Abstract
The germline of Caenorhabditis elegans derives from a single founder cell, the germline blastomere P4. P4 is the product of four asymmetric cleavages that divide the zygote into distinct somatic and germline (P) lineages. P4 inherits a specialized cytoplasm (“germ plasm”) containing maternally encoded proteins and RNAs. The germ plasm has been hypothesized to specify germ cell fate, but the mechanisms involved remain unclear. Three processes stand out: (1) inhibition of mRNA transcription to prevent activation of somatic development, (2) translational regulation of the nanos homolog nos-2 and of other germ plasm mRNAs, and (3) establishment of a unique, partially repressive chromatin. Together, these processes ensure that the daughters of P4, the primordial germ cells Z2 and Z3, gastrulate inside the embryo, associate with the somatic gonad, initiate the germline transcriptional program, and proliferate during larval development to generate ~2,000 germ cells by adulthood.
Keywords: Germ plasm, Polarity, Germ granules, Cell fate, Transcriptional repression, Germline blastomeres, Primordial germ cells, P lineage, Maternal RNA
2.1 Introduction to the Embryonic Germ Lineage (P Lineage)
2.1.1 Embryonic Origin of the Germline
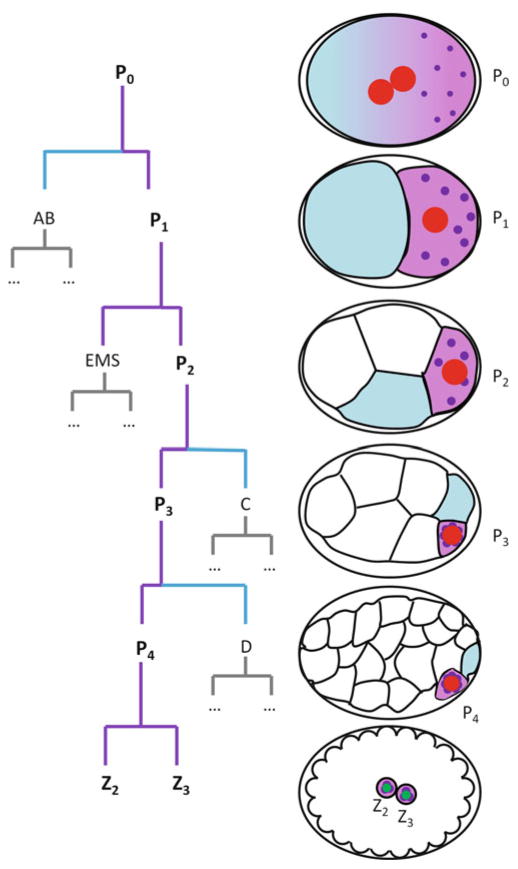
P4 arises in the 24-cell stage from a series of four asymmetric divisions starting in the zygote (P0) (Fig. 2.1). Each division generates a larger, somatic blastomere (AB, EMS, C and D) and a smaller, germline blastomere (P1, P2, P3, P4). Laser ablation of the P4 nucleus yields sterile worms with no germ cells (Sulston et al. 1983), confirming that P4 is the sole founder of the germline and that no other cell can replace P4.
Fig. 2.1.
Embryonic origin of the germline. Abbreviated embryonic lineage from the 1-cell stage to the ~88-cell stage and embryo schematics corresponding to each stage shown in the lineage tree. Germ plasm is denoted in purple, germ granules are darker purple dots. High levels of MEX-5/6 inherited by somatic blastomeres are denoted in blue. Red nuclei are not competent for mRNA transcription
In the 88-cell stage, P4 divides once to generate two daughters: the primordial germ cells, Z2 and Z3. Soon after their birth, Z2 and Z3 gastrulate into the embryo interior (Harrell and Goldstein 2011). Z2 and Z3 do not divide further during embryogenesis, and remain close to each other and to the intestine. By the 2-fold stage, Z2 and Z3 extend protrusions towards two intestinal cells (Sulston et al. 1983). Intestinal cells have been suggested to provide sustenance to Z2 and Z3 until the gonad is formed.
In mid-embryogenesis, the somatic gonadal precursors Z1 and Z4 migrate towards Z2 and Z3 to form the gonad primordium (Sulston et al. 1983). Z2 and Z3 resume divisions only in the first (L1) larval stage after the larva begins feeding. Z2 and Z3 will eventually generate ~2,000 germ cells by adulthood (Kimble and White 1981).
2.1.2 Characteristics of the P Blastomeres
2.1.2.1 Asymmetric Divisions
P0, P1, P2, P3 all divide asymmetrically. Before each division, the spindle becomes displaced towards one side of the cell. The P granules, RNA-rich organelles specific to the germline, and several associated cytoplasmic proteins and RNAs (collectively referred to as “germ plasm”; Table 2.1) also accumulate on that same side. As a result, each division generates daughters of unequal size with the smaller daughter inheriting most of the germ plasm (Gönczy and Rose 2005; Strome 2005).
Table 2.1.
Proteins in germ plasm
| Protein | Domains | Protein localization | Loss-of-function phenotype | Proposed function | References |
|---|---|---|---|---|---|
| PIE-1 | CCCH fingers | P blastomeres Nuclear, cytoplasmic, P granules, centrosomes |
Maternal effect embryonic lethality P2 transformed to EMS fate No germ cells |
Repression of RNA Polymerase II Translational control of mRNAs |
Mello et al. (1992), Seydoux et al. (1996), Tenenhaus et al. (2001) |
| POS-1 | CCCH fingers | P blastomeres Cytoplasmic, P granules |
Maternal effect embryonic lethality Complex cell fate transformations No germ cells |
Translational control of mRNAs | Tabara et al. (1999) |
| MEX-1 | CCCH fingers | P blastomeres Cytoplasmic, P granules |
Maternal effect embryonic lethality Complex cell fate transformations No germ cells |
Unknown | Mello et al. (1992), Guedes and Priess (1997) |
| MEX-3 | KH domain | Present in oocytes through 4-cell stage Cytoplasmic in multiple cell types P granules in P1 through P4 |
Maternal effect embryonic lethality D transformed to P4, as a result more than two Z2/Z3 like cells are formed |
Translational control of mRNAs | Draper et al. (1996) |
| MES-1 | Receptor tyrosine kinase related | In P2 at junction with EMS contact In P3 at junction with E contact |
Maternal effect sterility P4 transformed to D fate No germ cells |
Signaling from EMS to P2 to reverse polarity in P2 | Strome et al. (1995), Berkowitz and Strome (2000) |
| Sm proteins | LSm fold | P granules | Embryonic arrest at 50- to 100-cell stage Mislocalization of PIE-1, P granules Symmetric divisions and short cell cycles |
Regulation of mRNAs | Barbee et al. (2002), Barbee and Evans (2006) |
| PAR-1 | KIN1/MARK kinase | Cortical—P blastomeres and Z2 and Z3 until morphogenesis | Maternal effect embryonic lethality No germ cells |
Polarization of P blastomeres | Kemphues et al. (1988), Guo and Kemphues (1995) |
| MES-2/3/6 | Polycomb orthologs | Nuclear in both somatic and germ cells in embryos. Persists in Z2 and Z3 in embryos and L1 larvae. | Maternal effect sterility Germ cell death at L3 and L4 stages |
Repression of X chromosome transcription | Bender et al. (2004), Strome (2005) |
| MES-4 | SET domain | Chromatin, preferentially autosomes. Present in somatic and germ cells in early embryogenesis, persists in Z2 and Z3 | Maternal effect sterility Germ cell death at L3 and L4 stages |
Transmission of germline transcriptional program from mother to progeny Repression of X chromosome transcription |
Fong et al. (2002), Bender et al. (2006), Rechtsteiner et al. (2010) |
| NOS-1/2 | Nanos orthologs | NOS-1: expressed in Z2 and Z3 from zygotic mRNA, cytoplasmic NOS-2: expressed in P4 from maternal mRNA, cytoplasmic |
Z2 and or Z3 outside somatic gonad Germ cell death at L3 stage |
Regulation of mRNAs | Subramaniam and Seydoux (1999) |
| MEG-1/2 | Novel | P granules from P2 through P4 | Maternal effect sterility Germ cell death at L3 stage |
Unknown | Leacock and Reinke (2008) |
| PGL-1/2/3 | RGG box | Constitutive P granule components | Sterility with variable phenotypes | Unknown, primarily post-embryonic | Kawasaki et al. (2004) |
| GLH-1/2/3/4 | DEAD box helicase | Constitutive P granule components | Sterility with variable phenotypes | Unknown, primarily post-embryonic | Spike et al. (2008) |
| Anti-germ plasm: | |||||
| MEX-5/6 | CCCH fingers | High levels in somatic founder blastomeres—cytoplasmic High/low levels in Ant/Post cytoplasm of P blastomeres—cytoplasmic and enriched on P granules and centrosomes |
Embryonic lethality Complex cell fate transformations No germ cells |
Dispersal of P granules in dividing P blastomeres Degradation of germ plasm in somatic blastomeres |
Schubert et al. (2000) Gallo et al. (2010) |
In the first two divisions, the spindle becomes displaced towards the posterior pole of the embryo, such that P1 and P2 are born in the posterior. The posterior pole is defined in the zygote P0 by the position of the sperm centrosome, which orients the distribution of the PAR polarity regulators (Gönczy and Rose 2005). In the P2 blastomere, the polarity axis is reversed by signaling from the somatic blastomere EMS, and P3 and P4 are born towards the anterior (Schierenberg 1987; Arata et al. 2010). As a result, P4 is born next to the descendants of the E (intestinal) lineage. Unlike P0–P3, P4 divides symmetrically into two equal size daughters (Z2 and Z3) that both inherit germ plasm.
2.1.2.2 Long Cell Cycle Times
P blastomeres have longer cell cycle times than their somatic sisters. For example, P1 divides 2 min after AB, in part due to enhanced activity of a DNA replication checkpoint in P1 (Encalada et al. 2000; Brauchle et al. 2003), and in part due to higher levels of cell cycle regulators (PLK-1 and Cdc25.1) in AB (Rivers et al. 2008; Budirahardja and Gönczy 2008). P4 divides about 70 min after its birth (Sulston et al. 1983). Z2 and Z3 duplicate their DNA and centrosomes, but remain arrested in G2 until after hatching (Fukuyama et al. 2006).
2.1.2.3 No mRNA Transcription
mRNA transcription begins in the 3- to 4-cell stage in somatic blastomeres, but appears to remain off in the germline blastomeres until gastrulation. In a survey of 16 mRNAs, no newly transcribed mRNAs were detected in P0–P4 by in situ hybridization (Seydoux et al. 1996). During the transcription cycle, the serine-rich repeats in the carboxy-terminal tail of RNA polymerase II become phosphorylated, first on Serine 5 during initiation and then on Serine 2 during elongation. These phosphoepitopes are reduced (Pser5) or completely absent (Pser2) in the germline blastomeres (Seydoux and Dunn 1997). Both phosphoepitopes appear transiently in Z2 and Z3 shortly after their birth, but return to low/background levels by the 1.5-fold stage and do not reappear until after hatching (Furuhashi et al. 2010). Z2 and Z3 also lose the active chromatin marks H3K4me2, H3K4me3, and H4K8ac (Schaner et al. 2003). Z2 and Z3 are not completely transcriptionally silent, however: zygotic expression of several germline genes have been detected in Z2 and Z3. These include P granule components (pgl-1, glh-1, and glh-4), the nanos ortholog nos-1, and meiotic genes (htp-3, rec-8) (Subramaniam and Seydoux 1999; Kawasaki et al. 2004; Takasaki et al. 2007; Spencer et al. 2011). In contrast to mRNA transcription, transcription of ribosomal RNAs has been detected in all P blastomeres with the possible exception of P4 (Seydoux and Dunn 1997).
2.1.2.4 Maintenance of Maternal mRNAs
In situ hybridization and RNA profiling studies have uncovered two classes of maternal mRNAs in early embryos: maternal mRNAs that are maintained in all blastomeres, and maternal mRNAs that are rapidly turned over in somatic blastomeres and maintained only in germline blastomeres (Seydoux and Fire 1994; Seydoux et al. 1996; Baugh et al. 2003). Some in the latter class are also enriched in P granules. For example, the Nanos homolog nos-2 is partitioned to both germline and somatic blastomeres during the first two divisions. Between the 4- and 8-cell stages, nos-2 is turned over in somatic blastomeres and maintained in the P lineage, where it is enriched in P granules. By the 28-cell stage, nos-2 RNA remains only in P4, where it is finally translated (Subramaniam and Seydoux 1999; Tenenhaus et al. 2001).
2.2 Cellular Mechanisms of Germ Cell Specification
Two general modes of germline specification have been described in animals: induction by extracellular signals and induction by germ plasm, a specialized cytoplasm inherited from the oocyte (Seydoux and Braun 2006). In this section, we describe evidence for each of these mechanisms acting in C. elegans.
2.2.1 Asymmetric Segregation of the Germ Plasm
Several lines of evidence suggest that C. elegans embryos possess germ plasm. As described above, the germline-specific P granules and associated RNAs and RNA-binding proteins co-segregate to the same side of the P blastomere before each asymmetric cleavage (Table 2.1). P or “germ” granules have been reported in the germline of many different animals, including mammals, and are considered to be intimately associated with germ cell fate (Strome and Lehmann 1997).
Embryo manipulations support the view that at least some aspects of P cell fate are specified by factors that are asymmetrically localized in the zygote. Using a laser microbeam to create holes in the eggshell, Schierenberg (1988) extruded “partial embryos” containing cytoplasm from only the anterior or posterior of the zygote. Partial embryos containing anterior cytoplasm divided symmetrically, whereas partial embryos containing posterior cytoplasm divided asymmetrically, similar to the P blastomeres. However, mixing of posterior cytoplasm into anterior cytoplasm was not sufficient to induce asymmetric divisions. Delaying cell division eliminated the ability of posterior cytoplasm to support asymmetric divisions. Together these observations suggest that the germ plasm is required for germ cell fate but is not sufficient to induce germ cell fate when diluted with “somatic cytoplasm.” In contrast, in Drosophila, injection of germ plasm in the anterior pole of the embryo is sufficient to create ectopic germ cells (Mahowald and Illmensee 1974).
Asymmetric distribution of the germ plasm is controlled by the PAR network of polarity regulators, which regulates anterior–posterior polarity in P0 and most likely also in P1, P2, and P3 (see below). The PAR proteins PAR-1 and PAR-2 segregate with the germ plasm, and both are maintained in the P lineage through the asymmetric divisions leading to P4 (Guo and Kemphues 1995; Boyd et al. 1996). PAR-1 and PAR-2 become enriched at the cell periphery on the side of the germ plasm during each asymmetric division. Strong mutations in the par genes disrupt all polarity in the 1-cell stage and lead to embryonic lethality. Hypomorphic par mutations, however, lead to viable but sterile worms that lack all germ cells (Kemphues et al. 1988; Guo and Kemphues 1995; Spilker et al. 2009). These observations suggest that asymmetric segregation of the germ plasm is required to specify P4 as the germline founder cell.
2.2.1.1 MEX-5 and MEX-6: Germ Plasm Antagonists
The PAR network regulate germ plasm asymmetry through the action of the PAR-1 kinase and its substrates MEX-5 and MEX-6, two highly related and partially redundant RNA-binding proteins that segregate opposite to the germ plasm. Phosphorylation by PAR-1 stimulates MEX-5 (and presumably MEX-6) diffusion in the posterior cytoplasm of the zygote, causing MEX-5 to become enriched in the anterior (Tenlen et al. 2008; Griffin et al. 2011). As a result, the AB blastomere inherits high levels of MEX-5/6 and low levels of PAR-1, and the P1 blastomere inherit low levels of MEX-5/6 and high levels of PAR-1. This pattern is repeated during the divisions of P1, P2, and P3 (Schubert et al. 2000; Guo and Kemphues 1995). MEX-5 and MEX-6 promote both asymmetric partitioning of the germ plasm to germ cells during cell division and asymmetric degradation of the germ plasm from the soma after cell division.
2.2.1.2 Asymmetric Partitioning of the Germ Plasm During Division
Examination of P granule dynamics in live zygotes has revealed that P granule partitioning depends both on MEX-5/6-driven granule disassembly in the anterior cytoplasm and PAR-1-driven granule assembly in the posterior cytoplasm (Cheeks et al. 2004; Brangwynne et al. 2009; Gallo et al. 2010). P granule proteins that become dispersed in the anterior cytoplasm are reincorporated into granules in the posterior cytoplasm. As a result, P1 inherits more P granule proteins than AB (Gallo et al. 2010). After polarity reversal in P2, P granules appear to segregate using a different mechanism involving association with the P cell nuclei (Hird et al. 1996). PAR-1 and MEX-5/6 also promote the posterior enrichment of germ plasm proteins that are only loosely associated with P granules, such as PIE-1 and POS-1 (Table 2.1), but the mechanisms involved are not known (Schubert et al. 2000). MEX-5/6 also promotes anterior enrichment of PLK-1 and CDC-25, which contribute to the fast cell cycle of the AB blastomere (Rivers et al. 2008; Budirahardja and Gönczy 2008).
2.2.1.3 Asymmetric Degradation of the Germ Plasm After Division
Asymmetric enrichment of the germ plasm during division is not absolute and low levels of germ plasm RNAs and proteins are inherited by all somatic blastomeres. These low levels are rapidly turned over, and this degradation depends on MEX-5 and MEX-6. In mex-5;mex-6 embryos, germ plasm proteins are uniformly partitioned to all blastomeres. Heat shock-induced expression of MEX-5 in single blastomere is sufficient to degrade germ plasm proteins in that cell (Schubert et al. 2000). The potent anti-germ plasm effect of MEX-5 may explain why, in the cytoplasmic mixing experiments described above (Schierenberg 1988), anterior cytoplasm “suppresses” the potential for asymmetric division.
In somatic blastomeres, MEX-5 and MEX-6 are required for their own degradation and the degradation of other CCCH zinc finger proteins (POS-1, PIE-1, and MEX-1). CCCH protein degradation depends on ZIF-1, a substrate recognition subunit for the CUL-2 E3 ubiquitin ligase. ZIF-1 recognizes specific CCCH fingers in MEX-5, MEX-1, POS-1, and PIE-1. A fusion between GFP and the PIE-1 first zinc finger (GFP:ZF1) is symmetrically segregated to somatic and germline blastomeres, but degraded in each somatic lineage in a ZIF-1-dependent manner (DeRenzo et al. 2003). The distribution of ZIF-1 protein is not known, but a reporter containing the zif-1 3′ UTR is activated in each somatic lineage, suggesting that ZIF-1 activity is restricted to somatic blastomeres by translational regulation of the zif-1 mRNA. Recent studies indicate that zif-1 translation is controlled combinatorially by several RNA-binding proteins that all bind directly to the zif-1 3′ UTR. In oocytes, zif-1 is silenced by OMA-1 and OMA-2 (Guven-Ozkan et al. 2010; Robertson and Lin 2012, Chap. 12), two redundant RNA-binding proteins that interact with the eIF4E-binding protein and translational repressor SPN-2 (Li et al. 2009). In zygotes, OMA-1/2 are phosphorylated by the kinase MBK-2 (Nishi and Lin 2005; Shirayama et al. 2006; Stitzel et al. 2006), leading to the displacement of SPN-2 from the zif-1 3′ UTR and the eventual degradation of OMA-1 and OMA-2 during the first cleavage (Pellettieri et al. 2003; Nishi and Lin 2005; Shirayama et al. 2006; Guven-Ozkan et al. 2010). zif-1 continues to be silenced, however, through the combined action of MEX-3 and SPN-4 in zygotes and POS-1 in later stages (Oldenbroek et al. 2012). This repression is lifted in somatic blastomeres by MEX-5 and MEX-6, which compete with POS-1 for binding to the zif-1 3′ UTR (Oldenbroek et al. 2012). Thus, MEX-5 and MEX-6 promote their own degradation and the degradation of other CCCH-binding proteins by promoting the translation of the E3 ligase subunit that targets them for ubiquitination. MEX-5 activity requires phosphorylation by the Polo kinases PLK-1 and PLK-2, which directly bind to, and segregate with, MEX-5. Phosphorylation by PLK-1 and PLK-2 is primed by MBK-2, which is active in zygotes but not oocytes. This requirement may explain why MEX-5 promotes germ plasm turnover in embryos, but not in oocytes where MEX-5 is also present (Nishi et al. 2008).
The mechanisms by which MEX-5 and MEX-6 also promote RNA degradation in somatic blastomeres are less well understood. Activation of mRNA degradation in the 4-cell stage is temporally correlated with the recruitment of LSM-1 and CCF-1 (CAF1/Pop2 subunit of the CCR4/NOT deadenylase complex) to P bodies, cytoplasmic granules that have been implicated in the decapping and deadenylation of mRNAs. In mex-5; mex-6 (RNAi) embryos, LSM-1 is not recruited to P bodies and maternal mRNAs are stabilized. Consistent with a role for deadenylation, RNAi depletion of let-711/Not-1, a component of CCR4/NOT deadenylase, also interferes with LSM-1 recruitment and mRNA degradation (Gallo et al. 2008). Whether LSM-1 is required for this process, however, has not yet been examined.
2.2.1.4 Self-propagation of Germ Plasm and Anti-germ Plasm?
The properties of MEX-5 and MEX-6 suggest that in C. elegans the distinction between soma and germline depends both on maintenance of the germ plasm in the P lineage, and on the active degradation of germ plasm in somatic lineages (“anti-germ plasm activity”). In par-1 mutants, MEX-5 and MEX-6 remain uniform and germ plasm RNAs and CCCH proteins are degraded in all cells by the 4-cell stage. Presumably, in wild-type embryos, PAR-1 maintains MEX-5 and MEX-6 at low enough levels in the P blastomeres to avoid degradation of the germ plasm. PAR-1 is maintained in all germline blastomeres and in Z2 and Z3, suggesting that PAR-1 is required continuously in the embryonic germ lineage to maintain the germ plasm. Intriguingly, in the zygote, MEX-5/6 activity is required for maximal enrichment of PAR-1 in the posterior (Cuenca et al. 2003). One possibility is that mutual regulation/exclusion by PAR-1 and MEX-5/6 functions in a continuous loop to ensure that germ plasm asymmetry is reestablished in each P blastomere.
2.2.2 Asymmetric Segregation of P Granules: Not Essential?
The P granules are the only components of the germ plasm that persist in all germ cells throughout the development (except in sperm, Updike and Strome 2010). P or “germ” granules have been observed in the germ plasm and/or germ cells of all animals examined (Strome and Lehmann 1997). By electron microscopy in zygotes, P granules appear as round, electron-dense structures without membranes and dispersed throughout the cytoplasm (Wolf et al. 1983). Starting in P2, P granules associate with the cytoplasmic face of the nuclear envelope, where they will remain until gametogenesis. P granules exclude macromolecules larger than 70 kDa and greater, and have been proposed to extend the nuclear pore environment of the nuclear membrane into the cytoplasm (Updike et al. 2011).
P granules contain both constitutive components present at all stages of development and stage-specific components. Constitutive components include the RGG domain RNA-binding proteins PGL-1 and PGL-3 (Kawasaki et al. 1998, 2004) and the Vasa-related RNA helicases GLH-1,2,3 and 4 (Roussell and Bennett 1993; Kuznicki et al. 2000). PGL-1/3 are the core scaffolding components of P granules and can assemble into granules when expressed on their own in tissue culture cells (Hanazawa et al. 2011). Mutations in pgl and glh genes interfere with larval germ cell proliferation and gamete formation (Kawasaki et al. 2004; Spike et al. 2008). The most severe defects are seen when the worms are raised at high temperature or when mutations in multiple genes are combined. For example, pgl-1 mutants are fertile at 20 °C but sterile with underproliferated germlines at 26 °C. Double loss of pgl-1 and pgl-3 leads to sterility even at low temperature (Kawasaki et al. 2004). In all mutant combinations, however, germ cells are still formed, suggesting that P granule proteins are required primarily for germ cell proliferation and/or differentiation, but not for germ cell fate specification (Kawasaki et al. 2004; Spike et al. 2008). The redundancy and strong maternal contribution of PGL and GLH proteins, however, has made it difficult to exclude a potential role for P granules in germ cell fate specification in embryos.
In embryos, several germ plasm proteins are enriched on P granules (e.g., PIE-1, POS-1, MEX-1, MEX-3, MEG-1, MEG-2, Sm proteins), raising the possibility that P granules organize the germ plasm. Dynamic association of PIE-1 with P granules has been suggested to drive PIE-1 partitioning into P blastomeres by slowing down PIE-1 diffusion in the cytoplasm destined for P blastomeres (Daniels et al. 2009). Mutants that mislocalize P granules to somatic blastomeres or misexpress P granule components in somatic cells, however, do not make extra germ cells, suggesting that P granules on their own are not sufficient to assemble germ plasm and/or specify germ cell fate (Strome et al. 1995; Tabara et al. 1999; Mello et al. 1992). Mutants that mislocalize P granules often fail to form primordial germ cells (i.e., mes-1), but because these mutants also missegregate other germ plasm components, a specific requirement for P granules could not be inferred.
Recently, a gene required specifically for the asymmetric partitioning of P granules was identified. pptr-1 codes for a regulatory subunit of the phosphatase PP2A. In pptr-1 mutants, P granules disassemble during each embryonic cell division. As a result, P granule components, including PGL-1/3, GLH-1/2/4 and the P granule-associated mRNAs cey-2 and nos-2 are partitioned equally to somatic and germline blastomeres. Surprisingly, other germ plasm components (including PAR-1, MEX- 5/6 and PIE-1) still segregate asymmetrically in pptr-1 mutants, demonstrating that P granules are in fact not essential to organize germ plasm. Consistent with normal MEX-5 and MEX-6 partitioning, nos-2 and cey-2 mRNAs are quickly degraded in each somatic blastomere in pptr-1 mutants. After MEX-5 and MEX-6 turnover in the somatic lineages, PGL and GLH proteins reassemble into granules during inter-phase, but these granules appear in all cells and become progressively smaller with each division. By the time of the birth of Z2 and Z3, all cells have either very small or undetectable granules (Gallo et al. 2010).
The PGL granules inherited by somatic blastomeres in pptr-1 mutants are eventually eliminated by autophagy after gastrulation (Zhang et al. 2009). During midembryogenesis, when zygotic transcription of P granule components begins, Z2 and Z3 assemble new P granules. At that time, Z2 and Z3 also initiate expression of the nos-2 paralog nos-1, as they do in wild-type (Subramaniam and Seydoux 1999). Consistent with proper specification of Z2 and Z3, 100 % of pptr-1 mutants are fertile when raised at 20 °C (Gallo et al. 2010). These observations demonstrate that P granule partitioning is not essential to distinguish soma from germline. If P granules harbor factors that promote germ cell fate, these factors must be quickly inactivated in somatic cells, possibly by MEX-5 and MEX-6.
When raised at 26 °C, 20 % of pptr-1 mutants grow into sterile adults with underproliferated germlines. The pptr-1 phenotype is reminiscent of the phenotype of pgl and glh mutants, and is exacerbated by mutations in pgl-1: 15 % of pptr-1;pgl-1 double mutants are sterile at 20 °C (Gallo et al. 2010). These observations suggest that asymmetric inheritance of maternal P granules, although not essential, ensures that Z2 and Z3 have sufficient P granule material before starting to divide in the larva. Because pptr-1 mutants missegregate but do not eliminate all maternal P granule components, the possibility remains that P granules also contribute to germ cell fate specification, perhaps as permissive rather than instructive cues.
2.2.3 Cell-to-Cell Signaling: Also Required?
Specification of the embryonic germ lineage also depends on at least one cell–cell interaction. MES-1 is a transmembrane protein that functions with SRC-1 to mediate bidirectional signaling between EMS and P2. This signaling is required to polarize the EMS spindle and to reverse the polarity of P2 to ensure that P3 arises in the anterior (Strome et al. 1995; Berkowitz and Strome 2000; Bei et al. 2002). In the absence of MES-1, P3 divides symmetrically, and P4 adopts the somatic fate of its sister D. Both cells inherit P granules and other germ plasm components (Strome et al. 1995). The P4 to D transformation could be due to “dilution” of the germ plasm below a certain threshold necessary to induce germ cell fate. If so, MES-1 signaling could contribute to germ cell fate indirectly by promoting P3 polarity. Consistent with this possibility, MES-1 has been shown to be required for the proper localization of PAR-2 (Arata et al. 2010). Another possibility, however, is that signaling by MES-1 also induces other changes in P2 and P3 required directly to specify or maintain “germ cell fate.” Because no experiment has yet shown that the germ plasm is sufficient to induce germ cell fate in C. elegans, the possibility that other mechanisms are involved, including induction by cell–cell interactions, cannot be excluded at this time.
2.3 Molecular Mechanisms of Germ Cell Specification
While no single molecular mechanism has been shown yet to be sufficient to induce germ cell fate, several have been suggested to be required for the proper development of P blastomeres and/or Z2 and Z3. We consider each of these in turn below.
2.3.1 Translational Regulation of Maternal RNAs
Several germ plasm components are RNA-binding proteins (Table 2.1). Mutations in these proteins lead to embryonic lethality and cell fate transformations affecting both somatic and germline blastomeres. POS-1 and MEX-3 regulate the translation of several mRNAs and are required to maintain germ plasm asymmetry (Tabara et al. 1999; Jadhav et al. 2008; Mello et al. 1992; Draper et al. 1996). The complex phenotypes of these mutants make it difficult to evaluate their direct contribution to germ cell fate. Because each RNA-binding protein exhibits a unique pattern of perdurance within the germ plasm, one possibility is that they function combinatorially to specify the fate of each germline blastomere and their somatic daughters.
As described above, combinatorial control involving multiple RNA-binding proteins has been demonstrated to restrict the translation of zif-1 RNA to somatic blastomeres. Analysis of the nos-2 mRNA supports the view that similar mechanisms cooperate to regulate the translation of mRNAs in the germ plasm. As described above, nos-2 mRNA is maintained throughout the P lineage but translated only in P4. Silencing of nos-2 translation requires SPN-4, OMA-1, OMA-2, MEX-3, 5, and 6, and activation requires PIE-1 and POS-1 (Jadhav et al. 2008; Tenenhaus et al. 2001; D’agostino et al. 2006). OMA-1, OMA-2 and MEX-3 silence nos-2 during oogenesis, whereas SPN-4 is required primarily to silence nos-2 in embryos. POS-1 and SPN-4 compete for binding to the nos-2 3′ UTR; when SPN-4 levels fall below a threshold in P4, POS-1 prevails and activates nos-2 translation (Jadhav et al. 2008).
The role of PIE-1 in the translational activation of nos-2 is less understood, but is distinct from PIE-1’s role in transcriptional repression (described below). A pie-1 transgene with mutations in the second zinc finger (PIE-1ZF2−) rescues the transcriptional defects of a pie-1 null mutation, but is not sufficient to activate nos-2 translation in P4 (see below). In embryos expressing PIE-1ZF2−, Z2 and Z3 form normally, but do not gastrulate efficiently. In some embryos, Z2 and Z3 are never incorporated into the embryo proper, and are left behind when the larva crawls out of the egg shell at hatching (Tenenhaus et al. 2001).
These observations support the view that germ plasm proteins, such as PIE-1, promote the translation of mRNAs required for the proper development and/or specification of Z2 and Z3. The identity of these mRNAs is not yet known. In embryos where nos-2 is depleted by RNAi, Z2 and Z3 gastrulate normally, and only occasionally fail to associate with the somatic gonad, suggesting that PIE-1 also regulates other mRNAs besides nos-2.
Analysis of MEG-1 and MEG-2 supports the view that regulation of germ plasm mRNAs is essential for the proper specification of Z2 and Z3. MEG-1 and MEG-2 are two partially redundant novel proteins that associate with P granules specifically in the P2, P3, and P4 blastomeres. Loss of meg-1 and meg-2 leads to germ cell death in the L3 stage (Leacock and Reinke 2008). Interestingly, meg-1 interacts genetically with nos-2. nos-2(RNAi);meg-1(vr10) animals show the most severe phenotype reported for Z2 and Z3: the cells never proliferate, lose perinuclear P granules, and die by the first larval stage in an apoptosis-independent manner (Kapelle and Reinke 2011). Since MEG-1 and NOS-2 expression overlaps only in P4, events critical for germ cell fate specification likely occur first in this cell.
NOS-2 levels are partially reduced in meg-1 embryos, raising the possibility that like other germ plasm components, MEG-1 regulates the expression of germ plasm RNAs. MEG-1 does not contain any recognizable RNA-binding motif, but shows complex genetic interactions with RNA-binding proteins that function during larval germline development (Leacock and Reinke 2008; Kapelle and Reinke 2011). One possibility is that RNA regulation by the MEGs and other germ plasm components initiates the network of protein–RNA regulation that drives germ cell proliferation (see Chap. 8, Nousch and Eckmann 2012).
By the mid-embryogenesis, Z2 and Z3 initiate the transcription of nos-1, another Nanos homolog which functions partially redundantly with nos-2. Embryos lacking both nos-1 and nos-2 do not downregulate marks of active transcription in Z2 and Z3 and all germ cells degenerate during the L3 and L4 larval stages (Subramaniam and Seydoux 1999; Furuhashi et al. 2010). Nanos family members are RNA-binding proteins that often function with the PUF family of translational regulators (Parisi and Lin 2000), so nos-2 and nos-1 likely function by regulating the translation of other mRNAs, but the identity of these targets is not known.
Biochemical experiments have begun to define the RNA-binding specificity of some germ plasm proteins (POS-1, MEX-3, MEX-5, Pagano et al. 2007; Farley et al. 2008; Pagano et al. 2009). These types of approaches, together with the identification of RNAs bound by germ plasm proteins in vivo, may help elucidate the complex network of protein–RNA interactions that specify the fate of Z2 and Z3.
2.3.2 Inhibition of mRNA Transcription
As described above, the germline blastomeres P0–P4 maintain many maternally inherited mRNAs, but do not transcribe any mRNAs de novo. RNA polymerase II is present in the P blastomeres, but kept inactive by two distinct mechanisms.
2.3.2.1 Inhibition of TAF-4 by OMA-1 and OMA-2
In addition to their role as translational regulators (see above), OMA-1 and OMA-2 also inhibit transcription in the zygote. OMA-1 and OMA-2 interact with TAF-4, a component of the TFIID transcription complex. To activate transcription, TAF-4 must bind to TAF-12 in the nucleus. OMA-1 and 2 compete with TAF-12 for binding to TAF-4, and sequester TAF-4 in the cytoplasm (Guven-Ozkan et al. 2008). OMA-1 and OMA-2 are made during oogenesis, but become competent to bind TAF-4 only in the zygote due to phosphorylation by MBK-2, a kinase activated during the oocyte-to-embryo transition (see above). Phosphorylation by MBK-2 also induces degradation of OMA-1/2 by the two-cell stage (Pellettieri et al. 2003; Stitzel et al. 2006). Regulation by MBK-2 ensures that OMA-1/2 inhibit zygotic transcription specifically in the zygote and early 2-cell stage. OMA-1/2 turnover in the 2-cell stage releases TAF-4 and activates mRNA transcription in the somatic blastomeres ABa and Abp by the three-cell stage (Guven-Ozkan et al. 2008, also see Robertson and Lin 2012, Chap. 12).
2.3.2.2 Inhibition of RNA Polymerase II Phosphorylation by PIE-1
In the germline blastomeres P2, P3, and P4, transcription remains repressed through the action of PIE-1. Unlike other germ plasm components, which are primarily cytoplasmic, PIE-1 also accumulates in the nuclei of each P blastomere (Mello et al. 1996). In pie-1 mutants, high levels of CTD phosphorylation appear prematurely in P2, P3, and P4 (Seydoux and Dunn 1997). Studies in mammalian cells have shown that PIE-1 inhibits P-TEF-b, the cyclin T-Cdk9 complex that phosphorylates Serine 2 in the CTD repeats of RNA polymerase. PIE-1 binds to cyclin T and inhibits P-TEF-b kinase activity using a pseudo-substrate motif that resembles a nonphosphorylatable version of the CTD (Batchelder et al. 1999). Genetic studies have shown that this activity, although functional in the germline blastomeres, is not essential to promote germ cell fate. A pie-1 transgene with mutations in the pseudo-substrate motif fails to repress Serine 2 phosphorylation as expected, but still inhibits Serine 5 phosphorylation and mRNA transcription. In fact, such a transgene is sufficient to rescue a pie-1 loss-of-function mutant to viability and fertility (Ghosh and Seydoux 2008). These observations suggest that PIE-1 uses redundant mechanisms to inhibit RNA polymerase II activity and promote germ cell fate.
Why inhibit mRNA transcription in germline blastomeres? The phenotype of pie-1 null mutants provides one clue. In pie-1 mutants, P2 adopts the fate of its somatic sister EMS. pie-1 embryos die as disorganized embryos with excess intestine and pharyngeal cells (EMS fates) and no germ cells (Mello et al. 1992). This cell fate transformation depends on the transcription factor SKN-1. SKN-1 is maternally encoded and present at high levels in both P2 and EMS (Bowerman et al. 1993). One hypothesis therefore is that repression of mRNA transcription serves to protect germline blastomeres from transcription factors like SKN-1 that would otherwise induce somatic development (Seydoux et al. 1996).
Since the original observations in C. elegans, inhibition of RNA polymerase II phosphorylation has been observed in the embryonic germlines of Drosophila, Xenopus, ascidians, and mice (Nakamura and Seydoux 2008; Hanyu-Nakamura et al. 2008; Shirae-Kurabayashi et al. 2011; Kumano et al. 2011; Venkatarama et al. 2010). The factors responsible have been identified in Drosophila and ascidians and, remarkably, bear no resemblance to OMA-1/2 or PIE-1 (Hanyu-Nakamura et al. 2008; Shirae-Kurabayashi et al. 2011; Kumano et al. 2011). Inhibition of RNA polymerase II appears, therefore, to be conserved characteristic of germline development that depends on multiple mechanisms that have diverged during animal evolution.
2.3.3 Chromatin Regulation
While the chromatin of P0–P3 resembles that of somatic blastomeres, the chromatin of P4, Z2, and Z3 adopts a distinct compact configuration. PSer2 and PSer5 appear in Z2 and Z3 at birth coincident with degradation of PIE-1 at that time (Seydoux and Dunn 1997). By mid-embryogenesis, however, PSer2 and PSer5 levels are low again and Z2 and Z3 also become negative for the “active” chromatin marks H3K4me2, H3K4me3, and H4K8ac (Furuhashi et al. 2010; Schaner et al. 2003). PSer2, PSer5, and H3K4me reappear in Z2 and Z3 after hatching (Furuhashi et al. 2010). These observations suggest that Z2 and Z3 remain in a relatively transcriptionally repressed state during embryogenesis, although unlike P0–P4, they are capable of transcribing at least a few messages (see Sect. 2.1.2.3). Loss of H3K4me depends on nos-1 and nos-2 (Schaner et al. 2003). Whether the unique chromatin of Z2 and Z3 depends on their arrest in G2 is also not known (Fukuyama et al. 2006).
Genetic screens designed to identify maternal factors required for fertility identified four genes coding for chromatin regulators: MES-2, 3, 4, and 6. Mutations in these genes are maternal effect sterile (MES): homozygous mothers are fertile but give rise to sterile progeny (“grandchildless” phenotype). Z2 and Z3 cells are made in embryos derived from mes/mes mothers, and proliferate during the first two larval stages but die by necrosis in the L3 and L4 stages (Capowski et al. 1991; Paulsen et al. 1995). In mes-4 mutants, Z2 and Z3 retain pSer 2 (Furuhashi et al. 2010), suggesting that these cells are already compromised during embryogenesis. mes germ cells are also unable to differentiate: ablation of somatic gonadal cells in the L2 stage, which causes wild-type germ cells to differentiate prematurely, only causes mes-3 germ cells to stop proliferating (Paulsen et al. 1995).
MES-2/3/6 forms a complex related to Enhancer of Zeste that methylates Lys 27 of histone H3, a repressive mark that accumulates on the X chromosome (Xu et al. 2001; Bender et al. 2004). Consistently, the X is mostly inactive in germ cells (with the exception of oocytes; Schaner and Kelly 2006; Reinke 2006; Spencer et al. 2011). MES-4 methylates Lys 36 of histone H3, and MES-4 accumulates preferentially on autosomes (Bender et al. 2006). This specificity depends on MES-2/3/6: in mes-2, 3, and 6 mutants, MES-4 binds all along the X chromosome and the X is inappropriately activated in germ cells (Fong et al. 2002; Bender et al. 2006). Chromatin immunoprecipitation experiments revealed that, in embryos, MES-4 associates preferentially with genes that were active in the maternal germ line. For example, MES-4 associates with meiotic genes that are transcribed in germ cells but not in embryos, and does not associate with genes that are transcribed in embryos but not in the maternal germline (Rechtsteiner et al. 2010). H3K36 methylases typically mark genes in a transcription-dependent manner. Surprisingly, MES-4 appears unable to establish the H3K36 mark de novo, but is able to maintain the mark in the embryonic germ lineage even though RNA polymerase II is not active in the P blastomeres (Furuhashi et al. 2010; Rechtsteiner et al. 2010). Although further analysis is necessary to clarify the link between genes bound by mes-4 and those that are misregulated in mes-4 mutants, the results so far suggest that MES-4 functions as an “epigenetic memory factor” that marks genes expressed in the maternal germline for the next generation. Maternal contribution of another chromatin-associated protein, MRG-1, is also required for robust germ cell proliferation in the progeny (Takasaki et al. 2007), suggesting that inheritance of a specific chromatin state is key for germ cell development.
MES-4 is inherited maternally and segregated to all blastomeres. After the 100-cell stage, MES-4 is maintained primarily in Z2 and Z3 (Fong et al. 2002). The mechanisms that allow high levels of MES-4 to persist only in the germline are not known. Genetic evidence suggests that MES-4 is also active, at least transiently, in somatic lineages and is antagonized there by the synMuv B class of chromatin regulators. In synMuvB mutants, intestinal cells express germline genes and this ectopic expression requires MES-4 (Unhavaithaya et al. 2002; Wang et al. 2005). When grown at high temperatures, synMuv B mutants arrest as starved larvae, perhaps because germline gene expression compromises intestinal function (Petrella et al. 2011). One possibility is that maternal MES-4 initially confers competence for the germline transcriptional program to all blastomeres, including the intestinal founder cell (E blastomere). During embryogenesis, this competence is erased by the synMuv B complex in somatic lineages, but not in the P lineage, perhaps because that lineage activates transcription later and maintains maternal MES-4 for longer.
2.3.4 Epigenetic Licensing by Maternal RNA
A recent report suggests that activation of the germline transcriptional program also depends on maternal inheritance of specific germline transcripts. The fem-1 gene is required for masculinization of the germline and soma (Doniach and Hodgkin 1984). Mothers homozygous for deletions that remove the fem-1 gene produce progeny with feminized germlines, even when these progeny inherit a wild-type copy of the fem-1 gene from their father. This maternal effect can be rescued by injecting fem-1 RNA in the maternal germline. Remarkably, rescue is observed even when the injected RNA lacks a start codon, spans only short sub-regions of the fem-1 gene, or is antisense to the fem-1 transcript, indicating that inheritance of maternal fem-1 RNA, but not FEM-1 protein, is needed to “ license ” zygotic expression of the fem-1 gene (Johnson and Spence 2011). One possibility is that new germline transcripts are continuously compared to maternally inherited transcripts to avoid expression of potentially toxic “intruder genes.” Whether this phenomenon is specific to fem-1 or extends to other germline genes remains to be determined.
2.4 Conclusions and Remaining Questions
While the precise molecular mechanisms that specify germ cell fate remain elusive, several themes have emerged. First key to the delineation of distinct soma and germ lineages is the PAR-1-MEX-5/6 polarity axis. MEX-5/6 promotes the disassembly and degradation of germ plasm components in somatic lineages and PAR-1 stabilizes the germ plasm in the germ lineage, in part by physically excluding MEX-5 and MEX-6. The distinction between soma and germline, therefore, involves both active turnover of the germ plasm in somatic cells and protection of the germ plasm in the P blastomeres. Second, although P granules contribute to the proliferation and viability of germ cells during post-embryonic development, P granules are unlikely to be sufficient to specify germ cell fate during embryogenesis. We suggest instead that germ cell fate is specified by the collective action of RNAs and RNA-binding proteins found throughout the germ plasm. In the germline blastomeres, these factors mediate two important functions: (1) inhibition of mRNA transcription which prevents somatic transcription factors from activating somatic development and (2) translation of nos-2 and other maternal mRNAs whose products promote gastrulation of the primordial germ cells, adhesion to the intestine, and a unique partially repressive chromatin configuration. In Z2 and Z3, the chromatin regulator MES-4, perhaps with the help of “licensing RNAs” in the germ plasm, transmits the “memory” of the maternal germline transcriptional program.
The task of germ cell specification in the embryo may be viewed as a careful balancing act between the need to generate new (somatic) cell types and the need to preserve the germ cell program of the oocyte. In this context, the P0–P3 blastomeres may be considered an intermediate cell type, similar to the epiblast cells of the mammalian embryo, where the potential for soma and germline fates temporarily co-exist. Global silencing of transcription and of the translation of certain germline mRNAs (e.g., nos-2) in these cells ensures that neither program takes over. P4 in contrast may be considered the first cell where the germ cell fate program is returned to its original state, but how this program is implemented to modify the chromatin of P4 is not known.
We also do not yet know when P4 and/or Z2 and Z3 first activate the germline-specific transcription program. In many studies, “germ cell fate” is evaluated using markers present in germ plasm (such as P granules), but such markers do not necessarily indicate active commitment to germ cell fate. For example, Subramaniam et al. concluded that nos-1 and nos-2 are not required for germ cell fate because in nos-1;nos-2 larvae, the dying “germ cells” still expressed certain germline-specific markers, but whether these markers were maternally inherited or expressed de novo in those cells was not determined (Subramaniam and Seydoux 1999). Because maternal products can perdure in the germline into larval stages (Kawasaki et al. 1998), it will be important in future studies to use markers indicative of an “active germline program” such as germline-specific chromatin marks or zygotic transcripts (as in Schaner et al. 2003; Takasaki et al. 2007). Sequencing of RNAs isolated from Z2 and Z3 dissected from mid-stage embryos has confirmed that these cells already produce several germline-specific transcripts (Gerstein et al. 2010; Spencer et al. 2011). Analyses of the zygotic transcriptome of Z2 and Z3 may provide further insights into the molecular mechanisms that specify germ cell fate.
Acknowledgments
We thank members of the Seydoux lab for helpful discussions. We gratefully acknowledge funding from NIH (T32 HD007276 to J.W. and HD037047 to G.S.) and the Howard Hughes Medical Institute.
References
- Arata Y, Lee J-Y, Goldstein B, Sawa H. Extracellular control of PAR protein localization during asymmetric cell division in the C. elegans embryo. Development. 2010;137:3337–3345. doi: 10.1242/dev.054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SA, Evans TC. The Sm proteins regulate germ cell specification during early C. elegans embryogenesis. Dev Biol. 2006;291:132–143. doi: 10.1016/j.ydbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Barbee SA, Lublin A, Evans TC. A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr Biol. 2002;12:1502–1506. doi: 10.1016/s0960-9822(02)01111-9. [DOI] [PubMed] [Google Scholar]
- Batchelder C, Dunn MA, Choy B, et al. Transcriptional repression by the Caenorhabditis elegans germ-line protein PIE-1. Genes Dev. 1999;13:202–212. doi: 10.1101/gad.13.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh LR, Hill AA, Slonim DK, et al. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- Bei Y, Hogan J, Berkowitz LA, et al. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev cell. 2002;3:113–125. doi: 10.1016/s1534-5807(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Bender L, Cao R, Zhang Y, Strome S. The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr Biol. 2004;14:1639–1643. doi: 10.1016/j. [DOI] [PubMed] [Google Scholar]
- Bender LB, Suh J, Carroll CR, et al. MES-4: an autosome-associated histone methyltransferase that participates in silencing the X chromosomes in the C. elegans germ line. Development. 2006;133:3907–3917. doi: 10.1242/dev.02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz LA, Strome S. MES-1, a protein required for unequal divisions of the germline in early C. elegans embryos, resembles receptor tyrosine kinases and is localized to the boundary between the germline and gut cells. Development. 2000;127:4419–4431. doi: 10.1242/dev.127.20.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman B, Draper BW, Mello CC, Priess JR. The maternal gene skn-1 encodes a protein that is distributed unequally in early C. elegans embryos. Cell. 1993;74:443–452. doi: 10.1016/0092-8674(93)80046-h. [DOI] [PubMed] [Google Scholar]
- Boyd L, Guo S, Levitan D, et al. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 1996;122:3075–3084. doi: 10.1242/dev.122.10.3075. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Baumer K, Gönczy P. Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr Biol. 2003;13:819–827. doi: 10.1016/S. [DOI] [PubMed] [Google Scholar]
- Budirahardja Y, Gönczy P. PLK-1 asymmetry contributes to asynchronous cell division of C. elegans embryos. Development. 2008;135:1303–1313. doi: 10.1242/dev.019075. [DOI] [PubMed] [Google Scholar]
- Capowski EE, Martin P, Garvin C, Strome S. Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics. 1991;129:1061–1072. doi: 10.1093/genetics/129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeks RJ, Canman JC, Gabriel WN, et al. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol. 2004;14:851–862. doi: 10.1016/j. [DOI] [PubMed] [Google Scholar]
- Cuenca AA, Schetter A, Aceto D, et al. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development. 2003;130:1255–1265. doi: 10.1242/dev.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino I, Merritt C, Chen P-L, et al. Translational repression restricts expression of the C. elegans Nanos homolog NOS-2 to the embryonic germline. Dev Biol. 2006;292:244–252. doi: 10.1016/j.ydbio.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Daniels BR, Perkins EM, Dobrowsky TM, et al. Asymmetric enrichment of PIE-1 in the Caenorhabditis elegans zygote mediated by binary counter diffusion. J Cell Biol. 2009;184:473–479. doi: 10.1083/jcb.200809077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRenzo C, Reese KJ, Seydoux G. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature. 2003;424:685–689. doi: 10.1038/nature01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniach T, Hodgkin J. A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev Biol. 1984;106:223–235. doi: 10.1016/0012-1606(84)90077-0. [DOI] [PubMed] [Google Scholar]
- Draper BW, Mello CC, Bowerman B, et al. MEX-3 is a KH domain protein that regulates blastomere identity in early C. elegans embryos. Cell. 1996;87:205–216. doi: 10.1016/s0092-8674(00)81339-2. [DOI] [PubMed] [Google Scholar]
- Encalada SE, Martin PR, Phillips JB, et al. DNA replication defects delay cell division and disrupt cell polarity in early Caenorhabditis elegans embryos. Dev Biol. 2000;228:225–238. doi: 10.1006/dbio.2000.9965. [DOI] [PubMed] [Google Scholar]
- Farley BM, Pagano JM, Ryder SP. RNA target specificity of the embryonic cell fate determinant POS-1. RNA. 2008;14:2685–2697. doi: 10.1261/rna.1256708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Bender L, Wang W, Strome S. Regulation of the different chromatin states of autosomes and X chromosomes in the germ line of C. elegans. Science. 2002;296:2235–2238. doi: 10.1126/science.1070790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Rougvie AE, Rothman JH. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol. 2006;16:773–779. doi: 10.1016/j.cub.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Furuhashi H, Takasaki T, Rechtsteiner A, et al. Trans-generational epigenetic regulation of C. elegans primordial germ cells. Epigenetics chromatin. 2010;3:15. doi: 10.1186/1756-8935-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CM, Munro E, Rasoloson D, et al. Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev Biol. 2008;323:76–87. doi: 10.1016/j.ydbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Gallo CM, Wang JT, Motegi F, Seydoux G. Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans. Science. 2010;330:1685–1689. doi: 10.1126/science.1193697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein MB, Lu ZJ, Van Nostrand EL, et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–1787. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Seydoux G. Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics. 2008;178:235–243. doi: 10.1534/genetics.107.083212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P, Rose LS. Wormbook, editor. The C elegans Research Community. Wormbook; 2005. Asymmetric cell division and axis formation in the embryo; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EE, Odde DJ, Seydoux G. Regulation of the MEX-5 gradient by a spatially segregated kinase/phosphatase cycle. Cell. 2011;146:955–968. doi: 10.1016/j.cell.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes S, Priess JR. The C. elegans MEX-1 protein is present in germline blastomeres and is a P granule component. Development. 1997;124:731–739. doi: 10.1242/dev.124.3.731. [DOI] [PubMed] [Google Scholar]
- Guo S, Kemphues K. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Guven-Ozkan T, Nishi Y, Robertson SM, Lin R. Global transcriptional repression in C. elegans germline precursors by regulated sequestration of TAF-4. Cell. 2008;135:149–160. doi: 10.1016/j.cell.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven-Ozkan T, Robertson SM, Nishi Y, Lin R. zif-1 translational repression defines a second, mutually exclusive OMA function in germline transcriptional repression. Development. 2010;137:3373–3382. doi: 10.1242/dev.055327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M, Yonetani M, Sugimoto A. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J Cell Biol. 2011;192:929–937. doi: 10.1083/jcb.201010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, et al. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature. 2008;451:730–733. doi: 10.1038/nature06498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JR, Goldstein B. Internalization of multiple cells during C. elegans gastrulation depends on common cytoskeletal mechanisms but different cell polarity and cell fate regulators. Dev Biol. 2011;350:1–12. doi: 10.1016/j.ydbio.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird SN, Paulsen JE, Strome S. Segregation of germ granules in living Caenorhabditis elegans embryos: cell-type-specific mechanisms for cytoplasmic localisation. Development. 1996;122:1303–1312. doi: 10.1242/dev.122.4.1303. [DOI] [PubMed] [Google Scholar]
- Jadhav S, Rana M, Subramaniam K. Multiple maternal proteins coordinate to restrict the translation of C. elegans nanos-2 to primordial germ cells. Development. 2008;135:1803–1812. doi: 10.1242/dev.013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Spence AM. Epigenetic licensing of germline gene expression by maternal RNA in C. elegans. Science. 2011;333:1311–1314. doi: 10.1126/science.1208178. [DOI] [PubMed] [Google Scholar]
- Kapelle WS, Reinke V. C. elegans meg-1 and meg-2 differentially interact with nanos family members to either promote or inhibit germ cell proliferation and survival. Genesis. 2011;49:380– 391. doi: 10.1002/dvg.20726. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Shim YH, Kirchner J, et al. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Amiri A, Fan Y, et al. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics. 2004;167:645– 661. doi: 10.1534/genetics.103.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kumano G, Takatori N, Negishi T, et al. A maternal factor unique to ascidians silences the germline via binding to P-TEFb and RNAP II regulation. Curr Biol. 2011;21:1308–1313. doi: 10.1016/j.cub.2011.06.050. [DOI] [PubMed] [Google Scholar]
- Kuznicki KA, Smith PA, Leung-Chiu WM, et al. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 2000;127:2907–2916. doi: 10.1242/dev.127.13.2907. [DOI] [PubMed] [Google Scholar]
- Leacock SW, Reinke V. MEG-1 and MEG-2 are embryo-specific P-granule components required for germline development in Caenorhabditis elegans. Genetics. 2008;178:295–306. doi: 10.1534/genetics.107.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeBella LR, Guven-Ozkan T, Lin R, Rose LS. An eIF4E-binding protein regulates katanin protein levels in C. elegans embryos. J Cell Biol. 2009;187:33–42. doi: 10.1083/jcb.200903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald A, Illmensee K. Transplantation of posterior polar plasm in drosophila. Induction of germ cells at the anterior pole of the egg. PNAS. 1974;71:1016–1020. doi: 10.1073/pnas.71.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Draper BW, Weintraub H, Priess JF. The pie-1 and mex-1 genes and maternal control of blastomere in early C. elegans embryos. Cell. 1992;70:163–176. doi: 10.1016/0092-8674(92)90542-k. [DOI] [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, et al. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Seydoux G. Less is more: specification of the germline by transcriptional repression. Development. 2008;135:3817–3827. doi: 10.1242/dev.022434. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Lin R. DYRK2 and GSK-3 phosphorylate and promote the timely degradation of OMA-1, a key regulator of the oocyte-to-embryo transition in C. elegans. Dev Biol. 2005;288:139–149. doi: 10.1016/j.ydbio.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Rogers E, Robertson SM, Lin R. Polo kinases regulate C. elegans embryonic polarity via binding to DYRK2-primed MEX-5 and MEX-6. Development. 2008;135:687–697. doi: 10.1242/dev.013425. [DOI] [PubMed] [Google Scholar]
- Nousch M, Eckmann CR. Advances in Experimental Medicine and Biology. Vol. 757. Springer; New York: 2012. Translational control in the C. elegans germ line; pp. 205–247. (Chap. 8, this volume) [DOI] [PubMed] [Google Scholar]
- Oldenbroek M, Robertson SM, Guven-Ozkan T, et al. Multiple RNA-binding proteins function combinatorially to control the soma-restricted expression pattern of the E3 ligase subunit ZIF-1. Dev Biol. 2012;363:388–398. doi: 10.1016/j.ydbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano JM, Farley BM, McCoig LM, Ryder SP. Molecular basis of RNA recognition by the embryonic polarity determinant MEX-5. J Biol Chem. 2007;282:8883–8894. doi: 10.1074/jbc.M700079200. [DOI] [PubMed] [Google Scholar]
- Pagano JM, Farley BM, Essien KI, Ryder SP. RNA recognition by the embryonic cell fate determinant and germline totipotency factor MEX-3. Proc Natl Acad Sci USA. 2009;106:20252–20257. doi: 10.1073/pnas.0907916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M, Lin H. Translational repression: a duet of Nanos and Pumilio. Curr Biol. 2000;10:R81–R83. doi: 10.1016/s0960-9822(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Paulsen JE, Capowski EE, Strome S. Phenotypic and molecular analysis of mes-3, a maternal- effect gene required for proliferation and viability of the germ line in C. elegans. Genetics. 1995;141:1383–1398. doi: 10.1093/genetics/141.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, Reinke V, Kim SK, Seydoux G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev Cell. 2003;5:451–462. doi: 10.1016/s1534-5807(03)00231-4. [DOI] [PubMed] [Google Scholar]
- Petrella LN, Wang W, Spike CA, et al. synMuv B proteins antagonize germline fate in the intestine and ensure C. elegans survival. Development. 2011;138:1069–1079. doi: 10.1242/dev.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtsteiner A, Ercan S, Takasaki T, et al. The histone H3K36 methyltransferase MES-4 acts epigenetically to transmit the memory of germline gene expression to progeny. PLoS Genetics. 2010 doi: 10.1371/journal.pgen.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V. WormBook, editor. The C elegans Research Community. Wormbook; 2006. Germline genomics; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers DM, Moreno S, Abraham M, Ahringer J. PAR proteins direct asymmetry of the cell cycle regulators Polo-like kinase and Cdc25. J Cell Biol. 2008;180:877–885. doi: 10.1083/jcb.200710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Lin R. Advances in Experimental Medicine and Biology. Vol. 757. Springer; New York: 2012. The oocyte-to-embryo transition; pp. 351–372. (Chap. 12, this volume) [DOI] [PubMed] [Google Scholar]
- Roussell DL, Bennett KL. glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc Natl Acad Sci USA. 1993;90:9300–9304. doi: 10.1073/pnas.90.20.9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaner CE, Kelly WG. Wormbook, editor. The C elegans Research Community. Wormbook; 2006. Germline chromatin; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaner CE, Deshpande G, Schedl PD, Kelly WG. A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev Cell. 2003;5:747–757. doi: 10.1016/s1534-5807(03)00327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenberg E. Reversal of cellular polarity and early cell-cell interaction in the embryo of Caenorhabditis elegans. Dev Biol. 1987;122:452–463. doi: 10.1016/0012-1606(87)90309-5. [DOI] [PubMed] [Google Scholar]
- Schierenberg E. Localization and segregation of lineage-specific cleavage potential in embryos of Caenorhabditis elegans. Roux’s Arch Dev Biol. 1988;197:282–293. doi: 10.1007/BF00380022. [DOI] [PubMed] [Google Scholar]
- Schubert CM, Lin R, de Vries CJ, et al. MEX-5 and MEX-6 function to establish soma/germline asymmetry in early C. elegans embryos. Mol Cell. 2000;5:671–682. doi: 10.1016/s1097-2765(00)80246-4. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127:891–904. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development. 1997;124:2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994;120:2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Mello CC, Pettitt J, et al. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382:713–716. doi: 10.1038/382713a0. [DOI] [PubMed] [Google Scholar]
- Shirae-Kurabayashi M, Matsuda K, Nakamura A. Ci-Pem-1 localizes to the nucleus and represses somatic gene transcription in the germline of Ciona intestinalis embryos. Development. 2011;138:2871–2881. doi: 10.1242/dev.058131. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Soto MC, Ishidate T, et al. The conserved kinases CDK-1, GSK-3, KIN-19, and MBK-2 promote OMA-1 destruction to regulate the oocyte-to-embryo transition in C. elegans. Curr Biol. 2006;16:47–55. doi: 10.1016/j.cub.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Spencer WC, Zeller G, Watson JD, et al. A spatial and temporal map of C. elegans gene expression. Genome Res. 2011;21:325–341. doi: 10.1101/gr.114595.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike C, Meyer N, Racen E, et al. Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins. Genetics. 2008;178:1973–1987. doi: 10.1534/genetics.107.083469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker AC, Rabilotta A, Zbinden C, et al. MAP kinase signaling antagonizes PAR-1 function during polarization of the early Caenorhabditis elegans embryo. Genetics. 2009;183:965–977. doi: 10.1534/genetics.109.106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel ML, Pellettieri J, Seydoux G. The C. elegans DYRK kinase MBK-2 marks oocyte proteins for degradation in response to meiotic maturation. Curr Biol. 2006;16:56–62. doi: 10.1016/j.cub.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Strome S. Wormbook, editor. The C elegans Research Community. Wormbook; 2005. Specification of the germ line; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 1997;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- Strome S, Martin P, Schierenberg E, Paulsen J. Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development. 1995;121:2961–2972. doi: 10.1242/dev.121.9.2961. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126:4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Tabara H, Hill RJ, Mello CC, et al. pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development. 1999;126:1–11. doi: 10.1242/dev.126.1.1. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Liu Z, Habara Y, et al. MRG-1, an autosome-associated protein, silences X-linked genes and protects germline immortality in Caenorhabditis elegans. Development. 2007;134:757–767. doi: 10.1242/dev.02771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhaus C, Subramaniam K, Dunn MA, Seydoux G. PIE-1 is a bifunctional protein that regulates maternal and zygotic gene expression in the embryonic germ line of Caenorhabditis elegans. Genes Dev. 2001;15:1031–1040. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenlen JR, Molk JN, London N, et al. MEX-5 asymmetry in one-cell C. elegans embryos requires PAR-4- and PAR-1-dependent phosphorylation. Development. 2008;135:3665–3675. doi: 10.1242/dev.027060. [DOI] [PubMed] [Google Scholar]
- Unhavaithaya Y, Shin TH, Miliaras N, et al. MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell. 2002;111:991–1002. doi: 10.1016/s0092-8674(02)01202-3. [DOI] [PubMed] [Google Scholar]
- Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. J Androl. 2010;31:53–60. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL, Hachey SJ, Kreher J, Strome S. P granules extend the nuclear pore complex environment in the C. elegans germ line. J Cell Biol. 2011;192:939–948. doi: 10.1083/jcb.201010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatarama T, Lai F, Luo X, et al. Repression of zygotic gene expression in the Xenopus germline. Development. 2010;137:651–660. doi: 10.1242/dev.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Kennedy S, Conte D, et al. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–597. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- Wolf N, Priess J, Hirsh D. Segregation of germline granules in early embryos of Caenorhabditis elegans: an electron microscopic analysis. J Embryol Exp Morphol. 1983;73:297–306. [PubMed] [Google Scholar]
- Xu L, Fong Y, Strome S. The Caenorhabditis elegans maternal-effect sterile proteins, MES-2, MES-3, and MES-6, are associated in a complex in embryos. Proc Natl Acad Sci USA. 2001;98:5061. doi: 10.1073/pnas.081016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan L, Zhou Z, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]