Abstract
Purpose
Four US National Clinical Trials Network components (Southwest Oncology Group, Cancer and Leukemia Group B/Alliance, Eastern Cooperative Oncology Group, and the AIDS Malignancy Consortium) conducted a phase II Intergroup clinical trial that used early interim fluorodeoxyglucose positron emission tomography (FDG-PET) imaging to determine the utility of response-adapted therapy for stage III to IV classic Hodgkin lymphoma.
Patients and Methods
The Southwest Oncology Group S0816 (Fludeoxyglucose F 18-PET/CT Imaging and Combination Chemotherapy With or Without Additional Chemotherapy and G-CSF in Treating Patients With Stage III or Stage IV Hodgkin Lymphoma) trial enrolled 358 HIV-negative patients between July 1, 2009, and December 2, 2012. A PET scan was performed after two initial cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and was labeled PET2. PET2-negative patients (Deauville score 1 to 3) received an additional four cycles of ABVD, whereas PET2-positive patients (Deauville score 4 to 5) were switched to escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (eBEACOPP) for six cycles. Among 336 eligible and evaluable patients, the median age was 32 years (range, 18 to 60 years), with 52% stage III, 48% stage IV, 49% International Prognostic Score 0 to 2, and 51% score 3 to 7.
Results
Three hundred thirty-six of the enrolled patients were evaluable. Central review of the interim PET2 scan was performed in 331 evaluable patients, with 271 (82%) PET2-negative and 60 (18%) PET2-positive. Of 60 eligible PET2-positive patients, 49 switched to eBEACOPP as planned and 11 declined. With a median follow-up of 39.7 months, the Kaplan-Meier estimate for 2-year overall survival was 98% (95% CI, 95% to 99%), and the 2-year estimate for progression-free survival (PFS) was 79% (95% CI, 74% to 83%). The 2-year estimate for PFS in the subset of patients who were PET2-positive after two cycles of ABVD was 64% (95% CI, 50% to 75%). Both nonhematologic and hematologic toxicities were greater in the eBEACOPP arm than in the continued ABVD arm.
Conclusion
Response-adapted therapy based on interim PET imaging after two cycles of ABVD seems promising with a 2-year PFS of 64% for PET2-positive patients, which is much higher than the expected 2-year PFS of 15% to 30%.
INTRODUCTION
One of the great medical triumphs of the last century has been improvement in the survival of patients with advanced-stage classic Hodgkin lymphoma (HL) as a result of improved diagnosis, staging, and therapy.1 For almost two decades, the doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen has been the standard chemotherapy in the United States, with an expected cure rate of approximately 70% for patients with stage III to IV disease.2,3 Studies from the German Hodgkin Study Group suggest that an intensified regimen of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (escalated-dose BEACOPP [eBEACOPP]) may cure more patients4 but is more toxic and causes infertility in most recipients.5 Many physicians believe that it is desirable to avoid the toxicities of overtreatment for the 70% of patients who are cured with ABVD; however, it is also desirable to improve the cure rate. Positron emission tomography (PET) scans showing persistent fluorodeoxyglucose (FDG) uptake after two cycles of ABVD (PET2-positive) seems highly predictive of treatment failure with ABVD.6-9 Only 15% to 45% of PET2-positive patients achieve long-term progression-free survival (PFS) if they continue on treatment with ABVD.10 Therefore, the four major US National Clinical Trials Network components (Southwest Oncology Group [SWOG], Cancer and Leukemia Group B [CALGB]/Alliance, Eastern Cooperative Oncology Group, and the AIDS Malignancy Consortium) decided to assess the use of interim FDG-PET for intensifying chemotherapy in PET2-positive patients unlikely to be cured with continued ABVD. The two coprimary objectives were to (1) improve the 2-year PFS of patients with stage III to IV disease from the expected survival of 70% with ABVD to 78% with response-adapted therapy and (2) improve the 2-year PFS of PET2-positive patients with stage III to IV disease from the historical survival rate of 15% to 30% (if continued on ABVD) to at least 48% with response-adapted therapy. Secondary objectives included estimating the response and overall survival (OS) rates and evaluating toxicities. The trial was approved by applicable institutional review boards, and informed consent was obtained from all participants.
PATIENTS AND METHODS
Study Design
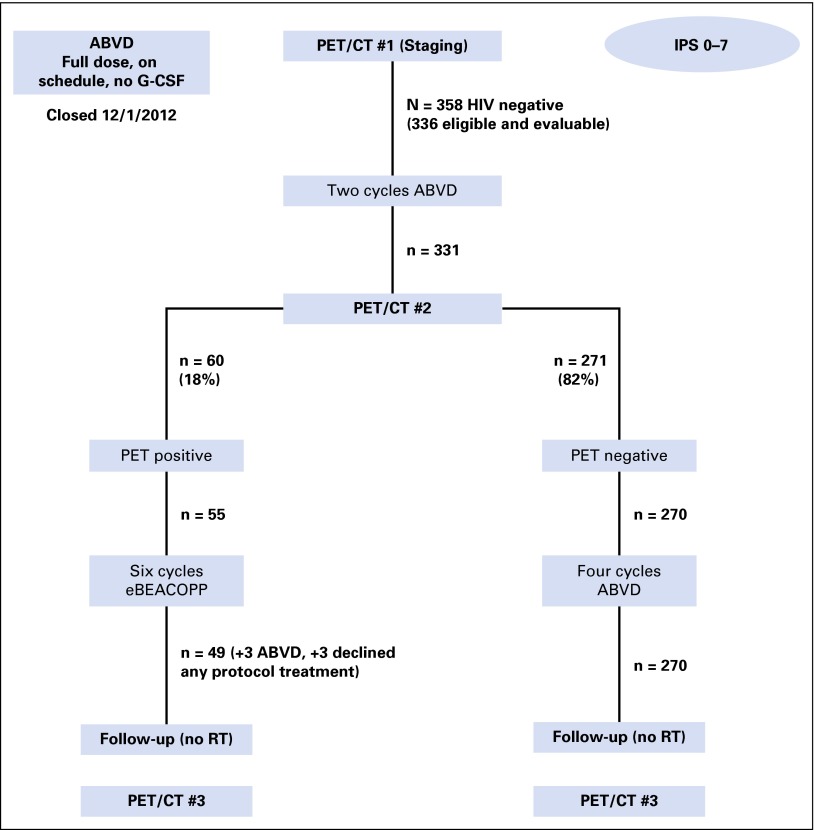
Eligible patients with stages III or IV classic HL were initially treated with two cycles of ABVD followed by interim PET/computed tomography (CT) imaging. PET2-negative patients received an additional four cycles of ABVD, and PET2-positive patients were switched to eBEACOPP for six cycles (Fig 1).
Fig 1.
CONSORT diagram demonstrating patient flow of 358 patients enrolled in the Southwest Oncology Group S0816 trial. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; CT, computed tomography; eBEACOPP, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; G-CSF, granulocyte colony-stimulating factor; IPS, International Prognostic Score; PET, positron emission tomography; RT, radiotherapy.
Eligibility
Patients age 18 to 60 years were eligible if they had measurable stage III to IV HL as documented by excisional or core needle biopsy, no prior therapy for lymphoma, a Zubrod performance status of 0 to 2, and no other serious medical ailments. Full inclusion and exclusion criteria are listed in the Data Supplement. A second registration was performed after interim PET/CT scans were submitted for centralized review to the CALGB Imaging Core Laboratory (CALGB ICL) after the first two cycles of ABVD.
Patient Evaluation and Follow-Up Testing
Patients were required to have a baseline history, physical examination, and laboratory testing. Marrow biopsies were performed at baseline and again after completion of therapy (day 197) if they were initially positive. Physical examination and laboratory tests were repeated with each cycle on days 197, 276, and 365, and then every 6 months or whenever symptoms or signs of relapse occurred.
Imaging Studies
Combined PET/CT imaging was performed at baseline, after two cycles of ABVD (PET2), and 6 to 8 weeks after the end of therapy. Each scan was transmitted electronically in Digital Imaging and Communications in Medicine format to the CALGB ICL for real-time, centralized review (see Data Supplement for details of image acquisition and central review). Briefly, all scans underwent central review using the 5-point Deauville scale. Scans given Deauville scores 1 to 3 were considered PET2-negative, and scans given Deauville scores 4 to 5 were considered PET2-positive. Contrast enhanced, diagnostic quality CT scans were performed at baseline, at the time of interim PET/CT, 6 to 8 weeks after the last cycle, every 6 months in year 2, annually in years 3 to 5, and whenever relapse was suspected.
Chemotherapy
ABVD was administered at standard doses on days 1 and 15 with cycles repeated once every 28 days. Investigators were advised to administer full doses on time without growth factor support, regardless of blood counts, unless fever or infection occurred.11,12 The eBEACOPP regimen was administered as defined by the German Hodgkin Study Group (Data Supplement).4 The relative dose delivered of each planned drug was calculated for all patients according to the formula:
The total actual dose for each agent was counted as 0 for patients who did not register for step 2 or withdrew. No radiotherapy was administered.
Outcome Assessment
Objective disease response status was recorded at each evaluation time point according to the 2007 Cheson criteria13 (Data Supplement). PFS was measured from the date of registration to the first observation of progressive disease, relapse, or death. Patients last known to be alive and progression free were censored at date of last contact. OS was measured from the date of registration to the date of death.
Statistical Analysis
The two coprimary objectives of this study were to (1) estimate the 2-year PFS rate in patients with advanced-stage HL who were treated with response-adapted therapy and (2) estimate the 2-year PFS rate in PET2-positive patients who were subsequently treated with eBEACOPP. The goal was to enroll 60 eligible patients in the PET2-positive subgroup. To estimate the 2-year PFS rate to within 6%, 278 eligible HIV-negative patients were judged to be a sufficient number assuming an FDG-PET–positive rate of 22%. With 60 patients in the PET2-positive group, the 2-year PFS rate could be estimated in this subgroup to within 13%. With 278 total patients, the overall rates of response, toxicity, and PET positivity could be estimated to within 6%.
Any toxicity occurring with at least 5% probability was likely to be seen at least once (> 99% chance). Details are given in the Data Supplement. Ultimately, 371 patients were enrolled to achieve the PET-positive accrual goal with the lower observed PET2-positive rate of 18%. PFS and OS estimates (with 95% CIs) were calculated by using the Kaplan-Meier method.14 The two-sided Fisher’s exact test was used to compare toxicity rates between treatment arms. All analyses presented here focus on HIV-negative patients. Data regarding the HIV-positive cohort will be reported separately. Data as of September 22, 2015, were included in the analysis.
RESULTS
Accrual
Three hundred seventy-one patients were enrolled (21 ineligible and two not evaluable) between September 2010 and December 2012, including 358 HIV-negative patients (Table 1). Of those 358 patients, 336 were considered eligible and evaluable after centralized pathology review (including 308 with definite clinical HL and 28 most consistent with clinical HL). Two initial cycles of ABVD were completed as planned in 332 patients. After two cycles of ABVD, 331 patients underwent centralized PET2 review (Fig 1).
Table 1.
Demographic Characteristics of Eligible and Evaluable Patients on SWOG S0816 (n = 336)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 32 | |
| Range | 18-60 | |
| Sex | ||
| Male | 56 | |
| Female | 44 | |
| Race | ||
| White | 82 | |
| Other | 18 | |
| Stage | ||
| III | 52 | |
| IV | 48 | |
| “B” symptoms | 62 | |
| Bulk > 10 cm | 18 | |
| IPS | ||
| 0-2 | 49 | |
| 3-7 | 51 |
Abbreviations: IPS, International Prognostic Score; SWOG, Southwest Oncology Group.
Centralized FDG-PET/CT Review
Baseline, interim (PET2), and end-of-treatment scans were analyzed by a panel of experts through the CALGB ICL. PET2 scans were submitted for centralized review for 331 active patients. The central PET2 review was completed in less than 2 days in 78% of patients and in less than 4 days in 95% of patients (Data Supplement). Of 331 eligible and evaluable patients, 271 (82%) were PET2-negative and 270 received four more cycles of ABVD as planned. The other 60 patients (18%) were PET2-positive; 55 registered for the second part of the study, but six declined because they were reluctant to receive eBEACOPP. Of the 55 PET2-positive eligible patients who registered, only 49 actually received eBEACOPP; three others received ABVD, and another three declined any protocol treatment.
Outcomes of Therapy
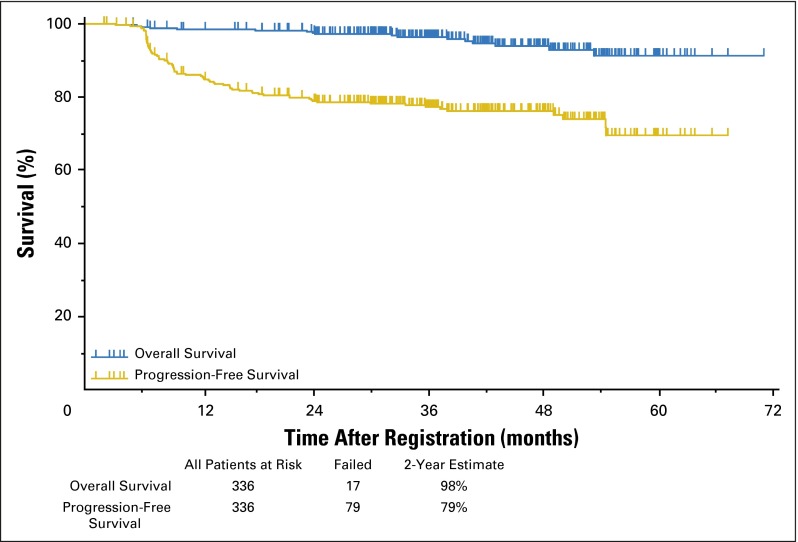
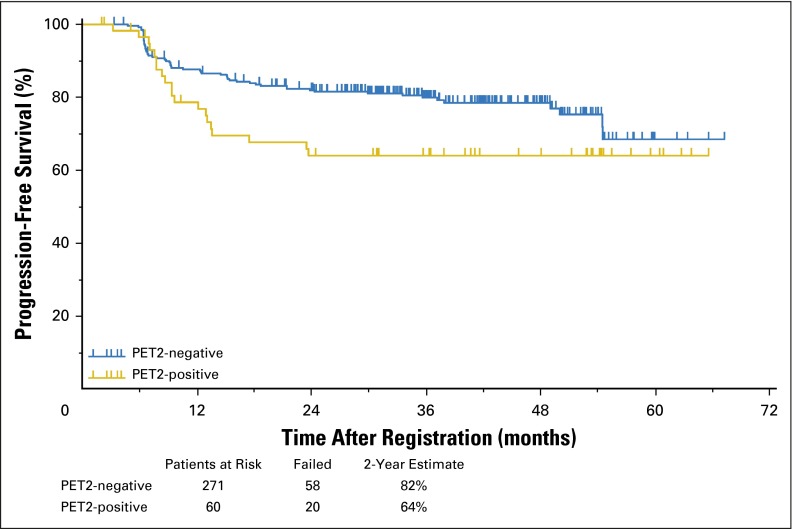
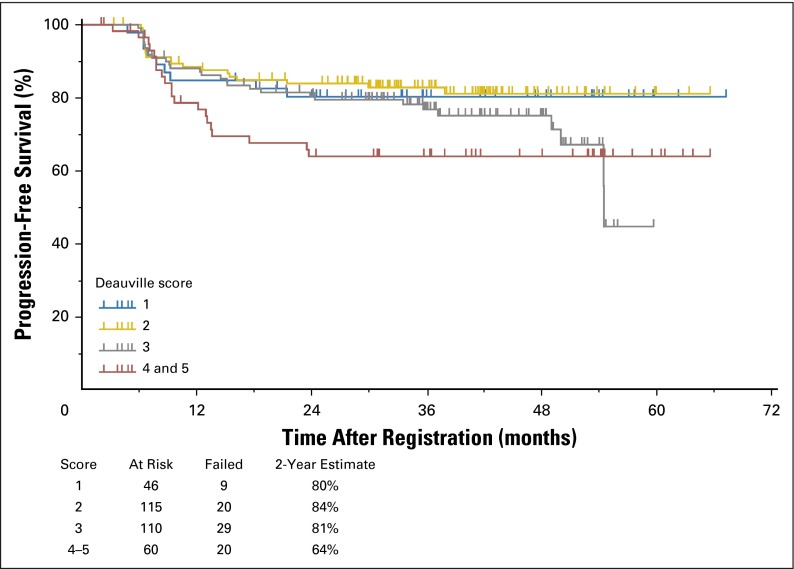
Of 325 patients treated with two cycles of ABVD followed by response-adapted therapy, 96% of patients on the ABVD arm achieved a complete remission (CR; Data Supplement), whereas 4% were designated partial responders despite a PET2-negative scan because they did not submit to follow-up marrow biopsies. In the ABVD → eBEACOPP arm, 55% achieved CR, 38% partial response, 5% stable disease, and 2% unknown response (as a result of inadequate data submission). The OS and PFS of the entire cohort of 336 evaluable patients with a median follow-up of 3.3 years are shown in Figure 2. The estimated 2-year OS was 98% with 17 deaths, including six as a result of HL, two as a result of bleomycin toxicity, and one each as a result of sepsis, cervical adenocarcinoma, anaplastic large-cell lymphoma, brain mass, or graft vs host disease; four patients had unknown cause of death. The estimated 2-year PFS was 79% (95% CI, 74% to 83%), surpassing the target of 78% prespecified as a success in the protocol. The PFS by allocation to treatment arm, analyzed on an intent-to-treat basis, is shown in Figure 3. The 2-year estimate of PFS for 271 PET2-negative patients was 82% (95% CI, 77% to 86%) with 58 patients experiencing treatment failure. The 2-year estimate of PFS for the 60 PET2-positive patients was 64% (95% CI, 50% to 75%) with 20 patients experiencing treatment failure, which exceeded the desired target of 48% prespecified as the protocol goal. Five patients did not have a PET2 assessment and are not included in the PFS curves in Figure 3. The 2-year PFS and OS rates for compliant patients (270 PET2-negative v 49 PET2-positive) are comparable to those in the intent-to-treat analysis. Two of the 20 relapses in 60 PET2-positive patients occurred in the 11 patients who did not receive eBEACOPP. Among 271 PET2-negative patients, 60 patients (22%) had an International Prognostic Score (IPS) of 4 to 7, whereas 28 (47%) of 60 PET2-positive patients had an IPS of 4 to 7. PET2 status is significantly associated with PFS. The risk of disease progression for PET2-positive patients is 1.7 times the risk for PET2-negative patients (two-sided P =.0442). The risk of disease progression for International Prognostic Index high-risk patients (IPS score 4 to 7) is 1.35 times the risk for International Prognostic Index low-risk patients (IPS score 0 to 3). However, the association is not statistically significant (two-sided P = .2191). Figure 4 depicts the 2-year PFS estimates for 331 evaluable patients stratified by Deauville score, which supports our decision to consider Deauville score 1 to 3 scans as negative and Deauville score 4 to 5 scans as positive. The outcome of the HIV-positive cohort will be reported separately.
Fig 2.
Overall and progression-free survival for 336 patients with Hodgkin lymphoma treated with response-adapted therapy on the Southwest Oncology Group S0816 trial, regardless of interim positron emission tomography/computed tomography scan result or treatment arm.
Fig 3.
Progression-free survival of 331 evaluable patients with Hodgkin lymphoma treated with response-adapted therapy on the Southwest Oncology Group S0816 trial.
Fig 4.
Progression-free survival of 331 evaluable patients with Hodgkin lymphoma treated with response-adapted therapy on the Southwest Oncology Group S0816 trial. Patients were stratified by Deauville score assessed via centralized positron emission tomography (PET) review of the fluorodexoxyglucose-PET interim scan performed after two cycles of chemotherapy with doxorubicin, bleomycin, vinblastine, and dacarbazine. Only five patients had a Deauville score of 5 after two cycles of ABVD, so they are combined with the 55 patients with a Deauville score of 4.
Relative Dose Delivery
The doses of drugs delivered to patients closely adhered to the planned doses for most patients. For the first two cycles of ABVD, 98.4% to 99.4% of the planned doses of drugs were administered to 336 HIV-negative patients (Data Supplement). Dose delivery was slightly lower for cycles 3 to 6 of ABVD delivered to 271 PET2-negative patients with 93.4% to 96.1% of planned doses of doxorubicin, vinblastine, and dacarbazine delivered, but only 86.6% ± 24.1% of the planned bleomycin dose delivered because of pulmonary toxicities (Data Supplement). Compliance was worse for the 60 PET2-positive patients intended to receive eBEACOPP, with delivery of only 72.1% to 82.3% of planned doses of drugs (Data Supplement). These figures were significantly impacted by noncompliance with this intense regimen: 11 PET-positive patients refused to receive eBEACOPP, and the dose delivered for each agent was counted as 0 for these patients. If only patients who actually received at least one cycle of eBEACOPP are included, the dose delivery for this regimen varied from 84.0% (for bleomycin) to 95.0% (for doxorubicin) of planned doses.
Toxicity
Significant toxicities experienced by eligible HIV-negative patients are provided in the Data Supplement. As expected, eBEACOPP was much more toxic than ABVD (85.7% v 36.7% grade 4 to 5 toxicities. P < .001). There were three treatment-related deaths that included one (0.4%) of 270 evaluable HIV-negative patients on ABVD and two (4%) of 49 on the eBEACOPP arm (P = .06). Six (1.4%) of the 336 eligible patients developed secondary malignancies (two non-HLs, two kidney cancers, one melanoma, and one skin cancer), including three (1%) of 270 patients who received ABVD and three (6.1%) of 49 who received at least one cycle of eBEACOPP (P = .0487).
Patterns of Relapse
With a median follow-up of 3.3 years, 74 relapses were documented among 336 patients. Twenty (32%) of the evaluable 62 relapses occurred only at sites of disease identified at initial presentation, 33 relapses (53%) occurred only at new sites, and four relapses (6%) occurred at both old and new sites. (Data were unavailable for 9% of relapsed patients.) The median baseline size of lesions that recurred at sites of previous involvement was 3.5 cm; only 24% of lesions with measurements available that recurred at previous sites were > 5 cm and only two (9.5%) were > 10 cm at initial presentation.
DISCUSSION
As recently as 1950, HL was incurable, but today approximately 70% of patients with stage III to IV HL are cured with ABVD.3 eBEACOPP seems to be more effective, with approximately 90% failure-free survival (FFS) reported in the HD15 trial,15 but it is considerably more toxic.4,16 Our goal was to reserve eBEACOPP for patients at greatest risk of recurrence after ABVD. Determining the risk of treatment failure at diagnosis in advanced-stage HL has been challenging. The IPS is of limited utility, because only 19% of patients with HL fall into the poor-prognosis group (IPS 4 to 7),17 although a preliminary publication on a newer scoring system may provide refined clinical risk estimation for patients treated in the modern era.18 More recently, infiltration of HL tumors with macrophages (detected by immunohistochemical staining for CD68) has been associated with poor outcomes.19 Genomic approaches have also been proposed; for example, a 23-gene expression signature demonstrated major prognostic power in patients with HL treated with ABVD.20 These findings await further clinical validation and, for now, the most promising approach for identifying patients with poor-risk HL seems to be interim FDG-PET/CT.6-9,21-24 Although at least seven phase II and III cooperative group studies are currently underway testing this approach in advanced-stage HL (Data Supplement), to the best of our knowledge, ours is the first large, multicenter, prospective study to publish detailed findings. Our results suggest that interim PET/CT is of prognostic value and can also predict patients with a higher likelihood of achieving durable CR by switching to a more intense regimen. The results of our study are consistent with a retrospective series in which patients with advanced-stage HL who were PET2-positive and were switched to eBEACOPP23 achieved a 2-year PFS of 65%. Furthermore, preliminary results of the similar RATHL (Response-Adjusted Therapy for Hodgkin Lymphoma) study presented as an abstract provide similar findings.25 In this trial, patients with high-risk stage II or stage III to IV HL were treated with two cycles of ABVD followed by interim PET/CT. PET2-negative patients continued ABVD, and PET2-positive patients were switched to eBEACOPP or BEACOPP14 (intensified treatment given every 14 days). The 3-year OS of the entire group (which included 41% patients with advanced-stage II) was 95% and the PFS was 82.5%.25 PFS in the 16% of patients who were PET2-positive and were switched to eBEACOPP was 70%. The value of interim PET2 response-adapted therapy has already been assessed in patients with stage I to II HL in attempts to eliminate consolidative radiotherapy, but conflicting conclusions were reached in the two published studies. A European Organisation for Research and Treatment of Cancer study26 was stopped early by an independent data monitoring committee because of inferior PFS in the PET response-adapted group.26
Preliminary results for the PET2-positive patients suggest a benefit for escalation of therapy compared with continued ABVD.27 Investigators of the RAPID (Randomized Phase III Trial to Determine the Role of FDG-PET Imaging in Clinical Stage IA/IIA Hodgkins Disease) study concluded that the inferior PFS in the group receiving chemotherapy alone was acceptable because their OS was not different, and late complications associated with radiotherapy were avoided.28
Our results suggest an improvement in PFS for PET2-positive stage III to IV patients switched to eBEACOPP compared with the historical experience with continued ABVD in PET2-positive patients.6,8 The PFS of the entire cohort of patients with stage III to IV HL exceeded the prespecified goal of ≥ 78% stated in the protocol, suggesting that the strategy of using eBEACOPP for patients with a Deauville score of 4 or 5 on interim FDG-PET/CT after two cycles of ABVD improves outcomes in the overall group. Furthermore, the 64% 2-year estimate for PFS for the PET2-positive patients is far superior to the 15% to 30% 2-year PFS reported in the literature, and it surpasses the 48% 2-year PFS threshold set as a goal in this trial. When comparing the results of this study with those of previous reports, it is vital to recognize that enrollment in this trial was restricted to patients with stage III to IV disease.
In contrast, most comparator studies included patients with unfavorable early-stage disease. For example, the last US Intergroup study E2496 (Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin’s Lymphoma) included 281 (35%) of 794 patients with unfavorable stage I to II disease.3 These early-stage patients, although their disease was considered unfavorable, fared much better (approximately 85% 5-year FFS) compared with the stage III to IV patients, who experienced approximately 65% 5-year FFS.3 Therefore, the lower FFS of 65% reported for only stage III to IV patients should be used as the comparator for this study. Similar considerations apply for other studies, as shown in the Data Supplement.4,16
The relative dose of ABVD delivered in this study was outstanding (98% to 99% in cycles 1 to 2 and 87% to 96% in cycles 3 to 6), demonstrating that administration of full doses on time without growth factor support was safe and feasible, even though neutrophil counts were often low on the day of treatment. Only a single death occurred in the 270 patients enrolled on the protocol, and rates of serious infection were low (one patient with grade 3 pneumonia, 18 patients [6%] with febrile neutropenia). Only bleomycin was administered at significantly lower doses than planned (86.6% ± 24.1%) because of pulmonary toxicities. In contrast, the relative dose of eBEACOPP delivered was poor (72% to 82% of planned dosages delivered) by comparison. Nevertheless, outcomes of the overall PET2-positive group were excellent, suggesting that even better results might be achieved in the future if compliance is improved.
The role of consolidative radiotherapy in advanced-stage HL is controversial. Radiotherapy was not permitted in this trial; however, analysis of the patterns of relapse suggest that omission of radiotherapy contributed little to relapses, because only 32% of progressions occurred at sites of previous involvement, and only two relapses occurred at sites of previous bulk (> 10 cm) where consolidative radiotherapy would be expected to confer the most benefit.
Although the results of SWOG S0816 argue strongly for a response-adapted approach for advanced-stage HL using early interim FDG-PET/CT, it must be acknowledged that the outcomes are being compared with historical figures with their inherent limitations.
Longer follow-up is essential for confirming that the reported findings will stand the test of time. The results of ongoing, randomized, phase III trials testing this hypothesis are needed. Finally, some treatment failures were observed, even in PET2-negative patients, which indicates that interim PET is not a perfect test. We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than are achievable with current technology.20 Until that time, our results suggest that the response-adapted strategy of increasing treatment to eBEACOPP in PET2-positive patients is a reasonable option for advanced-stage HL therapy.
Supplementary Material
Acknowledgment
We thank all investigators, data coordinators, and patients for making the study possible.
Footnotes
Supported in part by Grants No. CA180888, CA180819, CA180821, CA180820, CA180799, CA180816, CA180801, CA180835, CA180858, CA180801, CA180846, CA180835, CA180818, CA180828, CA180826, and CA180834 from the Public Health Service, Department of Health and Human Services, National Cancer Institute (NCI), National Clinical Trials Network; by Grants No. CA189830, CA189971, CA189808, CA189854, CA189821, CA189848, CA189858, CA189860, CA189872, and CA189856 from the NCI Community Oncology Research Program; by Grant No. CA31946 from the National Institutes of Health (NIH)-NCI; by Grant No. 3U10CA032102-30S1 from the NIH American Recovery and Reinvestment Act of 2009 for the interim FDG-PET imaging; by Grant No. CA121947 from the NCI AIDS Malignancy Clinical Trials Consortium; and by the David and Patricia Giuliani Family Foundation (O.W.P.), The Lymphoma Foundation (D.J.S.), the Adam Spector Fund for Hodgkin’s Research (D.J.S.), and the Ernest and Jeanette Dicker Charitable Foundation (D.J.S.).
Presented at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-22, 2013.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Contributions are found at the end of this article.
See accompanying editorial on page 1975
AUTHOR CONTRIBUTIONS
Conception and design: Oliver W. Press, Hongli Li, Heiko Schöder, David J. Straus, Michael LeBlanc, Nancy L. Bartlett, Andrew M. Evens, Ann S. LaCasce, John W. Sweetenham, Paul M. Barr, Bruce D. Cheson, Richard I. Fisher, Jonathan W. Friedberg
Provision of study materials or patients: Oliver W. Press, Heiko Schöder, David J. Straus, Craig H. Moskowitz, Nancy L. Bartlett, Andrew M. Evens, John W. Sweetenham, Michelle A. Fanale, Ariela Noy, Randy D. Gascoyne, John P. Leonard, Brad S. Kahl, Bruce D. Cheson, Jonathan W. Friedberg
Collection and assembly of data: Oliver W. Press, Hongli Li, Heiko Schöder, David J. Straus, Craig H. Moskowitz, Michael LeBlanc, Nancy L. Bartlett, Erik S. Mittra, Ann S. LaCasce, Paul M. Barr, Michelle A. Fanale, Michael V. Knopp, Ariela Noy, Eric D. Hsi, Mary Jo Lechowicz, Randy D. Gascoyne, John P. Leonard, Brad S. Kahl, Bruce D. Cheson, Richard I. Fisher, Jonathan W. Friedberg
Data analysis and interpretation: Oliver W. Press, Hongli Li, Heiko Schöder, David J. Straus, Craig H. Moskowitz, Michael LeBlanc, Lisa M. Rimsza, Nancy L. Bartlett, Erik S. Mittra, Ann S. LaCasce, Paul M. Barr, Michelle A. Fanale, Michael V. Knopp, Ariela Noy, Eric D. Hsi, James R. Cook, Mary Jo Lechowicz, Randy D. Gascoyne, John P. Leonard, Brad S. Kahl, Bruce D. Cheson, Richard I. Fisher, Jonathan W. Friedberg
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose–Positron Emission Tomography Imaging: Southwest Oncology Group S0816
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Oliver W. Press
Stock or Other Ownership: PhaseRx, Emergent Bio Solutions
Consulting or Advisory Role: BIND Biosciences, Adaptive Biotechnologies, Roche
Research Funding: Genentech (Inst), Presage Biosciences (Inst)
Travel, Accommodations, Expenses: Roche
Hongli Li
No relationship to disclose
Heiko Schöder
No relationship to disclose
David J. Straus
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb
Craig H. Moskowitz
Consulting or Advisory Role: Seattle Genetics, Merck, Celgene, Novartis
Research Funding: Seattle Genetics, Merck
Michael LeBlanc
No relationship to disclose
Lisa M. Rimsza
Consulting or Advisory Role: Celgene
Speakers’ Bureau: Ventana Medical Systems, Celgene
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Gilead Sciences
Research Funding: Seattle Genetics (Inst), Millennium Pharmaceuticals (Inst), Pfizer (Inst), Pharmacyclics (Inst), Novartis (Inst), MedImmune (Inst), Celgene (Inst), ImaginAB (Inst), Genentech (Inst), Janssen Research and Development (Inst), AstraZeneca (Inst)
Andrew M. Evens
Honoraria: Seattle Genetics, Genentech, Celgene, Millennium Pharmaceuticals
Consulting or Advisory Role: Celgene, Millennium Pharmaceuticals
Speakers’ Bureau: Celgene
Research Funding: Millennium Pharmaceuticals
Erik S. Mittra
Consulting or Advisory Role: Calithera Biosciences
Research Funding: Piramal Life Sciences
Ann S. LaCasce
No relationship to disclose
John W. Sweetenham
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, Sandoz
Paul M. Barr
Consulting or Advisory Role: Pharmacyclics, Gilead Sciences, Genentech, Abbott/AbbVie, Seattle Genetics
Michelle A. Fanale
Honoraria: Seattle Genetics, Takeda Pharmaceuticals, Research to Practice, PleXus Communications
Consulting or Advisory Role: Spectrum Pharmaceuticals, Acetylon Pharmaceuticals, Clarient, Amgen
Research Funding: Millennium Pharmaceuticals, Seattle Genetics, Novartis, MedImmune, Bristol-Myers Squibb, Celgene, Molecular Templates, Genentech, Gilead Sciences
Travel, Accommodations, Expenses: Takeda Pharmaceuticals, Spectrum Pharmaceuticals, Research to Practice, PleXus Communications
Michael V. Knopp
No relationship to disclose
Ariela Noy
Research Funding: Pharmacyclics (Inst)
Travel, Accommodations, Expenses: Pharmacyclics
Eric D. Hsi
Consulting or Advisory Role: HTG Molecular Diagnostics, Onyx Pharmaceuticals, AbbVie, Cellerant Therapeutics
Speakers’ Bureau: Seattle Genetics
Research Funding: AbbVie (Inst), Eli Lilly (Inst), Cellerant Therapeutics (Inst)
James R. Cook
Research Funding: Abbott Molecular
Patents, Royalties, Other Intellectual Property: US Provisional Patent Application No. 61/900,553, filed November 6, 2013: Methods for Selecting and Treating Lymphoma Types (coinventor)
Mary Jo Lechowicz
Consulting or Advisory Role: Seattle Genetics, Spectrum Pharmaceuticals, Janssen, Millennium Pharmaceuticals
Travel, Accommodations, Expenses: Janssen
Randy D. Gascoyne
Honoraria: Seattle Genetics
Consulting or Advisory Role: Genentech, Celgene, Janssen, Seattle Genetics
Speakers’ Bureau: Seattle Genetics
John P. Leonard
Consulting or Advisory Role: Celgene, ADC Therapeutics, Biotest Pharmaceuticals, MedImmune, Hospira, Bayer, Juno Therapeutics, OncoTracker, Gilead Sciences, Mirati Therapeutics, Eisai, Pfizer, Novartis, Seattle Genetics, ProNAi Therapeutics, Vertex Pharmaceuticals, Boehringer Ingelheim, Spectrum Pharmaceuticals, Genentech, Cornerstone Pharmaceuticals, Pharmacyclics
Brad S. Kahl
Consulting or Advisory Role: Seattle Genetics, Millennium Pharmaceuticals
Bruce D. Cheson
Consulting or Advisory Role: Celgene, Seattle Genetics, Pharmacyclics, Gilead Sciences, Astellas Pharma, Roche/Genentech, Amgen
Research Funding: Gilead Sciences, Pharmacyclics, Celgene, Seattle Genetics, Acerta Pharma, Teva, MedImmune
Richard I. Fisher
Consulting or Advisory Role: Johnson & Johnson, Celgene, Seattle Genetics, Gilead Sciences
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer
Research Funding: Seattle Genetics (Inst), Millennium Pharmaceuticals (Inst)
REFERENCES
- 1.Press OW. Hodgkin lymphoma, in Kaushansky K, Lichtman M, Prchal J, et al (eds): Williams’ Hematology (ed 9). New York, NY, McGraw Hill, 2016, pp 1603-1624. [Google Scholar]
- 2.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 3.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An Intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 5.Sieniawski M, Reineke T, Nogova L, et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: A report of the German Hodgkin Study Group (GHSG). Blood. 2008;111:71–76. doi: 10.1182/blood-2007-02-073544. [DOI] [PubMed] [Google Scholar]
- 6.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 7.Gallamini A, Kostakoglu L. Interim FDG-PET in Hodgkin lymphoma: A compass for a safe navigation in clinical trials? Blood. 2012;120:4913–4920. doi: 10.1182/blood-2012-03-403790. [DOI] [PubMed] [Google Scholar]
- 8.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 9.Kostakoglu L, Gallamini A. Interim 18F-FDG PET in Hodgkin lymphoma: Would PET-adapted clinical trials lead to a paradigm shift? J Nucl Med. 2013;54:1082–1093. doi: 10.2967/jnumed.113.120451. [DOI] [PubMed] [Google Scholar]
- 10.Oki Y, Chuang H, Chasen B, et al. The prognostic value of interim positron emission tomography scan in patients with classical Hodgkin lymphoma. Br J Haematol. 2014;165:112–116. doi: 10.1111/bjh.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boleti E, Mead GM. ABVD for Hodgkin’s lymphoma: Full-dose chemotherapy without dose reductions or growth factors. Ann Oncol. 2007;18:376–380. doi: 10.1093/annonc/mdl397. [DOI] [PubMed] [Google Scholar]
- 12.Evens AM, Cilley J, Ortiz T, et al. G-CSF is not necessary to maintain over 99% dose-intensity with ABVD in the treatment of Hodgkin lymphoma: Low toxicity and excellent outcomes in a 10-year analysis. Br J Haematol. 2007;137:545–552. doi: 10.1111/j.1365-2141.2007.06598.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 16.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386–2395. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 17.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease: International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 18.Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015;171:530–538. doi: 10.1111/bjh.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700. doi: 10.1200/JCO.2012.43.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrington SF, Mikhaeel NG, Kostakoglu L, et al: Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048-3058, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152:551–560. doi: 10.1111/j.1365-2141.2010.08485.x. [DOI] [PubMed] [Google Scholar]
- 24.Kostakoglu L, Cheson BD. Current role of FDG PET/CT in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1004–1027. doi: 10.1007/s00259-013-2686-2. [DOI] [PubMed] [Google Scholar]
- 25. Johnson PW, Federico M, Fossa A, et al: Response-adapted therapy based on interim FDG-PET scans in advanced Hodgkin lymphoma: First analysis of the safety of de-escalation and efficacy of escalation in the international RATHL study (CRUK/07/033). Hematol Oncol 33, 2015 (suppl; abstr 108)
- 26.Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32:1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 27.Raemaekers JM. Early FDG-PET adapted treatment improves the outcome of early FDG-PET positive patients with stages I/II Hodgkin lymphoma (HL): Final results of the randomized intergroup EORTC/LYSA/FIL H10 trial. Presented at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 17-21, 2015, 2015. [Google Scholar]
- 28.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.