Abstract
Hexavalent chromium is a marine pollutant of concern, both for the health of ocean ecosystems and for public health. Hexavalent chromium is known to induce genotoxicity in human and other terrestrial mammals. It is also known to be present in both water and air in the marine environment. However, currently there are limited data concerning both chromium levels and its toxicological effects in marine mammals. This study investigated the cytotoxic and genotoxic effects of soluble and particulate hexavalent chromium in sperm whale skin fibroblasts. Both forms of hexavalent chromium induced concentration-dependent increases in cytotoxicity and genotoxicity indicating that these compounds can be a health risk if the whales are exposed to them. These data support a hypothesis that chromium is a concern in the marine environment in general and for the health of sperm whales in particular.
Keywords: chromium, chromate, sperm whale, marine mammal, genotoxicity, cytotoxicity, lead chromate, sodium chromate
INTRODUCTION
Exposure to hexavalent chromium [(Cr(VI)] is both an occupational and environmental concern as Cr(VI) is heavily used in industry and commercial products. Substantial amounts of Cr-contaminated waste are released into the environment which affect both the atmosphere and oceans, putting both terrestrial and aquatic species at risk [Chung, 1986; Hixon et al., 2001; ATSDR, 2002]. In the ocean, Cr(VI) is the predominant state for Cr [Pettine and Millero, 1990; Geisler and Schmidt, 1992].
The toxic effects of Cr(VI) have been studied in several fish species as Cr is known to accumulate in fish [Sivaperumal et al., 2007; Agah et al., 2009]. These in vivo studies have shown that Cr(VI) induces a wide range of sublethal effects [Thaker et al., 1996; Roberts and Oris, 2004; Prabakaran et al., 2006] and genotoxicity [Leonard and Lauwerys, 1980; Krishnaja and Rege, 1982; Torres de Lemos et al., 2001; Kuykendall et al., 2006]. Recently, we showed that Cr(VI) induces cell death, chromosomal aberrations and DNA double strand breaks in a medaka fish cell culture model [Goodale et al., 2008].
For whales, recent data show that some have very high Cr skin levels [Wise et al., 2008, 2009]. For example, a recent study of 361 free-ranging sperm whales collected from around the globe had Cr skin levels that ranged from 0.9 to 122.6 μg Cr/g tissue with a global mean of 8.8 μg/g [Wise et al., 2009]. The data also show that the levels vary by region indicating local pollution is a likely source [Wise et al., 2009). Similarly, a study of seven North Atlantic right whales (Eubalaena glacialis) from the Gulf of Maine had mean Cr skin levels of 7.1 μg/g [Wise et al., 2008]. These levels appear to be high because previously levels of this magnitude had only been reported in occupationally exposed humans with Cr-induced lung cancer. For example, the range of Cr lung levels in such workers was 0.4–132 μg/g with a median level of 20.4 [Tsuneta et al., 1980]. For the sperm whales, almost 8% of them had Cr levels above the median level for these exposed workers [Wise et al., 2009]. Another suggestion that these levels are high comes from data showing that mean Cr skin levels in humans without occupational exposure was 0.31 μg/g, which is 23–28 times lower than the levels in whales [Schroeder et al., 1970].
The route of exposure to Cr(VI) for whales is uncertain. Cr(VI) can enter the body through dermal, oral, and inhalational routes [ATSDR, 2002] and it seems likely that all three routes could occur in whales though the oral and inhalation routes seem the most likely [Wise et al., 2008, 2009]. The form of Cr(VI) the whales are exposed to also is uncertain and exposure to both particulate and soluble forms probably occur.
The toxic effects of Cr(VI) in whales are poorly understood. In humans, Cr(VI) is a well-known genotoxic agent [Holmes et al., 2008; Wise et al., 2008; IARC, 1990]. The most potent carcinogenic form of Cr(VI) is the water insoluble or “particulate” form. This increased potency results from partial dissolution of the particles in the extracellular environment chronically releasing Cr(VI) ions with the toxic effects resulting from the uptake and intracellular reduction of the Cr(VI) ions to Cr(III) [Wise et al., 2004c; Xie et al., 2004]. The genotoxic effects can then cause carcinogenic, teratogenic, and developmental problems, and therefore, Cr(VI) is also a concern for the reproductive system of mammals. The potential developmental and reproductive toxicity is a particular concern for whales, given that many species are threatened or endangered. Human and rodent studies show that Cr(VI) accumulates in the testes and causes a reduction in testicular weights, seminiferous tubule degeneration, decreased sperm counts, and altered reproductive behaviors, although the contribution of genotoxicity to these specific events has not been determined [Witmer et al., 1989, 1991; Chowdhury et al., 1995; Bataineh et al., 1997; Mancuso, 1997; Al-Hamood et al., 1998].
Only two studies have considered Cr(VI) genotoxicity in a whale model. The data showed that both particulate and soluble Cr(VI) were genotoxic to North Atlantic right whale lung, skin, and testes cells inducing chromosomal aberrations [Wise et al., 2008; Li Chen et al., 2009]. However, it is unknown if these effects in right whales reflect all whales or are specific to either just right whales or perhaps just related whales species in their suborder. Different whale species do occupy different ecological niches, so it is certainly possible that they have evolved different cellular response to stressors. For example, right whales are baleen whales (suborder Mysticeti) that feed mostly on tiny copepods and dive to comparatively shallower depths. By contrast, sperm whales are toothed whales (suborder Odontoceti) that feed on squid and fish and dive to very deep depths. It is unknown if these different types of whales respond similarly or differently to contaminant levels as there are currently no data concerning metal toxicity in toothed whales or cells from them. This data gap is important because several toothed whales are also threatened and endangered. Accordingly, in this investigation, we studied the cytotoxic and genotoxic effects of both particulate and soluble Cr(VI) in sperm whale skin fibroblasts as a first step for building a model system for studying this important groups of cetaceans (odontocetes).
MATERIALS AND METHODS
Cells and Cell Culture
Sperm whale skin fibroblasts were isolated from a tissue biopsy obtained from a free-ranging sperm whale off the coast of North Carolina during the voyage of the research vessel Odyssey. The biopsy was placed in L-15 medium supplemented with 100 μg/ml streptomycin, 100 U/ml penicillin, and 10 mg/ml gentamicin and then shipped on cold packs to the Wise Laboratory. Upon receipt, tissue explants were rinsed several times in phosphate-buffered saline (PBS) with penicillin-streptomycin and gentamicin. Explants were then sliced with a scalpel into small pieces (≤1 mm), rinsed repeatedly and placed into T-25 flasks containing DMEM/F-12 (50:50 mixture of Dulbecco’s minimal essential medium and Ham’s F-12) supplemented with 15% CCS (Cosmic calf serum), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM sodium pyruvate. Flasks with cells were placed in a 33°C humidified incubator with 5% CO2 and fibroblast cells were observed growing out of the explants. Cells were maintained as adherent subconfluent monolayers. They were fed at least twice a week and subcultured at least once a week. Cells were tested routinely for Mycoplasma contamination. All experiments were conducted on logarithmically growing cells with a doubling time of 36 hr. DMEM/F-12 was purchased from Mediatech Inc. (Herndon, VA). CCS was purchased from Hyclone, (Logan, UT). Sodium pyruvate, penicillin/streptomycin, Gurr’s buffer, trypsin/EDTA, and glutamax were purchased from Invitrogen Corporation (Grand Island, NY). Tissue culture dishes, flasks, and plasticware were purchased from Corning Inc. (Acton, MA).
Preparation of Chemicals
Lead chromate (CAS# 7758-97-6, ACS reagent minimum 98% purity), a particulate Cr(VI) compound was administered as a suspension in acetone as previously described [Wise et al., 2002]. Sodium chromate (CAS #7775-11-3, ACS reagent minimum 98% purity), a soluble hexavalent chromium compound was administered as a solution in water as previously described [Wise et al., 2002]. Lead chromate is an insoluble compound and therefore treatments are expressed as weight per surface area (μg/cm2). Sodium chromate is fully soluble and therefore treatments doses expressed as μM. Lead chromate and sodium chromate were purchased from Sigma/Aldrich.
Cytotoxicity
Cytotoxicity was determined using a clonogenic assay based on our published methods with minor modifications [Wise et al., 2008]. Briefly, cells in log phase were seeded in each well of a 6-well tissue-culture dish. Cells were then treated for 24 hr with either suspensions of particulate Cr(VI), ranging from 0 to 5 g/cm2, or solutions of soluble Cr(VI), ranging from 0 to 25 μM. After treatment, cells were resuspended in fresh medium and reseeded at 1,000 cells per 100 mm dish with four dishes per treatment group. Colonies formed in about 14 days and were fixed and stained with crystal violet and then each dish was counted and averaged together to get a mean value for each dose in each experiment. Each experiment was repeated at least three times. These data were used to set the concentrations for the chromosome damage assay. Crystal violet and methanol were purchased from J.T. Baker (Phillipsburg, NJ). Tissue culture dishes, flasks, and plasticware were purchased from Corning Inc. (Acton, MA).
Clastogenicity
Clastogenicity was determined using a chromosomal aberration assay based on our published methods with minor modifications [Wise et al., 2008]. Briefly, log phase cells were seeded into 100-mm dishes. Cells were then treated for 24 hr with either suspensions of particulate Cr(VI), ranging from 0 to 5 g/cm2, or solutions of soluble Cr(VI), ranging from 0 to 25 μM. Each experiment consisted of a control and several doses and was repeated at least three times. Five hours before the end of treatment, 0.1 g/ml demecolchicine was added to arrest the cells in metaphase. After treatment the cells were resuspended in a 0.075 M potassium chloride (KCl) hypotonic solution for 17 min followed by fixation in 3:1 methanol:acetic acid. The fixative was changed twice; cells dropped onto clean wet slides and stained with 5% Giemsa stain in Gurr’s buffer. One hundred metaphases per treatment were analyzed in each experiment and chromosome aberrations were scored by standard criteria [Wise et al., 2008]. Crystal violet, acetic acid, and methanol were purchased from J.T. Baker (Phillipsburg, NJ). Demecolchicine and KCl and were purchased from Sigma/Aldrich. Tissue culture dishes, flasks, and plasticware were purchased from Corning Inc. (Acton, MA). Giemsa stain was purchased from Biomedical Specialties Inc. (Santa Monica, CA).
Statistics
One-way ANOVA was used to evaluate whether there were differences in cytotoxicity and genotoxicity as a function of concentration of chromium. The Tukey’s test was used to calculate P-values for evaluating the statistical significance of the difference in mean values for cytotoxicity and genotoxicity between each pair of chromium concentrations. Ninety-five percent confidence intervals for these differences in mean values were constructed based on the studentized range distribution.
RESULTS
Particulate and Soluble Cr(VI) Cytotoxicity in Sperm Whale Skin Cells
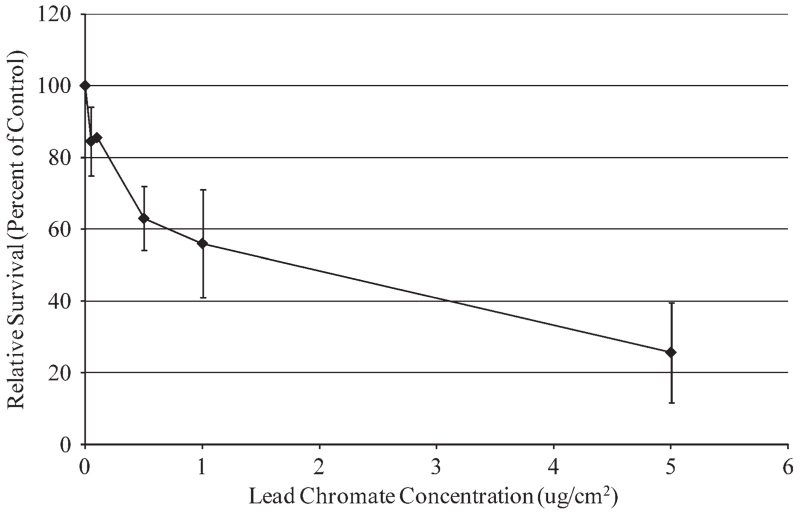
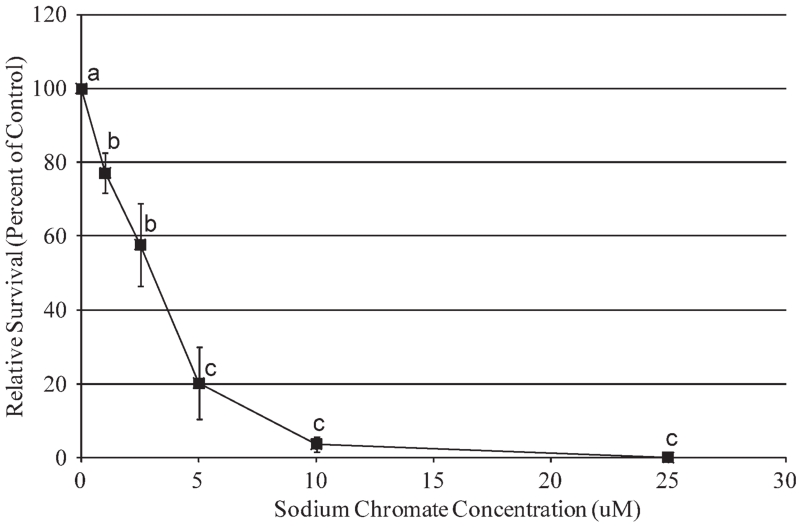
Particulate Cr(VI) induced a clear concentration-dependent decrease in cell survival over a range of 0.05 to 0.5 μg/cm2 (Fig. 1). Concentrations of 0.05, 0.1, 0.5, 1, and 5 μg/cm2 lead chromate induced 85, 86, 63, 36, and 26% relative survival; however, this overall pattern was not statistically significant based on ANOVA analysis (F(0.05,5,18) = 1.03, P = 0.429). Given the clear trend towards increased cytotoxicity, this lack of significance is probably due to insufficient power given the sample size (n = 4). Soluble Cr(VI) did induce a statistically significant concentration-dependent increase (F(0.05,5,18) = 40.92; P = 0.000) in cytotoxicity in sperm whale skin fibroblasts over a range of 1 to 25 μM (Fig. 2). Concentrations of 1, 2.5, 5, 10, and 25 μM sodium chromate induced 77, 58, 20, 4, and 0% relative survival, respectively.
Fig. 1.
Particulate Cr(VI) cytotoxicity in sperm whale skin cells. This figure shows the cytotoxic responses in sperm whale skin cells following exposure to particulate Cr(VI). No statistically significant difference (P > 0.05) was observed. Data represent the average of relative cell survival ± S. E.; n = 4.
Fig. 2.
Soluble Cr(VI) cytotoxicity in sperm whale skin cells. This figure shows the cytotoxic responses in sperm whale skin cells following exposure to soluble Cr(VI). Data represent the average of relative cell survival ± S. E.; n = 3; (a–c) = denotes doses that are statistically significant from each other (P < 0.05) and from the control (P < 0.05); the same letter denotes no statistical differences between doses.
Particulate and Soluble Cr(VI) Clastogenicity in Sperm Whale Skin Cells
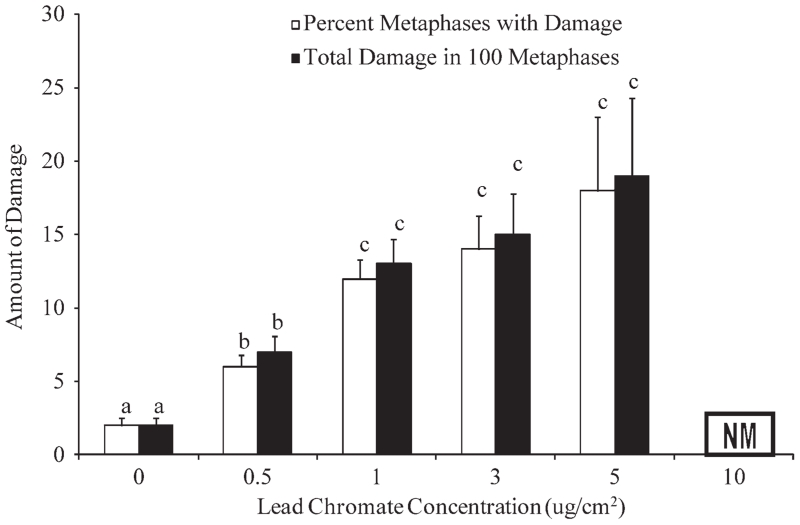
Particulate Cr(VI) induced a significant concentration-dependent increase in genotoxicity measured as the percent of metaphases with damage (F(0.05,4,17) = 11.08, P = 0.000) or total amount of damage in 100 metaphases (F((0.05,4,17) = 9.31, P = 0.000) (Fig. 3). Concentrations of 0.05, 1, 3, and 5 μg/cm2 lead chromate damaged 6, 12, 13, and 18% of metaphases and induced 7, 13, 15, and 19 aberrations per 100 metaphases respectively. At 10 μg/cm2 lead chromate there was significant cell cycle arrest and no metaphases were seen. The percent of metaphases with damage measure indicates the frequency that a metaphase cell incurs at least one damaging event. By contrast, the total damage in 100 metaphases with damage measure accounts for multiple aberrations in a cell. Thus, comparing the two sets of data show there are few cells with multiple aberrations as the two measures are similar.
Fig. 3.
Particulate Cr(VI) clastogenicity in sperm whale skin cells. This figure shows the clastogenic responses in sperm whale skin cells following exposure to particulate Cr(VI). Genotoxicity is presented as the average percent of metaphases with damage and total aberrations in 100 metaphases for each cell line, except at 3 μg/cm2 which one experiment only yielded 85 metaphases, ± S. E.; n = 4, except for 5 μg/cm2 where n = 2; NM denotes no metaphases; (a–c) denotes doses which are statistically significant from each other (P < 0.05) and from the control (P < 0.05); the same letter denotes no statistical significance between doses.
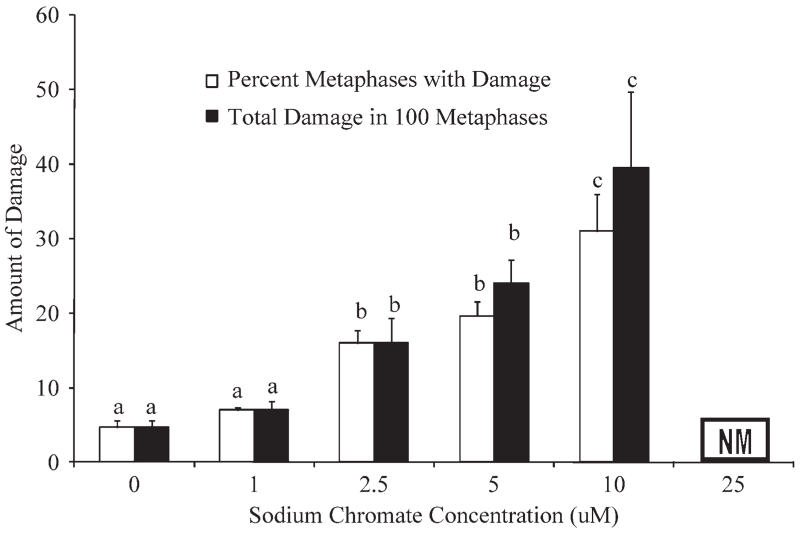
Soluble Cr(VI) also induced a significant concentration-dependent increase in genotoxicity in sperm whale skin fibroblasts for both measures; percent of metaphases with damage (F(0.05,4,9) = 28.79, P = 0.000) and total amount of damage in 100 metaphases (F(0.05,4,9) = 10.83, P = 0.002) (Fig. 4). Specifically, concentrations of 1, 2.5, 5, and 10 μM sodium chromate damaged 7, 16, 19.7, and 31% of metaphases and induced 7, 16, 24, and 39.5 aberrations per 100 metaphases, respectively. Sodium chromate (25 μM) induced substantial cell cycle arrest and no metaphases were seen. The difference in magnitude between the two genotoxicity measures at 5 and 10 μM sodium chromate indicates an increasing number of cells with multiple aberrations.
Fig. 4.
Soluble Cr(VI) clastogenicity in sperm whale skin cells. This figure shows the clastogenic responses in sperm whale skin cells following exposure to soluble Cr(VI). Genotoxicity is presented as the average percent of metaphases with damage and total aberrations in 100 metaphases for each cell line ± S. E.; n = 3; NM denotes no metaphases; (a–c) denotes doses with are statistically significant from each other (P < 0.05) and from the control (P < 0.05); the same letter denotes no statistical significance between doses.
The spectrum of damage for both compounds consisted predominately of chromatid and isochromatid lesions (Table I). Dicentrics and double minutes occurred at a low frequency (<1%), though these more complex lesions were never seen in untreated control cells.
TABLE I. Spectrum of Chromosome Aberrations.
| Chromatid lesion |
Isochromatid lesion |
Chromatid exchange |
Ring | Double minute |
Acentric fragment |
Dicentric | |
|---|---|---|---|---|---|---|---|
| Sodium chromate concentrationa | |||||||
| 0 | 11 ± 1.2 | 3 ± 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 17 ± 0.9 | 5 ± 1.5 | 0 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 2.5 | 43 ± 3.5 | 8 ± 2 | 0 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 5 | 53 ± 5.2 | 5 ± 1.5 | 0 | 0 | 1 ± 0 | 1 ± 0 | 0 |
| 10 | 90 ± 11 | 7 ± 1.5 | 0 | 0 | 0 | 0 | 1 ± 0 |
| Lead chromate concentration | |||||||
| 0b | 0.57 ± 0.2 | 0.29 ± 0.2 | 0 | 0 | 0 | 0 | 0 |
| 0.5c | 3 ± 0.7 | 0.25 ± 0.2 | 0 | 0 | 0 | 0 | 0 |
| 1c | 7.4 ± 1 | 1.6 ± 0.7 | 0 | 0 | 0 | 0 | 0 |
| 3c | 8.5 ± 2.5 | 1 ± 0.6 | 0 | 0 | 0 | 0 | 0 |
| 5d | 14 ± 3.8 | 1.5 ± 1.5 | 0 | 0 | 0 | 0 | 0 |
Values represent the number of occurrences for each type of chromosome aberration.
Average of three experiments.
Average of seven experiments.
Average of four experiments.
Average of two experiments (two others yielded no metaphases due to cell cycle arrest).
DISCUSSION
Cr(VI) is a well-known genotoxicant in humans and terrestrial animals with both the respiratory and reproductive systems as major targets [ATSDR, 2002]. In marine mammals, however, the toxicity of Cr(VI) is poorly understood. Recent data show that sperm whales are significantly exposed to Cr. Skin Cr tissue levels in the sperm whales ranged from 0.9 to 122.6 μg/g with a mean level of 8.8 μg/g [Wise et al., 2009]. These levels are comparable with the levels found in human tissues of occupationally exposed chromate workers with lung cancer [Tsuneta et al., 1980; Kishi et al., 1987; Wise et al., 2009] and suggest that Cr(VI) exposure may be a significant risk factor for sperm whales. However, Cr(VI) toxicity has not been previously studied in sperm whales. We report here that Cr(VI) is cytotoxic and genotoxic to sperm whale skin fibroblasts, consistent with a hypothesis that Cr(VI) may be a toxic concern for toothed whales, particularly when considering the high Cr levels observed in this species.
Cr(VI)-induced lung cancer is a major concern in humans, but due to the lifestyle of sperm whales, it is unknown if this is a concern for this species. Environmental contaminants are thought to be a cause of cancer in whales. For example, polycyclic aromatic hydrocarbons (PAHs) are considered to be the cause of cancer in the St. Lawrence Estuary (Canada) beluga whales [Martineau et al., 2002]. However, Cr(VI) has not been studied as a possible etiological factor for cancer in the beluga or any other whale species. Thus, it remains unknown if Cr(VI) is contributing to a cancer burden in a whale species, though our data suggest that it could for sufficiently exposed whales.
It can be difficult to directly compare cell culture doses to tissue levels and environmental exposures. One approach is to express all levels in units of ppm. With this approach, our administered doses of 1, 2.5, 5, 10, and 25 μM sodium chromate convert to 0.052, 0.130, 0.260, 0.520, and 1.3 ppm (treatment dose × M(Cr)/1,000) total Cr, respectively. For lead chromate, the administered doses of 0.1, 0.5, 1, and 5 μg/cm2 convert to 0.068, 0.340, 0.681, and 3.4 ppm (treatment dose × A(culture dish) × M(Cr)/M(PbCrO4)/V(media)) total Cr, respectively. Thus our treatments ranged from 0.052 to 3.4 ppm. The published sperm whale skin Cr tissue level is μg/g [Wise et al., 2009] which directly converts to ppm such that total Cr levels in whale skin range from 0.9 to 101.9 ppm with a mean level of 8.8 ppm. By this approach, our cell culture treatments were lower than the mean Cr tissue levels, and at the low end of the range of Cr found in whale tissue.
A second approach is to calculate a hypothetical whale inhalation exposure. Whales have large lung volumes and can hold their breaths for lengthy periods of time, allowing for possible increased deposition and absorption of particles [Rawson et al., 1991]. Whales have an estimated lung volume of 2 m3 and an approximate respiration rate of 24 breaths per hour [Brodie, 1975; New England Aquarium, unpublished data]. The reported atmospheric chromium for Hawaii is 0.067 μg/m3 total Cr [Bowen, 1979]. On the basis of this value, and whale lung capacity, a whale in the waters around Hawaii for 24 hr could inhale ~77 μg of total Cr (0.067 μg total Cr/m3 × 2 m3 air/breath × 24 breaths/hr × 24 hr = 77 μg total Cr). If 35% of this total amount was hexavalent as has been predicted [ATSDR, 2000], then a whale would inhale 26.95 μg Cr(VI) in 24 hr. In our studies we dosed cells for 24 hr with 0.88–44 μg Cr(VI). Thus, by this measure our doses also model a potential environmental exposure.
Of course it is tempting to speculate that the exposures are still different because a sperm whale lung is much bigger than a culture dish. This observation is true, and we can only partially model a lung-type exposure in a culture dish. However, Cr particles, when inhaled do not uniformly diffuse throughout the lung. Instead, human studies indicate that Cr particles deposit and persist at bronchial bifurcation sites causing much higher localized exposures in a much smaller surface area [Ishikawa et al., 1994a,b]. Thus, while our doses cannot precisely mimic an environmental exposure; given this focal particle distribution and the dose of particles that whales may be inhaling, genotoxicity does seem to be a realistic and meaningful concern for these animals. Such concern is consistent with previous reports that cetaceans may be significantly exposed to airborne particles [Rawson et al., 1991, 1995]. These reports found a correlation between particle deposition in the lungs and particle concentration in the atmosphere. One report suggested that exposure to atmospheric pollution may be greater in marine mammals than in humans due to the concentration of particles in the air-water interface [Rawson et al., 1991].
Our data are consistent with previous studies of chromate in baleen whale cells [Wise et al., 2008; Li Chen et al, 2009]. While this outcome is perhaps not surprising, they are important as right whales and sperm whales have evolved to occupy very different ecological niches. Right whales and sperm whales are both model species for the two suborders of whales representing 85 different species, and thus it is important to ascertain how they are both similar and different. However, while Cr(VI) was genotoxic and cytotoxic to both species, the response is not exactly the same and there are some interesting differences in potency. For example, sodium chromate appears to be more cytotoxic to sperm whale cells than right whale cells. Specifically, we found that 10 μM sodium chromate induced 2% survival in sperm whale skin cells, compared to a our previous reported value of 32% in right whale lung cells [Wise et al., 2008] indicating that sperm whales were almost 10-fold more sensitive to soluble Cr(VI) cytotoxicity. By contrast, the genotoxicity levels of sodium chromate between the whales were similar.
The explanation for these differences is uncertain. The current paradigm for Cr(VI)-induced toxicity indicates that Cr-DNA adducts cause a stalled replication fork, which lead to DNA double strand breaks and these breaks then become chromosomal aberrations [Wise et al., 2008]. One possibility, albeit untested, is that sperm whale cells may be more efficient in recognizing this damage and then signaling to the apoptosis machinery more effectively than the right whale cells leading to more cell death in the presence of less damage. A second possibility is that the difference may be due to a difference in response because of the tissue of origin (skin and lung, respectively). Such a possibility has not been directly tested, but a previous report indicated that human lung cells appeared to be less sensitive than human skin cells to the cytotoxic effects of sodium chromate [Wise et al., 2002]. However, this comparison considered data published by slightly different methods in two laboratories. Also, our report of particulate Cr(VI) in right whales cells found no differences in cytotoxicity between lung and skin fibroblasts [Li Chen et al., 2009]. Alternatively, it could be that the different whale species may have evolved different intracellular metabolism pathways that affect the amount of the adducting Cr species produced.
In contrast to soluble Cr(VI), comparing the two species for particulate Cr(VI) genotoxicity shows that the cytotoxic and genotoxic responses to lead chromate are similar as 1 μg/cm2 induced damage in 13% of metaphases and 87% survival in sperm whale skin fibroblasts compared with our reported values of 15% of metaphases and 78% survival in right whale skin cells [Li Chen et al., 2009]. This difference is surprising because both lead chromate and sodium chromate cytotoxicity and genotoxicity are mediated by released Cr(VI) ions [Wise et al., 2004c; Xie et al., 2004; Holmes et al., 2005]. Thus, one would expect the cytotoxic effects for lead chromate to mimic sodium chromate and be different in the two species. However, the cytotoxic response to lead chromate is different suggesting that lead ions may play a role. In human cells, lead ions play no role in particulate Cr(VI) cytotoxicity [Holmes et al., 2005], but perhaps in right whale cells the lead ions can enter the cells and block the toxic effects of the chromate ions released by lead chromate. Further research is needed to determine the mechanisms that underlie these differences.
In summary, our data show that particulate and soluble Cr(VI) are cytotoxic and clastogenic to sperm whale skin cells and when combined with published data that Cr skin tissue levels in sperm whales are very high, support the possibility that Cr(VI) is a health risk factor for sperm whales. Our data also show that toothed whale response to Cr(VI) differs from baleen whales indicating the need to study both groups of whales. The data suggest future research should consider potentially important and interesting adaptations of the cell death mechanisms in the response to lead ions in sperm whales cells.
ACKNOWLEDGMENTS
The authors thank David Kirstein and Christy Gianios Jr. for administrative and technical support on this project.
Grant sponsor: NMFS; Grant number: #1008-1637-00; Grant sponsor: Maine Center for Toxicology and Environmental Health.
REFERENCES
- Agah H, Leermakers M, Elskens M, Fatemi SMR, Baeyens W. Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess. 2009;157:499–514. doi: 10.1007/s10661-008-0551-8. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Chromium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2000. [Google Scholar]
- Al-Hamood MH, Elbetieha A, Bataineh H. Sexual maturation and fertility of male and female mice exposed prenatally and postnatally to trivalent and hexavalent chromium compounds. Reprod Fertil Dev. 1998;10:179–183. doi: 10.1071/r97001. [DOI] [PubMed] [Google Scholar]
- Bataineh H, Al-Hamood MH, Elbetieha A, Bani Hani I. Effect of long-term ingestion of chromium compounds on aggression, sex behavior and fertility in adult male rat. Drug Chem Toxicol. 1997;20:133–149. doi: 10.3109/01480549709003875. [DOI] [PubMed] [Google Scholar]
- Bowen HJM. Environmental Chemistry of the Elements. Academic Press; London: 1979. [Google Scholar]
- Brodie PF. Cetacean energetics, an overview of intraspecific size variation. Ecology. 1975;56:152–161. [Google Scholar]
- Chowdhury AR, Mitra C. Spermatogenic and steroidogenic impairment after chromium treatment in rats. Indian J Exp Biol. 1995;33:480–484. [PubMed] [Google Scholar]
- Chung YS. Air pollution detection by satellites: The transport and deposition of air pollutants over oceans. Atmos Environ. 1986;20:617–630. [Google Scholar]
- Geisler CD, Schmidt D. An overview of chromium in the marine environment. Dt Hydrogr Z. 1991;44:185–196. [Google Scholar]
- Hartwig A, Schlepegrell R, Beyersmann D. Indirect mechanism of lead-induced genotoxicity in cultured mammalian cells. Mutat Res. 1990;241:75–82. doi: 10.1016/0165-1218(90)90110-n. [DOI] [PubMed] [Google Scholar]
- Hixon MA, Boersma PD, Hunter ML, Jr, Micheli F, Norse EA, Possingham HP, Snelgrove PVR. Oceans at risk: Research priorities in marine conservation biology. In: Soulé ME, Orians GH, editors. Conservation Biology: Research Priorities for the Next Decade. Island Press; Washington, D.C.: 2001. pp. 125–154. [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol Appl Pharmacol. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Sandwick SJ, Lingle WL, Negron VC, Thompson WD, Wise JP., Sr. Chronic exposure to lead chromate causes centrosome abnormalities and aneuploidy in human lung cells. Cancer Res. 2006;66:4041–4048. doi: 10.1158/0008-5472.CAN-05-3312. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Wise JP., Sr. Carcinogenicity of hexavalent chromium. Ind J Med Res. 2008;128:353–372. [PubMed] [Google Scholar]
- IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Vol. 49. International Agency for Research on Cancer; Lyons, France: 1990. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers’ cancers, chromium lung deposition and precancerous bronchial lesions: An autopsy study. Br J Cancer. 1994a;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Res. 1994b;54:2342–2346. [PubMed] [Google Scholar]
- Kishi R, Tarumi T, Uchino E, Miyake H. Chromium content of organs of chromate workers with lung cancer. Am J Ind Med. 1987;11:67–74. doi: 10.1002/ajim.4700110107. [DOI] [PubMed] [Google Scholar]
- Krishnaja AP, Rege MS. Induction of chromosomal aberrations in fish Boleophthalmus dussumieri after exposure in vivo to mitomycin C, heavy metals mercury, selenium and chromium. Mutat Res. 1982;102:71–82. doi: 10.1016/0165-1218(82)90147-1. [DOI] [PubMed] [Google Scholar]
- Kuykendall JR, Miller KL, Mellinger KN, Cain AV. Waterborne and dietary hexavalent chromium exposure causes DNA-protein crosslink (DPX) formation in erythrocytes of largemouth bass (Micropterus salmoides) Aquat Toxicol. 2006;78:27–31. doi: 10.1016/j.aquatox.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Leonard A, Lauwerys RR. Carcinogenicity and mutagenicity of chromium. Mutat Res. 1980;76:227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Li Chen T, Wise SS, Kraus S, Shaffiey F, Grau M, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T, Wise JP., Sr. Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts. Environ Mol Mutagen. 2009;50:387–393. doi: 10.1002/em.20471. [DOI] [PubMed] [Google Scholar]
- Mancuso TF. Chromium as an industrial carcinogen. II. Chromium in human tissues. Am J Ind Med. 1997;2:140–147. doi: 10.1002/(sici)1097-0274(19970204)31:2<140::aid-ajim2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Martineau D, Lemberger K, Dallaire A, Labelle P, Lipscomb TP, Michel P, Mikaelian I. Cancer in wildlife, a case study: Beluga from the St. Lawrence Estuary, Québec, Canada. Environ Health Perspect. 2002;110:285–292. doi: 10.1289/ehp.02110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettine M, Millero FJ. Chromium speciation in seawater: The probable role of hydrogen peroxide. Limnol Oceanogr. 1990;35:730–736. [Google Scholar]
- Prabakaran M, Binuramesh C, Steinhagen D, Michael RD. Immune response and disease resistance of Oreochromis mossambicus to Aeromonas hydrophila after exposure to hexavalent chromium. Dis Aquat Organ. 2006;68:189–196. doi: 10.3354/dao068189. [DOI] [PubMed] [Google Scholar]
- Roberts AP, Oris JT. Multiple biomarker response in rainbow trout during exposure to hexavalent chromium. Compar Biochem Physiol C. 2004;138:221–228. doi: 10.1016/j.cca.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rawson J, Anderson HF, Patton GW, Beecher T. Anthracosis in the Atlantic Bottlenose Dolphin (Tursiops truncatus) Marine Mammal Sci. 1991;7:413–416. [Google Scholar]
- Rawson AJ, Bradley JP, Teetsov A, Rice SB, Haller EM, Patton GW. A role for airborne particulates in high mercury levels of some cetaceans. Ecotoxicol Environ Saf. 1995;30:309–14. doi: 10.1006/eesa.1995.1035. [DOI] [PubMed] [Google Scholar]
- Roy NK, Rossman TG. Mutagen and comutagenesis by lead compounds. Mutat Res. 1992;298:97–103. doi: 10.1016/0165-1218(92)90034-w. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS/STAT 9.1 User’s Guide. SAS Institute, Inc.; Cary, NC: 2004. p. 5136. [Google Scholar]
- Sivaperumal P, Sankar TV, Nair Viswanathan PG. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chem. 2007;102:612–620. [Google Scholar]
- Thaker J, Chhaya J, Nuzhat S, Mittal R, Mansuri AP, Kundu R. Effects of chromium(VI) on some ion-dependent ATPases in gills, kidney and intestine of a coastal teleost Periophthalmus dipes. Toxicology. 1996;112:237–244. doi: 10.1016/0300-483x(96)86481-x. [DOI] [PubMed] [Google Scholar]
- Torres de Lemos C, Rödel PM, Terra NR, Erdtmann B. Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish erythrocytes. Environ Toxicol Chem. 2001;20:1320–1324. doi: 10.1897/1551-5028(2001)020<1320:eobmfa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tsuneta Y, Ohsaki Y, Kimura K, Mikami H, Abe S, Murao M. Chromium content of lungs of chromate workers with lung cancer. Thorax. 1980;35:294–297. doi: 10.1136/thx.35.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer CM, Harris R, Shupack SI. Oral bioavailability of chromium from a specific site. Environ Health Perspect. 1991;92:105–110. doi: 10.1289/ehp.92-1519374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer CM, Park H-S, Shupack SI. Mutagenicity and disposition of chromium. Sci Total Environ. 1989;86:131–148. doi: 10.1016/0048-9697(89)90200-3. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Ketterer ME, Hartsock WJ, Fomchenko E, Katsifis SP, Thompson WD, Wise JP., Sr. Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat Res. 2004c;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutat Res. 2006;610:2–7. doi: 10.1016/j.mrgentox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr. Hexavalent chromium-induced DNA damage and repair mechanisms. Rev Environ Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Kraus S, Shaffiey F, Grau M, Li Chen T, Perkins C, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T. Hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and testes fibroblasts. Mutat Res. 2008;650:30–38. doi: 10.1016/j.mrgentox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr, Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75:1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Gordon N, Wise JP., Sr. Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chem Res Toxicol. 2004;17:1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]