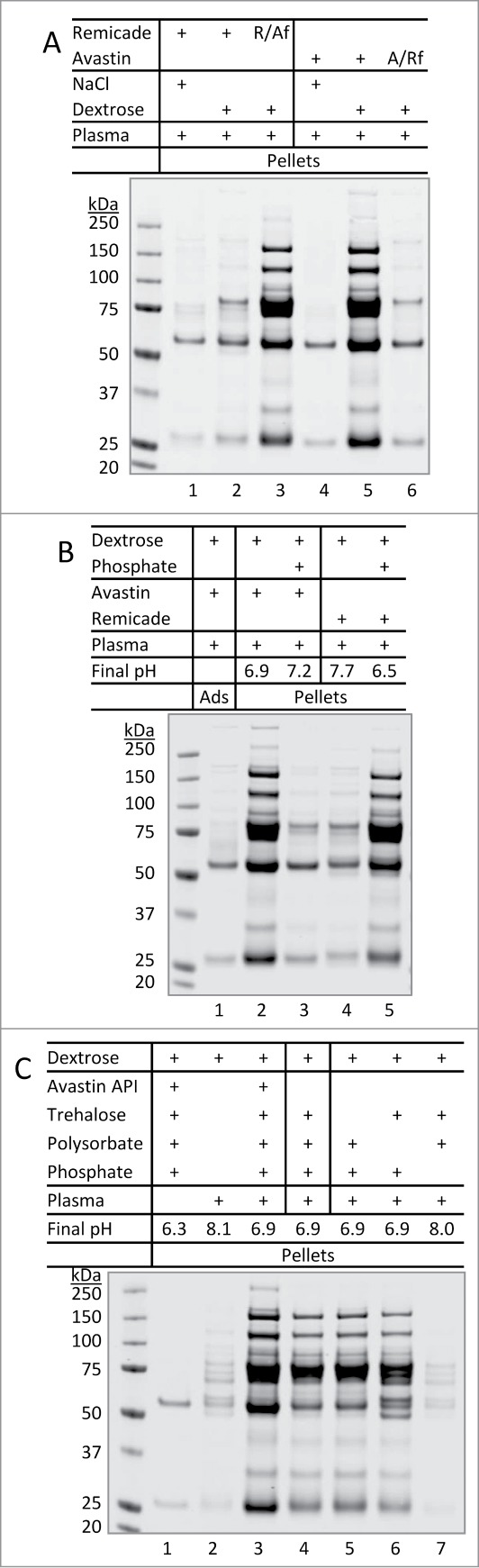
Formulation-dependent aggregation of therapeutic mAbs when encountering dextrose and human plasma. (A) Reversal of aggregation patterns of mAbs after switching formulation buffers. Buffer exchange was achieved using centrifugal filter units as described in Materials and Methods. R/Af and A/Rf: Remicade was placed in Avastin's formulation buffer or vice versa. (B) Effects of altering formulation pH. Lanes 3 and 5: aliquot of phosphate buffer (0.2 M, pH 8.0 or 5.9) 7.5 µL was added to 0.3 mL of dextrose prior to mixing with 10 µL of Avastin or Remicade and then 10 µL of plasma, respectively, resulting in the pH values as indicated. (C) Effects of alterations to formulation excipients. A solution of 0.3 mL dextrose was mixed with 10 µL of either Avastin (lane 1 and 3), Avastin formulation (lane 4, no API), or artificial formulation buffers lacking the specific excipients (lane 5–7). After mixing with 10 µL of human plasma (lane 2–7), resulting in the final pH as indicated, the mixtures were incubated for 10 min at 25°C, pellets were collected and resolved on SDS-PAGE.
A major difference between the approved formulations for the therapeutic mAbs is noted to be the pH values of the formulation buffers. Avastin and Herceptin are formulated at pH 6.0 – 6.2 and Remicade at pH 7.2 (Table 2). In comparing the observed aggregation patterns, it became evident that only the products formulated at lower pH (Avastin and Herceptin) formed insoluble aggregates in the presence of dextrose and plasma. We then tested if mAb aggregation could be affected by alteration of formulation pH while maintaining other formulation excipients. When the pH of Avastin-dextrose-plasma mixture was raised from 6.9 to 7.2, protein aggregation was effectively prevented (Fig. 4B; lanes 2–3). A similar result was obtained for Herceptin-dextrose-plasma mixture (data not shown). In contrast, when the pH of Remicade-dextrose-plasma mixture was lowered from 7.7 to 6.5, massive insoluble protein aggregates were detected (lanes 4–5).
We tested the potential effects of each formulation excipient on dextrose-mediated mAb aggregation. To this end, we prepared artificial formulation samples by omitting each individual component of the Avastin product formulation and also the mAb active pharmaceutical ingredient (API) itself (Table 2). All the samples prepared were confirmed to be at pH 6.2 as the approved formulation, except the sample lacking phosphate-buffering agent. Compared to the native Avastin-dextrose-plasma mixture (Fig. 4C; lane 3), a similar pattern of protein aggregates was detected when Avastin API was omitted from the formulation (lane 4). In the absence of mAb API, SDS-PAGE still showed the 2 protein bands corresponding to IgG heavy and light chains, which presumably came from endogenous immunoglobulins present in human plasma. Removal of trehalose (lane 5) or polysorbate (lane 6) from the formulation had no significant effect on aggregate formation. Notably, protein aggregation was effectively abolished when phosphate was eliminated from the formulation buffer, which raised the pH to ˜8.0 (lane 7). In agreement with data in Fig. 4A and 4B, the altered aggregation patterns were closely associated with changes in the pH of each mixture (Fig. 4C, final pH).
Given that the plasma proteins present in the insoluble aggregates are characterized by a pI value in the range of 5.5 – 6.7 (Fig. 2B), it is reasonable to propose that dextrose-induced aggregation of Avastin or Herceptin likely results from the decreased solubility of these abundant plasma proteins whose pI values are close to the pH of the mixture. These abundant plasma proteins appear to undergo isoelectric precipitation when encountering dextrose at a pH close to their pI values. To support this notion, plasma proteins were found to display a pH-dependent aggregation in the presence of dextrose (Fig. 5A). In this experiment, aliquots of human plasma were mixed with dextrose and a phosphate buffer with a pH ranging from 5.9 to 8.0. Massive insoluble protein aggregates were detected at pH 6.5 and steadily decreased as the pH increased. No apparent insoluble aggregates were detected at pH 7.4 or above. The protein components of aggregates that were formed in plasma alone were found to be almost identical to those identified in the Avastin-containing aggregates.
Figure 5.
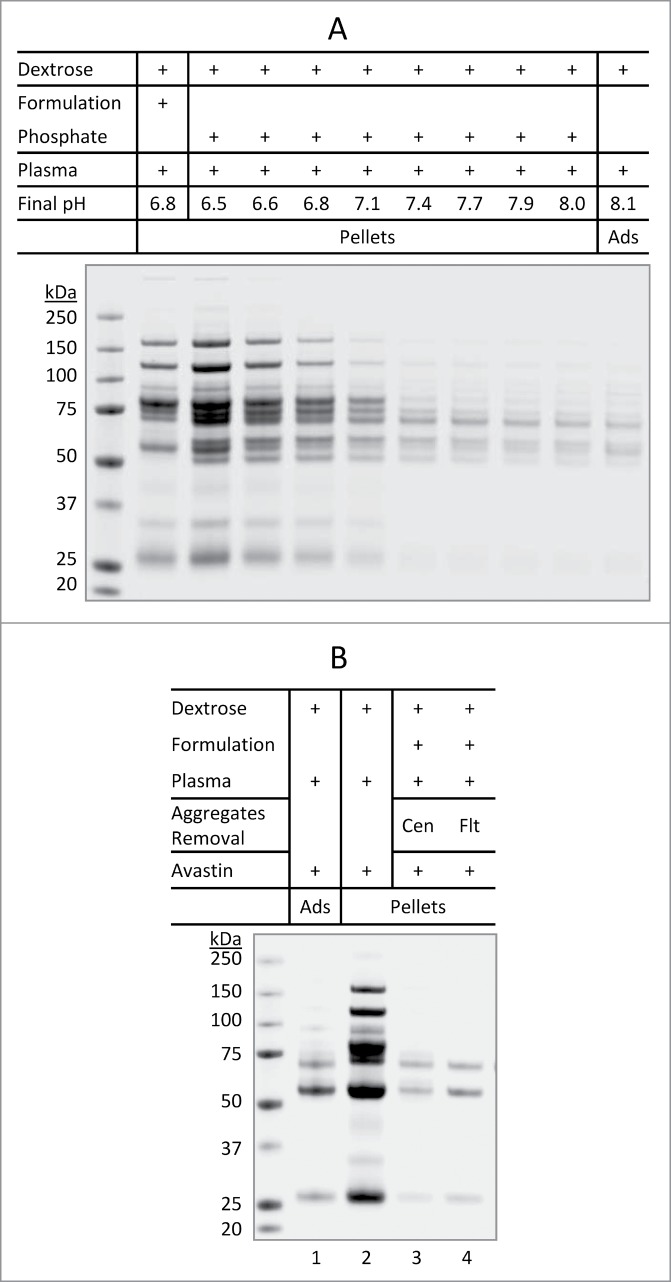
Aggregation of plasma proteins as a prerequisite of Avastin aggregation in the presence of dextrose. (A) pH-dependent aggregation of plasma proteins. Aliquots of dextrose (0.3 mL) were mixed with either 10 µL of Avastin formulation (no mAb API) or 7.5 µL of phosphate buffer (0.2 M) with different starting pH (5.9 to 8.0). After mixing with 10 µL of plasma, the mixture at the indicated final pH was incubated for 10 min at 25°C, the pellets were collected and analyzed. (B) Removal of pre-formed aggregates of plasma proteins prevented dextrose mediated aggregation of Avastin. Aggregates formed in the mixture of Avastin formulation (no mAb API) and dextrose-plasma solution were removed by either centrifugation (Cen) or filtration (Flt) through 0.22 µm filter. Avastin was then added and the pellets were analyzed.
We further showed that Avastin was no longer able to form aggregates in a plasma sample in which the pre-formed aggregates of plasma proteins were removed. In this experiment, aliquots of human plasma were incubated with dextrose in the formulation buffer (no mAb API) of Avastin (pH 6.2) at 25 °C for 30 min. The aggregates formed by plasma proteins were removed using either centrifugation or membrane filtration (0.22 µm). The resulting supernatant was incubated with Avastin, which showed little or no insoluble aggregates (Fig. 5B; lanes 3–4). This data supports the notion that aggregation of plasma proteins, likely those with pI values close to 6, is a prerequisite for a therapeutic mAb to be co-precipitated with abundant plasma proteins (e.g., complement proteins C3/C4). Collectively, dextrose-mediated aggregation of therapeutic mAbs in plasma appears to involve isoelectric precipitation of several abundant plasma proteins (Fig. 2B) whose pI values are proximate to the pH of the mixture under the specified in vitro conditions.
We also tested if the aggregation of plasma proteins in dextrose solution could trigger co-precipitation of non-mAb therapeutic proteins. Pulmozyme® (dornase alfa, inhalation solution), a recombinant human deoxyribonuclease I formulated in sodium chloride solution (pH 6.3), was chosen for this study. When Pulmozyme was mixed with dextrose and plasma, protein aggregates were detected, presumably formed by abundant plasma proteins. However, no Pulmozyme protein (˜37 kDa) was detected in the aggregates of plasma proteins (Fig. 6; lane 3 versus lane 2). This data suggests that aggregates of plasma proteins (Fig. 2B) may only lead to co-precipitation of therapeutic mAbs, but not other recombinant proteins.
Figure 6.
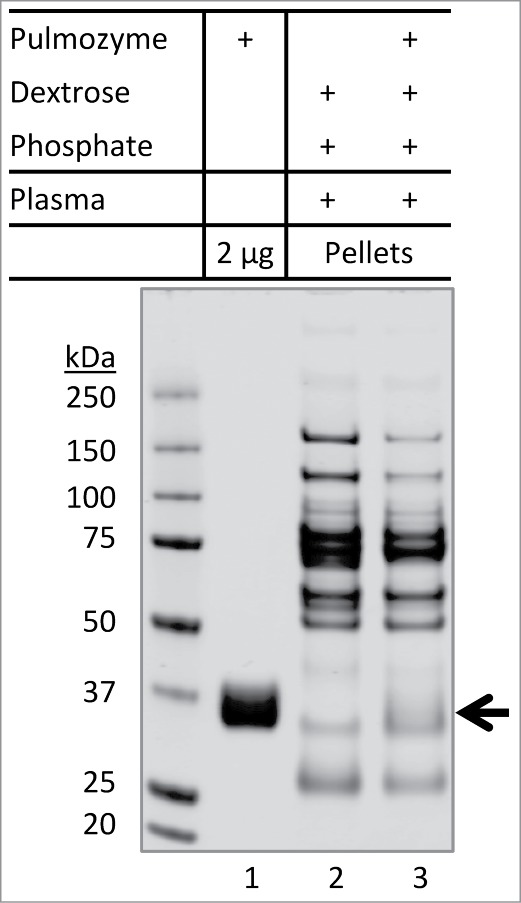
Inability of Pulmozyme to co-precipitate with aggregates of plasma proteins. Pulmozyme was exchanged into dextrose-phosphate solution prior to mixing with plasma (lane 3). Briefly, Pulmozyme (250 µL) was added to 3 mL of 5% dextrose containing 75 µL of 0.2 M phosphate buffer pH 5.9. The solution was concentrated to 0.3 mL at 5°C using an Amicon ultra-4 membrane unit (3 kDa molecular weight cutoff). The membrane integrity was confirmed by SDS-PAGE showing a complete recovery of total proteins and that no protein was detected in the filtrate. A solution of 10 µL plasma was then added to the Pulmozyme-dextrose-phosphate mixture, followed by 30 min incubation at 25°C. The pellets were collected and analyzed by SDS-PAGE. Lane 1: Pulmozyme solution (2 µg) alone. Lane 2: plasma protein aggregates formed in dextrose-phosphate-plasma mixture. Arrow indicates potential location of Pulmozyme protein band (˜37 kDa), if any, presented in the pellets.
Discussion
The presence of particulate matters in pharmaceuticals used in IV injection can adversely affect patient safety and product efficacy; 1 therefore, the levels of particulates in these products must be adequately assessed and controlled. This is especially true for therapeutic mAbs because of the inherent tendency of proteins to form aggregates. To add complexity, many therapeutic mAbs are clinically administered through IV infusion after mixing with a diluent (e.g., 5% dextrose or 0.9% saline). Such a clinical setting increases the likelihood of interactions among mAb molecules, diluent, and plasma components, which may further enhance protein aggregation. For instance, use of dextrose is prohibited as a diluent for Herceptin and Avastin according to the FDA-approved product labels. In consistence with Arvinte et al,13 here we show that both Avastin and Herceptin undergo rapid aggregation when mixing with 5% dextrose and human plasma under in vitro conditions. This data provides a mechanistic basis for the incompatibility between dextrose and the therapeutic mAb products.
The insoluble aggregates from dextrose-Avastin-plasma mixture are composed of mAb itself and complement proteins from plasma, including complement protein C3, C4, factor H, fibronectin, and apolipoprotein B-100. These proteins are characterized by a pI ∼5.5 – 6.7 (Fig. 2), and became precipitated when incubated with a mixture of product formulation and dextrose whose pH lies within this specified range (Fig. 5). Complement proteins are known to interact with IgG immunoglobulins.18 Therefore, it is reasonable to propose that dextrose-mediated aggregation of mAb in plasma involves isoelectric precipitation of abundant plasma proteins (Fig. 7). As a result, the concurrent mAb molecules are co-precipitated, leading to the formation of insoluble protein aggregates containing mAb itself and complement proteins. In support of this notion, plasma proteins underwent a pH-dependent aggregation, with the most severity being observed at pH˜6.5 (Fig. 5A). Dextrose-mediated aggregation of mAb was only observed for products formulated at pH 6.0 – 6.2, but not for Remicade formulated at pH 7.2. Notably, switching formulation buffers for Avastin and Remicade reversed their aggregation patterns (Fig. 4A). Further, dextrose-mediated aggregation of Avastin was effectively blocked when buffer pH was raised to 7.2 or higher (Fig. 4B). These data for the first time demonstrate that plasma proteins are involved in the aggregation of a therapeutic mAb in dextrose. The insoluble aggregates were only detected in the mixture with dextrose, while none of the products tested showed aggregation when mixed with saline and plasma, although the Avastin-saline-plasma mixture had an acidic pH 6.6. Additional studies are underway in our laboratory to characterize the potential effects of ionic strength on aggregate formation when a therapeutic mAb encounters a diluent and human plasma.
Figure 7.
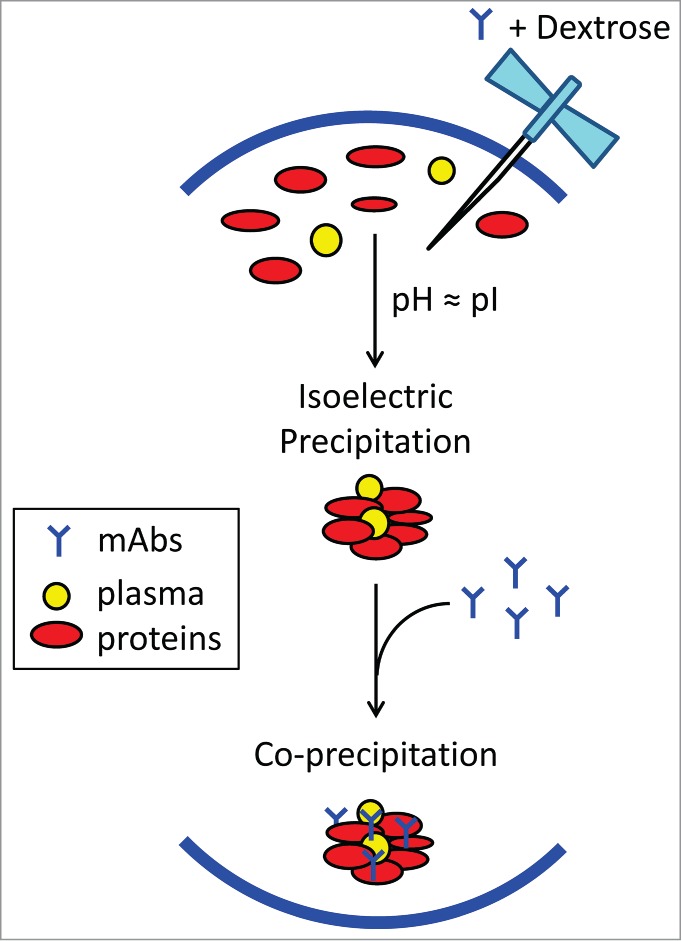
Schematic view of possible biochemical pathways facilitating dextrose-mediated aggregation of therapeutic monoclonal antibodies in human plasma. Dextrose appears to induce isoelectric precipitation of abundant plasma proteins, namely complement protein C3/C4, complement factor H, fibronectin, and apolipoprotein B-100, whose pI values are proximate to the pH of product formulation (in the range of 6.0 to 6.2). Subsequently, the concurrent mAb becomes co-precipitated, possibly through interactions with complement proteins,18 resulting in insoluble protein aggregates containing both mAb and plasma proteins.
It should be noted that our observations were made under in vitro conditions where the ratios of mAb with diluent and plasma were not reflective of the clinical settings. The data presented only suggest a possibility of adverse aggregation arising from interactions between a therapeutic mAb and plasma proteins when an inappropriate diluent is being used. Moreover, it remains to be determined if the observation is generally applicable to other mAb products under similar in vitro conditions. More rigorous studies are required to assess risk factors causing aggregation of a particular therapeutic mAb product under clinical relevant conditions involving diluent and human plasma. Nonetheless, our findings highlight the importance of assessing the compatibility of a mAb product formulation with diluent and human plasma during product development.
This report of the pH-dependent aggregation of Avastin or Herceptin in mixing with dextrose and plasma should not be interpreted to imply a suggestion of changes to the approved product formulations. Avastin and Herceptin are formulated at pH 6.2 and 6.0, respectively, and both molecules are known to have unique stability issues at higher pH. For instances, the light chain Asn 30 of Herceptin deamidates at pH >5, which lowers product bioactivity.19 Avastin forms noncovalent dimers at pH >5.5 or “reversible self-association” that is concentration, pH, ionic strength, and temperature dependent.20 These product-specific issues must be taken into consideration when developing formulations in order to ensure product stability. By contrast, this study addresses unique issues concerning compatibility between a formulated therapeutic mAb and diluent for IV infusion into patients. The acquired information has potential implications in guiding the selection of appropriate diluents for particular therapeutic mAbs.
Materials and Methods
Therapeutic proteins and chemicals
Therapeutic proteins including Avastin (Roche), Herceptin (Roche), Remicade (Janssen Biotech), and Pulmozyme (Roche) were purchased from the Division of Veterinary Resources pharmacy, Office of Research Services, National Institutes of Health. Dextrose, sodium chloride, α,α-trehalose, sucrose, sodium phosphate, polysorbate, acetonitrile (Chromasolv® Plus), ammonium bicarbonate, trypsin (Singles, Proteomics Grade), and 50% formic acid were purchased from Sigma-Aldrich. Stock solutions of 5% dextrose and 9% NaCl (1.54 M) were prepared using purified water, passed through a 0.22 micron syringe filter (EMD Millipore Millex-GS), and stored at room temperature and at 5 °C, respectively. The 5% dextrose solution and a working NaCl solution (0.9%) were used within a week. Human plasma samples (EDTA treated) from healthy volunteers were obtained from National Institutes of Health. Human serum control and serum samples immunodepleted with complement proteins C1q, C2, C3, C4, factor B, or factor D, were purchased from Complement Technology. All plasma and serum samples were aliquoted and stored at −80°C, and before use, vials were thawed and centrifuged at 5°C and 21,000 g for 5 min. Fresh serum from a complement C3 knocked out mouse21 and a wild-type mouse were kindly provided by Qi Qiu and Hui Xu of FDA, respectively.
SDS-PAGE analysis
Aliquots of therapeutic protein solution (10 µL Avastin or Herceptin, or 25 µL Remicade) were diluted in duplicate with 0.3 mL of diluent (0.9% NaCl or 5% dextrose) in 1.5 mL tubes, and followed by addition of 10 µL human plasma. The mixture was incubated at 25°C for 30 min and then subjected to centrifugation at 21,000 g for 3 min at 25°C. The insoluble protein pellets, if any, were washed 3 times by gentle and brief vortex in 0.3 mL of the same diluent used for the mixture (Note: supernatant was removed with caution to avoid disturbing the pellets at the bottom of the microcentrifuge tube). After washing, the pellets were suspended in 13 µL water followed by addition of 5 µL NuPAGE LDS sample buffer 4× (Life Technologies) and 2 µL of freshly prepared dithiothreitol (DTT) 0.5 M solution (Pierce, No-Weigh Format). The samples were incubated at 70°C for 10 min. The resultant samples were resolved onto 1.0 mm 12-well NuPAGE Novex 4–12% Bis-Tris SDS-PAGE gels (Life Technologies), and stained with SimplyBlue SafeStain (Life Technologies) Coomassie. The protein size marker was 3–5 µL of Odyssey One-Color Protein Molecular Weight Marker (LI-COR). The stained gels were imaged with a LI-COR Odyssey scanner.22 As a negative control, samples were prepared following the same procedures but omitting centrifugation steps in order to determine the levels of protein adsorption to the microcentrifuge tube surface.
Particulate counting
The number of particulates in a testing sample was determined using Micro-Flow Imaging (MFI) 5200 Particle Counter (ProteinSimple) with a capacity of detecting particulates of 1–70 microns and up to 900,000 particles/mL.
Liquid chromatography–tandem mass spectrometry
Insoluble protein aggregates were characterized using LC-MS/MS analysis. Briefly, protein aggregates were harvested by centrifugation and then resolved onto SDS-PAGE with Coomassie Blue staining. Protein bands were retrieved and destained with 50% acetonitrile in 25 mM ammonium bicarbonate (AB). Cysteine residues were reduced with 100 µL of 10 mM DTT, and then alkylated with 100 µL of 50 mM iodoacetamide (Pierce, Single-Use) in 25 mM AB at 25°C for 30 min. Excess iodoacetamide was washed away with 100 µL of 50 mM AB and then 50% acetonitrile in 25 mM AB. Gel pieces were dehydrated with 200 µL neat acetonitrile for 5 min followed by air drying for 10 min. A solution of 15 µL trypsin in 25 mM AB at 25 ng/µL concentration was added to rehydrate the gel pieces for 15 min, excess trypsin solution was removed. A solution of 25 mM AB 20 µL was added to completely cover the gel pieces, in-gel proteins were digested overnight at 37°C in a CO2 incubator. The digestion was stopped by acidification with 1 µL of 10% formic acid to reach pH 2–3, and the solution was transferred to a new microtube. After extraction of remaining peptides from the gel pieces with 20 µL of 60% acetonitrile in 0.1% formic acid for 15 min, the peptide-containing solutions were combined and the samples were dried in a Savant SpeedVac concentrator (Thermo Scientific). The tryptic peptides were dissolved in 8–20 µL of 3% acetonitrile in 0.1% formic acid based on the band intensity, injected onto an Agilent C18 ProtID-Chip-43 (G4240–62005), and then sequenced by an Agilent 1260 HPLC-Chip nano-electrospray-ionization 6520 Q-TOF tandem MS spectrometry using the auto-MS/MS acquisition method and the Agilent Spectrum Mill data analysis software as described previously.23
Buffer exchange
Formulation buffers were prepared according to the approved excipient formulas for the therapeutic mAb products (Table 2). To exchange formulation buffer, 100 µL of drug A (e.g., Remicade) was diluted into 500 µL of drug B (e.g., Avastin) formulation buffer and then concentrated at 5°C in an Amicon ultra-0.5 centrifugal filter unit (EMD Millipore) with ultracel-10 (10 kDa molecular weight cutoff) membrane. The procedures were repeated until reaching a dilution factor of 1:800. The resulting samples were adjusted to a final volume of 100 µL with drug B formulation buffer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The comments in this paper reflect the views of the author and should not be construed to represent FDA's views or policies.
Acknowledgments
We thank Drs. Bingjie Li, Qi Qiu, and Hui Xu for kindly providing human plasma and mouse serum samples. We also thank our labmates (Drs. William Bozza, Yaqin Zhang, Kory Hallett, William Hallett, and Julianne Twomey) for technical support and helpful discussions. This work was supported by FDA intramural funding.
References
- 1.Langille SE. Particulate matter in injectable drug products. PDA J Pharm Sci Technol 2013; 67:186-200; PMID:23752747; http://dx.doi.org/ 10.5731/pdajpst.2013.00922 [DOI] [PubMed] [Google Scholar]
- 2.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, Fan YX, Kirshner S, Verthelyi D, Kozlowski S, el al. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci 2009; 98:1201-5; PMID:18704929; http://dx.doi.org/ 10.1002/jps.21530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Roberts CJ. Aggregation of Therapeutic Proteins. John Wiley & Sons, Inc; 2010. [Google Scholar]
- 4.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J 2006; 8:E501-E507; PMID:17025268; http://dx.doi.org/ 10.1208/aapsj080359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill SE, Heldman LS, Goo ED, Whippo PE, Perkinson JC. Fatal microvascular pulmonary emboli from precipitation of a total nutrient admixture solution. JPEN J Parenter Enteral Nutr 1996; 20:81-7; PMID:8788269; http://dx.doi.org/ 10.1177/014860719602000181 [DOI] [PubMed] [Google Scholar]
- 6.Jack T, Brent BE, Boehne M, Muller M, Sewald K, Braun A, Wessel A, Sasse M. Analysis of particulate contaminations of infusion solutions in a pediatric intensive care unit. Intensive Care Med 2010; 36:707-11; PMID:20165942; http://dx.doi.org/ 10.1007/s00134-010-1775-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehne M, Jack T, Koditz H, Seidemann K, Schmidt F, Abura M, Bertram H, Sasse M. In-line filtration minimizes organ dysfunction: new aspects from a prospective, randomized, controlled trial. BMC Pediatr 2013; 13:21; PMID:23384207; http://dx.doi.org/ 10.1186/1471-2431-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack T, Boehne M, Brent BE, Hoy L, Koditz H, Wessel A, Sasse M. In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med 2012; 38:1008-16; PMID:22527062; http://dx.doi.org/ 10.1007/s00134-012-2539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puntis JW, Wilkins KM, Ball PA, Rushton DI, Booth IW. Hazards of parenteral treatment: do particles count? Arch Dis Child 1992; 67:1475-7; PMID:1489228; http://dx.doi.org/ 10.1136/adc.67.12.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shay DK, Fann LM, Jarvis WR. Respiratory distress and sudden death associated with receipt of a peripheral parenteral nutrition admixture. Infect Control Hosp Epidemiol 1997; 18:814-7; PMID:9442405; http://dx.doi.org/ 10.2307/30141339 [DOI] [PubMed] [Google Scholar]
- 11.Bradley JS, Wassel RT, Lee L, Nambiar S. Intravenous ceftriaxone and calcium in the neonate: assessing th10e risk for cardiopulmonary adverse events. Pediatrics 2009; 123:e609-e613; PMID:19289450; http://dx.doi.org/ 10.1542/peds.2008-3080 [DOI] [PubMed] [Google Scholar]
- 12.Cant AJ, Lenney W, Kirkham N. Plastic material from a syringe causing fatal bowel necrosis in a neonate. Br Med J (Clin Res Ed.) 1988; 296:968-9; PMID:3129111; http://dx.doi.org/ 10.1136/bmj.296.6627.968-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvinte T, Palais C, Green-Trexler E, Gregory S, Mach H, Narasimhan C, Shameem M. Aggregation of biopharmaceuticals in human plasma and human serum: implications for drug research and development. mAbs 2013; 5:491-500; PMID:23571158; http://dx.doi.org/ 10.4161/mabs.24245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demeule B, Palais C, Machaidze G, Gurny R, Arvinte T. New methods allowing the detection of protein aggregates: a case study on trastuzumab. mAbs 2009; 1:142-50; PMID:20061815; http://dx.doi.org/ 10.4161/mabs.1.2.7632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters BJ, Capelle MA, Arvinte T, van de Garde EM. Validation of an automated method for compounding monoclonal antibody patient doses: case studies of Avastin (bevacizumab), Remicade (infliximab) and Herceptin (trastuzumab). mAbs 2013; 5:162-70; PMID:23255057; http://dx.doi.org/ 10.4161/mabs.22873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. New York: Garland Science; 2001. [Google Scholar]
- 17.Soltis RD, Hasz D, Morris MJ, Wilson ID. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology 1979; 36:37-45; PMID:422227 [PMC free article] [PubMed] [Google Scholar]
- 18.Miletic VD, Frank MM. Complement-immunoglobulin interactions. Curr Opin Immunol 1995; 7:41-7; PMID:7772281; http://dx.doi.org/ 10.1016/0952-7915(95)80027-1 [DOI] [PubMed] [Google Scholar]
- 19.Harris RJ, Kabakoff B, Macchi FD, Shen FJ, Kwong M, Andya JD, Shire SJ, Bjork N, Totpal K, Chen AB. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J Chromatogr B Biomed Sci Appl 2001; 752:233-45; PMID:11270864; http://dx.doi.org/ 10.1016/S0378-4347(00)00548-X [DOI] [PubMed] [Google Scholar]
- 20.Moore JM, Patapoff TW, Cromwell ME. Kinetics and thermodynamics of dimer formation and dissociation for a recombinant humanized monoclonal antibody to vascular endothelial growth factor. Biochemistry 1999; 38:13960-7; PMID:10529242; http://dx.doi.org/ 10.1021/bi9905516 [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T, Byrnes AP. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med 2013; 19:452-7; PMID:23524342; http://dx.doi.org/ 10.1038/nm.3107 [DOI] [PubMed] [Google Scholar]
- 22.Luo S, Wehr NB, Levine RL. Quantitation of protein on gels and blots by infrared fluorescence of Coomassie blue and Fast Green. Anal Biochem 2006; 350:233-8; PMID:16336940; http://dx.doi.org/ 10.1016/j.ab.2005.10.048 [DOI] [PubMed] [Google Scholar]
- 23.Luo S, Uehara H, Shacter E. Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation. Free Radic Biol Med 2014; 75:84-94; PMID:25058340; http://dx.doi.org/ 10.1016/j.freeradbiomed.2014.07.018 [DOI] [PubMed] [Google Scholar]


