OVERVIEW
Ampullary cancers are rare, accounting for only 0.2% of gastrointestinal cancers and approximately 7% of all periampullary cancers.1 They arise from the ampullary complex, distal to the confluence of the common bile and pancreatic duct (Fig. 1). In contrast to other periampullary malignancies, true ampullary cancers present earlier in their disease course with symptoms that result from biliary obstruction. It is often difficult to distinguish primary ampullary cancers from other periampullary cancers preoperatively. In early stages, ampullary cancers are surgically treated, similar to pancreatic cancers, and typically with a pancreatico-duodenoectomy (or Whipple procedure). Because of their earlier presentation, resection rates for all patients are much higher than other periampullary carcinomas. Moreover, their prognosis tends to be better than those with other periampullary- and pancreatic-originating cancers. In patients with true ampullary cancer, there is very limited data to guide physicians on the choice of therapy, largely because of the rarity of the disease and the paucity of related research. Herein, we provide an overview of the biology, histology, current therapeutic strategies, and potential future therapies for carcinomas arising from the ampulla of Vater.
BIOLOGY AND HISTOLOGY
Most ampullary carcinomas are adenocarcinomas, but the histology varies with tumors comprising sub-types including papillary, adenosquamous, mucinous, and adenocarcinomas. Recent studies helped identify two main distinct histologic sub-types of adenocarcinoma based on their epithelium of origin: intestinal and pancreatobiliary.2 Intestinal histology originates from the intestinal epithelium overlying the ampulla, however pancreaticobiliary histology originates from the epithelium of the distal common bile duct and distal pancreatic duct.
Evidence suggests that histologic sub-types also differ in biologic behavior, bearing implications on prognosis and outcome. Differing outcomes are presumably related to the originating epithelia. Notably, ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology (median overall survival of 16 vs. 115.5 months; p < 0.001 respectively).3 When controlled for other risk factors, in resectable periampullary cancers, biologic behavior appears to be the most important prognostic indicator for patient outcome.4
KEY POINTS.
Ampullary cancers represent a small subset of periampullary cancers and represent only 0.2% of all gastrointestinal malignancies.
Ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology.
The management of locoregional disease is primarily a surgical intervention by a pancreaticoduodenectomy (or Whipple’s procedure) followed by the administration of adjuvant chemotherapy (preferably gemcitabine).
The management of unresectable and metastatic disease is primarily through the administration of systemic therapy with an anti-metabolite (fluoropyrimidine and/or gemcitabine) combined with a platinum compound, (usually cisplatin or oxaliplatin).
EARLY STAGE DISEASE: IS ADJUVANT THERAPY INDICATED?
Curative surgery is possible in approximately 50% of ampullary cancer compared to that of less than 10% in pancreatic adenocarcinoma.5 Despite the high rate of potentially curative resection, the majority of patients with ampullary carcinomas will eventually succumb to recurrent disease.6 Given the rarity of this disease, there is absence of randomized clinical trials focused on ampullary carcinomas and treatment recommendations are mainly derived from results of adjuvant clinical trials conducted in pancreaticobiliary cancers where ampullary cancers may represent a sub-group of patients. Given many of the similarities between ampullary and pancreas cancers, patients who have undergone resection are often offered adjuvant chemotherapy with or without the addition of radiotherapy.7
The Role Of Chemoradiation
Although there is no clear guidance in regards to adjuvant therapy, patients with resected ampullary carcinomas and stage IB or higher often receive concurrent chemotherapy with radiation. These findings are based on the results of several randomized controlled trials, which investigated the use of adjuvant concurrent chemoradiation in resected pancreas or biliary cancers. The group at Mayo Clinic published its experience in 2006 suggesting a potential benefit from concurrent adjuvant chemoradiotherapy. In this retrospective study, 29 patients with ampullary cancer who received adjuvant concurrent chemoradiotherapy showed a trend toward the improvement of their overall survival in comparison to the surgery alone group. In the multivariate analysis, lymph node involvement was the only major predictor of outcome, suggesting a potential benefit to adjuvant chemoradiation in those high-risk patients.8 In a larger study that included 113 patients with ampullary cancers who had undergone pancreatico-duodenectomy, adjuvant chemoradiotherapy did not improve the long-term survival or the incidence of loco-regional recurrence.9 The role of adjuvant radiotherapy with 5-fluorouracil (5FU) in resected pancreatic and periampullary cancers was previously examined in a prospective randomized phase III trial. The EORTC trial (40891) examined the role of adjuvant chemoradiation and found no considerable benefit from the concurrent administration of chemoradiation.10 Another study investigated the role of adjuvant intra-arterial chemotherapy in conjunction with radiotherapy, but did not demonstrate a survival benefit.11
Unfortunately, most of the studies examining the role of adjuvant chemoradiation were either retrospective or had a limited sample size with a potential for selection bias or confounding variables that potentially may confound the observed results. Overall, most studies did not suggest the presence of a survival benefit from adjuvant chemoradiation, except perhaps for a subset of patients with adverse risk factors (T stage, lymph node involvement, histologic grade) that may potentially benefit from chemoradiation.8,12,13
The Role of Chemotherapy
The role of adjuvant chemotherapy in pancreatic cancer has been investigated in several randomized trials suggesting a consistent benefit for gemcitabine of 5FU.14,15 One of the first randomized controlled trials investigating the use of adjuvant chemotherapy in periampullary cancers was an international trial from Japan evaluating the role of 5-FU and mitomycin C following resection versus surgery alone. In the ampullary cancer subgroup (n = 56), no significant survival benefit was observed with the addition of chemotherapy.16 ESPAC-3, the largest phase III randomized study to evaluate the role of adjuvant chemotherapy in periampullary carcinomas, included 428 patients that were randomly assigned to one of three arms: observation, 5-FU/leucovorin, or gemcitabine. The use of adjuvant chemotherapy demonstrated a trend toward improving overall survival favoring the chemotherapy group versus observation (median overall [mOS] of 43 vs. 35 months, p =0.25). There was no difference between the two chemotherapy arms. In patients with ampullary cancer only (n = 297), patients who received gemcitabine had a significant improvement in their median overall survival versus the observation group (mOS = 70.8 months in the gemcitabine arm vs. 40.6 months in the observation arm; mOS = 57.8 in the 5-FU arm). Post-hoc analysis did not demonstrate a difference in outcome based on histology with the effectiveness of chemotherapy seen in ampullary cancer in both the adenocarcinoma and pancreaticobiliary histology.17
Early Stage Disease: Conclusions
The majority of patients with resected ampullary carcinomas will eventually die from recurrent disease. Multiple studies suggest a potential survival benefit from the administration of adjuvant chemotherapy, with less support for the use of radiation. Our institutional bias defaults to our pancreatic cancer practice, and patients tend to receive adjuvant chemotherapy with gemcitabine for 24 weeks.
LOCALLY ADVANCED AND METASTATIC DISEASE: WHAT IS THE EVIDENCE FOR SYSTEMIC THERAPY?
Systemic chemotherapy remains the mainstay in the treatment of patients with locally advanced unresectable and metastatic disease. Given the rarity of this disease, our reliance is on limited published data sets including a variety of trials including basket trials that allowed the inclusion of ampullary cancer in addition to small intestinal or biliary duct cancers. Agents that have been examined in this disease include anti-metabolites (fluoropyrimidine and/or gemcitabine) with or without the addition of a platinum compound (usually cisplatin or oxaliplatin). Published work suggests variability in response rates ranging from 10% to 40% and reported median overall survival up to 20 months (Table 1).18–23 A recent phase II trial investigating the combination of capecitabine and oxaliplatin in patients with advanced small bowel or ampullary adenocarcinoma, showed that in the subset analysis of ampullary cancers, patients achieved a response rate of 33%, although the median time to progression and overall survival were reported to be 11.3 and 20.4 months, respectively.19 Another study, ABC-02, which represents the largest randomized controlled trial in biliary tract cancers, investigated the use of cisplatin with gemcitabine versus gemcitabine alone in 410 patients with advanced or metastatic biliary tract cancer. Benefits in overall survival (11.7 vs. 8.1 months) and progression-free survival (8 vs. 5 months) were observed in patients treated with the combination therapy versus gemcitabine alone.18 Subset analysis of patients with ampullary cancers, approximately 5% of the study population, suggested a similar survival benefit with the combination compared to the rest of the study population.
TABLE 1.
Studies Evaluating the Role of Palliative Chemotherapy in Advanced Ampullary Cancer
| Type of Cancer | N | Study Design | Treatment Group | RR (%) | OS (months) | Author |
|---|---|---|---|---|---|---|
| Small Bowel + Ampullary | 30 | Phase II | Capecitabine + oxaliplatin | 33 | 20.4 | Overman et al 2009 |
| Biliary Tract + Ampullary | 410 | Phase III | Gemcitabine + cisplatin | CR: 0.6 | 11.7 | Valle et al 2010 |
| PR: 25.5 | ||||||
| Small Bowel + Ampullary | 38 | Phase II | 5-FU + doxorubicin + mitomycin | 18 | 8 | Gibson et al 2005 |
| Ampullary | 29 | Phase II | Cisplatin + 5-FU, cisplatin + capecitabine, gemcitabine + cisplatin | 27.5 | 12.5 | Kim et al 2010 |
| Biliary Tract + Ampullary | 56 | Phase II | Gemcitabine + oxaliplatin | 7.6–15.4 | Andre et al 2004 | |
| Biliary Tract + Ampullary | 37 | Phase II | Cisplatin + epirubicin + gemcitabine + 5-FU | 43 | 12.1 | Cereda et al 2010 |
Local Recurrence: Is There a Role for Local Approaches?
Recurrence patterns following resection in ampullary cancer include locoregional and distant disease. There is no known role for localized therapy approaches in cases of recurrence. One recent study suggests that palliative reoperation was associated with an unacceptable rate of postoperative mortality of 86% and a median survival of no more than 45 days,24 although another study suggested that reirradiation may have a potential benefit with improved local control.25 At this time, there is no evidence for localized approaches in patients with recurrent disease, with systemic therapy remaining the treatment of choice for those with a good performance status.
CONCLUSION AND FUTURE DIRECTIONS
Despite numerous advances in cancer care and research, efforts in rare malignancies such as ampullary cancer remain very challenging with a clear absence of an evidence-based standard of care treatment paradigm. At this time, data are available to us from a multitude of retrospective studies and subset analysis of larger studies suggest that for loco-regional disease, surgical resection followed by the administration of adjuvant chemotherapy (preferably gemcitabine) represents the preferred mainstay of treatment. The role of radiation is less clear. For unresectable and metastatic disease, systemic therapy with an antimetabolite (fluoropyrimidine and/or gemcitabine) combined with a platinum compound, (usually cisplatin or oxaliplatin) should be considered.
Given the rarity of the disease, it is very unlikely to perform well-powered randomized controlled clinical trials to understand further the role of the various modalities or establish the role of new therapies. Perhaps, as our understanding of the molecular and genetic origins of this cancer will improve, more targeted therapeutic strategies will help us achieve a better outcome for patients with ampullary cancer. In addition to histology, recent studies examining the genomic sequences in ampullary cancers have identified many relevant aberrations, including deletions of KRAS, SMAD4, and PTEN. Findings suggest a distinct oncogenesis, which may be important in the therapeutic context for the development of targeted therapies, including PI3K inhibition.26 Additionally, studies investigating microRNA expression profiles confirm ampullary cancers are distinct compared to pancreatic adenocarcinoma. Despite the limitations of sample size, and based on recent findings, every attempt for future therapeutic agent development should be made specific to ampullary cancers and made distinct from malignancies in adjacent organs.27
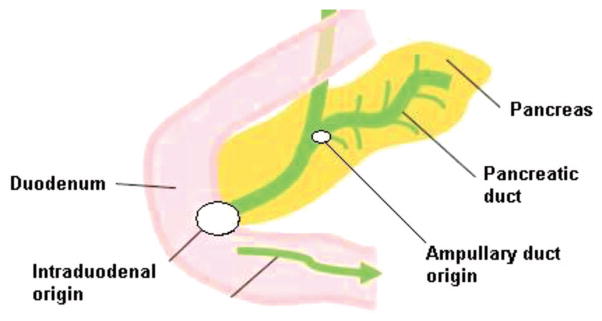
FIG 1.
Diagram of the sites of origin in ampullary cancer (Ampullary vs. Intraduodenal).
Footnotes
Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Agoff SN, Crispin DA, Bronner MP, et al. Neoplasms of the ampulla of vater with concurrent pancreatic intraductal neoplasia: a histological and molecular study. Mod Pathol. 2001;14:139–146. doi: 10.1038/modpathol.3880270. [DOI] [PubMed] [Google Scholar]
- 3.Chang DK, Jamieson NB, Johns AL, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348–1356. doi: 10.1200/JCO.2012.46.8868. [DOI] [PubMed] [Google Scholar]
- 4.Hatzaras I, George N, Muscarella P, et al. Predictors of survival in peri-ampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell JB, Maggard MA, Manunga J, Jr, et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820–1827. doi: 10.1245/s10434-008-9886-1. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. [Accessed January 10, 2014];Pancreatic Adenocarcinoma. http://www.nccn.org.
- 8.Bhatia S, Miller RC, Haddock MG, et al. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514–519. doi: 10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Sikora SS, Balachandran P, Dimri K, et al. Adjuvant chemoradiotherapy in ampullary cancers. Eur J Surg Oncol. 2005;31:158–163. doi: 10.1016/j.ejso.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morak MJ, van der Gaast A, Incrocci L, et al. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Hsu CC, Winter JM, et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of Vater. Radiother Oncol. 2009;92:244–248. doi: 10.1016/j.radonc.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Whittington R, Williams NN, et al. Outcome of pancreaticoduo-denectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 2000;47:945–953. doi: 10.1016/s0360-3016(00)00537-x. [DOI] [PubMed] [Google Scholar]
- 14.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 15.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 16.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 18.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 19.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 20.Cereda S, Passoni P, Reni M, et al. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer. 2010;116:2208–2214. doi: 10.1002/cncr.24970. [DOI] [PubMed] [Google Scholar]
- 21.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 22.Kim ST, Lee J, Lee KT, et al. The efficacy of frontline platinum-based combination chemotherapy in advanced adenocarcinoma of the ampulla of Vater. Med Oncol. 2010;27:1149–1154. doi: 10.1007/s12032-009-9351-4. [DOI] [PubMed] [Google Scholar]
- 23.André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 24.Plastaras JP, Berman A, Apisarnthanarax S, et al. Proton reirradiation of locally recurrent pancreatic and ampullary adenocarcinomas. J Clin Oncol. 2012;30(suppl 34) abstr 317. [Google Scholar]
- 25.Boone BA, Moser AJ, Mock BK, et al. Palliative reoperation for recurrent perampullary adenocarcinoma: Primum non nocer? J Clin Oncol. 2012;30(suppl 34) abstr 257. [Google Scholar]
- 26.Demeure MJ, Craig DW, Sinari S, et al. Cancer of the ampulla of Vater: Analysis of the whole genome sequence exposes a potential therapeutic vulnerability. Genome Med. 2012;4:56. doi: 10.1186/gm357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz NA, Werner J, Willenbrock H, et al. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol. 2012;25:1609–1622. doi: 10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]