Abstract
Intracellular antibodies (intrabodies) are recombinant antibody fragments that bind to target proteins expressed inside of the same living cell producing the antibodies. The molecules are commonly used to study the function of the target proteins (i.e., their antigens). The intrabody technology is an attractive alternative to the generation of gene-targeted knockout animals, and complements knockdown techniques such as RNAi, miRNA and small molecule inhibitors, by-passing various limitations and disadvantages of these methods. The advantages of intrabodies include very high specificity for the target, the possibility to knock down several protein isoforms by one intrabody and targeting of specific splice variants or even post-translational modifications. Different types of intrabodies must be designed to target proteins at different locations, typically either in the cytoplasm, in the nucleus or in the endoplasmic reticulum (ER). Most straightforward is the use of intrabodies retained in the ER (ER intrabodies) to knock down the function of proteins passing the ER, which disturbs the function of members of the membrane or plasma proteomes. More effort is needed to functionally knock down cytoplasmic or nuclear proteins because in this case antibodies need to provide an inhibitory effect and must be able to fold in the reducing milieu of the cytoplasm. In this review, we present a broad overview of intrabody technology, as well as applications both of ER and cytoplasmic intrabodies, which have yielded valuable insights in the biology of many targets relevant for drug development, including α-synuclein, TAU, BCR-ABL, ErbB-2, EGFR, HIV gp120, CCR5, IL-2, IL-6, β-amyloid protein and p75NTR. Strategies for the generation of intrabodies and various designs of their applications are also reviewed.
Keywords: endoplasmic reticulum, inhibitor, intrabody, KDEL, knockdown, knockout
Abbreviations
- CDR
complementarity determining region
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- IgG
immunoglobulin
- G MBP
maltose binding protein
- NusA
N utilization substance A
Introduction
Various strategies are used to study the functions of newly discovered proteins, including gene-targeted knockout animals, targeted gene disruption in mammalian cells and knockdown techniques such as siRNA, shRNA, miRNA, CRISPR and TALEN. In addition to these methods, which are active on the nucleotide level, the use of dominant negative mutants or inhibitor molecules were the most widely used methods to interfere directly with protein function. Another method with fast growing relevance that avoids some of the problems encountered with the above technologies is the use of intracellular antibodies (intrabodies). The molecules can be selected and designed to be very specific to the target, which are advantages of the technology. Because they can be thoroughly characterized for specificity in biochemical assays before use in cells, they avoid off-target effects known from nucleotide-based methods, and they can be designed to selectively target splice variants, different isoforms or even one post-translational modification of a protein. Furthermore, the function of target proteins can be knocked down in specific cellular compartments exclusively, while their function remains intact in other cellular compartments, as recently demonstrated by the knockdown of Sec61 in endosomes while maintaining its function in the ER.1 Although intrabodies are typically seen as an experimental tool to reveal the function of proteins by interfering with their function, this approach is also reported to have therapeutic potential against viral infections2-6 brain diseases7-10 or cancer.11-14 Here, the various protein knockdown strategies mediated by intrabodies (Fig. 1) are reviewed.
Figure 1.
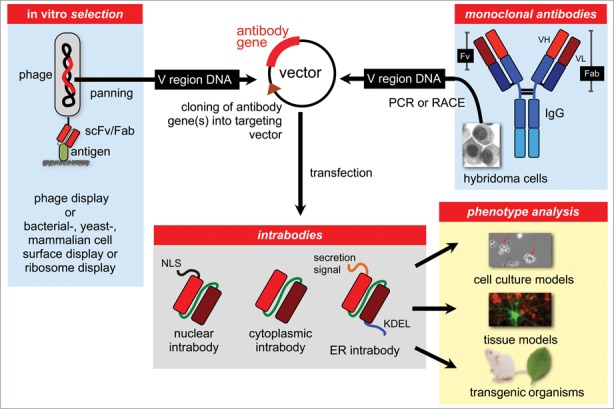
The intrabody approach for the generation of protein interference phenotypes. Antibody fragments containing the antigen binding domains (V regions) specific for a particular protein can be selected from antibody phage display libraries or other in vitro selection systems like bacterial, yeast, mammalian or ribosomal display libraries. Alternatively, the variable region genes can be obtained from hybridoma antibodies by PCR with consensus primers, RACE or PCR with adaptor ligated cDNA. Antibody fragments, typically in scFv format, are then cloned into a specific targeting vector allowing expression of the intrabody in the nucleus, cytoplasm or ER.
Types of Intrabodies
Intrabodies are antibodies that bind intracellularly to their antigen after being produced in the same cell,15-21 in contrast to antibodies delivered to a living cell from the outside.22-24 Intracellular antibodies can be subdivided into 2 main subgroups according to their mechanism of action. These are: 1) cytosolic intrabodies (cyto-intrabodies) and 2) endoplasmic reticulum (ER) retained intrabodies (ER intrabodies). While cyto-intrabodies block targets in a neutralizing fashion in the cytosol, ER intrabodies knock down proteins in the secretory pathway even without the requirement for the antibody to be neutralizing. By addition of a suitable signal peptide, intrabodies can also be targeted to the nucleus or mitochondria (Fig. 2).15,18, 21
Figure 2.
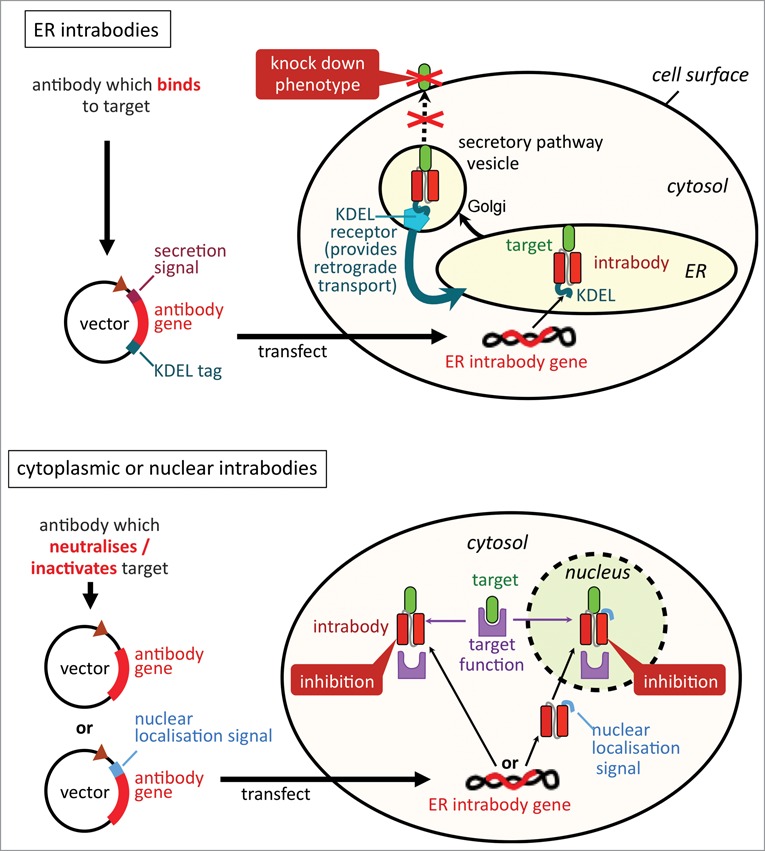
Differences between cytoplasmic/nuclear intrabodies and ER intrabodies. Via their retention signal KDEL, ER intrabodies (A) retain antigens passing the ER by binding to them. As antibodies are naturally produced in the ER, no particular selection for special folding/stability properties is required. In contrast, cytoplasmic/nuclear intrabodies (B) need to fold correctly in the reducing milieu of the cytoplasm. Further, they need to be tested and screened to identify antibodies which are capable, in addition to binding, to neutralise or inactivate their target's activity in the cytoplasmic biochemical milieu.
As antibodies usually are produced in an oxidizing biochemical milieu and folded with the help of ER based chaperones, only a fraction of the repertoire of naïve IgGs can be correctly folded in the reducing environment of the cytoplasm, which does not allow the formation of disulfide bridges.25,26 However, there are numerous examples where intrabodies function against cytosolic targets including examples against EGFR,11 BCR-ABL,13 NSP5 from rotavirus,27 Clostridium botulinum neurotoxin proteases,28,29 core antigen of HBV,6 Bax,30 HIV-1 Vif,2 Etk-kinase12 or hungtingtin protein.7,8 It has also been shown that cyto-intrabodies can trace cellular components in living cells.31,32
Various approaches have been described for the selection and generation of cyto-intrabodies: 1) use of the intracellular antibody capture technology (IACT);33 2) construction of single domain intrabodies;12,34-36 3) expression of intrabody fusion proteins;37-42 4) complementarity-determining region (CDR) grafting or introduction of synthetic CDRs into suitable preselected frameworks;43-46 and 5) selection of single-chain variable fragments (scFv) without disulfide bonds.7,47 If the functional expression of a particular antibody in the cytoplasm fails, introduction of externally produced antibodies into the cytosol has been proposed using methods such as protein transfection (profection), peptides as protein transduction domains, fusion to targeting proteins or the use of translocation sequences and endosome escape domains.23,48, 49 However, it has been difficult to achieve endosomal escape and to reach the cytoplasm with most of these methods.24
In contrast to cyto-intrabodies, antibodies targeted to the ER are made in their native environment (Fig. 2), thus can be expected to be correctly folded with intact disulfide bridges forming in the oxidizing environment.50 ER intrabodies work by just retaining antigens that pass the secretory pathway. Typically, these are cell-surface molecules, secreted molecules, intravesicular receptors or Golgi-located glycosyltransferases (ref. 3).
ER intrabodies are targeted to the lumen of the ER by a secretory signal peptide, and fusion of the retention sequence KDEL or SEKDEL to their C-terminus prevents their secretion together with the antigen bound to it. The KDEL receptor substrate leaves the ER, is transported to the cis-Golgi apparatus where it binds to the ERD1 and ERD2 receptors which are then recycled back to the ER via COPI-coated vesicles.21,51,52 The ER intrabody-antigen complex may then be degraded via an ER-associated degradation (ERAD) pathway that is either proteasome-dependent or proteasome-independent.53-55
Nuts and Bolts: How to Make Intrabodies
Intrabodies can be generated by cloning the respective cDNA from an existing hybridoma clone56,57 or more conveniently, new scFvs/Fabs can be selected from in vitro display techniques such as phage display (Fig. 1),58,59 which provide the necessary gene encoding the antibody from the onset and allow a more detailed predesign of antibody fine specificity.60 In addition bacterial-, yeast-, mammalian cell surface display and ribosome display can be employed.61-64 However, the most commonly used in vitro display system for selection of specific antibodies is phage display.59,60,65-67 In a procedure called panning (affinity selection), recombinant antibody phages are selected by incubation of the antibody phage repertoire with the antigen. This process is repeated several times leading to enriched antibody repertoires comprising specific antigen binders to almost any possible target. To date, in vitro assembled recombinant human antibody libraries have already yielded thousands of novel recombinant antibody fragments.58,66,68,69 Antibo-dy phage repertoires can be divided into 4 different types according to the origin of their frameworks and CDRs: immune, naïve, synthetic and semi-synthetic antibody libraries.70 Immune antibody libraries are constructed from antibody genes of B cells from immunized animals or infected patients by PCR, using IgG primers for the heavy and light chain. Nonimmune (also named naïve or universal) libraries are built up from natural, unimmunized, rearranged V genes (e.g., from the IgM B-cell pool.71 Synthetic antibody libraries are constructed entirely in vitro using oligonucleotides that introduce areas of complete or tailored degeneracy into the CDRs of one or more V genes.72,73 Semi-synthetic libraries combine natural and synthetic gene fragments encoding different parts of the scFv or Fab molecule.74
Conversion of scFvs from in vitro selection methods into an ER intrabody only requires a single cloning step into the ER-targeting vector, e.g., pCMV/myc/ER to add the CMV promoter, a secretory leader, the ER retention sequence and a myc tag.56 Additional reporter functions, like green fluorescent protein (GFP), can also be added by using bicistronic vector designs.75
From hybridoma cells, the variable VH and VL domains can be amplified from the hybridoma cDNA using 1) PCR amplification with consensus primers,57,76-78 2) rapid amplification of cDNA-ends (RACE79), and 3) PCR amplification using adaptor-ligated antibody cDNA.80 RACE and adaptor-ligated antibody cDNA prevent mismatches that might occur if VH and VL are amplified by consensus primers. VH and VL can be assembled by overlapping PCR introducing a linker taken from phage sequences (Gly4Ser)356 or human proteins,59 and the assembled scFv will be cloned into a targeting vector to transport scFvs into specific cell compartments.81
The most commonly used format for intrabodies is the scFv, which consists of the H- and L-chain variable antibody domain (VH and VL) held together by a short, flexible linker sequence (frequently (Gly4Ser)3),82 to avoid the need for separate expression and assembly of the 2 antibody chains of a full IgG or Fab molecule.83,84 Alternatively, the Fab format comprising additionally the C1 domain of the heavy chain and the constant region of the light chain has been used. Recently, a new possible format for intrabodies, the scFab, has been described.85 The scFab format promises easier subcloning of available Fab genes into the intracellular expression vector, but it remains to be seen whether this provides any advantage over the well-established scFv format. In addition to scFv and Fab, bispecific formats (for review, see ref. 86) have been used as intrabodies. A bispecific Tie-2 x VEGFR-2 antibody targeted to the ER demonstrated an extended half-life compared to the monospecific antibody counterparts.87,88 A bispecific transmembrane intrabody has been developed as a special format to simultaneously recognize intra- and extracellular epitopes of the epidermal growth factor,89 combining the distinct features of the related monospecific antibodies, i.e., inhibition of autophosphorylation and ligand binding. Another intrabody format particularly suitable for cytoplasmic expression are single domain antibodies derived from camels36 or consisting of one human VH domain34,90 or human VL domain.7,8,12,91 These single domain antibodies often have advantageous properties, e.g., high stability; good solubility; ease of library cloning and selection; high expression yield in E.coli and yeast.
The intrabody gene is expressed inside the target cell after transfection with an expression plasmid or viral transduction with a recombinant virus. With respect to the vector technologies used to supply the antibody mRNA to the target cell, there seem to be few limitations because, in principle, all the usually functional vector-, promoter- and transfection systems for heterologous expression could be employed. Typically, the choice is aimed at providing optimal intrabody transfection and production levels. Microinjection of hybridoma derived or in vitro transcribed mRNA was used in initial proof of principle studies,92-94 but, due to more delicate RNA handling and very small number of transfected cells, this approach has not been used frequently.
Successful transfection and subsequent intrabody production can be analyzed by immunoblot detection of the produced antibody, but, for the evaluation of correct intrabody/antigen-interaction, co-immunoprecipitation from HEK 293 cell extracts transiently cotransfected with the corresponding antigen and intrabody expression plasmids may be used, or the antigen intrabody complex can be confirmed to be localized in the ER by co-staining with the ER marker calnexin.4,5
Examples for Functional Studies with Cytoplasmic Intrabodies
A quite successful group of cytoplasmic intrabodies has been developed against proteins playing an important role in the brain and for Alzheimer's and Parkinson's disease. Using the intracellular antibody capture technology, antibodies against the microtubule-associated protein TAU found in neurofibrillary lesions of Alzheimer's disease brains has been selected.33 This large panel of anti-TAU intrabodies selected from a naïve human antibody library provides a useful tool to study TAU function in degenerating neurons and brains. Meli and colleagues10 selected conformation-sensitive intrabodies by IACT against Alzheimer's amyloid-β oligomers starting from a phage display library constructed from mice immunized with a truncated version of human amyloid-β peptide. Some antibodies recognized in vivo-produced amyloid-β peptide “deposits” in histological sections from human Alzheimer's disease brains and significantly inhibited amyloid-β peptide oligomer-induced toxicity. A human scFv against oligomeric α-synuclein that inhibits aggregation and prevents α-synuclein-induced toxicity was also isolated from a naïve antibody phage display library.9 α-Synuclein is a presynaptic neuronal protein that is linked genetically and neuropathologically to Parkinson's disease and other neurodegenerative disorders. Interestingly, an anti-α-synuclein clone selected from the same library by a novel biopanning technique based on atomic force microscopy showed an opposite effect compared with the scFv mentioned above. It bound to intracellular aggregates of misfolded ataxin-3 and a pathological fragment of huntingtin and accelerated aggregation of these 2 molecules and increased cytotoxicity.95 Intrabodies have furthermore been selected against the natural precursor of nerve growth factor (proNGF).96 These antibodies can be used for the analysis of intracellular trafficking and signaling of proNGF in living cells. Intrabodies against gephyrin playing a role in clustering receptors have also been selected.97 This strategy could be very useful because the gephyrin knockout mice lead to a lethal phenotype.
Other intrabodies are directed against kinases, for example against the cytoplasmic protein kinase Syk,98 or the respective domains of human epidermal growth factor receptor11 or against the BCR-ABL oncogenic protein.13 Blocking the function of the latter protein may neutralize the oncogenicity of BCR-ABL, and could be used as a potential therapeutic agent in Philadelphia chromosome-positive leukemias. An efficient antibody-caspase 3-mediated cell killing system based on antibody-caspase 3 fusion has been developed.99 In the future, this approach may be used to kill tumor cells expressing tumor-specific proteins. Intrabodies against intracellular kinase domains of cell surface receptors and intracellular kinases (in particular against phosphorylation sites) can also be useful to decipher signaling pathways.11,12, 98-100 Furthermore, oncogenic transcription factors may be attractive targets to inhibit the transcription of oncogenic genes. An example is a functional intrabody against the yeast transcription factor GCN4.46
Another intrabody selected from a naïve antibody phage display library neutralized aggrecanase-2, a factor crucial in the development of osteoarthritis, and dose-dependently improved disease progression in an osteoarthritis mouse model.101 A cytoplasmic intrabody used to investigate the role of the rotavirus non-structural protein NSP5 in the virus replication cycle has been described.27 It was demonstrated that NSP5 is an essential element for the assembly of functional viroplasms. These intrabodies were selected from mice that had been immunized with recombinant GST-NSP5. Intrabodies have also been employed as a strategy to target rabies.102
Selection and Engineering of Intrabodies Suitable for Cytoplasmic Expression
In contrast to ER intrabodies, the prerequisite for a specific protein knockdown by a cytoplasmatic intrabody is that the antigen is neutralized/inactivated through the antibody binding (Fig. 2). As said above, any cytoplasmic intrabody approach will be compromised by failure of the antibody to fold correctly.25,26 Consequently, specialized assays or selection conditions have been proposed to increase the success rates. While the selection of cytoplasmic intrabodies is still often a time-consuming trial and error approach and no reliable standard procedure has emerged, 5 different approaches to generate suitable antibodies are described in detail here and in Table 1: 1) In vivo selection of functional intrabodies in eukaryotes such as yeast and in prokaryotes such as E.coli (antigen-dependent and independent); 2) generation of antibody fusion proteins for improving cytosolic stability; 3) use of special frameworks for improving cytosolic stability (e.g., by grafting CDRs or introduction of synthetic CDRs in stable antibody frameworks); 4) use of single domain antibodies for improved cytosolic stability; and 5) selection of disulfide bond free stable intrabodies.
Table 1.
Strategies for the selection of functional cytoplasmic intrabodies
| Technique | Outcome | Reference |
|---|---|---|
| 1) In vivo selection of intrabodies in the absence/presence of antigen in yeast and in E.coli | ||
| Antigen independent selection for cytoplasmic expression in yeast and mammalian cells using interaction between transcription activator domain coupled to scFv and DNA binding domain | Selection of stable frameworks from a human naïve antibody library | 103 |
| Antigen independent intrabody selection after export into the cytoplasm mediated by Tat (ISELATE). | Isolation of soluble scFv variants from an insoluble parental sequence. | 106 |
| Modification of ISELATE method. Selection will be performed by analyzing inner membrane anchored Tat-scFv fusions of transfected spheroblasts with anti-FLAG antibody. | Selection and affinity maturation of scFvs using error prone PCR resulted in scFvs with higher affinity than the original clones. | 107 |
| Intracellular Antibody Capture Technology based on an antigen-dependent 2 hybrid system. Cotransfection of antigen fused to DNA binding domain lexA and scFv fused to transactivator domain VP16 leads to selection of cytoplasmic active intrabodies. | Selection of intrabodies against Tau, amyloid-β peptide,α-synuclein, proNGF, gephyrin, Syk, EGFR, BCR-ABL, caspase 3, aggrecanase-2, NSP5 from rotavirus | 33,10,9,96,97,98,11,13,99,101,27 |
| Antigen dependent selection of intrabodies in the E.coli cytoplasm based on co-expression of Tat signalpeptide-scFv and antigen-β-lactamase fusion | Selection of antigen specific binders from a library of randomized CDR-H3 sequences. | 108 |
| 2) In vivo selection of stable intrabody fusion proteins | ||
| Generation of scFv-protein fusions | Fusion to MBP, GFP or the Fc domain are the most reliable strategies. | 41,39,37,40,42,38 |
| 3) CDR grafting or introduction of synthetic CDRs into stable frameworks | ||
| Transplantation of CDRs from one antibody to a different stable antibody framework. Introduction of synthetic CDR loops into a stable human framework. Construction of a novel human VH domain antibody library with restricted randomizations at fixed positions in the CDR2 and CDR3, respectively. |
Generation of a framework stabilized version of an anti-GCN4 intrabody. Grafting several groups of residues in the CDRs important for antigen binding of scFv D1.3 into stable framework of scFvF8 Construction of a synthetic antibody library based on the human framework of scFv13R4 by introducing synthetic CDR3 loops. Construction of a synthetic single human VH domain antibody library based on the framework of antibody HEL4. Selection of thermodynamically stable anti-lysozyme clones expressed in E.coli. |
46, 43, 4544 |
| 4) Cytoplasmic intrabodies in the single domain format | ||
| Generation of Camelid VH single domain antibodies from naïve or immune-camelid antibody repertoires Selection of stable human VH domains from naïve or synthetic VH domain antibody repertoires Selection of human VL domains from naïve or synthetic VL domain antibody repertoires |
Camelid VH single domain antibodies against: F-actin capping protein CapG, β2-adrenergic receptor, Clostridium botulinum neurotoxin (BoNT) proteases, core antigen of HBV (HBcAg), 15-acetyldeoxynivalenol, caspase-3, Bax, nuclear polyA-binding protein, HIV-1 Vif Most of the VH domains isolated from a human germ-line VH library by the ISELATE method resulted in soluble clones expressed in E.coli. Construction of a large human VH domain antibody repertoire by combinatorial assembly of CDR building blocks from a smaller repertoire comprising aggregation-resistant clones Selection of a human VL domain against Etk kinase from a large VL domain phage display library, derived from a single human framework of light chain Construction of a naïve VL domain library and isolation of clones recognizing B cell super-antigen protein L. Selection of an α-hungtingtin human VL domain intrabody from an original non-functional scFv. Affinity maturation of the original scFv was performed using error prone PCR and yeast surface display. |
14,100,29,28, 6,122,123,30,124,2 90 34 12 91 8 |
| 5) Selection of scFvs without disulfide bonds | Isolation of cysteine free scFvs by phage display. After DNA-shuffling and Error prone PCR cysteine free variants of an α-levan antibody could be expressed functional in the cytoplasm of E.coli. Functional cysteine free VL domains recognizing hungtingtin protein could be selected by yeast surface display after Error prone PCR. |
47 7 |
Approach 1, intrabody selection strategies, in eukaryotes. An antigen-independent selection method termed “Quality Control” was developed to identify intrabodies that are soluble in the cytoplasm of yeast and mammalian cells.103 For selection in yeast, the scFvs were fused to a transcriptional activation domain and a peptide derived from Gal11P binding to the transcription factor Gal4 (1–100) fragment. Soluble expression of the scFv mediates the specific interaction of peptide Gal 11P with the DNA-bound Gal4 (1–100) fragment and then transcription of lacZ and HIS3 reporter genes can be activated, whereas insoluble scFvs are expected to result in non-functionality of the whole fusion protein and thus no activation of reporter genes occurs. Selection in mammalian cells, based on a similar principle, was implemented by employing scFv-VP16 fusions that bind to the DNA binding domain Gal4. Using the “Quality Control” approach, growth of colonies under selection conditions, indicating scFv clones that are stable and soluble in the cytosol, was below 1% among screened clones, which gives a rough initial estimate of the fraction of antibodies that can be expected to fold correctly in the cytoplasm.103
Yeast two-hybrid technology-based IACT is an antibody-dependent method employed to select suitable intrabodies from scFvs preselected by phage display. The pre-enriched antibody fragment library is fused to the VP16 transcription activation domain. Antigen-specific functional intrabodies are selected after co-transfection of the scFv-VP16 library with antigen coding sequence fused to the LexA DNA binding domain in yeast cells. Complex formation of the antigen and an antigen-specific scFv in the yeast cytoplasm leads to activation of yeast chromosomal reporter genes.33 A single pot library of intracellular antibodies (SPLINT) was generated by amplification of natural V regions from non-immunized mice and intrabodies selected against different antigens.104 A further modification of the original IACT technique was established to select for intrabodies that are able to interrupt protein-protein interactions.105 Concerning the IACT selection strategy, it is important that the source for intrabody selection (particularly naïve, immunized mouse antibody or naïve human antibody phage display repertoires have been employed) must have a very large diversity, because functional cytoplasmic intrabodies were described to be a very small subset of the total antibody repertoire.
Approach 1, intrabody selection strategies, in prokaryotes. Another in vivo selection strategy is the selection of functional cytoplasmic intrabodies fused to a selection marker in E.coli using the twin-arginine translocation (TAT) machinery. This strategy is referred to as intrabody selection after Tat export into the cytoplasm (ISELATE).106 Selection is provided by fusion of an N-terminal Tat-specific signal and C-terminal TEM1 β-lactamase to the coding region of the scFv. With the TAT signal providing the transport of folded proteins, only non-aggregated (therefore presumably correctly folded) intrabodies can be transported from the cytoplasm to the periplasm of E.coli where the fused ß-lactamase provides ampicillin resistance, hence positive selection of the respective clones. Recently, a variant of this selection approach for the isolation of correctly folded membrane-anchored scFvs was developed.107 Karlsson et al found that correctly folded scFvs fused to the Tat signal peptide are transported from the cytoplasm to the periplasm of E.coli, but remain N-terminally anchored with the Tat signal peptide to the inner membrane. Selection is performed with antibody expression plasmid transfected spheroplasts and anti-FLAG antibody, which can only recognize the FLAG epitope fused to the C-terminal end of the antibody if the N-terminal signal peptide is anchored in the inner membrane of E.coli and the scFv localized in the periplasm. Affinity maturation of selected scFvs using error prone PCR resulted in scFv clones expressed in the cytoplasm with higher affinity than the original clones.
A mixed strategy that combined elements described above was also developed in E.coli. In this case, the scFv is N-terminally fused to the Tat signal peptide ssTorA and coexpressed with the antigen C-terminally fused to the β-lactamase gene. It was observed that only those binding pairs that are correctly folded and functional in the cytoplasm could be exported to the periplasm and give rise to antibiotic resistant cells.108 Notably, intrabodies with high stability and high antigen-binding affinity could be selected from resistant bacterial clones on high concentrations of the antibiotic carbenicillin.109
Approach 2, antibody fusion proteins. Several publications proposed the addition of non-intrabody domains to enhance folding or stability of cytoplasmic intrabodies. One approach is to use GFP to improve folding of proteins, including recombinant antibody fragments, in E.coli and mammalian cells.37,39,40 It was shown that GFP variants fold differentially in prokaryotic and eukaryotic cells.110 Recently, scFv-GFP fusions were used to screen for intrabody solubility in mammalian cells by fluorescence activated cell sorting (FACS).39 Mammalian cells were transfected with GFP fusions by retroviral gene transfer, the GFP positive cells sorted and expression of the scFv-GFP fusions analyzed. The GFP signal correlated with soluble expression levels of the scFvs in the cytoplasm; however, only 2 different scFvs were tested. A GFP-tagged cytoplasmically expressed scFv was applied for in vivo labeling of tyrosinated α-tubulin and measurement of microtubule dynamics.37 It remains to be proven whether this strategy is broadly applicable.
In addition to GFP, a Cκ domain,111,112 maltose binding protein (MBP),41 N utilization substance A (NusA) 113 or Fc domain38,42 have been proposed as fusion partners, although few successful examples have been presented. Different labs have focused on the inhibition of p53 and activation of mutated p53 inside tumor cells.111,112,114 The anti-p53 intrabodies derived from hybridomas showed different stability inside the cytoplasm, as expected. One of the scFvs targeted to the nucleus or cytoplasm was expressed well as a fusion with the Cκ domain.111 Another scFv showed very low expression inside the cytoplasm after fusion with a Cκ domain.112 Interestingly, 2 intrabodies recognizing p53 mutants that were expressed in the nucleus and cytoplasm restored transcriptional activity of p53 mutants by themselves,114 which demonstrates that solubility depended on the properties of the intrabody rather than on the Cκ domain. Because degradation of proteins can be linked to the presence of regions rich in proline, glutamic acid, serine and threonine (PEST sequences),115 the low content of potential PEST sequences might explain the stability of some intrabodies such as the 2 anti-p53 mutated intrabodies.114 However, the fusion of a proteasome-targeting PEST motif to intrabodies recognizing Huntingtin or α-synuclein-mediated increase of intrabody stability and degradation of the corresponding antigen,116,117 demonstrating that no simple PEST sequence motif-related algorithm will predict the intrabody efficacy.
MBP has extensively been used to enhance folding of intrabodies inside the cytoplasm of E.coli and mammalian cells.41 It was found that the folding of some passenger proteins fused to MBP could be mediated by endogenous chaperones in vivo.118 It was proposed that the solubility-enhancing activity of MBP is mediated by its open conformation and it is assumed that the ligand-binding cleft is involved in the mechanism.119 However, the molecular mechanism of the process is not known, and some reports of improved folding may have been mistakenly based on the improved overall solubility of the fusion.
Approach 3, CDR grafting or introduction of synthetic CDRs in stable frameworks. Antigen-binding loops can be transplanted from one antibody to a different antibody framework, a procedure called CDR loop grafting.120 While the technology gained historical relevance by being used to de-immunize (“humanize”) therapeutic antibodies, it also can be used to transfer a given specificity to a preselected V region framework with optimized production / folding / stability properties. A stable framework-engineered stabilized version of an anti-GCN4 antibody was cytoplasmically expressed as an scFv intrabody in yeast.46 This variant inhibited the activity of β-galactosidase expressed from a GCN4-dependent reporter gene compared to the unfolded original scFv. Another approach used the scFv(F8), which showed very high in vitro stability and functional folding in both the prokaryotic and eukaryotic cytoplasm.43 Several groups of residues in the CDRs important for antigen binding of the poorly stable anti-hen egg lysozyme (HEL) scFv(D1.3) were grafted into the framework of scFv(F8). Five different variants were constructed, and 4 of those could be expressed in the cytoplasm of E.coli and bind to the antigen after purification. The donor framework of scFv(F8) seems to be able to tolerate extensive CDR substitutions without loss of stability in the cytoplasm.45 A synthetic antibody library based on the human framework of anti-β galactosidase scFv13R4 was expressed in the E.coli, yeast and mammalian cell cytoplasm. Randomized CDR 3 loops were introduced in the framework by PCR and a number of functional cytoplasmic intrabodies against different antigens selected, including an intrabody that identified a protein of yet unknown function involved in mast cell degranulation.121 Another approach used amino acid randomization at 4 and 7 different positions in the CDR2 and CDR3 loop of the single domain VH of anti-lysozyme antibody HEL4.44 The library was used for selection of antibodies with different specificities on both purified lysozyme and whole cells.
Approach 4, single domain antibodies. Single domain antibodies are composed of only one V region, which could be either a variable domain of the heavy or light chain. They can be produced from conventional human IgGs (VHs and VLs), from camelid heavy-chain IgGs (VHHs) and from cartilaginous fish IgNARs (VNARs). As for scFvs, libraries of single domain antibodies can be constructed from naïve, synthetic, semi-synthetic, transgenic animals or immunized sources and clone selection is typically done by phage panning or yeast cell surface display. Despite providing only half of the binding surface of an antibody, camelid VH single domain antibodies have demonstrated high affinity for many cognate antigens, high solubility and aggregation resistance. The camelid single domain antibodies are selected from camelid VHH naïve or immune libraries. Several camelid cytoplasmic single domain antibodies have been successfully generated, for example against F-actin capping protein CapG,14 β2-adrenergic receptor,100 Clostridium botulinum neurotoxin (BoNT) proteases,28,29 core antigen of HBV (HBcAg),6 15-acetyldeoxynivalenol,122 caspase-3,123 Bax,30 and nuclear polyA-binding protein.124 A nanobody recognizing the F-actin capping protein CapG was generated after immunization of a llama.14 It was demonstrated that expression of the intrabody in breast cancer cells restrained cell migration and lung metastasis in a xenograft tumor mouse model. Anti-β2-adrenergic receptor single domain intrabodies from the Camelid family inhibiting G protein activation, G protein-coupled receptor kinase-mediated receptor phosphorylation and β-arrestin recruitment have been published.100 An intrabody against caspase-3 was isolated from a naïve phage display llama antibody repertoire.123 Single-domain antibodies against the proapoptotic Bax protein have been selected.30 These anti-Bax VH intrabodies could be used as tools for studying the role of Bax in oxidative-stress-induced apoptosis and for developing novel therapeutics for the degenerative diseases involving oxidative stress.
Yeast surface display has also been used to select camelid VHHs as immune inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. VHHs were selected from an immunized llama single domain repertoire that remained functional when expressed within neurons and bound to BoNT proteases with high affinity.29 VHHs from a non-immune llama single-domain library have been isolated.28 The X-ray crystal structure of the most potent intrabody in complex with the protease was solved and the structure revealed that the VHH binds in the α-exosite of the enzyme, far from the active site for catalysis. Camelid VHH domain antibodies selected from a non-immune llama single-domain phage display library prevented oculopharyngeal muscular dystrophy associated aggregation of nuclear poly(A)-binding protein.124 Notably, intrabodies also reduce the presence of already existing aggregates. VHH intrabodies against the hepatitis B core antigen (HBcAg) were selected from a single domain phage display library constructed from llamas immunized with noninfectious HBV particles.6 Intrabodies targeted to the nucleus affected HBcAg expression and trafficking in HBV-transfected hepatocytes. Camelid VHH domains selected from a hyper-immunized phagemid library were selected against toxin 15-acetyldeoxynivale.122 Expression of the corresponding intrabodies in yeast resulted in significant resistance to the toxin. The authors suggested that VHH expression in plants may lead to enhanced tolerance to mycotoxins. A camelized rabbit – derived VH single-domain recognizing HIV-1 Vif was constructed starting from an original rabbit scFv.2 A set of 3 mutants of the derived VH single domain antibody with gradual increasing camelization was performed. There was a strong correlation between the improvement in protein solubility in mammalian cells and the gradual increasing camelization. The intrabodies neutralized HIV-1 infectivity. Anti-ß catenin llama single domain intrabodies that inhibit Wnt signaling were recently reported, providing a tool for further study of the Wnt pathway.125
In contrast to the VH domains of camels and llamas, human heavy chain variable domains and human light chain variable domains are more prone to dimerization and aggregation and exhibit poor solubility.35 Nevertheless, several human single domain antibodies have been selected, for example against Etk kinase,12 huntingtin protein7,8 and B cell super-antigen protein L.91 As for scFvs, a Tat signal peptide and β-lactamase-based strategy was used to isolate human VH domains from a human germ-line VH library, with increased level of thermodynamic stability, reversible folding and soluble expression in E.coli.90 In another approach, aggregation-resistant human heavy chain variable domains were selected on phage by heat denaturation.126 A large human VH domain antibody repertoire was constructed by combinatorial assembly of CDR building blocks from a smaller repertoire comprising a high frequency of aggregation-resistant antibody domains.34 Barthelmy and colleagues127 evolved and analyzed stable human VH domains from a phage display antibody library. By building libraries comprising the framework of humanized anti-HER2 scFv and different diverse synthetic VH CDR3 loops, they selected the most stably expressed VH domains secreted in E.coli and then introduced mutations that increased the hydrophilicity of the former light chain interface by replacing exposed hydrophobic residues with structurally compatible hydrophilic substitutions. The stability of many in vitro evolved VH domains seemed to be essentially independent from the CDR3 sequence and instead derived from mutations introduced in the second step.
A repertoire of stable human VL domains was isolated after panning with a human naïve VL library with B cell super-antigen protein L. Isolated clones exhibited improved reversibility of thermal unfolding after purification from the periplasm of E.coli.91 Nevertheless, it remains to be demonstrated whether the isolated variable domains can also be stably expressed in the cytoplasm as stable functional intrabodies. An α-hungtingtin single domain antibody comprising a human light chain variable domain (VL) was selected from an original nonfunctional scFv.8 Affinity maturation of the original scFv was performed using error-prone PCR and yeast surface display, and it was shown that the paratope was localized in the VL. However, this selection approach is only applicable when the binding energy of the scFv is provided predominantly by only one of the 2 V domains. A human VL against Etk kinase was selected from a large single domain phage display library, derived from a single human framework of light chain.12 When expressed in Src-transformed cells, the single-domain antibodies interacted with endogenous Etk in the cytoplasm and efficiently blocked its kinase activity, leading to partial inhibition of cellular transformation.
Approach 5, cyto-intrabodies without disulfide bridges. Many stabilizing and destabilizing mutations in the framework or CDRs have been described.128,129 In this context, stable cysteine-free scFvs have been selected and expressed in functional form in the E.coli cytoplasm starting from the levan binding antibody ABPC48. Affinity maturation was performed using DNA-shuffling and random mutagenesis.47 An engineered single domain VL antibody without disulfide-bridge and with high affinity preventing aggregation of huntingtin was selected after affinity maturation of the VL domain of an original anti-huntingtin scFv using error-prone PCR and yeast surface display.7 These intrabodies may have therapeutic potential for the treatment of hungtingtin disease. However, no naïve library strategy has yet emerged from this interesting approach, limiting this method to the tedious optimization of individual antibody clones.
Targeting the “Outside” from the “Inside”: The ER Intrabody Approach
The use of various signal sequences attached to antibody fragments allows its targeting specifically to different intracellular compartments. ER targeting in particular allows interference with the function of the members of the membrane and the plasma proteome (secretome), relying on a different mechanism than cytoplasmic antibodies. Hence, generation of suitable ER intrabodies is much easier because they do not require special folding or stability features, and, in particular, no neutralizing activity toward the antigen is needed. Reasonable antigen binding is sufficient (Fig. 2). This eliminates the necessity for any specialized selection strategy and allows immediate utilization of the vast and quickly growing resource of recombinant research antibodies currently generated.130
Reports of ER intrabodies published so far have shown protein knockdown in vitro and recently in vivo in a mouse model. As a step toward in vivo use, ER intrabodies have been applied in primary tissue explants (Zhang, Korte and Dübel, unpublished data) and against the oncogenic receptors VEGFR-2/KDR and Tie-2 in xenograft mouse tumor models.87,88,131 In addition ER intrabodies specific to amyloid-ß demonstrated an inhibition of amyloid-ß accumulation in an Alzheimer's disease mouse model.132 ER intrabodies have been applied for various purposes (Table 2).
Table 2.
ER intrabodies applied
| Function targeted | Targets |
|---|---|
| Oncogenic receptors | VEGFR 2,87,153,198,88 Tie-2,87,88,131 ErbB-2,145,147,150,155,199-202 EGFR,203 metalloproteinases MMP-1 and MMP-9,204 extracellular matrix metalloproteinase inducer,205,206 human α folate receptor,207 cathepsin L208 oncoprotein E7209 |
| Virus proteins to prevent virus assembly | HIV-1 gp120,210,211 HIV-1 gp 160,212 HBV precore antigen,213 HCV ApoB,214 HCV core protein,215 gp46 of maedivisna virus216 |
| Knockdown of cellular virus receptors to block virus entry | CCR5,217-219 CXCR4.220,221 |
| Receptors of the immune system | MHC I,222-224 integrins,225-227 VCAM-1,134,149 NCAM,56 TLR2,4 TLR9,5 IL-2,148,151,228 CD147,229 IL-6230 |
| Nervous system | Neurotrophin Receptor p75,75 β-amyloid protein,132 β-amyloid precursor protein,154 cellular prion protein231,232 |
Intrabodies have also been used in plants to interfere with cell functions and plant cell-pathogen interactions.133 The first transgenic ER intrabody mouse that constitutively expresses an α-VCAM1 intrabody, resulting in a clear phenotype (aberrant distribution of immature B cells in blood and bone marrow), has recently been described.134 In the future, by using inducible or cell- and tissue specific promoters, this important approach will facilitate the functional analysis of proteins in specific subpopulations of cells or tissues, or with different time kinetics. Moreover, this study hinted at possible quantitative effects, which would allow regulation of knockdown strength.
A therapeutic application of intrabodies would require transfection systems allowing gene therapy, typically viral or non-viral systems.135 Despite growing numbers of clinical studies using gene therapy, some issues are yet unresolved, like risks from insertional mutagenesis136 or low gene transfer efficiency of nonviral vectors.137 The construction of retroviruses with low safety risk138 and the development of transductional and transcriptional targeting for cell-specific gene transfer139 are new promising approaches. Furthermore, gene therapy with mRNA might be possible in the future.140
Parameters Influencing the Efficiency of ER Intrabodies
ER antibodies work by retaining their antigen in the ER. For this simple approach, and despite publication of many successful cases, our systematic understanding of the many parameters involved in a successful knockdown by intrabodies is still very limited. The influence of target and antibody expression levels, antibody affinity and epitope structures is still largely unexplored. For instance, a study by Beerli et al141 reported efficient target knockdown by only one of 2 ER intrabodies with nanomolar affinity,141-144 which suggests that high affinity is not sufficient as a predictive criterion for successful ER intrabody-mediated knockdown. This is further supported by a report on the lack of correlation between the affinity and antineoplastic effect of anti-erbB2 targeting ER intrabodies.145 Different knockdown efficiencies not correlated to monovalent affinities have also been reported by others.75 An anti-erbB2 antibody has been reported to bind to its target if transported to the cell surface but not within the cell146 and the reasons for this are not fully understood. The exact reasons for these differences are not yet fully clear, but it can be assumed that individual differences in the epitopes play a key role. Alternatively, the local biochemical milieus may influence individual epitope/idiotype combinations. Study of these parameters in the future is needed, as the results will allow pre-selection of the intrabodies accordingly. If, for instance, the different pH in the Golgi does not allow binding of the ER intrabody to its target in this compartment, in vitro antibody selection strategies such as phage display could easily be adapted by carrying out the selection procedure under buffer conditions that mimic those in the Golgi.
In spite of numerous examples for the successful application of ER intrabodies that can be found in literature (Table 3, ref. 3), the majority of these reports mainly focus on the analysis of phenotypical effects and on study of a particular target. Questions concerning the intrinsic properties of the ER intrabody technology and the underlying principles that determine success or failure of ER intrabody-mediated knockdowns have not been satisfactorily addressed so far. For wider and more systematic application of the ER intrabody technology, further insight into the parameters influencing the success of the method itself is needed. A more detailed characterization of the ER intrabody knockdown process will allow elimination of potential off-target effects when interpreting results and identification of specific properties of ER intrabody-mediated knockdowns. Opening new ways of analysis, in addition to the already known unique advantages of ER intrabodies over other methods, is critical.
Table 3.
B. ER intrabody-mediated knockdown: methodological aspects
| Target | expression target T/S/E* | expression ER intrabody T/S* | origin of binders | Affinity | Expression levels/degradation levels/half lives | Accumulation or accelerated degradation? | Knockdown efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| ErbB2 | S | S | Hybridoma, clone FRP5 | 4.2 nM for scFv(FRP5)-ETA construct (Wels et al. 1995), apparent affinity FRP5 (IgG) 0.82 nM (Harwerth et al. 1993) | Higher intracellular levels of retained antibodies than secreted antibodies, half-life of ErbB2* ∼1.5h, ErbB2 >7h (Reinman et al. 2003) | Accumulation of ER intrabodies in ER | Cell surface expression qualitatively analyzed ,NIH/ErbB2* cells: 95% growth inhibition | Beerli 1994b150 |
| ErbB2 | S | S | Hybridoma, clone FWP51 | Apparent affinity FWP51 (IgG) 1.3 nM (Harwerth et al. 1993) | Higher intracellular levels of retained antibodies than secreted antibodies, half-life of ErbB2* ∼1.5h, ErbB2 >7h (Reinman et al. 2003) | Accumulation of ER intrabodies in ER | Cell surface expression qualitatively analyzed ,NIH/ErbB2* cells: 80% growth inhibition | Beerli 1994b150 |
| EGFR | S | S | Hybridoma, mab225 | Nanomolar (Sato et al. 1983) | 1–2 x105 EGFR receptors per cell, scFv255 expression not detectable (potentially due to unavailability of optimal detection antibody) | Authors conclude retained scFv255 might accelerate degradation | Cell surface expression not analyzed, 31% EGFR reduction by secreted scFv225S, 9% EGFR reduction by retained scFv255R | Beerli 1994a155 |
| ErbB2 | E | S | Hybridoma, clone FRP5 | 4.2 nM for scFv(FRP5)-ETA construct (Wels et al. 1995), apparent affinity FRP5 (IgG) 0.82 nM (Harwerth et al. 1993) | qualitatively assessed by WB, NDF induced stimulation of cell growth 2.8 fold in control cells, 1.5 fold stimulation in ER-intrabody transfected cells | (Not quantified but WB seems to indicate similar levels of ErbB2 in presence and absence of ER-intrabody → there seems to be neither accumulation nor accelerated degradation) | Cell surface expression qualitatively analyzed | Graus-Porta 1995147 |
| IL-2 Rα | induced in Jurkat | S | Hybridoma HD245–332: anti-Tac, Hybridoma | Not determined in this paper | Half-life of scFv-KDEL more than 30h (pulse chase) | Antibody/IL-2Ra complexes are degraded by a nonlysosomal mechanism (= accelerated degradation) | Cell surface expression qualitatively analyzed, 16 of 16 cell clones stably transfected with ER-intrabody showed complete inhibition of target induction, 3of 15 cell clones stably transfected with secreted antibody version also showed downregulation of target but incomplete (remaining 12 clones showed normal or even high levels of the target) | Richardson 1995148 |
| Anti-Integrin VLA4 | S | S | Humanized form (hHP1/2, unpublished) of the mouse mAb mAbHP1/2) (hybridoma) | EC50 - 0.015 nM (Hanf et al. 2014) | Not quantified | Not determined | 52% of stably transfected RD cell clones and 8% of stably transfected Jurkat cells displayed reduced surface expression of the target. Target reduction on the surface of selected RD clones was ∼80%, on the surface of selected Jurkat cells 65–100% (MFI levels used for quantification of knockdown efficiency). 90% of mock transfected but < 1% of ER-intrabody transfected RD cells were spread. | Yuan 1996152 |
| EGFR | S | S2 | Hybridoma, mab225 | Nanomolar (Sato et al. 1983) | Expression levels of scFv225 were much lower than scFv-FRP5 in Cos-1 cells. scFv225 was mainly in the insoluble fraction. | Not determined for scFv225R | Not determined for scFv225R, EGF dependent cell growth reduced by 80% (FE5 cells) or 50% (NE1 cells) by scFv225S | Beerli 1996141 |
| ErbB2 | S 3 | S 2 | Hybridoma, clone FRP5 | 4.2 nM for scFv(FRP5)-ETA construct (Wels et al. 1995), apparent affinity FRP5 (IgG) 0.82 nM (Harwerth et al. 1993) | Expression levels of scFv-FRP5 were much higher than scFv255 in Cos-1 cells. | Not determined | 97% growth inhibition in soft agar, 55% reduced tumor volume after 13 d of growth in nude mice | Beerli 1996141 |
| IL-2 Rα | S | S and inducible | Hybridoma HD245–332: anti-Tac, Hybridoma | Not determined in this paper | Half-life of IL2Ralpha is greater than 24h (Levin et al. 1997), Full induction of scFv after 24h of tetracycline withdrawal.IL2Ralpha persisted for up to 72h at the cell surface after tetracycline withdrawal. Cell lines normally express more than 200 000 target molecules per cell. | While mature form (55 kDa) of the target vanishes, the immature (40kDa) form of the target accumulates | 2 or more than 2 log units of mean fluorescence intensity (cell surface staining) in all cell lines tested | Richardson 1997151 |
| alphaV integrin | E | S | Hybridoma, L230 | — | Not determined | Not determined | 70–100% decrease | Koistinen 1999225 |
| CCR5 | TTT | TTS | ST6 from phage display of a Fab rabbit immune library, converted to scFv format | 2.7 nM for ST6 8.5 nM for ST6/34 (humanized version of ST6) ST6 binds to a linear epitope in the first extracellular domain of CCR5236 | ST6 expressed at higher levels than its humanized version but the humanized version of ST6 led to a slightly higher knockdown efficiency (results from experiments with HEK293T cells transiently cotransfected with target and ER intrabody).236 | Not determined | Decrease from 66% target positive cells of controls to 5.9% target-positive cells for ST6 ER intrabody expressing cells or 2.7% target-positive cells for ST6/34 ER intrabody expressing cells.236 | Steinberger 2000217 |
| Procathepsin L | E | T | Hybridoma clone 3D8 | 5×10–8 M | Not quantified | Intracellular accumulation of target | Preliminary studies suggested susceptibility to complement lysis was 50% increased if ER intrabody was expressed | Guillaume-Rousselet 2002208 |
| Folate receptor | E | S | Hybridoma clone MOV19 Mab | 7.1×10–8 M by displacement study, 1.8×10–8 M by scatchard analysis | mRNA levels for the ER intrabody were highest in the IGROV-1 clone with the most efficient target knockdown | Accelerated degradation of the target upon ER intrabody expression (determined by western blot of whole cell lysates) | IGROV-1 clones with 60 (=DM60), 85 (=DM85) and 99% (=DM99) reduction, ER intrabody transfected SKOV3 were negative for surface target. Reduction of cell surface target remained stable over 1 y of culture in DM99, but spontaneous decrease in target expression was observed in DM60 and DM85 | Figini 2003207 |
| CCR5 | SE | S | Hybridoma, CTC8, antibody 2C7 | — | Very high expression in SupT/CCR5 cells, target expression in <75% of PM1 cells, target expression in <50% of MDMs | Not quantified | 50–60% reduction of surface target | Cordelier 2004219 |
| MHC I | E | ST | Hybridoma, clone OX-18 | — | With increasing ER intrabody expression, the cell surface expression of the target decreased (indicated by flow cytometric analysis). Down-regulation of cell surface target was only observed at ER intrabody expression levels of >200 U green fluorescence. | Intracellular accumulation of target | Reduction of specific cell lysis from more than 80% to less than 30% of cells. | Busch 2004223 |
| VEGF-R2 | S | S | Phage display,scFvA2, scFvA7 | 3.8 nM (Böldicke et al. 2001) | — | — | Not quantified | Böldicke 2005153 |
| VEGF-R2 and Tie-2 | E | S | Phage display from an immune library, clone S08b (Popkov et al. 2003) and VR05 (Popkov et al. 2004) | 2S08b: 1nM(Popkov et al. 2003)VR05: 3 nM (Popkov et al. 2004) | Not determined | Not determined | 98.6% reduction of VEGF-R2 surface expression and 91.2% reduction of Tie-2 surface expression in HUVEC ; 92.1% reduction of VEGF-R2 and 92.5% reduction of Tir-2 cell surface expression in HMEC-1 cells | Jendreyko 200588 |
| Tie-2 | E | S | Phage display from an immune library, clones 1S05 and 2S03 (Popkov et al. 2003) | — | Not determined | Not determined | 80–90% reduction of cell surface expression of the target | Popkov 2005131 |
| CCR5 | E | S | Phage display from an immune library, clone ST6/34 | 8.5 nM (Steinberger et al. 2000) | Not determined | Not determined | Not quantified | Swan 2006218 |
| vIL-6 | T | T | Phage display with naïve library (Tomlinson), monoclona l anti-vIL-6 (MAV) | 340 nM | Not quantified | Not quantified | 30% reduction or 69% reduction of phospho-STAT3 if 0.5 μg or 0.25 μg of target-vector DNA were transfected. | Kovaleva 2006230 |
| TfR | E | T | hybridoma | — | Not determined | Not determined | 83% reduced surface expression of the target at 72h after transfection with the ER intrabody | Peng 2007233 |
| ApoB | E | S | Hybridoma clone 1D1 and 1C4 | — | Not quantified | Not determined | Not quantified | Liao 2008214 |
| CD147 | E | T | Hybridoma, clone M6–1B9 | — | Not determined | Not determined | 13% of ER intrabody transduced cells were positive for the target while 28% of untransduced and 29% of mock-transduced cells were positive for the target (36h after transduction) | Tragoolpua 2008229 |
| CD147 | E | T | Hybridoma, clone M6–1B9 | — | not determined | Not determined | Not quantified | Intrasai 2009234 |
| VCAM1 | S | T | Hybridoma clone 6C7.1 | — | not determined | Not determined | 43% of cells were negative for VCAM1 48h after transfection with the ER intrabody, 94 % after 96h | Strebe 2009149 |
| TLR2 | TEE | TSS | Hybridoma clone T2.5 | — | — | Intracellular accumulation of target | Complete knockdown | Kirschning 20104 |
| HBV precore antigen | S | T | Immunoglobulin new antigen receptor (IgNAR) single domain antibody VNAR H6 isolated by phage display from a shark library | 53 nM for HBeAg | Not quantified | Slight intracellular accumulation of p25/p17 in AD38 cells, slight reduction of intracellular p25 in pTRE precore and HepG2.2.15 cells | In pTRE precore cells ER intrabody expression resulted in 0.9 fold reduced intracellular p25 levels and 0.3 fold reduction of p17 HBeAg. In secretion incompetent AD38 cells, ER intrabody expression led to a slight increase (1.1-1.2 fold) in p25. In HepG2.2.15 intracellular p25 was reduced 0.6-0.8 fold and secretion of p17 HBeAg was reduced 0.5 fold upon ER intrabody expression. | Walsh 2011213 |
| p75NTR | E | T | phage display from a naïve library, clones SH325-A11, SH325-B6, SH325-G7 | SH325-A11: 6.5×10–9SH325-B6: 3.9×10–9SH325-G7:3.9×10–8 | SH325-A11 and SH325-B6: 5–10 ng ER intrabodies per 106 cells, SH325-G7:>15 ng ER intrabodies per 106 cells | Not determined | 56% reduction of cell surface target after 96h (maximal knockdown efficiency was reached 96h after transfection with the ER intrabody plasmid) | Zhang 201275 |
| TLR9 | T and E | T | Hybridoma clone 5G5 | Not determined | Not determined | Not determined | Not determined | Reimer 20135 |
| VCAM1 | E | S | Hybridoma clone 6C7.1 | Not determined | Not quantified | Not determined | Cell surface stainings suggested complete depletion of cell surface VCAM1 in bone marrow cells but absence of the lethal phenotype expected for the complete knockout of VCAM1 hinted at the potential presence of traces of residual VCAM1cell surface expression | Marschall 2014a134 |
| Aβ oligomers | S | S | scFvA13 selected by IACT in yeast | Not determined | ER intrabody levels were unchanged after treatment with proteasome inhibitor for 6h suggesting degradation does not occur via ERAD, but the ER intrabody had a short half-life, being almost completely degraded after 6 h | No intracellular accumulation of Aβ. Steady state levels of APP and APP C-terminal fragments were similar in control and ER intrabody expressing cells. | 60–70% reduction of secreted Aβ dimers and trimers upon expression of the ER intrabody (detected in concentrated conditioned media), 70–80% reduction of secreted oligomeric conformers, increase in monomeric species was observed | Meli 2014235 |
| Z α antitrypsin | T | T | Hybridoma clone mAb4B12 | Not determined | ER Intrabodies were expressed at level comparable to those of the target | Target accumulates upon ER intrabody expression (compared to cells that express a secreted antibody). No marked ER stress was found upon ER intrabody expression and degradation of the ER-intrabody-target complex by proteasomal or autophagic pathways was not observed. | Intracellular polymerization of Z α antitrypsin was reduced by 60%, secretion of Z α antitrypsin was reduced in scFv4B12-KDEL expressing cells and secretion was increased in scFv4B12 expressing cells. | Ordonez 2015 |
1Transient expression (T), Stable expression (S), Endogenous expression (E).
2pool (polyclonal cells).
3of oncogenically activated form of target: ErbB2*.
Table 3.
A. Reports of ER intrabody mediated knockdown
| Target | Cell line | Biochemical knockdown detection | Physiological knockdown readout | Ref. |
|---|---|---|---|---|
| ErbB2 | ErbB2 transformed NIH/3T3 (clone 3.7) | Cell surface staining (Flow cytometry) | Phosphotyrosine analysis (WB), analysis of growth inhibition and morphology | Beerli 1994b150 |
| ErbB2 | ErbB2 transformed NIH/3T3 (clone 3.7) | Cell surface staining (Flow cytometry) | Phosphotyrosine analysis (WB), analysis of growth inhibition and morphology | Beerli 1994b150 |
| EGFR | EGFR transformed NIH/3T3 | No cell surface staining performed, effect of intrabodies on total cellular amount of target was analyzed | EGF induced colonies in soft agar | Beerli 1994a155 |
| ErbB2 | T47D cells | Cell surface staining (Flow cytometry) | ErbB2 tyrosine phosphorylation abolished after EGF treatment (no phosphorylated ErbB2 detectable in WB), 46% reduction of EGFR phosphotyrosine following EGF treatment, following NDF treatment ErbB-3 phosphotyrosine reduced by 89%. Phosphorylation states of various proteins. Reduction of NDF induced soft agar growth | Graus-Porta 1995147 |
| IL-2 Rα | Jurkat cells with induced IL-2 Rα expression | Cell surface staining with the same antibody clone like the ER-intrabody (Flow cytometry), pulse chase experiment – detection of only the immature target in the presence of the ER-intrabody, endoglycosidase H sensitivity of target (consistent with localization in pre- or early Golgi compartment) | None | Richardson 1995148 |
| Anti-Integrin VLA4 | RD rhabdomyosarcoma cells, Jurkat cells | Cell surface staining and flow cytometry | Number of adhering cells as functional assay, cell adhesion, cell spreading | Yuan 1996152 |
| EGFR | EGFR transformed NIH/3T3 fibroblast cells (NE1), IL-3 dependent FDC-P1 hematopoietic cell line (FE5) (note: activation of EGFR replaces IL-3 dependent growth) | Not determined for scFv225R | Not determined for scFv255R , EGF dependent cell growth reduced by 80% (FE5 cells) or 50% (NE1 cells) by scFv255S | Beerli 1996141 |
| ErbB2 | ErbB2 transformed NIH/3T3 (clone 3.7) | No cell surface staining performed | Anchorage independent growth, ability to form tumors in nude mice | Beerli 1996141 |
| IL-2 Rα | T cell line Kit225 HTLV-I transformed cell lines (C8166–45 and HUT102) Jurkat |
Cell surface staining with the same antibody clone like the ER-intrabody. Cell surface stainings were also repeated with an antibody that recognizes a different epitope than the ER intrabody. | Proliferative response | Richardson 1997151 |
| alphaV integrin | Saos-2 | Cell surface staining and analysis by flow cytometry | Cell adhesion, cell spreading | Koistinen 1999225 |
| CCR5 | HEK293T Cos 7 PM1 |
Cell surface staining and analysis by flow cytometry | CCR5 dependent cell fusion was prevented by the ER intrabody, protection of PM1 cells from R5 HIV-1 infection by the ER intrabody | Steinberger 2000217 |
| Procathepsin L | Human melanoma cell line A375SM | Immunoblots of cell culture supernatant (target is secreted) | Complement lysis assay | Guillaume-Rousselet 2002208 |
| Folate receptor | IGROV-1, SKOV3 | Cell surface staining and analysis by flow cytometry | Cell proliferation, morphology, cell adhesion | Figini 2003207 |
| CCR5 | SupT cells stably transfected with the target, PM1 cells, primary monocyte derived macrophages (MDMs) and microglial cells | Cell surface staining and analysis by flow cytometry | HIV infection assays | Cordelier 2004219 |
| MHC I | HEK293, Primary rat keratinocytes | Cell surface staining and analysis by flow cytometry | Cell lysis | Busch 2004223 |
| VEGF-R2 | PAE VEGR-R2 | Cell surface staining and analysis by flow cytometry | Angiogenesis | Böldicke 2005153 |
| VEGF-R2 and Tie-2 | in vivo gene delivery into nude mice, HUVEC, HMEC-1 | Cell surface staining and analysis by flow cytometry | Cell proliferation (in vitro), tumor growth (in vivo) | Jendreyko 200588 |
| Tie-2 | in vivo gene delivery into nude mice, HUVEC | Cell surface staining and analysis by flow cytometry | In vitro cell proliferation assay, Analysis of vessel density (87% reduction of vessel density upon ER intrabody treatment) | Popkov 2005131 |
| CCR5 | THP-1, PM1, primary CD4+ T cells | Cell surface staining and analysis by flow cytometry | Susceptibility of cells to HIV-1 infection | Swan 2006218 |
| vIL-6 | Cos-7 | Comparison of target content in cell lysate and cell culture supernatant, analysis for colocalization of ER intrabody and target by microscopy | Neutralization of the target signaling in the ER analyzed by monitoring STAT3 phosphorylation. | Kovaleva 2006230 |
| TfR | MCF-7 | Cell surface staining and analysis by flow cytometry | Analysis of proliferation and cell cycle | Peng 2007233 |
| ApoB | HepG2 | Analysis of the cell culture supernatant for the presence of secreted target, Co-IP | Analysis of albumin secretion into the cell culture medium | Liao 2008214 |
| CD147 | HEK293A | Cell surface staining and analysis by flow cytometry | — | Tragoolpua 2008229 |
| CD147 | HeLa | Cell surface staining and analysis by flow cytometry | — | Intasai 2009234 |
| VCAM1 | HEK293::VCAM-YFP | Cell surface staining and analysis by flow cytometry | Cell adhesion assessed by microscopy | Strebe 2009149 |
| TLR2 | HEK293, RAW264.7, primary macrophages | Cell surface staining and analysis by flow cytometry | TNF-alpha release | Kirschning 20104 |
| HBV precore antigen | pTRE AD38 HepG2.2.15 |
Analysis of ER intrabody and target localization by fluorescence microscopy | Reduction of extracellular HBeAg in pTRE precore and HepG2.2.15 cells. | Walsh 2011213 |
| p75NTR | PC12 and NSC19 cells | Cell surface staining and analysis by flow cytometry | Bcl-xL mRNA expression, cell differentiation (neurite outgrowth) | Zhang 201275 |
| TLR9 | HEK293, RAW264.7 | Co-IP, analysis by microscopy for colocalization with an ER marker | Analysis of TLR9 challenge by NF-kappa B reporter assay, TNF-alpha response upon treatment with TLR agonists analyzed by ELISA and intracellular flow cytometry | Reimer 20135 |
| VCAM1 | in vivo, mouse strain C57BL/6N | Cell surface staining and analysis by flow cytometry | analysis of B-cell distribution over blood and bone marrow | Marschall 2014a134 |
| Aβ oligomers | 7PA2 fADcells (CHO cells which express a fADhumanV717FAPP mutant) 7WD4 cells |
Co-immunoprecipitation | Dephosphorylation of ERK1/2 and CREB is reduced in hippocampal neurons treated with conditioned media of the ER intrabody expressing 7PA2-A13K cells. Effect of the ER intrabody on the mTor pathway in 7PA2-A13K cells, which plays a role in homeostasis and autophagy. | Meli 2014235 |
| Z α antitrypsin | Cos-7 | Co-immunoprecipitation | - | Ordonez 2015 |
1Transient expression (T), Stable expression (S), Endogenous expression (E).
2pool (polyclonal cells).
3of oncogenically activated form of target: ErbB2*.
In the following, we review ER intrabody-mediated knockdown studies with a particular focus on methodological aspects (Table 3A and 3B). The first example of in vivo efficacy of ER intrabodies has recently been described,134 but ER intrabody-mediated knockdowns have so far been employed in vitro3 in a large variety of setups. ER intrabody mediated knockdowns have been performed either in cell lines with constitutive75,147 or inducible148 endogenous expression of the target or in cell lines that had been either transiently149 or stably141,149, 150 transfected with the target. Correspondingly, transfection of ER intrabodies into these cell lines has been performed transiently,75,149 inducibly stable151 or constitutively stable.151-153 A significant quantitative biochemical analysis of the involved parameters is lacking in most studies published so far. Further, the information on the knockdown process has come from very diverse assays usually optimized for one target, which prevents a systematic analysis of these datasets to obtain general conclusions.
Hence, it is currently difficult to attribute knockdown efficiencies to the biochemical properties of the respective intrabody, e.g., affinity, because knockdown efficiency may also be influenced by the particular expression levels of the ER intrabody and its antigen. Individual developments of transient expression levels over time will also surely affect the knockdown efficiency and a respective time dependence of knockdown efficiency after transient transfection of ER intrabodies has been observed.149
Despite the lack of a systematic analysis of these important parameters, we can nevertheless gain some valuable insights from some examples. For instance, the analysis of 3 different ER intrabodies transiently expressed in the same cell line against the same target allowed some interesting observations.75 The ER intrabodies in this study were analyzed for affinity, expression levels and their ability to bind a linear or conformational epitope. Interestingly, the ER intrabody with the lowest affinity but the highest expression levels gave rise to the highest knockdown efficiency,75 underlining once more the need for further understanding of the knockdown process in a quantitative manner. Moreover, while all antibodies in this study recognized the native target on the cell surface, the ER intrabody with the highest knockdown efficiency also efficiently recognized a linear epitope.75 Recognition of a linear epitope of an unfolded or only partly folded protein, possibly already while the protein is still in the process of translation into the ER, can be imagined to efficiently interfere with the folding process and in this way accelerate degradation of this protein. A systematic characterization of ER intrabody epitopes, particularly of ER intrabodies that are known to accelerate degradation of the target, will in the future be required to evaluate this hypothesis. If the recognition of linear epitopes by ER retained antibodies can indeed promote accelerated degradation of target proteins, this mechanism may in the future be harnessed by selectively generating antibodies against short linear peptide sequences within the target protein instead of whole proteins, and would also allow an important acceleration of the method since the necessity for antigen protein production – the single most retarding process in today's antibody generating pipelines – can be completely avoided.
An important question to understand intrabody generated phenotypes and to avoid incorrect conclusions due to unspecific and off-target effects is for the fate of the intrabody/target protein complex. Only few studies are available so far which tried to assess these questions. It was shown that the ER intrabodies targeting ß-amyloid precursor protein (APP) and the anti-TLR2 ER intrabody are degraded by the proteasome (Ref. 154, Böldicke and Burgdorf, unpublished). Although ER intrabodies have been reported to cause accelerated degradation of a target protein,155 there are also reports on accumulation of the target within the cell, although it is depleted from the surface.150,151 The determining factors that are responsible for accelerated degradation, accumulation or even unchanged intracellular target levels are still not clear. However, no detectable ER stress response (unfolded protein response) was observed following substantial overexpression of an anti-p75NTR ER intrabody, confirming the specificity of this particular approach75 and recommending measurement of UPR as a good control allowing for rating the relevance of any future intrabody approaches. Another observation, with possible relevance to the question of the post-translational fate of intrabodies and their antigens, is the lack of target protein glycosylation upon expression of an ER intrabody as a result of the retention in the ER.148,150,153
To compare the results of different ER intrabody-mediated knockdowns, the choice of methods used for detecting knockdowns is critical. The heterogeneity of analysis methods used so far is therefore an obstacle for correlating antibody properties with knockdown efficiency. Methods for detection of ER intrabody-mediated knockdowns ranged from proof on the biochemical level by cell surface stainings followed by flow cytometry to a variety of functional assays including entirely qualitative analysis (see Table 3A and 3B). While flow cytometric analysis of cell surface stainings are preferable to other methods because they provide direct biochemical evidence for a potential membrane expression knockdown, cell surface stainings have also not been done in a standardized way. In some reports, the same antibody clone that was used as an ER intrabody was also used for the detection of the target on the cell surface,148 which bears the risk of artifacts due to ER intrabodies that still mask the target protein's epitope on the cell surface after being released by cell lysis. Although retention by the KDEL receptor has been described to be very efficient (retention of a 10-fold molar excess of substrate may be possible156), masking of target proteins on the cell surface due to secretion of ER intrabodies must be excluded. In order to avoid this artifact, cell surface stainings have therefore increasingly been performed with detection antibodies that have been mapped to bind to a different epitope than the ER intrabody.75,134, 149,151
In conclusion, although there are already initial findings that suggest a link between particular antibody properties and knockdown efficiency, a more systematic analysis of these links is required to allow better a priori predictions, and thus to allow the selection of optimal antibodies for future intrabody knockdown approaches.
The ER Intrabody Approach in Comparison to other Knockdown Methods
The cell surface and its receptors provide an interface for communication between the individual cell and its environment, and is therefore a crucial element of cellular decision processes, determining the organization and function of cells, tissues and organs. Complementary to cell surface receptors, secreted factors control cellular decisions by transducing signals via transmembrane receptors to the inside of the cell. Because both the soluble extracellular factors as well as cell surface receptors pass the secretory pathway, the ER intrabody technology can be employed to systematically target these crucial control elements at the protein level.
There have been other attempts to target these proteins at the post-translational level by pharmacological inhibition of receptor tyrosine kinases,157 blocking cell surface receptors or secreted factors extracellularly with antibodies158,159 or even by targeting the intracellular part of cell surface receptors or signaling molecules downstream of the cell surface receptors via cytosolic intrabodies11,23 or phosphopeptide mimetics.160,161 Most of these approaches have very narrow application ranges and require tedious individual developments for every case. For instance, not all of the cell surface receptors are receptor tyrosine kinases, and therefore not all of them are accessible to the respective pharmacological agents, and pharmacological inhibitors are only available for some of the receptor tyrosine kinases. The same is true for phosphopeptide mimetics: inhibitory and at the same time cell permeable drugs are only available for a very small number of the signaling molecules, allowing only specialized applications.
Another drawback of pharmacological agents for protein inhibition is the dependence of their effectiveness on biodistribution and bioavailability and the potential side effects that may occur due to lack of specificity, especially as tyrosine kinase inhibitors are generally less specific than antibodies,162,163 which for example, has been shown for protein kinase inhibitors and inhibitory oligonucleotides of TLRs. It was shown that the compounds KT5720, Rottlerin and quercetin inhibited many different protein kinases.164 Inhibitory CpG comprising oligonucleotides developed to inhibit the function of TLR9 inhibit TLR9 signaling, but also bind signal transducer and activator of transcription 1 and 4 (STAT1 and STAT4) and interact with TLRs 3, 7 and 8.165,166 Finally, there is no systematic way to generate them for any possible protein, very much in contrast to antibodies, which can be rationally and systematically generated to almost any target within a few weeks.167
Dominant negative mutants (for review see Ref. 168) interfere with protein function by competing with the wildtype, and usually require detailed knowledge of the role of individual amino acids in the protein function. In addition, its dependence on vast overexpression is prone to cause unspecific effects and cell stress. Intrabody approaches do not require any knowledge of the target protein, but even may be used to reveal phenotypes of yet completely unknown proteins, because they can be generated from the genomic sequence information of unknown open reading frames (ORFs), without requiring the antigen for their generation, e.g., by the well-established method of phage display based on synthetic peptides.169
The application range for antibody-based targeting of the cytosolic part of kinase receptors and of the corresponding signaling molecules downstream of cell surface receptors similarly suffers from limitations. As described above, antibodies that can fold correctly if expressed in the cytosol are not easily obtained19,23, 25 and, although the delivery of antibodies as proteins to the cytosol has been demonstrated in vitro,24 protein delivery from the outside still remains a challenge. ER intrabodies, in contrast, are generally applicable both in vitro and in vivo and can be generated with high specificity against any protein of the secretome, provided that the target protein or a sufficient fragment of it (e.g., synthetic peptide) are available for the selection procedure.60,130,169,170 In vivo studies by ER intrabody gene delivery include knockdown in xenograft tumor models and in an Alzheimer's disease mouse model.88,131,132 The successful in vivo protein knockdown in transgenic intrabody mice by ER intrabodies has opened new opportunities to study genetically lethal phenotypes.134 If the hypothesis can be confirmed that the strength of the in vivo knockdown can be influenced by the strength of an exogenous promoter, different or inducible promoters in mice will certainly facilitate the establishment of novel disease-related preventive and therapeutic mouse models. The potential for intrabody expression restricted to specific tissues by appropriate promoters will yield new insights into the in vivo function of new proteins in subpopulations of cells. Although blocking cell surface receptors by secretion of antibodies to the cellular environment is broadly applicable to cell culture and has also been performed in vivo in transgenic mice,159 this type of interference at the protein level is little defined because the location of the effect in vivo can only poorly be controlled. Since secreted antibodies can be transported to different locations in a transgenic mouse, a secreted antagonistic antibody may not only have an autocrine and paracrine, but also an endocrine effect, which renders tissue specific knockdowns at the protein level impossible by this method.
The most commonly used techniques to knock down proteins of the secretome are so far not those taking action at the post-translational level, but methods that take effect at the RNA or DNA level. RNA interference (RNAi) has become a widely used standard method for knocking down proteins as it is generally applicable provided that the sequence of the target mRNA is known. Only a short RNA sequence (short interfering RNA, siRNA) that can induce degradation of all the homologous cellular mRNA as part of an RNA induced silencing complex (RISC) is required.171,172 Generating siRNA technically was less challenging and less time-consuming than ER intrabody generation by the hybridoma technology, but this situation is currently changed due to the availability of automated miniaturized in vitro antibody selection methods, which provide the antibody gene in the correct format (scFv) for immediate subcloning.
Consequently, the increasing availability of antibody genes and efforts to generate databases with detailed descriptions for affinity reagents60,169, 173,174 can in the future provide a well-annotated resource of already existing antibody genes that are ready to use as ER intrabodies. For these “ready to use” antibody genes, which are available in the thousands already, generating a knockdown with the ER intrabody will not require any more time than is required for an RNAi-based approach. Although confirmation by a larger data set is needed, a claimed advantage of the ER intrabody technology over RNAi is the potentially longer half-life of the protein-based ER intrabodies compared to RNA-molecules.175 Another inherent property of ER intrabodies that represents an advantage over RNAi concerns specificity, which can be thoroughly assessed biochemically by a multitude of assays before applying an antibody intracellularly, while specificity and off-target effects of RNAi are often more difficult to predict.
RNAi, which is thought to have its origins in nature from an antiviral defense mechanism,176-178 has been found to cause several off-target effects, including an interferon response179-183 and aberrant expression of up to more than 1000 genes as found by Persengiev et al.181 Off-target silencing of siRNA/shRNA results from imperfect pairing of siRNA strands with sequence motifs that reside primarily in 3′ UTR regions of cellular mRNA. siRNA and miRNA share the same silencing machinery and the magnitude of regulation of siRNA off-target transcripts is similar to that of micro RNAs.184 Furthermore, siRNA recognize TLR3, TLR7 and TLR8 resulting in secretion of interferon-α and synthesis of pro-inflammatory cytokines.185 RNAi and the ER intrabody technology both have broad application areas as they both can be applied in vitro and in vivo. Applications in vivo have already been performed both for RNAi and the ER intrabody technology in the form of in vivo delivery88,131,186 and in the form of transgenic mice.134,187 However, therapeutic applications are hampered by the hurdle posed by the need for efficient in vivo delivery of nucleic acids, and are still subject to safety concerns for both RNAi and the ER intrabody technology.175,188-190 Consequently, applications have primarily focused on use of the technologies as research tools. The same is true for the classic knockout strategies at the DNA level.
Genetic knockouts in mice have greatly contributed to a better understanding of human disease, and, as a consequence, can yield new therapeutic approaches. However, in spite of substantial efforts aimed at cataloging the mammalian genome, e.g., the International Mouse Phenotyping Consortium (www.mousephenotype.org), genetic knockouts do not always allow complete analysis of essential genes because approximately 30% of genetic knockouts are embryonically lethal.191 If a complete knockout of a gene is lethal early in development, alternative strategies for functional knockdown are required to study functions of the gene in later developmental stages and adults. ER intrabodies offer a solution. Although the genetic knockout of the cell adhesion molecule VCAM1 leads to early embryonic death, mice with an anti-VCAM1 ER intrabody exhibited depletion of VCAM1 at the cell surface, but were nevertheless viable.134 This demonstrates the potential of the ER intrabody technology as an alternative strategy for studying the phenotype of embryonically lethal knockouts in adult mice.
In conclusion, the inherent properties of ER intrabodies implicate some limitations, but also specific strengths that can serve to compensate shortcomings of the other established technologies for functional studies of the secretome.
The Promising Future of Intrabody Approaches
Although intrabody approaches have been known for more than a quarter of a century, and the above examples are very encouraging, this approach is not as widely used for cell biological research as could be expected given the possible benefits. In particular, the technology had a very slow start. What is the reason for that? The answer is not simple, and comprises a multitude of factors. The first intrabody studies used microinjection of mRNA, a cumbersome and inefficient method of limited specificity, resulting in a first decade of very rare applications. In the early nineties, the method gained some momentum with the advent of essential technological elements like the single chain format and recombinant antibody technologies. Typical applications followed the cytoplasmic intrabody approach. In light of the quite substantial requirements of getting an antibody combining both functional neutralization of its target and correct folding in reducing milieu, it can be assumed that those candidates were not easily identified from the collection of available hybridomas. In the early days of intrabody technology, a substantial number of intrabody trials may have failed due to the limited availability of suitable monoclonal antibodies and the limited knowledge about the biochemical requirements. Further, generating recombinant scFv antibodies from hybridoma, and particular expressing it in E. coli for functional validation, was a technology not widespread among the typical cell biology lab of the time. Quite a number of scFvs generated from hybridoma showed very poor production yields in E. coli,192 generating substantial problems for the necessary biochemical characterizations. Another problem, which originated from the use of early hybridoma cell lines as sources for the isolation of antibody DNA, was the frequently observed expression of multiple antibody mRNAs in these cells due to the aneuploid/deregulated state of the hybridoma cells, lacking allelic exclusion and allowing accumulation of mutations.193-196 This mandated substantial experimental efforts to confirm that the predominant V region PCR bands found from any hybridoma indeed encoded the V region subset providing the specificity identified in the supernatant.197 These factors contributed to give the intrabody technology a mixed reputation among the typical possible users who were not acquainted with recombinant antibody technology. Nevertheless, successful projects using hybridoma cells as a starting point accumulated over time and provided a slow but steady development of the technology.
Only in the last decade have recombinant in vitro antibody selections from gene repertoires been utilized in substantial numbers, allowing much quicker access to the antibody and providing the V region encoding DNA without any additional experimental effort. This also substantially increases the chances of getting antibodies with the required complex property combination using appropriately tuned libraries and panning strategies from the start. The technology to generate research antibodies from phage display in large numbers is now robust and reliable,65-67, 69,169 and international research consortia like the “Affinomics” initiative (www.affinomics.org) in the EU and similar initiatives in the US have already generated several thousands of antibodies to hundreds of new potential intrabody targets together with their V region genes, providing right away a vast resource of scFv DNA for future intrabody approaches. Studies on introducing antibodies from the outside to interfere with intracellular functions show the limitations of this approach,24 so the quick availability and fast generation of recombinant antibodies for intrabody approaches may motivate more researchers to try this technology for their functional analysis. Protein functions of bioinformatically predicted ORFs of so far unknown function could be evaluated in respect of resulting knockout phenotypes, even without the availability of the resulting protein, because antibodies can efficiently be generated by phage display to chemically synthetized peptides based on the ORF sequence. In the future, this strategy allows a completely new systematic approach to identify protein functions of yet uncharacterized members of the proteome, particularly straightforward for the membrane proteome where most binders may be able to generate a phenotype after just a single subcloning step into an ER intrabody vector. Further, new methods for the rapid generation of transgenic organisms, like CRISPR/Cas9 or TALEN, are maturing, and will further facilitate the experiments required to screen for a phenotype in vivo.
The application of the intrabody technology, in particular the most facile ER intrabody approach, can thus be expected to grow much more rapidly in the near future than in its first 2 decades.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Michael Hust for providing graphic elements for the figures.
Funding
We are grateful for support by the EU consortium “Affinomics.”
References
- 1.Zehner M, Marschall AL, Bos E, Schloetel J-G, Kreer C, Fehrenschild D, Limmer A, Ossendorp F, Lang T, Koster AJ, et al.. Endosomal Sec61 mediates antigen translocation in the cytosol for cross-presentation. Immunity 2015; 42:850-63; PMID:25979419; http://dx.doi.org/ 10.1016/j.immuni.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 2.Aires da Silva F, Santa-Marta M, Freitas-Vieira A, Mascarenhas P, Barahona I, Moniz-Pereira J, Gabuzda D, Goncalves J. Camelized rabbit-derived VH single-domain intrabodies against Vif strongly neutralize HIV-1 infectivity. J Mol Biol 2004; 340:525-42; PMID:15210352; http://dx.doi.org/ 10.1016/j.jmb.2004.04.062 [DOI] [PubMed] [Google Scholar]
- 3.Böldicke T. Blocking translocation of cell surface molecules from the ER to the cell surface by intracellular antibodies targeted to the ER. J Cell Mol Med 2007; 11:54-70; PMID:17367501; http://dx.doi.org/ 10.1111/j.1582-4934.2007.00002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschning CJ, Dreher S, Maass B, Fichte S, Schade J, Koster M, Noack A, Lindenmaier W, Wagner H, Böldicke T. Generation of anti-TLR2 intrabody mediating inhibition of macrophage surface TLR2 expression and TLR2-driven cell activation. BMC Biotechnol 2010; 10:31; PMID:20388199; http://dx.doi.org/ 10.1186/1472-6750-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimer E, Somplatzki S, Zegenhagen D, Hanel S, Fels A, Bollhorst T, Hovest LG, Bauer S, Kirschning CJ, Boldicke T. Molecular cloning and characterization of a novel anti-TLR9 intrabody. Cell Mol Biol Lett 2013; 18:433-46; PMID:23893288; http://dx.doi.org/ 10.2478/s11658-013-0098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serruys B, Van Houtte F, Farhoudi-Moghadam A, Leroux-Roels G, Vanlandschoot P. Production, characterization and in vitro testing of HBcAg-specific VHH intrabodies. J Gen Virol 2010; 91:643-52; PMID:19889923; http://dx.doi.org/ 10.1099/vir.0.016063-0 [DOI] [PubMed] [Google Scholar]
- 7.Colby DW, Chu Y, Cassady JP, Duennwald M, Zazulak H, Webster JM, Messer A, Lindquist S, Ingram VM, Wittrup KD. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody. Proc Natl Acad Sci U S A 2004; 101:17616-21; PMID:15598740; http://dx.doi.org/ 10.1073/pnas.0408134101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colby DW, Garg P, Holden T, Chao G, Webster JM, Messer A, Ingram VM, Wittrup KD. Development of a human light chain variable domain (V(L)) intracellular antibody specific for the amino terminus of huntingtin via yeast surface display. J Mol Biol 2004; 342:901-12; PMID:15342245; http://dx.doi.org/ 10.1016/j.jmb.2004.07.054 [DOI] [PubMed] [Google Scholar]
- 9.Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric α-synuclein that inhibits aggregation and prevents α-synuclein-induced toxicity. J Mol Biol 2007; 368:1132-44; PMID:17391701; http://dx.doi.org/ 10.1016/j.jmb.2007.02.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meli G, Visintin M, Cannistraci I, Cattaneo A. Direct in vivo intracellular selection of conformation-sensitive antibody domains targeting Alzheimer amyloid-β oligomers. J Mol Biol 2009; 387:584-606; PMID:19361429; http://dx.doi.org/ 10.1016/j.jmb.2009.01.061 [DOI] [PubMed] [Google Scholar]
- 11.Hyland S, Beerli RR, Barbas CF, Hynes NE, Wels W. Generation and functional characterization of intracellular antibodies interacting with the kinase domain of human EGF receptor. Oncogene 2003; 22:1557-67; PMID:12629519; http://dx.doi.org/ 10.1038/sj.onc.1206299 [DOI] [PubMed] [Google Scholar]
- 12.Paz K, Brennan LA, Iacolina M, Doody J, Hadari YR, Zhu Z. Human single-domain neutralizing intrabodies directed against Etk kinase: a novel approach to impair cellular transformation. Mol Cancer Ther 2005; 4:1801-9; PMID:16276002; http://dx.doi.org/ 10.1158/1535-7163.MCT-05-0174 [DOI] [PubMed] [Google Scholar]
- 13.Tse E, Lobato MN, Forster A, Tanaka T, Chung GT, Rabbitts TH. Intracellular antibody capture technology: application to selection of intracellular antibodies recognising the BCR-ABL oncogenic protein. J Mol Biol 2002; 317:85-94; PMID:11916380; http://dx.doi.org/ 10.1006/jmbi.2002.5403 [DOI] [PubMed] [Google Scholar]
- 14.Van Impe K, Bethuyne J, Cool S, Impens F, Ruano-Gallego D, De Wever O, Vanloo B, Van Troys M, Lambein K, Boucherie C, et al.. A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res 2013; 15:R116; PMID:24330716; http://dx.doi.org/ 10.1186/bcr3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biocca S, Di Luzio A, Werge T, Cattaneo A. Intracellular immunization: Expression of antibody domains in the cytoplasm and in the nucleus of mammalian cells. Cytotechnology 1991; 5:49-50; PMID:AMBIGUOUS; http://dx.doi.org/ 10.1007/BF00736806 [DOI] [PubMed] [Google Scholar]
- 16.Biocca S, Neuberger MS, Cattaneo A. Expression and targeting of intracellular antibodies in mammalian cells. EMBO J 1990; 9:101-8; PMID:2153072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson JR. A new means of inducibly inactivating a cellular protein. Mol Cell Biol 1988; 8:2638-46; PMID:3136320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobato MN, Rabbitts TH. Intracellular antibodies and challenges facing their use as therapeutic agents. Trends Mol Med 2003; 9:390-6; PMID:13129705; http://dx.doi.org/ 10.1016/S1471-4914(03)00163-1 [DOI] [PubMed] [Google Scholar]
- 19.Stocks M. Intrabodies as drug discovery tools and therapeutics. Curr Opin Chem Biol 2005; 9:359-65; PMID:15979379; http://dx.doi.org/ 10.1016/j.cbpa.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Tavladoraki P, Benvenuto E, Trinca S, De Martinis D, Cattaneo A, Galeffi P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 1993; 366:469-72; PMID:8247156; http://dx.doi.org/ 10.1038/366469a0 [DOI] [PubMed] [Google Scholar]
- 21.Wheeler YY, Chen SY, Sane DC. Intrabody and intrakine strategies for molecular therapy. Mol Ther 2003; 8:355-66; PMID:12946308; http://dx.doi.org/ 10.1016/S1525-0016(03)00183-7 [DOI] [PubMed] [Google Scholar]
- 22.Freund G, Sibler AP, Desplancq D, Oulad-Abdelghani M, Vigneron M, Gannon J, Van Regenmortel MH, Weiss E. Targeting endogenous nuclear antigens by electrotransfer of monoclonal antibodies in living cells. mAbs 2013; 5:518-22; PMID:23765067; http://dx.doi.org/ 10.4161/mabs.25084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marschall AL, Frenzel A, Schirrmann T, Schüngel M, Dübel S. Targeting antibodies to the cytoplasm. mAbs 2011; 3:3-16; PMID:21099369; http://dx.doi.org/ 10.4161/mabs.3.1.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marschall AL, Zhang C, Frenzel A, Schirrmann T, Hust M, Perez F, Dübel S. Delivery of antibodies to the cytosol: debunking the myths. mAbs 2014; 6:943-56; PMID:24848507; http://dx.doi.org/ 10.4161/mabs.29268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biocca S, Ruberti F, Tafani M, Pierandrei-Amaldi P, Cattaneo A. Redox state of single chain Fv fragments targeted to the endoplasmic reticulum, cytosol and mitochondria. Biotechnology (N Y) 1995; 13:1110-5; PMID:9636285; http://dx.doi.org/ 10.1038/nbt1095-1110 [DOI] [PubMed] [Google Scholar]
- 26.Wörn A, Plückthun A. Stability engineering of antibody single-chain Fv fragments. J Mol Biol 2001; 305:989-1010; PMID:11162109; http://dx.doi.org/ 10.1006/jmbi.2000.4265 [DOI] [PubMed] [Google Scholar]
- 27.Vascotto F, Campagna M, Visintin M, Cattaneo A, Burrone OR. Effects of intrabodies specific for rotavirus NSP5 during the virus replicative cycle. J Gen Virol 2004; 85:3285-90; PMID:15483242; http://dx.doi.org/ 10.1099/vir.0.80075-0 [DOI] [PubMed] [Google Scholar]
- 28.Dong J, Thompson AA, Fan Y, Lou J, Conrad F, Ho M, Pires-Alves M, Wilson BA, Stevens RC, Marks JD. A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic α-exosite binding region. J Mol Biol 2010; 397:1106-18; PMID:20138889; http://dx.doi.org/ 10.1016/j.jmb.2010.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 2010; 56:990-8; PMID:20637220; http://dx.doi.org/ 10.1016/j.toxicon.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueorguieva D, Li S, Walsh N, Mukerji A, Tanha J, Pandey S. Identification of single-domain, Bax-specific intrabodies that confer resistance to mammalian cells against oxidative-stress-induced apoptosis. FASEB J 2006; 20:2636-8; PMID:17060401; http://dx.doi.org/ 10.1096/fj.06-6306fje [DOI] [PubMed] [Google Scholar]
- 31.Kaiser PD, Maier J, Traenkle B, Emele F, Rothbauer U. Recent progress in generating intracellular functional antibody fragments to target and trace cellular components in living cells. Biochim Biophys Acta 2014; 1844:1933-42; PMID:24792387; http://dx.doi.org/ 10.1016/j.bbapap.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi AS, Freund G, Desplancq D, Sibler AP, Baltzinger M, Rochel N, Mely Y, Didier P, Weiss E. The use of fluorescent intrabodies to detect endogenous gankyrin in living cancer cells. Exp Cell Res 2013; 319:838-49; PMID:23353833; http://dx.doi.org/ 10.1016/j.yexcr.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 33.Visintin M, Settanni G, Maritan A, Graziosi S, Marks JD, Cattaneo A. The intracellular antibody capture technology (IACT): towards a consensus sequence for intracellular antibodies. J Mol Biol 2002; 317:73-83; PMID:11916379; http://dx.doi.org/ 10.1006/jmbi.2002.5392 [DOI] [PubMed] [Google Scholar]
- 34.Christ D, Famm K, Winter G. Repertoires of aggregation-resistant human antibody domains. Protein Eng Des Sel 2007; 20:413-6; http://dx.doi.org/ 10.1093/protein/gzm037 [DOI] [PubMed] [Google Scholar]
- 35.Kim DY, Hussack G, Kandalaft H, Tanha J. Mutational approaches to improve the biophysical properties of human single-domain antibodies. Biochim Biophys Acta 2014; 1844:1983-2001; PMID:25065345; http://dx.doi.org/ 10.1016/j.bbapap.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 36.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 2013; 82:775-97; PMID:23495938; http://dx.doi.org/ 10.1146/annurev-biochem-063011-092449 [DOI] [PubMed] [Google Scholar]
- 37.Cassimeris L, Guglielmi L, Denis V, Larroque C, Martineau P. Specific in vivo labeling of tyrosinated α-tubulin and measurement of microtubule dynamics using a GFP tagged, cytoplasmically expressed recombinant antibody. PloS one 2013; 8:e59812; PMID:23555790; http://dx.doi.org/ 10.1371/journal.pone.0059812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 2012; 4:1015-28; PMID:22837174; http://dx.doi.org/ 10.1002/emmm.201201379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guglielmi L, Denis V, Vezzio-Vie N, Bec N, Dariavach P, Larroque C, Martineau P. Selection for intrabody solubility in mammalian cells using GFP fusions. Protein Eng Des Sel 2011; 24:873-81; http://dx.doi.org/ 10.1093/protein/gzr049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 2006; 24:79-88; PMID:16369541; http://dx.doi.org/ 10.1038/nbt1172 [DOI] [PubMed] [Google Scholar]
- 41.Shaki-Loewenstein S, Zfania R, Hyland S, Wels WS, Benhar I. A universal strategy for stable intracellular antibodies. J Immunol Methods 2005; 303:19-39; PMID:16045924; http://dx.doi.org/ 10.1016/j.jim.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 42.Strube RW, Chen SY. Enhanced intracellular stability of sFv-Fc fusion intrabodies. Methods 2004; 34:179-83; PMID:15312671; http://dx.doi.org/ 10.1016/j.ymeth.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 43.Donini M, Morea V, Desiderio A, Pashkoulov D, Villani ME, Tramontano A, Benvenuto E. Engineering stable cytoplasmic intrabodies with designed specificity. J Mol Biol 2003; 330:323-32; PMID:12823971; http://dx.doi.org/ 10.1016/S0022-2836(03)00530-8 [DOI] [PubMed] [Google Scholar]
- 44.Mandrup OA, Friis NA, Lykkemark S, Just J, Kristensen P. A novel heavy domain antibody library with functionally optimized complementarity determining regions. PloS One 2013; 8:e76834; PMID:24116173; http://dx.doi.org/ 10.1371/journal.pone.0076834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philibert P, Stoessel A, Wang W, Sibler AP, Bec N, Larroque C, Saven JG, Courtete J, Weiss E, Martineau P. A focused antibody library for selecting scFvs expressed at high levels in the cytoplasm. BMC Biotechnol 2007; 7:81; PMID:18034894; http://dx.doi.org/ 10.1186/1472-6750-7-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wörn A, Auf der Maur A, Escher D, Honegger A, Barberis A, Plückthun A. Correlation between in vitro stability and in vivo performance of anti-GCN4 intrabodies as cytoplasmic inhibitors. J Biol Chem 2000; 275:2795-803; PMID:10644744; http://dx.doi.org/ 10.1074/jbc.275.4.2795 [DOI] [PubMed] [Google Scholar]
- 47.Proba K, Wörn A, Honegger A, Plückthun A. Antibody scFv fragments without disulfide bonds made by molecular evolution. J Mol Biol 1998; 275:245-53; PMID:9466907; http://dx.doi.org/ 10.1006/jmbi.1997.1457 [DOI] [PubMed] [Google Scholar]
- 48.Kakimoto S, Tanabe T, Azuma H, Nagasaki T. Enhanced internalization and endosomal escape of dual-functionalized poly(ethyleneimine)s polyplex with diphtheria toxin T and R domains. Biomed Pharmacother 2010; 64:296-301; PMID:20347568; http://dx.doi.org/ 10.1016/j.biopha.2009.06.017 [DOI] [PubMed] [Google Scholar]
- 49.Qian Z, LaRochelle JR, Jiang B, Lian W, Hard RL, Selner NG, Luechapanichkul R, Barrios AM, Pei D. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry 2014; 53:4034-46; PMID:24896852; http://dx.doi.org/ 10.1021/bi5004102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol 2005; 40:191-228; PMID:16126486; http://dx.doi.org/ 10.1080/10409230591008161 [DOI] [PubMed] [Google Scholar]
- 51.Hardwick KG, Lewis MJ, Semenza J, Dean N, Pelham HR. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J 1990; 9:623-30; PMID:2178921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 1992; 68:353-64; PMID:1310258; http://dx.doi.org/ 10.1016/0092-8674(92)90476-S [DOI] [PubMed] [Google Scholar]
- 53.Donoso G, Herzog V, Schmitz A. Misfolded BiP is degraded by a proteasome-independent endoplasmic-reticulum-associated degradation pathway. Biochem J 2005; 387:897-903; PMID:15610068; http://dx.doi.org/ 10.1042/BJ20041312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol 2005; 7:766-72; PMID:16056268; http://dx.doi.org/ 10.1038/ncb0805-766 [DOI] [PubMed] [Google Scholar]
- 55.Schmitz A, Schneider A, Kummer MP, Herzog V. Endoplasmic reticulum-localized amyloid β-peptide is degraded in the cytosol by two distinct degradation pathways. Traffic 2004; 5:89-101; PMID:14690498; http://dx.doi.org/ 10.1111/j.1600-0854.2004.00159.x [DOI] [PubMed] [Google Scholar]
- 56.Böldicke T, Somplatzki S, Sergeev G, Mueller PP. Functional inhibition of transitory proteins by intrabody-mediated retention in the endoplasmatic reticulum. Methods 2012; 56:338-50; PMID:22037249; http://dx.doi.org/ 10.1016/j.ymeth.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 57.Dübel S, Breitling F, Fuchs P, Zewe M, Gotter S, Welschof M, Moldenhauer G, Little M. Isolation of IgG antibody Fv-DNA from various mouse and rat hybridoma cell lines using the polymerase chain reaction with a simple set of primers. J Immunol Methods 1994; 175:89-95; PMID:7930642; http://dx.doi.org/ 10.1016/0022-1759(94)90334-4 [DOI] [PubMed] [Google Scholar]
- 58.Hust M, Frenzel A, Tomszak F, Kügler J, Dübel S. Antibody Phage Display Handbook of Therapeutic Antibodies, 2nd ed. Weinheim, Germany: Wiley-VCH; 2014; 43-76. [Google Scholar]
- 59.Breitling F, Dübel S, Seehaus T, Klewinghaus I, Little M. A surface expression vector for antibody screening. Gene 1991; 104:147-53; PMID:1916287; http://dx.doi.org/ 10.1016/0378-1119(91)90244-6 [DOI] [PubMed] [Google Scholar]
- 60.Bradbury AR, Sidhu S, Dubel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol 2011; 29:245-54; PMID:21390033; http://dx.doi.org/ 10.1038/nbt.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beerli RR, Bauer M, Buser RB, Gwerder M, Muntwiler S, Maurer P, Saudan P, Bachmann MF. Isolation of human monoclonal antibodies by mammalian cell display. Proc Natl Acad Sci U S A 2008; 105:14336-41; PMID:18812621; http://dx.doi.org/ 10.1073/pnas.0805942105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol 1997; 15:553-7; PMID:9181578; http://dx.doi.org/ 10.1038/nbt0697-553 [DOI] [PubMed] [Google Scholar]
- 63.Samuelson P, Gunneriusson E, Nygren PA, Stahl S. Display of proteins on bacteria. J Biotechnol 2002; 96:129-54; PMID:12039531; http://dx.doi.org/ 10.1016/S0168-1656(02)00043-3 [DOI] [PubMed] [Google Scholar]
- 64.Zhao XL, Chen WQ, Yang ZH, Li JM, Zhang SJ, Tian LF. Selection and affinity maturation of human antibodies against rabies virus from a scFv gene library using ribosome display. J Biotechnol 2009; 144:253-8; PMID:19818816; http://dx.doi.org/ 10.1016/j.jbiotec.2009.09.022 [DOI] [PubMed] [Google Scholar]
- 65.Colwill K, Renewable Protein Binder Working G, Graslund S. A roadmap to generate renewable protein binders to the human proteome. Nat Methods 2011; 8:551-8; PMID:21572409; http://dx.doi.org/ 10.1038/nmeth.1607 [DOI] [PubMed] [Google Scholar]
- 66.Schofield DJ, Pope AR, Clementel V, Buckell J, Chapple S, Clarke KF, Conquer JS, Crofts AM, Crowther SR, Dyson MR, et al.. Application of phage display to high throughput antibody generation and characterization. Genome Biol 2007; 8:R254; PMID:18047641; http://dx.doi.org/ 10.1186/gb-2007-8-11-r254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sidhu SS. Antibodies for all: The case for genome-wide affinity reagents. FEBS Lett 2012; 586:2778-9; PMID:22664378; http://dx.doi.org/ 10.1016/j.febslet.2012.05.044 [DOI] [PubMed] [Google Scholar]
- 68.Hust M, Frenzel A, Schirrmann T, Dübel S. Selection of recombinant antibodies from antibody gene libraries. Methods Mol Biol 2014; 1101:305-20; PMID:24233787; http://dx.doi.org/ 10.1007/978-1-62703-721-1_14 [DOI] [PubMed] [Google Scholar]
- 69.Mersmann M, Meier D, Mersmann J, Helmsing S, Nilsson P, Gräslund S, Colwill K, Hust M, Dübel S. Towards proteome scale antibody selections using phage display. N Biotechnol 2010; 27:118-28; PMID:19883803; http://dx.doi.org/ 10.1016/j.nbt.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 70.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol 2005; 23:1105-16; PMID:16151404; http://dx.doi.org/ 10.1038/nbt1126 [DOI] [PubMed] [Google Scholar]
- 71.Hust M, Frenzel A, Meyer T, Schirrmann T, Dübel S. Construction of human naive antibody gene libraries. Methods Mol Biol 2012; 907:85-107; PMID:22907347; http://dx.doi.org/ 10.1007/978-1-61779-974-7_5 [DOI] [PubMed] [Google Scholar]
- 72.Hayashi N, Welschof M, Zewe M, Braunagel M, Dübel S, Breitling F, Little M. Simultaneous mutagenesis of antibody CDR regions by overlap extension and PCR. Biotechniques 1994; 17:310, 2, 4-5. [PubMed] [Google Scholar]
- 73.Rothe C, Urlinger S, Lohning C, Prassler J, Stark Y, Jager U, Hubner B, Bardroff M, Pradel I, Boss M, et al.. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol 2008; 376:1182-200; PMID:18191144; http://dx.doi.org/ 10.1016/j.jmb.2007.12.018 [DOI] [PubMed] [Google Scholar]
- 74.Hoet RM, Cohen EH, Kent RB, Rookey K, Schoonbroodt S, Hogan S, Rem L, Frans N, Daukandt M, Pieters H, et al.. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol 2005; 23:344-8; PMID:15723048; http://dx.doi.org/ 10.1038/nbt1067 [DOI] [PubMed] [Google Scholar]
- 75.Zhang C, Helmsing S, Zagrebelsky M, Schirrmann T, Marschall AL, Schüngel M, Korte M, Hust M, Dübel S. Suppression of p75 neurotrophin receptor surface expression with intrabodies influences Bcl-xL mRNA expression and neurite outgrowth in PC12 cells. PloS One 2012; 7:e30684; PMID:22292018; http://dx.doi.org/ 10.1371/journal.pone.0030684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nam CH, Moutel S, Teillaud JL. Generation of murine scFv intrabodies from B-cell hybridomas. Methods Mol Biol 2002; 193:301-27; PMID:12325520 [DOI] [PubMed] [Google Scholar]
- 77.Pope AR, Embleton MJ, Mernaugh R. Construction and use of antibody gene repertoires In: Mc Cafferty J, Hoogenboom HR, Chiswell DJ (Eds.), Antibody engineering: A practical approach. New York, NY: Oxford University Press; 1996. p. 1–40. [Google Scholar]
- 78.Toleikis L, Frenzel A. Cloning single-chain antibody fragments (ScFv) from hyrbidoma cells. Methods Mol Biol 2012; 907:59-71; PMID:22907345; http://dx.doi.org/ 10.1007/978-1-61779-974-7_3 [DOI] [PubMed] [Google Scholar]
- 79.Ruberti F, Cattaneo A, Bradbury A. The use of the RACE method to clone hybridoma cDNA when V region primers fail. J Immunol Methods 1994; 173:33-9; PMID:8034983; http://dx.doi.org/ 10.1016/0022-1759(94)90280-1 [DOI] [PubMed] [Google Scholar]
- 80.Ladiges W, Osman GE. Molecular characterization of immunoglobulin genes In: Howard GC, Bethell DR (Eds.), Basic Methods in Antibody Production and Characterization. Boca Raton, FL: CRC Press Ltd; 2000:169-91. [Google Scholar]
- 81.Persic L, Righi M, Roberts A, Hoogenboom HR, Cattaneo A, Bradbury A. Targeting vectors for intracellular immunisation. Gene 1997; 187:1-8; PMID:9073060; http://dx.doi.org/ 10.1016/S0378-1119(96)00627-0 [DOI] [PubMed] [Google Scholar]
- 82.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotny J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, et al.. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A 1988; 85:5879-83; PMID:3045807; http://dx.doi.org/ 10.1073/pnas.85.16.5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levin R, Mhashilkar AM, Dorfman T, Bukovsky A, Zani C, Bagley J, Hinkula J, Niedrig M, Albert J, Wahren B, et al.. Inhibition of early and late events of the HIV-1 replication cycle by cytoplasmic Fab intrabodies against the matrix protein, p17. Mol Med 1997; 3:96-110; PMID:9085253 [PMC free article] [PubMed] [Google Scholar]
- 84.Reinman M, Jantti J, Alfthan K, Keranen S, Soderlund H, Takkinen K. Functional inactivation of the conserved Sem1p in yeast by intrabodies. Yeast 2003; 20:1071-84; PMID:12961755; http://dx.doi.org/ 10.1002/yea.1027 [DOI] [PubMed] [Google Scholar]
- 85.Hust M, Jostock T, Menzel C, Voedisch B, Mohr A, Brenneis M, Kirsch MI, Meier D, Dübel S. Single chain Fab (scFab) fragment. BMC Biotechnol 2007; 7:14; PMID:17346344; http://dx.doi.org/ 10.1186/1472-6750-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kontermann RE. Dual targeting strategies with bispecific antibodies. mAbs 2012; 4:182-97; PMID:22453100; http://dx.doi.org/ 10.4161/mabs.4.2.19000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jendreyko N, Popkov M, Beerli RR, Chung J, McGavern DB, Rader C, Barbas CF 3rd. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J Biol Chem 2003; 278:47812-9; PMID:12947084; http://dx.doi.org/ 10.1074/jbc.M307002200 [DOI] [PubMed] [Google Scholar]
- 88.Jendreyko N, Popkov M, Rader C, Barbas CF 3rd. Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc Natl Acad Sci U S A 2005; 102:8293-8; PMID:15928093; http://dx.doi.org/ 10.1073/pnas.0503168102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller N, Hartmann C, Genssler S, Koch J, Kinner A, Grez M, Wels WS. A bispecific transmembrane antibody simultaneously targeting intra- and extracellular epitopes of the epidermal growth factor receptor inhibits receptor activation and tumor cell growth. Int J Cancer 2014; 134:2547-59; PMID:24243620; http://dx.doi.org/ 10.1002/ijc.28585 [DOI] [PubMed] [Google Scholar]
- 90.Kim DS, Song HN, Nam HJ, Kim SG, Park YS, Park JC, Woo EJ, Lim HK. Directed evolution of human heavy chain variable domain (VH) using in vivo protein fitness filter. PloS One 2014; 9:e98178; PMID:24892548; http://dx.doi.org/ 10.1371/journal.pone.0098178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim DY, To R, Kandalaft H, Ding W, van Faassen H, Luo Y, Schrag JD, St-Amant N, Hefford M, Hirama T, et al.. Antibody light chain variable domains and their biophysically improved versions for human immunotherapy. mAbs 2014; 6:219-35; PMID:24423624; http://dx.doi.org/ 10.4161/mabs.26844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biocca S, Pierandrei-Amaldi P, Cattaneo A. Intracellular expression of anti-p21ras single chain Fv fragments inhibits meiotic maturation of xenopus oocytes. Biochem Biophys Res Commun 1993; 197:422-7; PMID:8267576; http://dx.doi.org/ 10.1006/bbrc.1993.2496 [DOI] [PubMed] [Google Scholar]
- 93.Burke B, Warren G. Microinjection of mRNA coding for an anti-Golgi antibody inhibits intracellular transport of a viral membrane protein. Cell 1984; 36:847-56; PMID:6323023; http://dx.doi.org/ 10.1016/0092-8674(84)90034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valle G, Jones EA, Colman A. Anti-ovalbumin monoclonal antibodies interact with their antigen in internal membranes of Xenopus oocytes. Nature 1982; 300:71-4; PMID:7133132; http://dx.doi.org/ 10.1038/300071a0 [DOI] [PubMed] [Google Scholar]
- 95.Kvam E, Nannenga BL, Wang MS, Jia Z, Sierks MR, Messer A. Conformational targeting of fibrillar polyglutamine proteins in live cells escalates aggregation and cytotoxicity. PloS one 2009; 4:e5727; PMID:19492089; http://dx.doi.org/ 10.1371/journal.pone.0005727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paoletti F, Malerba F, Konarev PV, Visintin M, Scardigli R, Fasulo L, Lamba D, Svergun DI, Cattaneo A. Direct intracellular selection and biochemical characterization of a recombinant anti-proNGF single chain antibody fragment. Arch Biochem Biophys 2012; 522:26-36; PMID:22516657; http://dx.doi.org/ 10.1016/j.abb.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 97.Zacchi P, Dreosti E, Visintin M, Moretto-Zita M, Marchionni I, Cannistraci I, Kasap Z, Betz H, Cattaneo A, Cherubini E. Gephyrin selective intrabodies as a new strategy for studying inhibitory receptor clustering. J Mol Neurosci 2008; 34:141-8; PMID:18008186; http://dx.doi.org/ 10.1007/s12031-007-9018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dauvillier S, Merida P, Visintin M, Cattaneo A, Bonnerot C, Dariavach P. Intracellular single-chain variable fragments directed to the Src homology 2 domains of Syk partially inhibit Fc epsilon RI signaling in the RBL-2H3 cell line. J Immunol 2002; 169:2274-83; http://dx.doi.org/ 10.4049/jimmunol.169.5.2274 [DOI] [PubMed] [Google Scholar]
- 99.Tse E, Rabbitts TH. Intracellular antibody-caspase-mediated cell killing: an approach for application in cancer therapy. Proc Natl Acad Sci U S A 2000; 97:12266-71; PMID:11050246; http://dx.doi.org/ 10.1073/pnas.97.22.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Staus DP, Wingler LM, Strachan RT, Rasmussen SG, Pardon E, Ahn S, Steyaert J, Kobilka BK, Lefkowitz RJ. Regulation of beta2-adrenergic receptor function by conformationally selective single-domain intrabodies. Mol Pharmacol 2014; 85:472-81; PMID:24319111; http://dx.doi.org/ 10.1124/mol.113.089516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiusaroli R, Visentini M, Galimberti C, Casseler C, Mennuni L, Covaceuszach S, Lanza M, Ugolini G, Caselli G, Rovati LC, et al.. Targeting of ADAMTS5s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage 2013; 21:1807-10; PMID:23954517; http://dx.doi.org/ 10.1016/j.joca.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Sun L, Yu P, Li A, Li C, Tang Q, Li D, Liang M. Viral suppression function of intracellular antibody against C-terminal domain of rabies virus phosphoprotein. Acta Biochim Biophys Sin 2015; http://dx.doi.org/ 10.1093/abbs/gmv060 [DOI] [PubMed] [Google Scholar]
- 103.Auf der Maur A, Tissot K, Barberis A. Antigen-independent selection of intracellular stable antibody frameworks. Methods 2004; 34:215-24; PMID:15312674; http://dx.doi.org/ 10.1016/j.ymeth.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 104.Visintin M, Meli GA, Cannistraci I, Cattaneo A. Intracellular antibodies for proteomics. J Immunol Methods 2004; 290:135-53; PMID:15261577; http://dx.doi.org/ 10.1016/j.jim.2004.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Visintin M, Melchionna T, Cannistraci I, Cattaneo A. In vivo selection of intrabodies specifically targeting protein-protein interactions: a general platform for an “undruggable” class of disease targets. J Biotechnol 2008; 135:1-15; PMID:18395925; http://dx.doi.org/ 10.1016/j.jbiotec.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 106.Fisher AC, DeLisa MP. Efficient isolation of soluble intracellular single-chain antibodies using the twin-arginine translocation machinery. J Mol Biol 2009; 385:299-311; PMID:18992254; http://dx.doi.org/ 10.1016/j.jmb.2008.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karlsson AJ, Lim HK, Xu H, Rocco MA, Bratkowski MA, Ke A, DeLisa MP. Engineering antibody fitness and function using membrane-anchored display of correctly folded proteins. J Mol Biol 2012; 416:94-107; PMID:22197376; http://dx.doi.org/ 10.1016/j.jmb.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waraho D, DeLisa MP. Versatile selection technology for intracellular protein-protein interactions mediated by a unique bacterial hitchhiker transport mechanism. Proc Natl Acad Sci U S A 2009; 106:3692-7; PMID:19234130; http://dx.doi.org/ 10.1073/pnas.0704048106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Waraho-Zhmayev D, Meksiriporn B, Portnoff AD, DeLisa M. Optimizing recombinant antibodies for intracellular function using hitchhiker-mediated survival selection. Protein Eng Des Sel 2014; 27: 351-358; PMID:25225416; http://dx.doi.org/ 10.1093/protein/gzu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sacchetti A, Cappetti V, Marra P, Dell'Arciprete R, El Sewedy T, Crescenzi C, Alberti S. Green fluorescent protein variants fold differentially in prokaryotic and eukaryotic cells. J Cell Biochem Suppl 2001; Suppl 36:117-28; PMID:11455577; http://dx.doi.org/ 10.1002/jcb.1091 [DOI] [PubMed] [Google Scholar]
- 111.Cohen PA, Mani JC, Lane DP. Characterization of a new intrabody directed against the N-terminal region of human p53. Oncogene 1998; 17:2445-56; PMID:9824155; http://dx.doi.org/ 10.1038/sj.onc.1202190 [DOI] [PubMed] [Google Scholar]
- 112.Jannot CB, Hynes NE. Characterization of scFv-421, a single-chain antibody targeted to p53. Biochem Biophys Res Commun 1997; 230:242-6; PMID:9016757; http://dx.doi.org/ 10.1006/bbrc.1996.5930 [DOI] [PubMed] [Google Scholar]
- 113.Zheng L, Baumann U, Reymond JL. Production of a functional catalytic antibody ScFv-NusA fusion protein in bacterial cytoplasm. J Biochem 2003; 133:577-81; PMID:12801908; http://dx.doi.org/ 10.1093/jb/mvg074 [DOI] [PubMed] [Google Scholar]
- 114.Caron de Fromentel C, Gruel N, Venot C, Debussche L, Conseiller E, Dureuil C, Teillaud JL, Tocque B, Bracco L. Restoration of transcriptional activity of p53 mutants in human tumour cells by intracellular expression of anti-p53 single chain Fv fragments. Oncogene 1999; 18:551-7; PMID:9927212; http://dx.doi.org/ 10.1038/sj.onc.1202338 [DOI] [PubMed] [Google Scholar]
- 115.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 1986; 234:364-8; PMID:2876518; http://dx.doi.org/ 10.1126/science.2876518 [DOI] [PubMed] [Google Scholar]
- 116.Joshi SN, Butler DC, Messer A. Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti-synuclein intrabodies. mAbs 2012; 4:686-93; PMID:22929188; http://dx.doi.org/ 10.4161/mabs.21696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Butler DC, Messer A. Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PloS One 2011; 6:e29199; PMID:22216210; http://dx.doi.org/ 10.1371/journal.pone.0029199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raran-Kurussi S, Waugh DS. The ability to enhance the solubility of its fusion partners is an intrinsic property of maltose-binding protein but their folding is either spontaneous or chaperone-mediated. PloS One 2012; 7:e49589; PMID:23166722; http://dx.doi.org/ 10.1371/journal.pone.0049589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nallamsetty S, Waugh DS. Mutations that alter the equilibrium between open and closed conformations of Escherichia coli maltose-binding protein impede its ability to enhance the solubility of passenger proteins. Biochem Biophys Res Commun 2007; 364:639-44; PMID:17964542; http://dx.doi.org/ 10.1016/j.bbrc.2007.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986; 321:522-5; PMID:3713831; http://dx.doi.org/ 10.1038/321522a0 [DOI] [PubMed] [Google Scholar]
- 121.Mazuc E, Guglielmi L, Bec N, Parez V, Hahn CS, Mollevi C, Parrinello H, Desvignes JP, Larroque C, Jupp R, et al.. In-cell intrabody selection from a diverse human library identifies C12orf4 protein as a new player in rodent mast cell degranulation. PloS One 2014; 9:e104998; PMID:25122211; http://dx.doi.org/ 10.1371/journal.pone.0104998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doyle PJ, Saeed H, Hermans A, Gleddie SC, Hussack G, Arbabi-Ghahroudi M, Seguin C, Savard ME, Mackenzie CR, Hall JC. Intracellular expression of a single domain antibody reduces cytotoxicity of 15-acetyldeoxynivalenol in yeast. J Biol Chem 2009; 284:35029-39; PMID:19783651; http://dx.doi.org/ 10.1074/jbc.M109.045047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGonigal K, Tanha J, Palazov E, Li S, Gueorguieva-Owens D, Pandey S. Isolation and functional characterization of single domain antibody modulators of Caspase-3 and apoptosis. Appl Biochem Biotechnol 2009; 157:226-36; PMID:18553063; http://dx.doi.org/ 10.1007/s12010-008-8266-4 [DOI] [PubMed] [Google Scholar]
- 124.Verheesen P, de Kluijver A, van Koningsbruggen S, de Brij M, de Haard HJ, van Ommen GJ, van der Maarel SM, Verrips CT. Prevention of oculopharyngeal muscular dystrophy-associated aggregation of nuclear polyA-binding protein with a single-domain intracellular antibody. Hum Mol Genet 2006; 15:105-11; PMID:16319127; http://dx.doi.org/ 10.1093/hmg/ddi432 [DOI] [PubMed] [Google Scholar]
- 125.Newnham LE, Wright MJ, Holdsworth G, Kostarelos K, Robinson MK, Rabbitts TH, Lawson AD. Functional inhibition of β-catenin-mediatedWnt signaling by intracellular VHHantibodies. mAbs 2015; 7:180-91; PMID:25524068; http://dx.doi.org/ 10.4161/19420862.2015.989023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jespers L, Schon O, Famm K, Winter G. Aggregation-resistant domain antibodies selected on phage by heat denaturation. Nat Biotechnol 2004; 22:1161-5; PMID:15300256; http://dx.doi.org/ 10.1038/nbt1000 [DOI] [PubMed] [Google Scholar]
- 127.Barthelemy PA, Raab H, Appleton BA, Bond CJ, Wu P, Wiesmann C, Sidhu SS. Comprehensive analysis of the factors contributing to the stability and solubility of autonomous human VH domains. J Biol Chem 2008; 283:3639-54; PMID:18045863; http://dx.doi.org/ 10.1074/jbc.M708536200 [DOI] [PubMed] [Google Scholar]
- 128.Yasui H, Ito W, Kurosawa Y. Effects of substitutions of amino acids on the thermal stability of the Fv fragments of antibodies. FEBS Lett 1994; 353:143-6; PMID:7926039; http://dx.doi.org/ 10.1016/0014-5793(94)01027-7 [DOI] [PubMed] [Google Scholar]
- 129.Jager M, Plückthun A. Folding and assembly of an antibody Fv fragment, a heterodimer stabilized by antigen. J Mol Biol 1999; 285:2005-19; PMID:9925781; http://dx.doi.org/ 10.1006/jmbi.1998.2425 [DOI] [PubMed] [Google Scholar]
- 130.Dübel S, Stoevesandt O, Taussig MJ, Hust M. Generating recombinant antibodies to the complete human proteome. Trends Biotechnol 2010; 28:333-9; PMID:20538360; http://dx.doi.org/ 10.1016/j.tibtech.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 131.Popkov M, Jendreyko N, McGavern DB, Rader C, Barbas CF 3rd. Targeting tumor angiogenesis with adenovirus-delivered anti-Tie-2 intrabody. Cancer Res 2005; 65:972-81; PMID:15705898 [PubMed] [Google Scholar]
- 132.Sudol KL, Mastrangelo MA, Narrow WC, Frazer ME, Levites YR, Golde TE, Federoff HJ, Bowers WJ. Generating differentially targeted amyloid-β specific intrabodies as a passive vaccination strategy for Alzheimer disease. Mol Ther 2009; 17:2031-40; PMID:19638957; http://dx.doi.org/ 10.1038/mt.2009.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gahrtz M, Conrad U. Immunomodulation of plant function by in vitro selected single-chain Fv intrabodies. Methods Mol Biol 2009; 483:289-312; PMID:19183906; http://dx.doi.org/ 10.1007/978-1-59745-407-0_17 [DOI] [PubMed] [Google Scholar]
- 134.Marschall AL, Single FN, Schlarmann K, Bosio A, Strebe N, van den Heuvel J, Frenzel A, Dübel S. Functional knock down of VCAM1 in mice mediated by endoplasmatic reticulum retained intrabodies. mAbs 2014; 6:1394-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet 2011; 12:316-28; PMID:21468099; http://dx.doi.org/ 10.1038/nrg2971 [DOI] [PubMed] [Google Scholar]
- 136.Knight S, Collins M, Takeuchi Y. Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr Gene Ther 2013; 13:211-27; PMID:23590635; http://dx.doi.org/ 10.2174/1566523211313030006 [DOI] [PubMed] [Google Scholar]
- 137.Jin L, Zeng X, Liu M, Deng Y, He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics 2014; 4:240-55; PMID:24505233; http://dx.doi.org/ 10.7150/thno.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papayannakos C, Daniel R. Understanding lentiviral vector chromatin targeting: working to reduce insertional mutagenic potential for gene therapy. Gene Ther 2013; 20:581-8; PMID:23171920; http://dx.doi.org/ 10.1038/gt.2012.88 [DOI] [PubMed] [Google Scholar]
- 139.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet 2007; 8:573-87; PMID:17607305; http://dx.doi.org/ 10.1038/nrg2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kallen KJ, Thess A. A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide-based vaccines and drugs. Ther Adv Vaccines 2014; 2:10-31; PMID:24757523; http://dx.doi.org/ 10.1177/2051013613508729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beerli RR, Wels W, Hynes NE. Inhibition of signaling from Type 1 receptor tyrosine kinases via intracellular expression of single-chain antibodies. Breast Cancer Res Treat 1996; 38:11-7; PMID:8825118; http://dx.doi.org/ 10.1007/BF01803779 [DOI] [PubMed] [Google Scholar]
- 142.Harwerth IM, Wels W, Schlegel J, Muller M, Hynes NE. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. British J Cancer 1993; 68:1140-5; PMID:7903153; http://dx.doi.org/ 10.1038/bjc.1993.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med 1983; 1:511-29; PMID:6094961 [PubMed] [Google Scholar]
- 144.Wels W, Beerli R, Hellmann P, Schmidt M, Marte BM, Kornilova ES, Hekele A, Mendelsohn J, Groner B, Hynes NE. EGF receptor and p185erbB-2-specific single-chain antibody toxins differ in their cell-killing activity on tumor cells expressing both receptor proteins. Int J Cancer 1995; 60:137-44; PMID:7814146; http://dx.doi.org/ 10.1002/ijc.2910600120 [DOI] [PubMed] [Google Scholar]
- 145.Arafat W, Gomez-Navarro J, Xiang J, Siegal GP, Alvarez RD, Curiel DT. Antineoplastic effect of anti-erbB-2 intrabody is not correlated with scFv affinity for its target. Cancer Gene Ther 2000; 7:1250-6; PMID:11023197; http://dx.doi.org/ 10.1038/sj.cgt.7700228 [DOI] [PubMed] [Google Scholar]
- 146.Grim JE, Siegal GP, Alvarez RD, Curiel DT. Intracellular expression of the anti-erbB-2 sFv N29 fails to accomplish efficient target modulation. Bioch Biophys Res Commun 1998; 250:699-703; PMID:9784409; http://dx.doi.org/ 10.1006/bbrc.1998.9391 [DOI] [PubMed] [Google Scholar]
- 147.Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol 1995; 15:1182-91; PMID:7532277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Richardson JH, Sodroski JG, Waldmann TA, Marasco WA. Phenotypic knockout of the high-affinity human interleukin 2 receptor by intracellular single-chain antibodies against the α subunit of the receptor. Proc Natl Acad Sci U S A 1995; 92:3137-41; PMID:7724529; http://dx.doi.org/ 10.1073/pnas.92.8.3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Strebe N, Guse A, Schüngel M, Schirrmann T, Hafner M, Jostock T, Hust M, Müller W, Dübel S. Functional knockdown of VCAM-1 at the posttranslational level with ER retained antibodies. J Immunol Methods 2009; 341:30-40; PMID:19038261; http://dx.doi.org/ 10.1016/j.jim.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 150.Beerli RR, Wels W, Hynes NE. Intracellular expression of single chain antibodies reverts ErbB-2 transformation. J Biol Chem 1994; 269:23931-6; PMID:7929040 [PubMed] [Google Scholar]
- 151.Richardson JH, Waldmann TA, Sodroski JG, Marasco WA. Inducible knockout of the interleukin-2 receptor α chain: expression of the high-affinity IL-2 receptor is not required for the in vitro growth of HTLV-I-transformed cell lines. Virology 1997; 237:209-16; PMID:9356333; http://dx.doi.org/ 10.1006/viro.1997.8779 [DOI] [PubMed] [Google Scholar]
- 152.Yuan Q, Strauch KL, Lobb RR, Hemler ME. Intracellular single-chain antibody inhibits integrin VLA-4 maturation and function. Biochem J 1996; 318 ( Pt 2):591-6; PMID:8809051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Böldicke T, Weber H, Mueller PP, Barleon B, Bernal M. Novel highly efficient intrabody mediates complete inhibition of cell surface expression of the human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR). J Immunol Methods 2005; 300:146-59; PMID:15946674; http://dx.doi.org/ 10.1016/j.jim.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 154.Paganetti P, Calanca V, Galli C, Stefani M, Molinari M. β-site specific intrabodies to decrease and prevent generation of Alzheimer Abeta peptide. J Cell Biol 2005; 168:863-8; PMID:15767460; http://dx.doi.org/ 10.1083/jcb.200410047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beerli RR, Wels W, Hynes NE. Autocrine inhibition of the epidermal growth factor receptor by intracellular expression of a single-chain antibody. Biochem Biophys Res Commun 1994; 204:666-72; PMID:7980527; http://dx.doi.org/ 10.1006/bbrc.1994.2511 [DOI] [PubMed] [Google Scholar]
- 156.Pelham HR. The dynamic organisation of the secretory pathway. Cell Struct Funct 1996; 21:413-9; PMID:9118249; http://dx.doi.org/ 10.1247/csf.21.413 [DOI] [PubMed] [Google Scholar]
- 157.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron 2005; 46:13-21; PMID:15820690; http://dx.doi.org/ 10.1016/j.neuron.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 158.Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, Gonfloni S, Rossi G, Berardi N, Cattaneo A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J Neurosci 2000; 20:2589-601; PMID:10729339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Capsoni S, Tiveron C, Vignone D, Amato G, Cattaneo A. Dissecting the involvement of tropomyosin-related kinase A and p75 neurotrophin receptor signaling in NGF deficit-induced neurodegeneration. Proc Natl Acad Sci U S A 2010; 107:12299-304; PMID:20566851; http://dx.doi.org/ 10.1073/pnas.1007181107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shakespeare WC. SH2 domain inhibition: a problem solved? Curr Opin Chem Biol 2001; 5:409-15; PMID:11470604; http://dx.doi.org/ 10.1016/S1367-5931(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 161.Mandal PK, Gao F, Lu Z, Ren Z, Ramesh R, Birtwistle JS, Kaluarachchi KK, Chen X, Bast RC Jr., Liao WS, et al.. Potent and selective phosphopeptide mimetic prodrugs targeted to the Src homology 2 (SH2) domain of signal transducer and activator of transcription 3. J Med Chem 2011; 54:3549-63; PMID:21486047; http://dx.doi.org/ 10.1021/jm2000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hojjat-Farsangi M. Small-Molecule Inhibitors of the Receptor Tyrosine Kinases: Promising Tools for Targeted Cancer Therapies. Int J Mol Sci 2014; 15:13768-801; PMID:25110867; http://dx.doi.org/ 10.3390/ijms150813768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S, Kurzrock R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat Rev 2014; 40:883-91; PMID:24867380; http://dx.doi.org/ 10.1016/j.ctrv.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 164.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351:95-105; PMID:10998351; http://dx.doi.org/ 10.1042/0264-6021:3510095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Graham KL, Lee LY, Higgins JP, Steinman L, Utz PJ, Ho PP. Treatment with a toll-like receptor inhibitory GpG oligonucleotide delays and attenuates lupus nephritis in NZB/W mice. Autoimmunity 2010; 43:140-55; PMID:19845477; http://dx.doi.org/ 10.3109/08916930903229239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6:823-35; PMID:17063184; http://dx.doi.org/ 10.1038/nri1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Braden BC, Goldbaum FA, Chen BX, Kirschner AN, Wilson SR, Erlanger BF. X-ray crystal structure of an anti-Buckminsterfullerene antibody fab fragment: biomolecular recognition of C(60). Proc Natl Acad Sci U S A 2000; 97:12193-7; PMID:11035793; http://dx.doi.org/ 10.1073/pnas.210396197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sheppard D. Dominant negative mutants: tools for the study of protein function in vitro and in vivo. Am J Respir Cell Mol Biol 1994; 11:1-6; PMID:8018332; http://dx.doi.org/ 10.1165/ajrcmb.11.1.8018332 [DOI] [PubMed] [Google Scholar]
- 169.Hust M, Meyer T, Voedisch B, Rülker T, Thie H, El-Ghezal A, Kirsch MI, Schütte M, Helmsing S, Meier D, et al.. A human scFv antibody generation pipeline for proteome research. J Biotechnol 2011; 152:159-70; PMID:20883731; http://dx.doi.org/ 10.1016/j.jbiotec.2010.09.945 [DOI] [PubMed] [Google Scholar]
- 170.Geyer CR, McCafferty J, Dubel S, Bradbury AR, Sidhu SS. Recombinant antibodies and in vitro selection technologies. Methods Mol Biol 2012; 901:11-32; PMID:22723092; http://dx.doi.org/ 10.1007/978-1-61779-931-0_2 [DOI] [PubMed] [Google Scholar]
- 171.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411:494-8; PMID:11373684; http://dx.doi.org/ 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- 172.Schmidt FR. About the nature of RNA interference. Appl Microbiol Biotechnol 2005; 67:429-35; PMID:15703909; http://dx.doi.org/ 10.1007/s00253-004-1882-1 [DOI] [PubMed] [Google Scholar]
- 173.Cottingham K. Antibodypedia seeks to answer the question: “how good is that antibody?.” J Proteome Res 2008; 7:4213. [DOI] [PubMed] [Google Scholar]
- 174.Bourbeillon J, Orchard S, Benhar I, Borrebaeck C, de Daruvar A, Dubel S, Frank R, Gibson F, Gloriam D, Haslam N, et al.. Minimum information about a protein affinity reagent (MIAPAR). Nat Biotechnol 2010; 28:650-3; PMID:20622827; http://dx.doi.org/ 10.1038/nbt0710-650 [DOI] [PubMed] [Google Scholar]
- 175.Cao T, Heng BC. Intracellular antibodies (intrabodies) versus RNA interference for therapeutic applications. Ann Clin Lab Sci 2005; 35:227-9; PMID:16081577 [PubMed] [Google Scholar]
- 176.Wang QC, Nie QH, Feng ZH. RNA interference: antiviral weapon and beyond. World J Gastroenterol 2003; 9:1657-61; PMID:12918096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Montgomery MK. RNA interference: historical overview and significance. Methods Mol Biol 2004; 265:3-21; PMID:15103066 [DOI] [PubMed] [Google Scholar]
- 178.Zhou R, Rana TM. RNA-based mechanisms regulating host-virus interactions. Immunol Rev 2013; 253:97-111; PMID:23550641; http://dx.doi.org/ 10.1111/imr.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol 2003; 5:834-9; PMID:12942087; http://dx.doi.org/ 10.1038/ncb1038 [DOI] [PubMed] [Google Scholar]
- 180.Jackson AL, Linsley PS. Noise amidst the silence: off-target effects of siRNAs? Trends Genet 2004; 20:521-4; PMID:15475108; http://dx.doi.org/ 10.1016/j.tig.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 181.Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA (New York, NY) 2004; 10:12-8; http://dx.doi.org/ 10.1261/rna5160904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sledz CA, Williams BR. RNA interference and double-stranded-RNA-activated pathways. Biochem Soc Trans 2004; 32:952-6; PMID:15506933; http://dx.doi.org/ 10.1042/BST0320952 [DOI] [PubMed] [Google Scholar]
- 183.Snove O Jr., Holen T. Many commonly used siRNAs risk off-target activity. Biochem Biophys Res Commun 2004; 319:256-63; PMID:15158470; http://dx.doi.org/ 10.1016/j.bbrc.2004.04.175 [DOI] [PubMed] [Google Scholar]
- 184.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 2010; 9:57-67; PMID:20043028; http://dx.doi.org/ 10.1038/nrd3010 [DOI] [PubMed] [Google Scholar]
- 185.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 2005; 23:457-62; PMID:15778705; http://dx.doi.org/ 10.1038/nbt1081 [DOI] [PubMed] [Google Scholar]
- 186.de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther 2008; 19:125-32; PMID:18257677; http://dx.doi.org/ 10.1089/hum.2008.928 [DOI] [PubMed] [Google Scholar]
- 187.Kleinhammer A, Wurst W, Kuhn R. Constitutive and conditional RNAi transgenesis in mice. Methods 2011; 53:430-6; PMID:21184828; http://dx.doi.org/ 10.1016/j.ymeth.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 188.Paroo Z, Corey DR. Challenges for RNAi in vivo. Trends Biotechnol 2004; 22:390-4; PMID:15283982; http://dx.doi.org/ 10.1016/j.tibtech.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 189.Cejka D, Losert D, Wacheck V. Short interfering RNA (siRNA): tool or therapeutic? Clin Sci (Lond) 2006; 110:47-58; PMID:16336204; http://dx.doi.org/ 10.1042/CS20050162 [DOI] [PubMed] [Google Scholar]
- 190.Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene 2013; 525:162-9; PMID:23618815; http://dx.doi.org/ 10.1016/j.gene.2013.03.137 [DOI] [PubMed] [Google Scholar]
- 191.Dickerson JE, Zhu A, Robertson DL, Hentges KE. Defining the role of essential genes in human disease. PloS One 2011; 6:e27368; PMID:22096564; http://dx.doi.org/ 10.1371/journal.pone.0027368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li JY, Sugimura K, Boado RJ, Lee HJ, Zhang C, Duebel S, Pardridge WM. Genetically engineered brain drug delivery vectors: cloning, expression and in vivo application of an anti-transferrin receptor single chain antibody-streptavidin fusion gene and protein. Protein Eng 1999; 12:787-96; PMID:10506289; http://dx.doi.org/ 10.1093/protein/12.9.787 [DOI] [PubMed] [Google Scholar]
- 193.Blatt NB, Bill RM, Glick GD. Characterization of a unique anti-DNA hybridoma. Hybridoma 1998; 17:33-9; PMID:9523235; http://dx.doi.org/ 10.1089/hyb.1998.17.33 [DOI] [PubMed] [Google Scholar]
- 194.Bradbury A. Antibody Reproducibility challenges: The Solution lies in the sequence. Nature 2015; in press. [Google Scholar]
- 195.Toleikis L, Broders O, Dübel S. Cloning single chain antibody fragments (scFv) from hybridoma clones In Decker J, Reischl U (Eds.), Molecular Diagnosis of Infectious Diseases, 2nd ed. Totowa, NY: Humana Press Inc; 2004:447-58. [DOI] [PubMed] [Google Scholar]
- 196.Zack DJ, Wong AL, Stempniak M, Weisbart RH. Two kappa immunoglobulin light chains are secreted by an anti-DNA hybridoma: implications for isotypic exclusion. Mol Immunol 1995; 32:1345-53; PMID:8643104; http://dx.doi.org/ 10.1016/0161-5890(95)00112-3 [DOI] [PubMed] [Google Scholar]
- 197.Strebe N, Breitling F, Moosmayer D, Brocks D, Dübel S. Cloning of Variable domains from mouse hybridoma by PCR In Antibody Engineering (Vol. 1; 2nd ed.). Berlin: Springer Protocols; 2010:3-14. [Google Scholar]
- 198.Wheeler YY, Kute TE, Willingham MC, Chen SY, Sane DC. Intrabody-based strategies for inhibition of vascular endothelial growth factor receptor-2: effects on apoptosis, cell growth, and angiogenesis. FASEB J 2003; 17:1733-5; PMID:12958192 [DOI] [PubMed] [Google Scholar]
- 199.Alvarez RD, Barnes MN, Gomez-Navarro J, Wang M, Strong TV, Arafat W, Arani RB, Johnson MR, Roberts BL, Siegal GP, et al.. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin Cancer Res 2000; 6:3081-7; PMID:10955787 [PubMed] [Google Scholar]
- 200.Deshane J, Siegal GP, Wang M, Wright M, Bucy RP, Alvarez RD, Curiel DT. Transductional efficacy and safety of an intraperitoneally delivered adenovirus encoding an anti-erbB-2 intracellular single-chain antibody for ovarian cancer gene therapy. Gynecol Oncol 1997; 64:378-85; PMID:9062138; http://dx.doi.org/ 10.1006/gyno.1996.4566 [DOI] [PubMed] [Google Scholar]
- 201.Grim J, Deshane J, Siegal GP, Alvarez RD, DiFiore P, Curiel DT. The level of erbB2 expression predicts sensitivity to the cytotoxic effects of an intracellular anti-erbB2 sFv. J Mol Med 1998; 76:451-8; PMID:9625302; http://dx.doi.org/ 10.1007/s001090050237 [DOI] [PubMed] [Google Scholar]
- 202.Wright M, Grim J, Deshane J, Kim M, Strong TV, Siegal GP, Curiel DT. An intracellular anti-erbB-2 single-chain antibody is specifically cytotoxic to human breast carcinoma cells overexpressing erbB-2. Gene Ther 1997; 4:317-22; PMID:9176517; http://dx.doi.org/ 10.1038/sj.gt.3300372 [DOI] [PubMed] [Google Scholar]
- 203.Jannot CB, Beerli RR, Mason S, Gullick WJ, Hynes NE. Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene 1996; 13:275-82; PMID:8710366 [PubMed] [Google Scholar]
- 204.Wang W, Zhou J, Xu L, Zhen Y. Antineoplastic effect of intracellular expression of a single-chain antibody directed against type IV collagenase. J Environ Pathol Toxicol Oncol 2000; 19:61-8; PMID:10905509 [PubMed] [Google Scholar]
- 205.Sangboonruang S, Thammasit P, Intasai N, Kasinrerk W, Tayapiwatana C, Tragoolpua K. EMMPRIN reduction via scFv-M6-1B9 intrabody affects alpha3beta1-integrin and MCT1 functions and results in suppression of progressive phenotype in the colorectal cancer cell line Caco-2. Cancer Gene Ther 2014; 21:246-55; PMID:24924201; http://dx.doi.org/ 10.1038/cgt.2014.24 [DOI] [PubMed] [Google Scholar]
- 206.Thammasit P, Sangboonruang S, Suwanpairoj S, Khamaikawin W, Intasai N, Kasinrerk W, Tayapiwatana C, Tragoolpua K. Intracellular Acidosis Promotes Mitochondrial Apoptosis Pathway: Role of EMMPRIN Down-regulation via Specific Single-chain Fv Intrabody. J Cancer 2015; 6:276-86; PMID:25663946; http://dx.doi.org/ 10.7150/jca.10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Figini M, Ferri R, Mezzanzanica D, Bagnoli M, Luison E, Miotti S, Canevari S. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti folate receptor antibodies. Gene Ther 2003; 10:1018-25; PMID:12776159; http://dx.doi.org/ 10.1038/sj.gt.3301962 [DOI] [PubMed] [Google Scholar]
- 208.Guillaume-Rousselet N, Jean D, Frade R. Cloning and characterization of anti-cathepsin L single chain variable fragment whose expression inhibits procathepsin L secretion in human melanoma cells. Biochem J 2002; 367:219-27; PMID:12241546; http://dx.doi.org/ 10.1042/BJ20020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Accardi L, Paolini F, Mandarino A, Percario Z, Di Bonito P, Di Carlo V, Affabris E, Giorgi C, Amici C, Venuti A. In vivo antitumor effect of an intracellular single-chain antibody fragment against the E7 oncoprotein of human papillomavirus 16. Int J Cancer 2014; 134:2742-7; PMID:24226851; http://dx.doi.org/ 10.1002/ijc.28604 [DOI] [PubMed] [Google Scholar]
- 210.Rondon IJ, Marasco WA. Intracellular antibodies (intrabodies) for gene therapy of infectious diseases. Annu Rev Microbiol 1997; 51:257-83; PMID:9343351; http://dx.doi.org/ 10.1146/annurev.micro.51.1.257 [DOI] [PubMed] [Google Scholar]
- 211.Poznansky MC, Foxall R, Mhashilkar A, Coker R, Jones S, Ramstedt U, Marasco W. Inhibition of human immunodeficiency virus replication and growth advantage of CD4+ T cells from HIV-infected individuals that express intracellular antibodies against HIV-1 gp120 or Tat. Hum Gene Ther 1998; 9:487-96; PMID:9525310; http://dx.doi.org/ 10.1089/hum.1998.9.4-487 [DOI] [PubMed] [Google Scholar]
- 212.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins AL. Cells transfected with a non-neutralizing antibody gene are resistant to HIV infection: targeting the endoplasmic reticulum and trans-Golgi network. J Immunol 1998; 160:1489-96. [PubMed] [Google Scholar]
- 213.Walsh R, Nuttall S, Revill P, Colledge D, Cabuang L, Soppe S, Dolezal O, Griffiths K, Bartholomeusz A, Locarnini S. Targeting the hepatitis B virus precore antigen with a novel IgNAR single variable domain intrabody. Virology 2011; 411:132-41; PMID:21239030; http://dx.doi.org/ 10.1016/j.virol.2010.12.034 [DOI] [PubMed] [Google Scholar]
- 214.Liao W, Strube RW, Milne RW, Chen SY, Chan L. Cloning of apoB intrabodies: specific knockdown of apoB in HepG2 cells. Biochem Biophys Res Commun 2008; 373:235-40; PMID:18558087; http://dx.doi.org/ 10.1016/j.bbrc.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heintges T, zu Putlitz J, Wands JR. Characterization and binding of intracellular antibody fragments to the hepatitis C virus core protein. Biochem Biophys Res Commun 1999; 263:410-8; PMID:10491307; http://dx.doi.org/ 10.1006/bbrc.1999.1350 [DOI] [PubMed] [Google Scholar]
- 216.Blazek D, Celer V, Navratilova I, Skladal P. Generation and characterization of single-chain antibody fragments specific against transmembrane envelope glycoprotein gp46 of maedi-visna virus. J Virol Methods 2004; 115:83-92; PMID:14656464; http://dx.doi.org/ 10.1016/j.jviromet.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 217.Steinberger P, Andris-Widhopf J, Buhler B, Torbett BE, Barbas CF 3rd. Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci U S A 2000; 97:805-10; PMID:10639161; http://dx.doi.org/ 10.1073/pnas.97.2.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Swan CH, Buhler B, Steinberger P, Tschan MP, Barbas CF 3rd, Torbett BE. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther 2006; 13:1480-92; PMID:16738691; http://dx.doi.org/ 10.1038/sj.gt.3302801 [DOI] [PubMed] [Google Scholar]
- 219.Cordelier P, Kulkowsky JW, Ko C, Matskevitch AA, McKee HJ, Rossi JJ, Bouhamdan M, Pomerantz RJ, Kari G, Strayer DS. Protecting from R5-tropic HIV: individual and combined effectiveness of a hammerhead ribozyme and a single-chain Fv antibody that targets CCR5. Gene Ther 2004; 11:1627-37; PMID:15295615; http://dx.doi.org/ 10.1038/sj.gt.3302329 [DOI] [PubMed] [Google Scholar]
- 220.BouHamdan M, Strayer DS, Wei D, Mukhtar M, Duan LX, Hoxie J, Pomerantz RJ. Inhibition of HIV-1 infection by down-regulation of the CXCR4 co-receptor using an intracellular single chain variable fragment against CXCR4. Gene Ther 2001; 8:408-18; PMID:11313818; http://dx.doi.org/ 10.1038/sj.gt.3301411 [DOI] [PubMed] [Google Scholar]
- 221.Mukhtar M, Acheampong E, Khan MA, Bouhamdan M, Pomerantz RJ. Down-modulation of the CXCR4 co-receptor by intracellular expression of a single chain variable fragment (SFv) inhibits HIV-1 entry into primary human brain microvascular endothelial cells and post-mitotic neurons. Brain Res Mol Brain Res 2005; 135:48-57; PMID:15857668; http://dx.doi.org/ 10.1016/j.molbrainres.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 222.Beyer F, Doebis C, Busch A, Ritter T, Mhashilkar A, Marasco WM, Laube H, Volk HD, Seifert M. Decline of surface MHC I by adenoviral gene transfer of anti-MHC I intrabodies in human endothelial cells-new perspectives for the generation of universal donor cells for tissue transplantation. J Gene Med 2004; 6:616-23; PMID:15170732; http://dx.doi.org/ 10.1002/jgm.548 [DOI] [PubMed] [Google Scholar]
- 223.Busch A, Marasco WA, Doebis C, Volk HD, Seifert M. MHC class I manipulation on cell surfaces by gene transfer of anti-MHC class I intrabodies-a tool for decreased immunogenicity of allogeneic tissue and cell transplants. Methods 2004; 34:240-9; PMID:15312677; http://dx.doi.org/ 10.1016/j.ymeth.2004.03.017 [DOI] [PubMed] [Google Scholar]
- 224.Mhashilkar AM, Doebis C, Seifert M, Busch A, Zani C, Soo Hoo J, Nagy M, Ritter T, Volk HD, Marasco WA. Intrabody-mediated phenotypic knockout of major histocompatibility complex class I expression in human and monkey cell lines and in primary human keratinocytes. Gene Ther 2002; 9:307-19; PMID:11938450; http://dx.doi.org/ 10.1038/sj.gt.3301656 [DOI] [PubMed] [Google Scholar]
- 225.Koistinen P, Pulli T, Uitto VJ, Nissinen L, Hyypia T, Heino J. Depletion of alphaV integrins from osteosarcoma cells by intracellular antibody expression induces bone differentiation marker genes and suppresses gelatinase (MMP-2) synthesis. Matrix Biol 1999; 18:239-51; PMID:10429943; http://dx.doi.org/ 10.1016/S0945-053X(99)00022-0 [DOI] [PubMed] [Google Scholar]
- 226.Koistinen P, Heino J. The selective regulation of α Vbeta 1 integrin expression is based on the hierarchical formation of α V-containing heterodimers. J Biol Chem 2002; 277:24835-41; PMID:11997396; http://dx.doi.org/ 10.1074/jbc.M203149200 [DOI] [PubMed] [Google Scholar]
- 227.Koistinen P, Ahonen M, Kahari VM, Heino J. alphaV integrin promotes in vitro and in vivo survival of cells in metastatic melanoma. Int J Cancer 2004; 112:61-70; PMID:15305376; http://dx.doi.org/ 10.1002/ijc.20377 [DOI] [PubMed] [Google Scholar]
- 228.Richardson JH, Hofmann W, Sodroski JG, Marasco WA. Intrabody-mediated knockout of the high-affinity IL-2 receptor in primary human T cells using a bicistronic lentivirus vector. Gene Ther 1998; 5:635-44; PMID:9797868; http://dx.doi.org/ 10.1038/sj.gt.3300644 [DOI] [PubMed] [Google Scholar]
- 229.Tragoolpua K, Intasai N, Kasinrerk W, Mai S, Yuan Y, Tayapiwatana C. Generation of functional scFv intrabody to abate the expression of CD147 surface molecule of 293A cells. BMC Biotechnol 2008; 8:5; PMID:18226275; http://dx.doi.org/ 10.1186/1472-6750-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kovaleva M, Bussmeyer I, Rabe B, Grotzinger J, Sudarman E, Eichler J, Conrad U, Rose-John S, Scheller J. Abrogation of viral interleukin-6 (vIL-6)-induced signaling by intracellular retention and neutralization of vIL-6 with an anti-vIL-6 single-chain antibody selected by phage display. J Virol 2006; 80:8510-20; PMID:16912301; http://dx.doi.org/ 10.1128/JVI.00420-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cardinale A, Filesi I, Vetrugno V, Pocchiari M, Sy MS, Biocca S. Trapping prion protein in the endoplasmic reticulum impairs PrPC maturation and prevents PrPSc accumulation. J Biol Chem 2005; 280:685-94; PMID:15513919; http://dx.doi.org/ 10.1074/jbc.M407360200 [DOI] [PubMed] [Google Scholar]
- 232.Vetrugno V, Cardinale A, Filesi I, Mattei S, Sy MS, Pocchiari M, Biocca S. KDEL-tagged anti-prion intrabodies impair PrP lysosomal degradation and inhibit scrapie infectivity. Biochem Biophys Res Commun 2005; 338:1791-7; PMID:16288721; http://dx.doi.org/ 10.1016/j.bbrc.2005.10.146 [DOI] [PubMed] [Google Scholar]
- 233.Peng JL, Wu S, Zhao XP, Wang M, Li WH, Shen X, Liu J, Lei P, Zhu HF, Shen GX. Downregulation of transferrin receptor surface expression by intracellular antibody. Biochem Biophys Res Commun 2007; 354:864-71; PMID:17266924; http://dx.doi.org/ 10.1016/j.bbrc.2007.01.052 [DOI] [PubMed] [Google Scholar]
- 234.Intasai N, Tragoolpua K, Pingmuang P, Khunkaewla P, Moonsom S, Kasinrerk W, Lieber A, Tayapiwatana C. Potent inhibition of OKT3-induced T cell proliferation and suppression of CD147 cell surface expression in HeLa cells by scFv-M6-1B9. Immunobiology 2009; 214:410–21. [DOI] [PubMed] [Google Scholar]
- 235.Meli G, Lecci A, Manca A, Krako N, Albertini V, Benussi L, Ghidoni R, Cattaneo A. Conformational targeting of intracellular Abeta oligomers demonstrates their pathological oligomerization inside the endoplasmic reticulum. Nat Commun 2014; 5:3867; PMID:24861166; http://dx.doi.org/ 10.1038/ncomms4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steinberger P, Sutton JK, Rader C, Elia M, Barbas CF 3rd. Generation and characterization of a recombinant human CCR5-specific antibody. A phage display approach for rabbit antibody humanization. J Biol Chem 2000; 275:36073-8; PMID:10969070; http://dx.doi.org/ 10.1074/jbc.M002765200 [DOI] [PubMed] [Google Scholar]


