Abstract
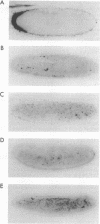
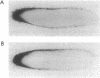
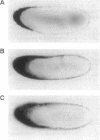
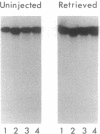
Messenger RNA (mRNA) localization is emerging as a means of regulating gene expression. This process is operational in fly and frog development, where a subset of maternally inherited RNAs are asymmetrically distributed and thought to impart axial polarity to the embryo. Since most maternal mRNAs are uniformly distributed, an apparatus must exist to recognize and specifically transport these rare localized species. Here I report the identification of a nine-nucleotide motif, YUGUUYCUG, common to the 3' untranslated regions of four sequenced messages of this class: Drosophila bicoid and nanos mRNAs and Xenopus An2 and Vg1 mRNAs. To test the role of this nonamer sequence in the localization process, a Drosophila transient assay has been established. The assay reveals that bicoid mRNA specifically lacking this nonamer is partially mislocalized. In contrast, nonamer deletion is inconsequential to message stability. The existence of specific and general mRNA localization signals is proposed and it is suggested that this conserved motif belongs to the latter category.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Nüsslein-Volhard C. Information for the dorsal--ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984 Sep 20;311(5983):223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- Baker N. E. Localization of transcripts from the wingless gene in whole Drosophila embryos. Development. 1988 Jun;103(2):289–298. doi: 10.1242/dev.103.2.289. [DOI] [PubMed] [Google Scholar]
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988 Jul 1;54(1):95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Driever W., Thoma G., Nüsslein-Volhard C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature. 1989 Aug 3;340(6232):363–367. doi: 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Dickinson L. K., Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991 Jul 12;66(1):37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb E. Messenger RNA transport and localization. Curr Opin Cell Biol. 1990 Dec;2(6):1080–1086. doi: 10.1016/0955-0674(90)90159-c. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J., Smith J. L., Macdonald P. M. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991 Jul 12;66(1):23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald P. M. bicoid mRNA localization signal: phylogenetic conservation of function and RNA secondary structure. Development. 1990 Sep;110(1):161–171. doi: 10.1242/dev.110.1.161. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987 Jul 2;328(6125):80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Pondel M. D., King M. L. Localized maternal mRNA related to transforming growth factor beta mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7612–7616. doi: 10.1073/pnas.85.20.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M. A., Kaufman T. C. Molecular analysis of the bicoid gene from Drosophila pseudoobscura: identification of conserved domains within coding and noncoding regions of the bicoid mRNA. EMBO J. 1990 Sep;9(9):2977–2987. doi: 10.1002/j.1460-2075.1990.tb07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991 Jul 12;66(1):51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Driever W., Berleth T., Richstein S., Nüsslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 1989;107 (Suppl):13–19. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- Stephenson E. C., Chao Y. C., Fackenthal J. D. Molecular analysis of the swallow gene of Drosophila melanogaster. Genes Dev. 1988 Dec;2(12A):1655–1665. doi: 10.1101/gad.2.12a.1655. [DOI] [PubMed] [Google Scholar]
- Struhl G., Struhl K., Macdonald P. M. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989 Jun 30;57(7):1259–1273. doi: 10.1016/0092-8674(89)90062-7. [DOI] [PubMed] [Google Scholar]
- Wang C., Lehmann R. Nanos is the localized posterior determinant in Drosophila. Cell. 1991 Aug 23;66(4):637–647. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the animal pole of Xenopus eggs encodes a subunit of mitochondrial ATPase. Proc Natl Acad Sci U S A. 1987 May;84(9):2798–2802. doi: 10.1073/pnas.84.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Whitfield W. G., González C., Sánchez-Herrero E., Glover D. M. Transcripts of one of two Drosophila cyclin genes become localized in pole cells during embryogenesis. Nature. 1989 Mar 23;338(6213):337–340. doi: 10.1038/338337a0. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Melton D. A. The material mRNA Vg1 is correctly localized following injection into Xenopus oocytes. Nature. 1988 Dec 8;336(6199):592–595. doi: 10.1038/336592a0. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]