Abstract
Purpose
Trastuzumab is a key component of adjuvant therapy for stage I to III human epidermal growth factor receptor 2 (HER2)–positive breast cancer. The rates and patterns of trastuzumab use have never been described in a population-based sample. The recent addition of HER2 information to the SEER-Medicare database offers an opportunity to examine patterns of trastuzumab use and to evaluate possible disparities in receipt of trastuzumab.
Methods
We examined a national cohort of Medicare beneficiaries with incident stage I to III HER2-positive breast cancer diagnosed in 2010 and 2011 (n = 1,362). We used insurance claims data to track any use of trastuzumab in the 12 months after diagnosis as well as to identify chemotherapy drugs used in partnership with trastuzumab. We used modified Poisson regression analysis to evaluate the independent effect of race on likelihood of receiving trastuzumab by controlling for clinical need, comorbidity, and community-level socioeconomic status.
Results
Overall, 50% of white women and 40% of black women received some trastuzumab therapy. Among women with stage III disease, 74% of whites and 56% of blacks received trastuzumab. After adjustment for tumor characteristics, poverty, and comorbidity, black women were 25% less likely to receive trastuzumab within 1 year of diagnosis than white women (risk ratio, 0.745; 95% CI, 0.60 to 0.93).
Conclusion
Approxemately one half of patients 65 years of age and older with stage I to III breast cancer do not receive trastuzumab-based therapy, which includes many with locally advanced disease. Significant racial disparities exist in the receipt of this highly effective therapy. Further research that identifies barriers to use and increases uptake of trastuzumab could potentially improve recurrence and survival outcomes in this population, particularly among minority women.
INTRODUCTION
In the past two decades, the monoclonal antibody trastuzumab has dramatically changed the treatment paradigm and prognosis of human epidermal growth factor receptor 2 (HER2)–positive breast cancer.1-4 Since its approval for adjuvant use in the United States in 2004, trastuzumab has been rapidly adopted as a standard of care for women with stage II to III HER2-positive disease and is offered to many women with stage I breast cancer. Although the benefits of trastuzumab are uncertain among some patients (eg, those with very small cancers and frail elderly patients),5 most women with HER2-positive breast cancer benefit from trastuzumab-based systemic therapy, which has been shown in long-term follow-up studies to improve overall survival in this patient population by 37%.4 However, noninitiation and nonadherence studies have shown in the other primary targeted therapy—endocrine therapy in hormone receptor–positive breast cancer—that highly effective drug therapy is not always delivered consistently across patient populations. The actual uptake of trastuzumab in community settings and the extent to which trastuzumab is used or underused in US populations, particularly among vulnerable groups such as minority and elderly women, are unknown.
The study of disparities in trastuzumab use has been complicated by the lack of large population-based cohorts with known HER2 status. To date, available observational data sources have been limited to single-institution series and pooled data from academic centers and have been focused primarily on the impact of trastuzumab-based therapy on outcomes among women with stage I tumors.6-10 To our knowledge, no prior study has described predictors of trastuzumab use among eligible women with HER2-positive breast cancer in a large population-based cohort.
Beginning in 2010, HER2 status became a required data element for cancer registries that participate in the SEER network of the National Cancer Institute, and these data have been made available for cases diagnosed in 2010 and 2011. To date, this information has been used only to describe the epidemiology of breast cancer with regard to the triple phenotype (the combination of estrogen receptor [ER], progesterone receptor [PR], and HER2 status) in breast cancer.11 Linkage of SEER data with fee-for-service Medicare administrative claims offers an opportunity to examine potential racial disparities in trastuzumab use among a national cohort of women with HER2-positive breast cancer. We examined racial variation in trastuzumab use among Medicare beneficiaries with newly diagnosed nonmetastatic HER2-positive breast cancer who are candidates for adjuvant trastuzumab-based therapy.
METHODS
Data Source and Study Population
We used data from the SEER-Medicare–linked data set for the period 2009 to 2013. SEER data represent approximately 28% of the US cancer population. For those ages 65 years and older and insured by fee-for-service Medicare, approximately 97% are successfully linked by the National Cancer Institute. The SEER Medicare data are linked by using an iterative, deterministic approach that has been described previously.12 Data elements include demographic characteristics, timing and type of cancer diagnosis, staging, and insurance-reimbursed treatment. Our preliminary sample included female patients who received a diagnosis in 2010 and 2011 of a first primary stage I to III HER2-positive invasive breast cancer at age 66 years or older (to allow for assessment of comorbidity in the prior year) and who survived at least 365 days from diagnosis. SEER registries use an algorithm to assign HER2 status that combines available results from immunohistochemistry, fluorescence in situ hybridization, and chromogenic in situ hybridization tests.13
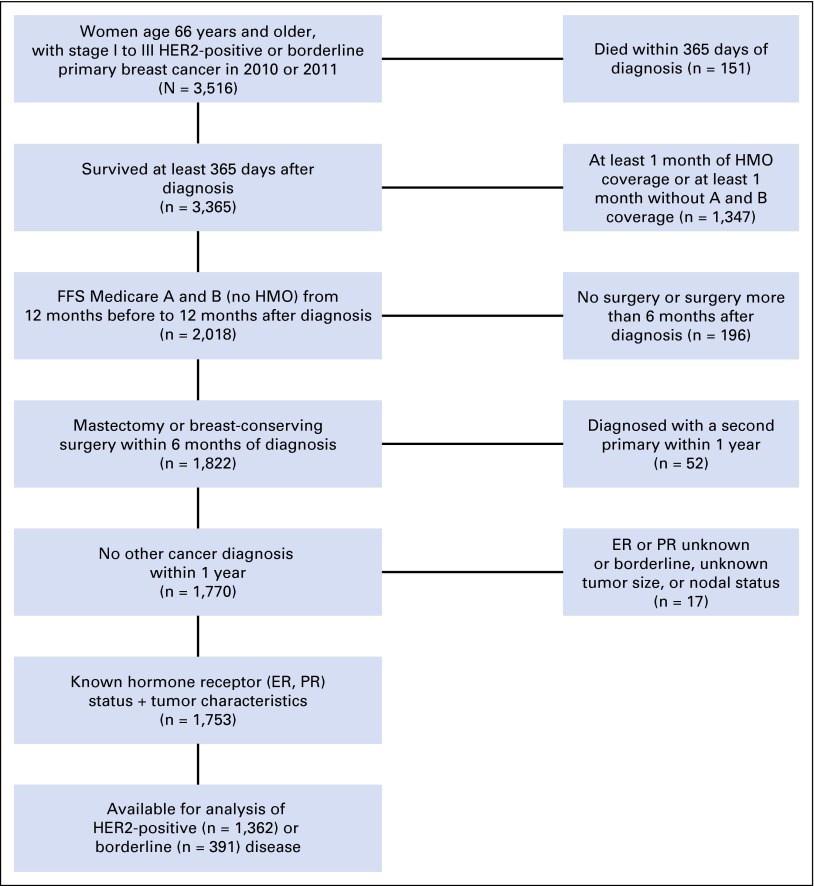
Patients were required to have continuous enrollment in fee-for-service Medicare parts A and B from 12 months before to 12 months after diagnosis to ensure full capture of all treatment of comorbid conditions as well as the first course of primary breast cancer treatment. Patients enrolled in health maintenance organization plans were excluded due to incomplete ascertainment of claims. Patients were required to have a primary breast cancer surgery (either breast-conserving surgery or mastectomy) within 6 months of diagnosis from a previous methodology14 (Data Supplement). Patients with borderline, unknown, or missing information for both ER and PR status were excluded because they could not be classified into a tumor phenotype (n ≤ 11). A CONSORT diagram is shown in Figure 1.
Fig 1.
CONSORT diagram for study cohort. ER, estrogen receptor; FFS, fee for service; HER2, human epidermal growth factor receptor 2; HMO, health maintenance organization; PR, progesterone receptor.
Primary Outcome
The primary outcome was receipt of trastuzumab, which was defined as at least one claim for trastuzumab within the year after diagnosis (Healthcare Common Procedure Classification System code J9355). Trastuzumab exposure could occur at any time between diagnosis and day 365 postdiagnosis, which included both neoadjuvant and adjuvant sequencing. Chemotherapies given concurrently with trastuzumab were examined and categorized by analyzing all claims for chemotherapy agents given to patients within 1 year of diagnosis and categorizing as follows: doxorubicin, cyclophosphamide, taxane (ACTH) regimen; docetaxel, carboplatin (TCH) regimen; some doxorubicin or carboplatin use that did not meet the definition of ACTH or TCH; taxane agents with or without cyclophosphamide; all other chemotherapy partners; and trastuzumab as a single agent. Detailed definitions of chemotherapy regimens can be found in the Data Supplement. For patients who did not receive trastuzumab, use of any chemotherapy within 1 year was also captured.
Primary Exposure and Covariates
Our primary exposure of interest was race as recorded by Medicare enrollment files, which are based on information from the Master Beneficiary Record of the Social Security Administration.15-17 We obtained key covariates from the SEER Patient Entitlement and Diagnosis Summary File, which included diagnosis date, age, race (white, black, other), tumor characteristics, and ER and PR status. Tumor grade, primary tumor size, and nodal involvement were defined according to American Joint Committee on Cancer Staging VII criteria.17a
Additional covariates defined based on claims-based algorithms were type of breast surgery, receipt of radiation,14 and comorbidity as defined by the Klabunde modification of the Charlson comorbidity index.18 Contextual socioeconomic variables were taken from American Communities Survey data and included the proportion of people living in poverty in the patients’ census tracts.19 We controlled for geographic variation in trastuzumab use by the four-category grouping of SEER regions (northeast, south, north central, and west).
Statistical Analysis
To identify predictors of trastuzumab use, we used modified multivariable Poisson regression analysis with sandwich standard errors20 by using the GENMOD procedure of SAS version 9.4 software (SAS Institute, Cary, NC). Covariates for the final model were determined a priori on the basis of clinical judgment and literature that has identified common factors associated with variations in breast cancer treatment. Patient-level covariates of interest were chosen based on literature that breast cancer treatment and outcomes may vary by marital status,21 comorbidity,22 socioeconomic status,23 and geographic region.24 Year of diagnosis was included based on literature that adoption of treatment innovations increases over time.25 Disease-related variables were chosen based on clinical judgment about their use in treatment decision making and prognostication, which includes tumor size, nodal status, tumor grade, and hormone receptor status. Local therapy approach was included based on clinical judgment that differences in local treatment preferences might be associated with differences in systemic treatment preferences. We present unadjusted and adjusted risk ratios (RRs) and 95% CIs for all models.
RESULTS
We identified 1,362 women with HER2-positive breast cancer (85% white, 7.6% black, 7% other races). Table 1 shows clinical and demographic characteristics of the cohort. Around one half of patients were younger than 75 years, and 69% had node-negative disease. Seventy-three percent of patients had triple-positive (hormone-receptor and HER2-positive) breast cancer. More than one half of patients (52%) had high-grade tumors, and 42% had tumors ≥ 2 cm, which reflects the relatively aggressive biology of HER2-positive cancer. In bivariable analyses, patients who received trastuzumab were significantly younger, more likely to have hormone receptor–negative disease, and more likely to be treated surgically with mastectomy than patients who did not receive trastuzumab.
Table 1.
Cohort Characteristics
| Total (n = 1,362) | Treated (n = 672) | Untreated (n = 690) | |||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | P | |
| Age-group | < .001 | ||||||
| 65-69 years | 354 | 26.0 | 212 | 31.5 | 142 | 20.6 | |
| 70-74 years | 362 | 26.6 | 222 | 33.0 | 140 | 20.3 | |
| 75-84 years | 501 | 36.8 | 216 | 32.1 | 285 | 41.3 | |
| ≥ 85 years | 145 | 10.6 | 22 | 3.3 | 123 | 17.8 | |
| Race | .1511 | ||||||
| Black | 104 | 7.6 | 42 | 6.3 | 62 | 9.0 | |
| Other | 96 | 7.0 | 50 | 7.4 | 46 | 6.7 | |
| White | 1,162 | 85.3 | 580 | 86.3 | 582 | 84.3 | |
| State buy-in for 12 months before diagnosis | .0861 | ||||||
| No | 1,163 | 85.4 | 585 | 87.1 | 578 | 83.8 | |
| Yes | 199 | 14.6 | 87 | 12.9 | 112 | 16.2 | |
| SEER region | .797 | ||||||
| North central | 172 | 12.6 | 81 | 12.1 | 91 | 13.2 | |
| Northeast | 274 | 20.1 | 141 | 21.0 | 133 | 19.3 | |
| South | 350 | 25.7 | 169 | 25.1 | 181 | 26.2 | |
| West | 566 | 41.6 | 281 | 41.8 | 285 | 41.3 | |
| Marital status | < .001 | ||||||
| Married | 554 | 40.7 | 255 | 37.9 | 299 | 43.3 | |
| Other | 808 | 59.3 | 417 | 62.1 | 391 | 56.7 | |
| Comorbidity score | < .001 | ||||||
| 0 | 844 | 62.0 | 451 | 67.1 | 393 | 57.0 | |
| 1 | 288 | 21.1 | 126 | 18.8 | 162 | 23.5 | |
| ≥ 2 | 230 | 16.9 | 95 | 14.1 | 135 | 19.6 | |
| Residents below poverty level | .1397 | ||||||
| < 5.0% | 289 | 21.2 | 160 | 23.8 | 129 | 18.7 | |
| 5.0%-9.99% | 373 | 27.4 | 178 | 26.5 | 195 | 28.3 | |
| 10.0%-19.99% | 439 | 32.2 | 212 | 31.5 | 227 | 32.9 | |
| ≥ 20.0% | 261 | 19.2 | 122 | 18.2 | 139 | 20.1 | |
| Diagnosis year | .0581 | ||||||
| 2010 | 684 | 50.2 | 320 | 47.6 | 364 | 52.8 | |
| 2011 | 678 | 49.8 | 352 | 52.4 | 326 | 47.2 | |
| AJCC tumor size | < .001 | ||||||
| T1a | 139 | 10.2 | 25 | 3.7 | 114 | 16.5 | |
| T1b | 203 | 14.9 | 71 | 10.6 | 132 | 19.1 | |
| T1c | 452 | 33.2 | 235 | 35.0 | 217 | 31.4 | |
| T2 | 452 | 33.2 | 271 | 40.3 | 181 | 26.2 | |
| T3 | 67 | 4.9 | 35 | 5.2 | 32 | 4.6 | |
| T4 | 49 | 3.6 | 35 | 5.2 | 14 | 2.0 | |
| AJCC nodes involved | < .001 | ||||||
| N0 | 942 | 69.2 | 386 | 57.4 | 556 | 80.6 | |
| N1 | 282 | 20.7 | 185 | 27.5 | 97 | 14.1 | |
| N2/N3 | 138 | 10.1 | 101 | 15.0 | 37 | 5.4 | |
| Tumor grade | < .001 | ||||||
| Well differentiated | 124 | 9.1 | 25 | 3.7 | 99 | 14.3 | |
| Moderately differentiated | 484 | 35.5 | 192 | 28.6 | 292 | 42.3 | |
| Poorly differentiated | 703 | 51.6 | 439 | 65.3 | 264 | 38.3 | |
| Unknown | 51 | 3.7 | 16 | 2.4 | 35 | 5.1 | |
| Hormone receptor status | .0091 | ||||||
| Positive | 990 | 72.7 | 467 | 69.5 | 523 | 75.8 | |
| Negative | 372 | 27.3 | 205 | 30.5 | 167 | 24.2 | |
| Surgery type | < .001 | ||||||
| Mastectomy | 574 | 42.1 | 305 | 45.4 | 269 | 39.0 | |
| Breast conserving | 324 | 23.8 | 201 | 29.9 | 123 | 17.8 | |
| Breast conserving and radiation | 397 | 29.1 | 127 | 18.9 | 270 | 39.1 | |
| Mastectomy and radiation | 67 | 4.9 | 39 | 5.8 | 28 | 4.1 | |
Abbreviation: AJCC, American Joint Committee on Cancer.
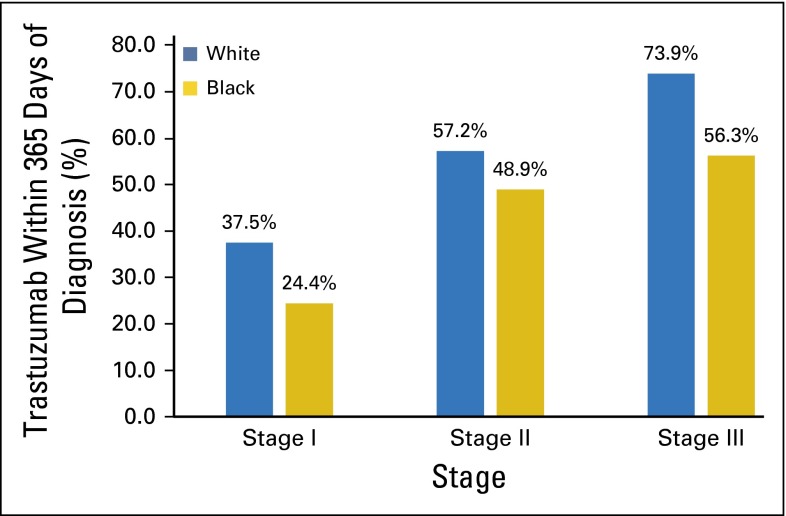
We examined unadjusted rates of trastuzumab use by race. Overall, trastuzumab use within 1 year of diagnosis was 50% among whites (n = 1,162), 40% among blacks (n = 104), and 52% among other minorities (n = 96; data not shown). Use among blacks compared with whites lagged at every stage (Fig 2), with 56% of blacks and 74% of whites receiving trastuzumab for stage III disease. Due to cell sizes <11 in black women, exact numbers are not displayed.
Fig 2.
Receipt of trastuzumab among women with human epidermal growth factor receptor 2–positive breast cancer by stage and race.
Predictors of Trastuzumab Use
After adjustment for the covariates age, comorbidity, and disease characteristics as well as type of locoregional therapy and neighborhood-level poverty, black women were 25% less likely to receive trastuzumab within 1 year of diagnosis than white women (RR, 0.75; 95% CI, 0.60 to 0.93; Table 2). Disease characteristics that predicted a greater likelihood of receiving trastuzumab were large tumor size (all T sizes greater than T1a), node positivity, and moderately or poorly differentiated tumors compared with well-differentiated tumors. Age older than 74 years and the presence of medical comorbidity measured by a Charlson comorbidity score > 1 were associated with a lower likelihood of receiving trastuzumab. Hormone receptor positivity was not an independent predictor of trastuzumab use after controlling for other tumor characteristics. Women who received breast-conserving surgery and radiation as their locoregional therapy were somewhat less likely to receive trastuzumab (RR, 0.73; 95% CI, 0.63 to 0.86) than those treated with mastectomy.
Table 2.
Results of Multivariable Model
| Parameter and Level | Risk Ratio | 95% CI | P |
|---|---|---|---|
| Age-group | |||
| 65-69 years | 1.00 | Reference | |
| 70-74 years | 0.98 | 0.88 to 1.09 | .7349 |
| 75-84 years | 0.74 | 0.65 to 0.84 | < .001* |
| ≥ 85 years | 0.25 | 0.17 to 0.37 | < .001* |
| Race | |||
| Black | 0.75 | 0.60 to 0.93 | .0097* |
| Other | 1.01 | 0.86 to 1.20 | .8855 |
| White | 1.00 | Reference | |
| Marital status | |||
| Married | 1.00 | Reference | |
| Not married | 0.93 | 0.84 to 1.03 | .1537 |
| SEER region | |||
| North central | 0.90 | 0.77 to 1.06 | .2038 |
| Northeast | 1.04 | 0.91 to 1.17 | .5917 |
| South | 0.98 | 0.87 to 1.11 | .7772 |
| West | 1.00 | Reference | |
| Below poverty | |||
| < 5.0% | 1.00 | Reference | |
| 5.0%-9.99% | 0.85 | 0.75 to 0.97 | .0155† |
| 10.0%-19.99% | 0.87 | 0.77 to 0.99 | .0377† |
| ≥ 20.0% | 0.88 | 0.76 to 1.02 | .0965 |
| Comorbidity score | |||
| 0 | 1.00 | Reference | |
| 1 | 0.90 | 0.80 to 1.02 | .1063 |
| ≥ 2 | 0.84 | 0.73 to 0.97 | .0187† |
| Diagnosis year | |||
| 2010 | 1.00 | Reference | |
| 2011 | 1.12 | 1.02 to 1.23 | .0176† |
| Hormone receptor status | |||
| Positive | 1.00 | Reference | |
| Negative | 1.04 | 0.94 to 1.15 | .4832 |
| AJCC tumor size | |||
| T1a | 1.00 | Reference | |
| T1b | 1.72 | 1.18 to 2.51 | .0047* |
| T1c | 2.19 | 1.54 to 3.12 | < .001* |
| T2 | 2.30 | 1.62 to 3.28 | < .001* |
| T3 | 2.04 | 1.36 to 3.05 | < .001* |
| T4 | 2.58 | 1.75 to 3.81 | < .001* |
| AJCC nodes involved | |||
| N0 | 1.00 | Reference | |
| N1 | 1.27 | 1.14 to 1.41 | < .001* |
| N2/N3 | 1.36 | 1.19 to 1.55 | < .001* |
| Tumor grade | |||
| Well differentiated | 1.00 | Reference | |
| Moderately differentiated | 1.68 | 1.19 to 2.38 | .0034* |
| Poorly differentiated | 2.30 | 1.63 to 3.23 | < .001* |
| Unknown | 1.48 | 0.90 to 2.44 | .1199 |
| Surgery type | |||
| Mastectomy | 1.00 | Reference | |
| Breast conserving only | 1.15 | 1.04 to 1.28 | .0075* |
| Breast conserving and radiation | 0.73 | 0.63 to 0.86 | < .001* |
| Mastectomy and radiation | 1.02 | 0.83 to 1.25 | .8797 |
Abbreviation: American Joint Committee on Cancer.
Significant at P < .01.
Significant at P < .05.
To address the concern that cardiac as opposed to overall comorbidity may exert a stronger effect on the likelihood of trastuzumab receipt and may attenuate the independent effects of race and age, we performed sensitivity analyses for measures of cardiac comorbidity derived from the Medicare Chronic Conditions Warehouse in combination with and substituted for the modified Charlson comorbidity score. The estimated effect of race and age on likelihood of trastuzumab receipt remained stable regardless of the approach to comorbidity modeling. Full results of this analysis are presented in the Data Supplement.
Patterns of Chemotherapy Use With Trastuzumab
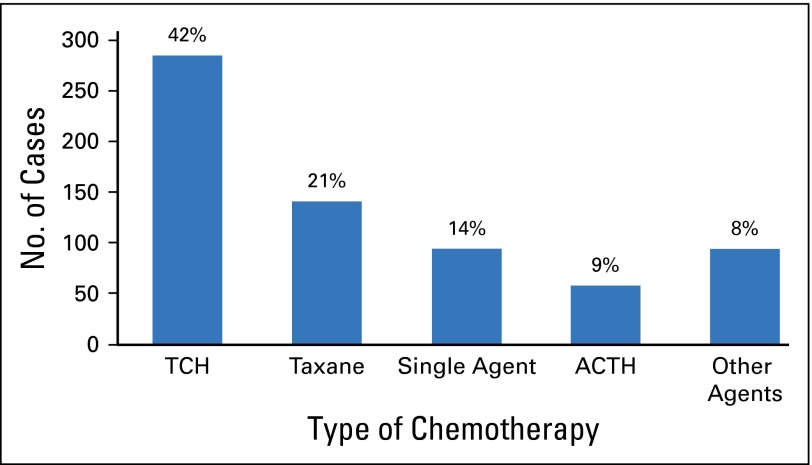
We performed descriptive analyses to determine the most common chemotherapy partners for patients who received trastuzumab. By using their pattern of chemotherapy drug claims, we categorized patients as having received one of the following: ACTH OR TCH, some exposure to anthracycline or carboplatin-based agents without meeting the predetermined criteria for ACTH or TCH, taxane agents only, single-agent trastuzumab, and other chemotherapy agents. Complete descriptions of the chemotherapy algorithms are provided in the Data Supplement. Among trastuzumab users (n = 672), the most common chemotherapy regimen was TCH (42% of treated patients [n = 285]) followed by taxane-only regimens (21% [n = 141]) and ACTH (9% [n = 58]). A small number of women had one or more claims for an anthracycline (3% [n = 20]) or carboplatin (8% [n = 55]) but did not clearly receive ACTH or TCH. In this sample, 94 women received trastuzumab as a single agent, and 19 received it in combination with another nonstandard chemotherapeutic agent. The distribution of chemotherapy regimens is illustrated in Figure 3. Due to small sample sizes for most regimens in minority women, racial differences in chemotherapy type were not systematically examined.
Fig 3.
Chemotherapies received with trastuzumab (n = 672). ACTH, doxorubicin, cyclophosphamide, taxane; TCH, docetaxel, carboplatin.
DISCUSSION
This study found that approximately one half of women with HER2-positive breast cancer insured by Medicare did not receive adjuvant HER2-targeted therapy and that black women were 25% less likely than clinically similar white women to receive such therapy. These findings have several troubling implications. First, although this study did not examine survival outcomes, the low overall rates of use and low rates among patients with stage II and III disease raise concerns for widespread underuse of trastuzumab in this age-group. Second, the racial findings suggest that the presence of a clear biologic predictor of treatment benefit, in this case the HER2 marker, does not mitigate the overall pattern of treatment disparity observed among black women with breast cancer.26 Finally, this pattern is observed during a recent time period (2010 to 2011) and suggests that gaps in breast cancer treatment continue to be problematic despite widespread awareness of the problem and efforts to improve access to care among minority women.27-30
We found that older patients and those with a higher comorbidity burden were less likely to receive trastuzumab-based therapy following a long-standing pattern observed in studies of systemic breast cancer treatment. In some cases, such variation may be appropriate because small gains in recurrence-free survival from targeted therapy may be attenuated or lost in patients with significant competing mortality risks. However, given the sizeable benefit of trastuzumab, the overall aggressive biology of this subtype, and extensive literature documenting undertreatment of even healthy older patients,31 the hypothesis that age is used inappropriately to withhold trastuzumab-based therapy deserves further exploration. The availability of large patient cohorts that represent the entire age spectrum of patients with HER2-positive disease will be key to such research.
Several aspects of trastuzumab therapy may contribute to its underuse among minority women. First, standard use of this biologic agent requires combination with chemotherapy, and evidence for the effectiveness of less-intensive single-agent taxane regimens for use in patients with HER2-positive breast cancer has only recently been presented32 and was not available during this study period. The pairing of trastuzumab with intensive chemotherapy and the long duration of treatment may be a barrier to the treatment of patients with unreliable sources of transportation, job insecurity, or poor social support, and providers may perceive such women as less able to tolerate prolonged intensive treatment. Although we controlled for poverty at the neighborhood level, a complete accounting for such complex social factors is not possible in a claims-based analysis. Second, trastuzumab is a costly infused therapy. Although the patients in the present sample were uniformly insured by Medicare, the generosity of supplemental plans for outpatient expenses and the affordability of shared costs likely vary by race and could influence uptake. Third, the decision to use trastuzumab-based adjuvant therapy in patients with stage I breast cancer is made in the context of limited evidence,5 with many patients falling into a gray zone of risk and benefit. Patients’ ways of considering and weighing this imperfect evidence may vary by race, and minority patients may also be treated by providers who are less comfortable with applying the current evidence to recommend treatment with uncertain or marginal benefit. Fourth, trastuzumab therapy is a lengthy course of treatment that involves 1 year of infused therapy. Previous studies have suggested that black women are more likely to discontinue adjuvant trastuzumab therapy early,10 and this protracted treatment course may similarly be a disincentive to initiation of treatment of minority patients with limited resources for obtaining and adhering to treatment. Finally, trastuzumab is a relatively new adjuvant therapy for breast cancer, and we and others have previously demonstrated that the adoption of cancer treatment innovations lags among minority patients.25,33,34
Not surprisingly, we found that clinically appropriate information, such as tumor size and nodal status, influenced the receipt of trastuzumab. We also found an indication of increasing use at a tumor size as small as 6 mm (T1b disease), which suggests that providers may define this as the appropriate cut point for considering HER2-directed therapy. Furthermore, hormone receptor status was not an independent predictor of trastuzumab use in adjusted analysis despite known differences in recurrence risk between patients with hormone receptor–positive/HER2-positive and hormone receptor–negative/HER2-positive disease and the availability of additional targeted therapy, specifically endocrine therapy in the former. However, given that hormone receptor status was associated with trastuzumab use in bivariable analysis, the inclusion of multiple other disease characteristics, such as grade and tumor size, in the model likely attenuated the independent effect of hormonal status because hormone receptor–positive tumors would also be expected to present at a lower grade and smaller size.
In descriptive analysis, we found that trastuzumab often was paired with chemotherapeutic agents other than ACTH and TCH, including a significant proportion of single-agent taxane regimens. Although weekly paclitaxel with trastuzumab is increasingly recognized as a regimen with good short-term outcomes in low-risk HER2-positive disease on the basis of data published in 2014,32 the current findings suggest that a significant number of providers adopted such regimens in advance of the clinical evidence possibly as an attempt to compromise between clinical need and toxicity concerns. More of concern, we found that 14% of trastuzumab recipients received no chemotherapeutic agent of any type, an approach that has no evidence of efficacy in this setting. Although sample size did not permit exploration of chemotherapy partners by race or other patient-level factors, concerns that there may be disparities in treatment intensity as well as trastuzumab receipt deserve further exploration.
This study has several important limitations. First, we focused the analysis on women with HER2-positive cancer and excluded 391 with SEER-defined HER2 borderline phenotype. We hypothesized that in the early period of adoption of this mandatory data element, the borderline group would include both patients with truly borderline clinical results and patients for whom the registry has incomplete information with regard to HER2 status (eg, an immunohistochemical result of 2+ without a subsequent confirmatory test result). To explore this, we examined rates of trastuzumab use among the HER2-positive and HER2 borderline groups and found that overall use was 49% among HER2-positive group but only 6% among the borderline group, which reinforces concerns that the borderline group might include many patients who were not ultimately determined by their treating provider to have HER2-positive disease. Second, although we measured comorbidity by using a validated claims-based instrument for patients with cancer, this measure may not reflect less critical but relevant comorbidities, such as cardiac risk factors, that might affect chemotherapy decisions and vary by race. However, all patients were required to have primary breast surgery, which indicated at least some level of fitness for cancer therapy. As with other analyses that used SEER-Medicare data, the current findings may not represent treatment patterns among younger women or those in Medicare-managed care plans. Finally, in a claims-based analysis, we were not able to ascertain whether HER2-directed therapy was offered to a patient and in what context it was discussed. Further investigations that examine prescribing behavior at the provider level may help to clarify provider-level factors that influence disparities.
In conclusion, trastuzumab is an innovative and nontoxic biologic therapy with a clear biomarker for prediction of response and ample evidence of significant improvements in survival of patients with HER2-positive breast cancer. Its underuse among older, particularly black, women is a concerning finding that merits further study. System-level interventions that highlight patients eligible for HER2-directed therapy objectively and consistently as well as interventions that focus on removing barriers to therapy at the patient level are needed to ensure that this effective treatment reaches all women who will benefit.
Supplementary Material
Footnotes
Supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant award number UL1TR001111/CERR11402 and by the Integrated Cancer Information and Surveillance System, University of North Carolina Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund through the State of North Carolina. K.R.-H. receives support through career development grants from the Conquer Cancer Foundation and Susan G. Komen. Also supported in part by career development and project funds to K.R.-H. from the University of North Carolina Breast Cancer Specialized Programs of Research Excellence grant P50CA058223-20. S.B.D. is supported by the NIH Building Interdisciplinary Research Careers in Women’s Health K12 program.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Katherine Reeder-Hayes, Stacie B. Dusetzina
Collection and assembly of data: Katherine Reeder-Hayes, Sharon Peacock Hinton
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disparities in Use of Human Epidermal Growth Hormone Receptor 2–Targeted Therapy for Early-Stage Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Katherine Reeder-Hayes
No relationship to disclose
Sharon Peacock Hinton
No relationship to disclose
Ke Meng
No relationship to disclose
Lisa A. Carey
Research Funding: GlaxoSmithKline, Genentech
Stacie B. Dusetzina
No relationship to disclose
REFERENCES
- 1.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeder-Hayes KE, Carey LA. How low should we go? The search for balance in management of small human epidermal growth factor receptor 2-positive breast cancers. J Clin Oncol. 2014;32:2122–2124. doi: 10.1200/JCO.2014.55.7249. [DOI] [PubMed] [Google Scholar]
- 6.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehrenbacher L, Capra AM, Quesenberry CP, Jr, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: A cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–2158. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 9.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J Clin Oncol. 2014;32:2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. (suppl) [DOI] [PubMed] [Google Scholar]
- 13. SEER: Derived HER2 Recode (2010+). http://seer.cancer.gov/seerstat/databases/ssf/her2-derived.html.
- 14.Reeder-Hayes KE, Meyer AM, Dusetzina SB, et al. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145:743–751. doi: 10.1007/s10549-014-2957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaslavsky AM, Ayanian JZ, Zaborski LB. The validity of race and ethnicity in enrollment data for Medicare beneficiaries. Health Serv Res. 2012;47:1300–1321. doi: 10.1111/j.1475-6773.2012.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arday SL, Arday DR, Monroe S, et al. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 17.McBean AM. Improving Medicare’s data on race and ethnicity. Medicare Brief. 2006;15:1–7. [PubMed] [Google Scholar]
- 17a.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 19.United States Census Bureau: Geographic terms and concepts - census tract. 2010.. https://www.census.gov/geo/reference/gtc/gtc_ct.html.
- 20.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 23.Bigby J, Holmes MD. Disparities across the breast cancer continuum. Cancer Causes Control. 2005;16:35–44. doi: 10.1007/s10552-004-1263-1. [DOI] [PubMed] [Google Scholar]
- 24.Schootman M, Jeffe DB, Lian M, et al. The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: A geographic and multilevel analysis. Am J Epidemiol. 2009;169:554–561. doi: 10.1093/aje/kwn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care. 2011;49:172–179. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Vital signs: Racial disparities in breast cancer severity—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61:922–926. [PubMed] [Google Scholar]
- 30.Rust G, Zhang S, Malhotra K, et al. Paths to health equity: Local area variation in progress toward eliminating breast cancer mortality disparities, 1990-2009. Cancer. 2015;121:2765–2774. doi: 10.1002/cncr.29405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owusu C, Lash TL, Silliman RA. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102:227–236. doi: 10.1007/s10549-006-9321-x. [DOI] [PubMed] [Google Scholar]
- 32.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black DM, Jiang J, Kuerer HM, et al. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149:788–796. doi: 10.1001/jamasurg.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.