Abstract
Background
Cellular immunotherapy has been widely used in the treatment of solid tumors. However, the clinical application of cord blood-derived dendritic cells and cytokine-induced killer cells (CB-DC-CIK) for the treatment of gastric cancer has not been frequently reported. In this study, the efficacy and safety of CB-DC-CIK for the treatment of gastric cancer were evaluated both in vitro and in vivo.
Methods
The phenotypes, cytokines, and cytotoxicity of CB-DC-CIK were detected in vitro. Patients with advanced gastric cancer were divided into the following two groups: the experimental group (CB-DC-CIK combined with chemotherapy) and the control group (chemotherapy alone). The curative effects and immune function were compared between the two groups.
Results
First, the results showed that combination therapy significantly increased the overall disease-free survival rate (P=0.0448) compared with chemotherapy alone. The overall survival rate (P=0.0646), overall response rate (P=0.410), and disease control rate (P=0.396) were improved in the experimental group, but these changes did not reach statistical significance. Second, the percentage of T-cell subsets (CD4+, CD3−CD56+, and CD3+CD56+) and the levels of IFN-γ, TNF-α, and IL-2, which reflect immune function, were significantly increased (P<0.05) after immunotherapy. Finally, no serious side effects appeared in patients with gastric cancer after the application of cellular immunotherapy based on CB-DC-CIK.
Conclusion
CB-DC-CIK combined with chemotherapy is effective and safe for the treatment of patients with advanced gastric cancer.
Keywords: cord blood, gastric cancer, dendritic cells, cytokine-induced killer cells, immunotherapy
Introduction
Gastric cancer is a malignant tumor, the genesis of which is highly related to eating habits, genetic factors, and the presence of other stomach diseases.1 Gastric cancer has become the third leading cause of death among all types of cancer according to the World Cancer Report of 2014. The 5-year survival rate of patients with gastric cancer is less than 20% at an advanced stage if early gastric cancer is not diagnosed in a timely manner.2,3
Currently, surgery, radiotherapy, and chemotherapy are the three most widely used therapeutic approaches for cancer, including gastric cancer. Many studies have indicated that these treatments have little impact on patients with advanced malignant tumors because they fail to completely eradicate the tumorous tissues, including small lesions and metastatic cells, which may cause disease recurrence.4,5 Furthermore, drug resistance, immunosuppression, and other adverse effects have made chemotherapy and radiotherapy more difficult.4,5 Thus, more effective and safer treatments are urgently required.
In recent years, the rapid development of immunotherapy has compensated for the shortcomings of traditional therapies. Cellular immunotherapies, such as lymphokine-activated killer cells,6,7 tumor-infiltrating lymphocytes,8,9 cytokine-induced killer cells (CIK),5,10 and other immune cells,11,12 have rapidly developed into a fourth-line cancer therapy, ranked after surgery, radiotherapy, and chemotherapy.5,13 CIK, which consist primarily of the CD3+CD56+ subset and are induced in vitro by anti-CD3 monoclonal antibodies, IFN-γ, and IL-2, are more widely used in the treatment of solid tumors. Compared with other immune cells, CIK exhibit a higher proliferation rate, stronger antitumor activity, and a broader antitumor spectrum.5,14 Dendritic cells (DCs) are the most potent antigen-presenting cells, which function by presenting tumor antigens to T lymphocytes and by inducing antitumor immune responses. DCs also act as stimulators of effective T-cells via the promotion of the generation of helper and cytotoxic T-cells.15–17 Studies have shown that the combination of DCs and CIK leads to a remarkable increase in cytotoxic activity.18,19
Several studies have indicated that DC-CIK were effective in the treatment of multiple solid tumors including non-small-cell lung cancer, breast cancer, and colon cancer, among others, without any serious adverse reactions.18–20 At present, peripheral blood (PB) is the main source of DC-CIK. However, repeated collection of PB in older patients is difficult, and sufficient numbers of antitumor T lymphocytes cannot be obtained from cancer patients in poor health.5,21
Cord blood-derived CIK cells (CB-CIK) can be easily obtained and largely expanded in vitro.22,23 It has also been shown that CB-CIK can lead to tumor cell death in a variety of tumor types.5,24,25 Compared with the PB-derived CIK (PB-CIK), CB-CIK have led to increased proliferation rates, lower immunogenicity, a higher percentage of main functional fraction CD3+CD56+ cells, and more potent antitumor efficacy against various malignancies.26,27 These biological characteristics suggest that CB-CIK might be more effective in the treatment of patients with cancer. Nevertheless, few reports have been published on the clinical application of CB-DC-CIK. Therefore, the efficacy and tolerability of CB-DC-CIK combined with chemotherapy for the treatment of patients with gastric cancer were evaluated in our study.
Patients and methods
Gastric cancer cell culture
The human gastric cancer cell lines SGC7901 and BGC823 were obtained from the Shanghai Cell Research Institute of the Chinese Scientific Academy (Shanghai, People’s Republic of China) and cultured in the Department of Central Laboratory of Liaocheng People’s Hospital. Dulbecco’s Modified Eagle’s Medium (GIBCO, Invitrogen Co., New York, NY, USA) supplemented with 10% fetal bovine serum (HyClone, UT, Logan, USA) was used as the culture medium. All cell lines were cultured at 37°C in 5% CO2.
Culture of CB-DC-CIK
CB (80 mL), which was obtained from healthy term infants, was provided by the Department of Gynecology and Obstetrics of Liaocheng People’s Hospital. The parent(s) or guardian(s) of all participants involved in this study signed an informed consent, and this study was approved by the Ethics Committee of Liaocheng People’s Hospital. Mononuclear cells were isolated by Ficoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation and were cultured in AIM-V complete medium (GIBCO, Invitrogen Co.) at a concentration of 5×106 cells/mL. Then, the cells were incubated in 5% CO2 at 37°C for 2 hours.
Adherent cells were cultured in DC medium (AIM-V supplemented with 5% inactivated human serum, 1,000 U/mL of GM-CSF, and 500 U/mL of IL-4) for 5 days. Half the medium was replaced every other day and was supplemented with cytokines. Tumor necrosis factor-alpha (TNF)-α was added on the fifth day to induce maturation, and the cells were harvested on the seventh day.
Nonadherent cells were collected and washed with phosphate-buffered saline twice, and the cell concentration was adjusted to 2×106 cells/mL. rhIFN-γ (1,000 U/mL, PeproTech Inc., NJ, USA) was added on day 1, and a monoclonal antibody against CD3 (OKT-3, 50 ng/mL; PeproTech Inc.), IL-1α (100 U/mL; PeproTech Inc.), and recombinant human IL-2 (rhIL-2, 300 IU/mL; PeproTech Inc.) were added on day 2. Fresh complete medium with rhIL-2 was added to the cells every 2–3 days.
Mature DCs and CIK were mixed at a proportion of 1:10 on day 7 and cultured for another 8 days, and then, CB-DC-CIK were harvested for use on day 15.
Quality control of CB-DC-CIK
The phenotypes of CB-DC (CD40, CD80, CD86; BD, Franklin Lakes, NJ, USA) were detected on days 1, 3, and 7, while the phenotypes of CB-DC-CIK (CD3, CD56, HLA-DR; BD) were detected on days 5, 10, and 15 by flow cytometry (BD).
On day 15, the viability of CB-DC-CIK was detected by trypan blue staining. The safety of CB-DC-CIK was evaluated by microbiological assays including tests for mycoplasma, chlamydia, bacteria, viruses, and endotoxin. The proliferation of CB-DC-CIK was observed by cell counts on days 1, 5, 10, and 15. Then, CB-DC-CIK were washed three times with saline, resuspended in 100 mL saline containing 1% human serum albumin, and transfused into the patients within 1 hour.
Cytotoxicity activity assay of CB-DC-CIK against gastric cancer cells
SGC7901 and BGC823 cells in the logarithmic growth phase were seeded into 96-well plates (100 µL/well) as target cells at a density of 1×105/mL; then, 100 µL of CB-DC-CIK was added into each well (E:T =5:1, 10:1, 20:1) as effector cells. The coculture group was used as the experimental group, while the effector cells or target cells cultured alone were used as the control group. Five parallel wells were dedicated to each group. All cells were incubated at 37°C in 5% CO2. After 24 hours of incubation, 10 µL of CCK-8 were added, and the cells were cultured for another 2 hours. Then, the absorbance was measured at a wavelength of 450 nm in an automatic microplate reader. The cytotoxicity rate (CR) was calculated by the following formula: CR = [1−(Aexperiment−Aeffector)/Atarget] ×100%.
Cytokine production of CB-DC-CIK
CB-DC-CIK were adjusted to a final concentration of 2×106 cells/mL and cultured in complete medium for 48 hours. The supernatants were collected and analyzed for the secretion of IFN-γ, TNF-α, and IL-2 using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA).
Study patients
Patients with advanced gastric cancer were recruited through the Department of Gastroenterology at Liaocheng People’s Hospital from June 2010 to October 2013. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Liaocheng People’s Hospital. All patients signed an informed consent before treatment. In all, 28 patients were divided into two groups as follows: 15 patients in the control group were treated with chemotherapy alone and 13 patients in the experimental group were treated with CB-DC-CIK immunotherapy combined with chemotherapy.
Patients were recruited for the study according to the following criteria: 1) diagnosis of histologically confirmed stage III or IV gastric cancer according to the American Joint Committee on Cancer TNM Staging Classification for Carcinoma of the Stomach; 2) expected survival of more than 6 months and having received at least three cycles of chemotherapy based on 5-fluorouracil; 3) primary gastric cancer and no previous history of other tumors; 4) postoperative recurrence and metastasis or the patient was unable to be treated by surgery; 5) no serious heart, lung, liver, and kidney dysfunction or autoimmune disease; and 6) not pregnant or lactating.
Treatments
All patients had received at least three cycles of chemotherapy based on 5-fluorouracil including FOLFOX4 (oxaliplatin: 85 mg/m2, 2 hours on day 1; 5-florouracil: 400 mg/m2, 2 hours, 600 mg/m2, 22 hours on days 1 and 2; leucovorin: 200 mg/m2, 2 hours on days 1 and 2) and DCF (docetaxel: 75 mg/m2 on day 1, cisplatin: 75 mg/m2 on day 1; 5-florouracil: 750 mg/m2, 24 hours on days 1–5).
After chemotherapy, patients in the experimental group received at least three cycles of immunotherapy based on CB-DC-CIK at 1-month intervals. Patients in the control group did not receive immunotherapy. The first cycle began 1 month after the end of chemotherapy. More than 3×109 CB-DC-CIK were transfused via a superficial vein into patients within 1 hour every day on days 1–3; three transfusions were defined as one cycle.
Follow-up
All patients were followed up every 2 months after immunotherapy for 2 years. The curative effects were diagnosed mainly by physical examination and abdominal computed tomography examinations. The recurrence and metastasis of the tumor were assessed regularly. In all patients, the site and date of the first recurrence and the date of death were recorded. Patients were followed up until they were lost to follow-up, if they died, or until the follow-up period ended.
The curative effects were assessed in terms of the overall response rate (ORR), disease control rate (DCR), overall survival (OS), and disease-free survival (DFS). RECIST 1.1 (Response Evaluation Criteria in Solid Tumors 1.1) was applied to evaluate the short-term treatment efficacy.28 The RECIST system contains four grades, including CR (complete response), PR (partial response), SD (stable disease), and PD (progressive disease). The ORR was defined as the sum of CR and PR, and the DCR was defined as the sum of CR, PR, and SD.24,29 OS was defined from the date of surgery to the date of death from any cause. DFS was defined as the time that elapsed from the date of treatment to either the date of recurrence or the date of the last follow-up. Tumor markers including CEA, CA199, and CA724, which can predict the recurrence of gastric cancer, were also detected by electrochemiluminescence immunoassay (Hoffman-La Roche Ltd., Basel, Switzerland).
PB (5 mL) of patients in the two groups was collected before and 1 month after treatment to analyze the changes of lymphocyte subsets including CD3+, CD4+, CD8+, CD3-CD56+, and CD3+CD56+ and the levels of cytokines including IFN-γ, TNF-α, and IL-2.
Side effects such as fever, diarrhea, neutropenia, thrombocytopenia, nausea, and vomiting were observed by patient interview and physical examination in accordance with the criteria provided by the National Cancer Institute Common Toxicity during and after the administration of therapy in the two groups.30
Statistical analysis
Comparisons between the experimental and control groups were evaluated using the χ2 test and the Fisher’s exact probability test. The data are expressed as percentages and as the mean ± standard deviation. The OS and PFS curves were evaluated by the Kaplan–Meier method, and a log-rank test with 95% confidence intervals was performed to compare the two groups. All analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). A P-value <0.05 was considered statistically significant.
Results
Characteristics of CB-DC-CIK
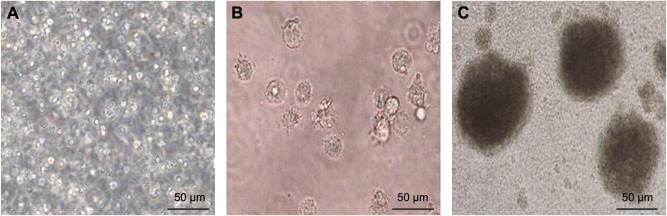
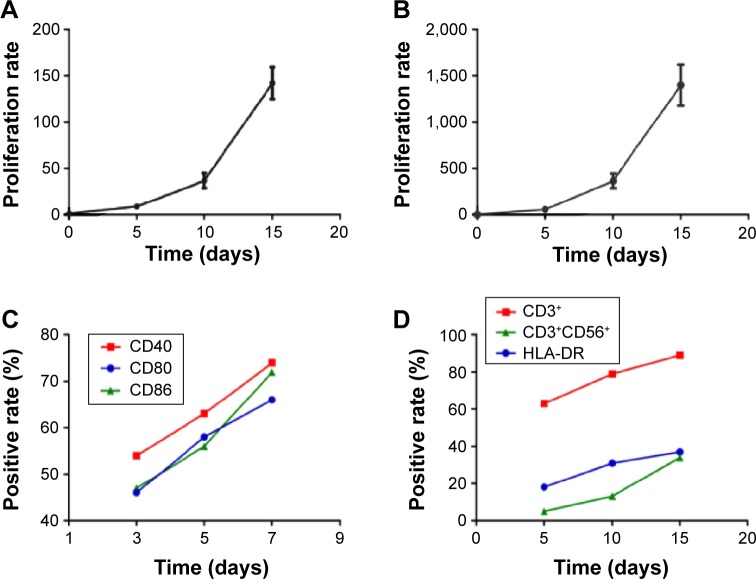
CB mononuclear cells were evenly dispersed in the medium at the beginning of the culture period (Figure 1A). The size of each CB-DC increased and was determined to have a round or irregular shape; the dendritic structure of the cells was also observed under a microscope after 7 days of culture (Figure 1B). CB-DC-CIK grew rapidly in a clonal manner as the culture period continued (Figure 1C). With the prolongation of the culture time, the number of CD3+ and CD3+CD56+ cell subsets increased ~150 times and 1,400 times, respectively, after 15 days of culture (Figure 2A and B). The positive rate of CD40+, CD80+, CD86+ for CB-DC and CD3+, CD3+CD56+ within CB-DC-CIK was significantly increased (P<0.05). The expression of HLA-DR for CB-DC-CIK was low and slightly increased after 15 days of culture (P>0.05; Figure 2C and D). This result suggests that CB-DC-CIK may have a lower immunogenicity and therefore may be associated with a lower risk for graft versus host disease.
Figure 1.
Morphological characteristics of immune cells.
Notes: (A) Morphology of CB mononuclear cells. (B) Morphology of mature CB-DC. (C) Morphology of CB-DC-CIK.
Abbreviations: CB, cord blood; DC, dendritic cells; CB-DC-CIK, cord blood-derived dendritic cells and cytokine-induced killer cells.
Figure 2.
The proliferation rate of CD3+ (A) and CD3+CD56+ (B) subsets and the changes in CB-DC and CB-DC-CIK cell phenotypes over different culture times (C and D).
Abbreviations: CB, cord blood; DC, dendritic cells; CB-DC-CIK, cord blood-derived dendritic cells and cytokine-induced killer cells.
All products were free of bacterial, fungal, and mycoplasma contamination, and the endotoxin level was <5 endotoxin units. After examination, at least 3×109 CB-DC-CIK with a viability of greater than 95% were harvested and transfused into patients within 1 hour every day three times a day (one cycle). Patients received at least three cycles of CB-DC-CIK therapy within a 4-week interval.
Cytotoxicity of CB-DC-CIK against gastric cancer cells in vitro
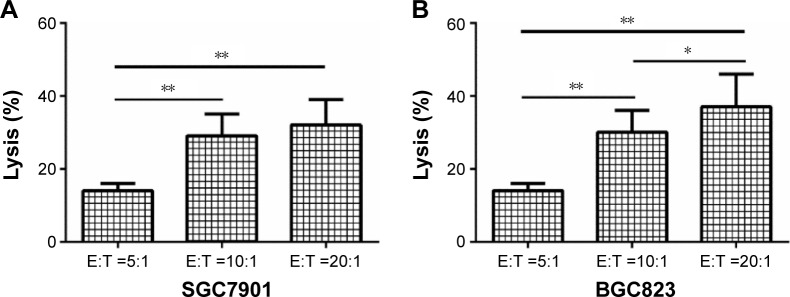
As shown in Figure 3, CB-DC-CIK exhibited a strong killing effect on the gastric cancer cell lines SGC7901 (3A) and BGC823 (3B) in vitro, and the killing effect was more significant with an increase in the effector–target ratio (P<0.05).
Figure 3.
Cytotoxicity of different numbers of CB-DC-CIK against the gastric cancer cell lines SGC7901 (A) and BGC823 (B) was tested by CCK-8 assay. *P<0.05, **P<0.01.
Abbreviations: CB-DC-CIK, cord blood-derived dendritic cells and cytokine-induced killer cells; E:T, effector cells:target cells.
Cytokine production of CB-DC-CIK
The cytokine production by CB-DC-CIK, which is listed in Table 1, indicated that the expression of IFN-γ, TNF-α, and IL-2 was significantly increased with the prolongation of the culture period (P<0.05). These results suggest that CB-DC-CIK can induce tumor cell death via the secretion of cytokines.
Table 1.
Expression of cytokines, including IFN-γ, TNF-α, and IL-2, of CB-DC-CIK after in vitro culture for 8 and 15 days
| Culture time (days) | Cytokines (pg/mL)
|
||
|---|---|---|---|
| IFN-γ | TNF-α | IL-2 | |
| 8 days | 568.5±129.5 | 58.5±15.7 | 623.8±161.5 |
| 15 days | 998.4±187.2* | 123.3±25.9* | 1,178.8±257.8* |
Note:
P<0.05, cultured for 15 days compared with 8 days.
Abbreviation: CB-DC-CIK, cord blood-derived dendritic cells and cytokine-induced killer cells.
Patient characteristics
The clinical characteristics of the patients are listed in Table 2. No significant differences were observed in the clinical characteristics in terms of age, sex, tumor site, histological differentiation, tumor stage, and chemotherapy regimen between the two groups (P>0.05).
Table 2.
Clinical characteristics of the patients with advanced gastric cancer in the two groups
| Characteristic | Total (n=28) | Control (n=15) | Experimental (n=13) | χ2 | P-value |
|---|---|---|---|---|---|
| Sex | 0.346 | 0.686 | |||
| Male | 20 | 10 | 10 | ||
| Female | 8 | 5 | 3 | ||
| Age (years) | 0.185 | 0.718 | |||
| <60 | 12 | 7 | 5 | ||
| >60 | 16 | 8 | 8 | ||
| Tumor site | 0.428 | 0.689 | |||
| Gastric cardia | 19 | 11 | 8 | ||
| Gastric body | 9 | 4 | 5 | ||
| Histologic differentiation | 0.597 | 0.476 | |||
| Moderately differentiated | 10 | 7 | 8 | ||
| Poorly differentiated | 18 | 8 | 5 | ||
| Tumor stage | 0.712 | 0.460 | |||
| III | 19 | 8 | 9 | ||
| IV | 9 | 7 | 4 | ||
| Chemotherapy regimen | 0.038 | 1.000 | |||
| FOLFOX4 | 22 | 12 | 10 | ||
| DCF | 6 | 3 | 3 |
Abbreviations: DCF, docetaxel, cisplatin, 5-florouracil; FOLFOX4, folinic acid, fluorouracil, oxaliplatin.
Curative effects
ORR and DCR
The ORR and DCR were 38.5% and 84.6%, respectively, in the experimental group compared with 20% and 66.7%, respectively, in the chemotherapy group (P=0.410 for ORR and P=0.396 for DCR). These results suggest that the response evaluation in the experimental group tended to be better than that in the chemotherapy alone group, but that the apparent advantages did not reach statistical significance (Table 3).
Table 3.
Comparison of the ORR and DCR between the two groups
| Group | ORR
|
DCR
|
CR | PR | SD | PD | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Exp (n=13) | 5 | 38.5 | 11 | 84.6 | 0 | 5 | 6 | 2 |
| Con (n=15) | 3 | 20 | 10 | 66.7 | 0 | 3 | 7 | 5 |
| χ2 | 1.122 | 1.154 | ||||||
| P-value | 0.410 | 0.396 | ||||||
Abbreviations: ORR, overall response rate; DCR, disease control rate; CR, cytotoxicity rate; PR, partial response; SD, stable disease; PD, progressive disease; Exp, experimental; Con, control.
OS and DFS
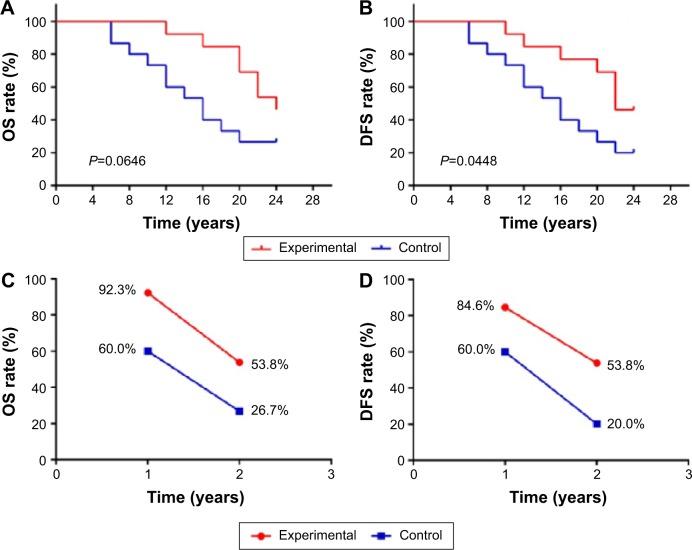
The OS and DFS curves for the immunotherapy group and the control group are presented in Figure 4A and B, respectively. The 1- and 2-year OS and the DFS rates of the patients in the two groups are shown in Figure 4C and D, respectively. These results suggest that immunotherapy combined with chemotherapy can prolong the survival time of patients compared with chemotherapy alone (OS P=0.0646, DFS P=0.0448).
Figure 4.
Kaplan–Meier estimates for OS and DFS for patients in the two groups (A and B), 1- and 2-year OS and the DFS rate in the two groups (C and D).
Abbreviations: OS, overall survival; DFS, disease-free survival.
Tumor markers
In Table 4, the results showed that the levels of CEA, CA199, and CA724 were significantly decreased after therapy in both groups (P<0.05) and that the reduction observed in the combined treatment group was more significant than that in the chemotherapy alone group (P<0.05).
Table 4.
Tumor markers including CEA, CA199, and CA724 in the two groups (mean ± sd, %)
| Group | CEA (ng/mL) | CA199 (ng/mL) | CA724 (ng/mL) |
|---|---|---|---|
| Exp (n=13) | |||
| Pretherapy | 23.7±15.8 | 47.2±32.5 | 22.5±14.6 |
| Posttherapy | 13.6±10.2*,** | 28.9±18.2*,** | 16.4±11.3*,** |
| Con (n=15) | |||
| Pretherapy | 22.6±14.1 | 46.8±33.6 | 24.1±15.2 |
| Posttherapy | 18.5±12.3* | 35.6±22.7* | 20.3±13.6* |
Notes:
P<0.05, comparisons between pretherapy and posttherapy in each group.
P<0.05, posttherapy comparisons between the two groups.
Abbreviations: Exp, experimental; Con, control; sd, standard deviation.
Immune function
A comparison of the T lymphocyte subsets in patients before and after therapy in the two groups is listed in Table 5. The percentages of CD4+, CD3−CD56+, and CD3+CD56+ cell subsets and the ratio of CD4+/CD8+ were significantly increased after therapy compared with pretherapy levels in the experimental group (P<0.05), while no significant differences were observed between pretherapy and posttherapy in the control group (P>0.05). In addition, all these cell subsets were significantly higher in the immunotherapy-treated patients than in those who were treated with chemotherapy alone (P<0.05).
Table 5.
T lymphocyte subsets in the two groups (mean ± sd, %)
| Group | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | CD‒CD56+ | CD3+CD56+ |
|---|---|---|---|---|---|---|
| Exp (n=13) | ||||||
| Pretherapy | 61.3±10.6 | 30.3±6.3 | 29.6±6.1 | 1.03±0.21 | 9.6±2.6 | 3.2±1.8 |
| Posttherapy | 65.2±11.5 | 36.1±7.5*,** | 28.2±6.3 | 1.33±0.28*,** | 14.6±3.3*,** | 6.3±2.3*,** |
| Con (n=15) | ||||||
| Pretherapy | 60.8±10.3 | 29.8±5.9 | 28.9±6.5 | 1.03±0.20 | 9.4±2.3 | 3.3±1.9 |
| Posttherapy | 60.2±10.8 | 29.2±5.2 | 29.6±7.3 | 0.97±0.19 | 9.7±2.5 | 3.1±1.8 |
Notes:
P<0.05, comparisons between pretherapy and posttherapy in each group.
P<0.05, posttherapy comparisons between the two groups.
Abbreviations: Exp, experimental; Con, control; sd, standard deviation.
Furthermore, the expression of IFN-γ, TNF-α, and IL-2 was significantly higher after immunotherapy than after treatment with chemotherapy alone (P<0.05; Table 6). The levels of these cytokines were significantly increased after therapy only in the experimental group (P<0.05). These results suggest that the immune status of these patients might be improved with this therapy.
Table 6.
Expression of cytokines including IFN-γ, TNF-α, and IL-2 in the two groups (mean ± sd, %)
| Group | IFN-γ (pg/mL) | TNF-α (pg/mL) | IL-2 (pg/mL) |
|---|---|---|---|
| Exp (n=13) | |||
| Pretherapy | 18.2±5.8 | 28.2±8.3 | 42.1±12.6 |
| Posttherapy | 45.4±13.7*,** | 39.5±12.7*,** | 84.2±23.7*,** |
| Con (n=15) | |||
| Pretherapy | 18.5±6.1 | 27.8±7.9 | 43.7±13.9 |
| Posttherapy | 17.8±5.9 | 28.3±7.4 | 41.9±14.1 |
Notes:
P<0.05, comparisons between pretherapy and posttherapy in each group.
P<0.05, posttherapy comparisons between the two groups.
Abbreviations: Exp, experimental; Con, control; sd, standard deviation.
Safety of CB-DC-CIK immunotherapy
Side effects including fever, diarrhea, neutropenia, thrombocytopenia, nausea, and vomiting were observed in the two groups. No serious adverse events or death occurred in any of the patients after treatment with immunotherapy. Most of the side effects subsided without intervention within 24 hours or were treated successfully by allopathic means.
As listed in Table 7, the incidence of side effects (except fever) was lower in the CB-DC-CIK group compared with the control group. These results suggest that immunotherapy was safe for patients with gastric cancer and may alleviate the adverse events caused by chemotherapy.
Table 7.
Comparison of the adverse effects between the two groups
| Group | Nausea and vomiting
|
Thrombocytopenia
|
Neutropenia
|
Diarrhea
|
Fever
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | No | % | |
| Exp (n=13) | 4 | 30.8 | 3 | 23.1 | 2 | 15.4 | 2 | 15.4 | 5 | 38.5 |
| Con (n=15) | 10 | 66.7 | 5 | 33.3 | 4 | 26.7 | 3 | 20.0 | 1 | 6.7 |
| χ2 | 3.462 | 0.346 | 0.508 | 0.098 | 4.032 | |||||
| P-value | 0.128 | 0.686 | 0.655 | 1.000 | 0.069 | |||||
Abbreviations: Exp, experimental; Con, control.
Discussion
In recent years, with the rapid development of cellular immunotherapy, studies have confirmed that the use of CIK, which usually consist of NKT cells, is effective and safe in the treatment of solid malignancies; NKT cells combine the powerful antitumor effects of T-cells with the nonmajor histocompatibility complex–restricted NK cells.5,10,24 CIK can induce apoptosis and inhibit the proliferation of tumor cells through direct contact and secreted cytokines, such as IL-2, IFN-γ, and TNF-α.26,27,31–33 In earlier research, Hoyle et al34 found that allogeneic CIK from healthy donors can suppress chronic myelogenous leukemia colony growth, but they did not inhibit the growth of normal hematopoietic colonies.34 Moreover, the combination of DC and CIK, which can promote the accumulation of effector T-cells around the cancer site through the secretion of chemokines, may improve the efficacy of tumor therapy.15 Studies have shown that DCs play an important role in the activation, proliferation, phenotypic expression, and cytokine secretion of CIK.16 Wei et al16 reported that the levels of cytokines such as IL-2, IFN-γ, TNF-α, and IL-12, and the rates of the main effector cells in CIK (CD3+CD56+ cells) were increased after coculture with DCs, which enhanced the cytotoxicity of CIK. Shan et al35 showed that CIK and DCs loaded with the protein lysate produced by radiofrequency ablation induced a specific antitumor response in vitro and in vivo.35 Other researchers found that CIK cells activated by DCs loaded with a tumor-specific antigen help achieve a better effect as a result of tumor therapy.36,37
Studies have indicated that CB-CIK have a similar phenotype, lower immunogenicity, stronger proliferation, and a similar antitumor activity compared with PB-CIK.26,27 In our study, CB mononuclear cells can be isolated from CB and easily introduced into CB-DC-CIK. The effector cells of CB-DC-CIK were easily expanded, and at least 1×1010 CB-DC-CIK could be infused in patients at the end of the culture period. CB-DC-CIK can induce gastric cancer cell death in vitro and produce high levels of cytokines such as IFN-γ, TNF-α, and IL-2, which can enhance the killing effect against tumors. These results are consistent with other reports on the effects of CB-CIK.
Until now, few reports have been published on the therapeutic effects of CB-CIK treatment in patients with gastric cancer. Thus, in this study, we evaluated the safety and antitumor capability of CB-DC-CIK infusion in patients with gastric cancer. The results of our study showed that immunotherapy combined with chemotherapy for the treatment of gastric cancer can achieve a better curative effect than treatment with chemotherapy alone. The OS (P<0.05), DFS, ORR, and DCR in the combined therapy group had improved compared with the control group. However, the latter three indicators did not reach statistical significance (P>0.05). These results indicate that the use of CB-DC-CIK in the treatment of gastric cancer was effective both in terms of reduction in the recurrence of cancer and prolongation of the survival time of patients.
The occurrence and metastasis of tumors are typically accompanied by disorders of the immune system.14,38 Therefore, a reconstruction of the immune system is vital for cancer therapy. In addition, many studies have demonstrated that the immune function of patients could be enhanced by CIK transfusion.14,38 The targeting of tumor cells by the human immune system mainly depends on cellular immunity.39 CD3−CD56+ (NK cells) and CD3+CD56+ cells (NKT cells) are the primary subsets of cells with antitumor activities. CD4+ T-cells, which can assist in the recruitment of CD8+ T-cells and in the activation of macrophages via the secretion of IFN-γ, are considered helper T-cells.38 Furthermore, it is well known that cytotoxicity against tumors is dependent on an appropriate CD4+ and CD8+ T-cell interaction.38 In our study, the percentages of CD4+, CD3−CD56+, and CD3+CD56+ subsets and the ratio of CD4+/CD8+ in the PB were significantly increased after CB-DC-CIK treatment. In contrast, the percentage of CD8+ T-cells was not significantly increased after the immunotherapy, which may be related to the immune suppression induced by CD8+ T-cells.
Finally, the balance between Th1 and Th2 cells plays an important role in immunotherapy, as Th1 cells can enhance the cytotoxicity of killer cells, and triggers the delayed-type hypersensitivity, whereas Th2 cells may lead to the immune escape of tumor cells.40 Th1-type cytokines such as IFN-γ and IL-2 play a critical role in the antitumor process.41 Other studies have suggested that CIK are also associated with the secretion of TNF-α.41,42 Our analysis showed that the levels of IFN-γ, TNF-α, and IL-2 (P<0.05) were significantly increased after immunotherapy. This result revealed that the immune function of patients with gastric cancer was significantly improved after immunotherapy, which might be related to the improvement in survival.
Tumor markers are mainly used for the detection of primary tumors, the evaluation of the therapeutic effect, and the prediction of recurrence and prognosis.43–46 Tumor markers for gastric cancer were analyzed in our study. We also concluded that the levels of tumor markers, such as CEA, CA199, and CA724 (P<0.05), were decreased in patients after CB-DC-CIK immunotherapy.
Safety is the key factor for clinical therapies, and our analysis showed that the use of immunotherapy based on CB-DC-CIK for the treatment of gastric cancer was safe. The side effects of CB-DC-CIK treatment were well tolerated by all of the patients, and no serious adverse events or death occurred after therapy. The adverse events caused by chemotherapy, including leukopenia, thrombocytopenia, nausea, vomiting, and diarrhea, seemed to be alleviated by immunotherapy, but this apparent advantage did not reach statistical significance (P>0.05).
Our study also has some limitations. The therapeutic effects of immunotherapy are variable due to numerous factors such as tumor stage, cell phenotype, and cell purity.47,48 After culture and expansion, the purity and phenotypes of the CB-DC-CIK varied among individuals. Furthermore, the total sample size was not large enough in this study. These factors may have led to variable therapeutic effects. Finally, the comparison of the cytotoxic activity against gastric cancer cells of chemotherapy combined with CB-DC-CIK therapy versus chemotherapy alone was not conducted in vitro. Therefore, we will perform the relevant research in the future, if possible.
Conclusion
Taken together, our results demonstrate that the use of immunotherapy for the treatment of gastric cancer is effective and safe. Therefore, CB-DC-CIK have great potential to be used as an effective therapy in the treatment of gastric cancer and may allow for the substitution of autologous CIK in clinical applications.
Acknowledgments
This research was supported by the Natural Science Foundation of Shandong (number 2015ZRA15027 to Chang-hui Zhou), the Medical Science Foundation of Shandong (number 2014WS0300 to Wei-hua Wang), and the Wu Jieping Medical Foundation (number 320.6750.15043 to Wei-hua Wang).
Footnotes
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Galvao de Azevedo I, Leal Muniz Carneiro IC, Oliveira Tomiya MT, Pessoa de Araujo Burgos MG. Gastric cancer and associated factors in hospitalized patients. Nutr Hosp. 2015;32(1):283–290. doi: 10.3305/nh.2015.32.1.9071. [DOI] [PubMed] [Google Scholar]
- 2.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalnina Z, Meistere I, Kikuste I, et al. Emerging blood-based biomarkers for detection of gastric cancer. World J Gastroenterol. 2015;21(41):11636–11653. doi: 10.3748/wjg.v21.i41.11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Wang L, Luo Z, et al. Efficacy and safety of cord blood-derived cytokine-induced killer cells in treatment of patients with malignancies. Cytotherapy. 2015;17(8):1130–1138. doi: 10.1016/j.jcyt.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Wang R, Tao Z, et al. Activation of T-LAK-cell-originated protein kinase-mediated antioxidation protects against focal cerebral ischemia-reperfusion injury. FEBS J. 2014;281(19):4411–4420. doi: 10.1111/febs.12948. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, Lv M, Sayimu W, et al. Therapeutic effect of lymphokine-activated killer cells treated with low-dose ionizing radiation on osteosarcoma. Oncol Lett. 2015;10(2):879–882. doi: 10.3892/ol.2015.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Gross CA, Somerville RP, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31(17):2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besser MJ, Shapira-Frommer R, Schachter J. Tumor-infiltrating lymphocytes: clinical experience. Cancer J. 2015;21(6):465–469. doi: 10.1097/PPO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10.Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–2259. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Xu Z, Zhou F, et al. The combination of dendritic cells-cytotoxic T lymphocytes/cytokine-induced killer (DC-CTL/CIK) therapy exerts immune and clinical responses in patients with malignant tumors. Exp Hematol Oncol. 2015;4:32. doi: 10.1186/s40164-015-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin T, Song C, Chuo DY, Zhang H, Zhao J. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol. 2016;37(4):4367–4372. doi: 10.1007/s13277-015-3957-2. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Nussenzweig MC. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 16.Wei XC, Yang DD, Han XR, et al. Bioactivity of umbilical cord blood dendritic cells and anti-leukemia effect. Int J Clin Exp Med. 2015;8(10):19725–19730. [PMC free article] [PubMed] [Google Scholar]
- 17.Wei XC, Zhai XH, Han XR, Yang DD, Wang QS. Influence of dendritic cells on biological activity of the homologous CIK cells and its anti-leukemia effect in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(4):946–951. [PubMed] [Google Scholar]
- 18.Zhu XP, Xu YH, Zhou J, Pan XF. A clinical study evaluating dendritic and cytokine-induced killer cells combined with concurrent radiochemotherapy for stage IIIB non-small cell lung cancer. Genet Mol Res. 2015;14(3):10228–10235. doi: 10.4238/2015.August.28.6. [DOI] [PubMed] [Google Scholar]
- 19.Mao Q, Li L, Zhang C, et al. Clinical effects of immunotherapy of DC-CIK combined with chemotherapy in treating patients with metastatic breast cancer. Pak J Pharm Sci. 2015;28(3 Suppl):1055–1058. [PubMed] [Google Scholar]
- 20.Chen B, Liu L, Xu H, et al. Effectiveness of immune therapy combined with chemotherapy on the immune function and recurrence rate of cervical cancer. Exp Ther Med. 2015;9(3):1063–1067. doi: 10.3892/etm.2015.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Zhu L, Wei J, et al. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: a clinical retrospective study. J Cancer Res Clin Oncol. 2012;138(6):1057–1062. doi: 10.1007/s00432-012-1179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Introna M, Franceschetti M, Ciocca A, et al. Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone Marrow Transplant. 2006;38(9):621–627. doi: 10.1038/sj.bmt.1705503. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Schmidt-Wolf IG, Wu YF, et al. Optimized protocols for generation of cord blood-derived cytokine-induced killer/natural killer cells. Anticancer Res. 2010;30(9):3493–3499. [PubMed] [Google Scholar]
- 24.Niu Q, Wang W, Li Y, et al. Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies. Int Immunopharmacol. 2011;11(4):449–456. doi: 10.1016/j.intimp.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Introna M, Pievani A, Borleri G, et al. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(11):1603–1607. doi: 10.1016/j.bbmt.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhao X, Zhang T, et al. Phenotypic characterization and anti-tumor effects of cytokine-induced killer cells derived from cord blood. Cytotherapy. 2015;17(1):86–97. doi: 10.1016/j.jcyt.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Wang L, Luo C, et al. Phenotypic and functional characterization of cytokine-induced killer cells derived from preterm and term infant cord blood. Oncol Rep. 2014;32(5):2244–2252. doi: 10.3892/or.2014.3457. [DOI] [PubMed] [Google Scholar]
- 28.Jang GS, Kim MJ, Ha HI, et al. Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer. Chin J Cancer Res. 2013;25(6):689–694. doi: 10.3978/j.issn.1000-9604.2013.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108958. doi: 10.1371/journal.pone.0108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kautio AL, Haanpaa M, Kautiainen H, et al. Oxaliplatin scale and National Cancer Institute-Common Toxicity Criteria in the assessment of chemotherapy-induced peripheral neuropathy. Anticancer Res. 2011;31(10):3493–3496. [PubMed] [Google Scholar]
- 31.Durrieu L, Lemieux W, Dieng MM, et al. Implication of different effector mechanisms by cord blood-derived and peripheral blood-derived cytokine-induced killer cells to kill precursor B acute lymphoblastic leukemia cell lines. Cytotherapy. 2014;16(6):845–856. doi: 10.1016/j.jcyt.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Qu HQ, Zhou XS, Zhou XL, Wang J. Effect of DC-CIK cell on the proliferation, apoptosis and differentiation of leukemia cells. Asian Pac J Trop Med. 2014;7(8):659–662. doi: 10.1016/S1995-7645(14)60111-5. [DOI] [PubMed] [Google Scholar]
- 33.Durrieu L, Dieng MM, Le Deist F, Haddad E. Cord blood-derived and peripheral blood-derived cytokine-induced killer cells are sensitive to Fas-mediated apoptosis. Biol Blood Marrow Transplant. 2013;19(9):1407–1411. doi: 10.1016/j.bbmt.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Hoyle C, Bangs CD, Chang P, et al. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92(9):3318–3327. [PubMed] [Google Scholar]
- 35.Shan CC, Shi LR, Ding MQ, et al. Cytokine-induced killer cells co-cultured with dendritic cells loaded with theprotein lysate produced by radiofrequency ablation induce a specific antitumor response. Oncol Lett. 2015;9(4):1549–1556. doi: 10.3892/ol.2015.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao D, Li C, Xie X, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One. 2014;9(4):e93886. doi: 10.1371/journal.pone.0093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marten A, Greten T, Ziske C, et al. Generation of activated and antigen-specific T cells with cytotoxic activity after co-culture with dendritic cells. Cancer Immunol Immunother. 2002;51(1):25–32. doi: 10.1007/s00262-001-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang ZX, Cao JX, Wang M, et al. Adoptive cellular immunotherapy for the treatment of patients with breast cancer: a meta-analysis. Cytotherapy. 2014;16(7):934–945. doi: 10.1016/j.jcyt.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Beavis PA, Slaney CY, Kershaw MH, Neeson PJ, Darcy PK. Enhancing the efficacy of adoptive cellular therapy by targeting tumor-induced immunosuppression. Immunotherapy. 2015;7(5):499–512. doi: 10.2217/imt.15.16. [DOI] [PubMed] [Google Scholar]
- 40.Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 41.Atan O, Aksu G, Ozgenc F, et al. Determination of intracellular Th1/Th2 type cytokines in lymphocytes of chronic hepatitis B patients treated with interferon-alpha. Turk J Gastroenterol. 2010;21(4):401–410. doi: 10.4318/tjg.2010.0127. [DOI] [PubMed] [Google Scholar]
- 42.Durrieu L, Gregoire-Gauthier J, Dieng MM, et al. Human interferon-alpha increases the cytotoxic effect of CD56(+) cord blood-derived cytokine-induced killer cells on human B-acute lymphoblastic leukemia cell lines. Cytotherapy. 2012;14(10):1245–1257. doi: 10.3109/14653249.2012.714864. [DOI] [PubMed] [Google Scholar]
- 43.Zheng TH, Zhao JL, Guleng B. Advances in Molecular Biomarkers for Gastric Cancer. Crit Rev Eukaryot Gene Expr. 2015;25(4):299–305. doi: 10.1615/critreveukaryotgeneexpr.2015014360. [DOI] [PubMed] [Google Scholar]
- 44.Cainap C, Nagy V, Gherman A, et al. Classic tumor markers in gastric cancer. Current standards and limitations. Clujul Med. 2015;88(2):111–115. doi: 10.15386/cjmed-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehena Z, Ghosh CK, Afroz F, et al. Comparison of serum CA72-4 and CEA levels in patient with endoscopically suspected gastric carcinoma. Mymensingh Med J. 2015;24(3):542–549. [PubMed] [Google Scholar]
- 46.Yin LK, Sun XQ, Mou DZ. Value of combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the diagnosis of gastric cancer. Asian Pac J Cancer Prev. 2015;16(9):3867–3870. doi: 10.7314/apjcp.2015.16.9.3867. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Guo ZQ, Shi CM, et al. Efficacy of adjuvant chemotherapy combined with immunotherapy with cytokine-induced killer cells for gastric cancer after d2 gastrectomy. Int J Clin Exp Med. 2015;8(5):7728–7736. [PMC free article] [PubMed] [Google Scholar]
- 48.Pan K, Wang QJ, Liu Q, et al. The phenotype of ex vivo generated cytokine-induced killer cells is associated with overall survival in patients with cancer. Tumour Biol. 2014;35(1):701–707. doi: 10.1007/s13277-013-1096-1. [DOI] [PubMed] [Google Scholar]