Abstract
In this study, nonhuman primates (NHPs) exposed to lethal doses of total body irradiation (TBI) within the gastrointestinal (GI) acute radiation syndrome range, sparing ~5% of bone marrow (TBI-BM5), were used to evaluate the mechanisms involved in development of the chronic GI syndrome. TBI increased mucosal permeability in the jejunum (12–14 Gy) and proximal colon (13–14 Gy). TBI-BM5 also impaired mucosal barrier function at doses ranging from 10–12.5 Gy in both small intestine and colon. Timed necropsies of NHPs at 6–180 days after 10 Gy TBI-BM5 showed that changes in small intestine preceded those in the colon. Chronic GI syndrome in NHPs is characterized by continued weight loss and intermittent GI syndrome symptoms. There was a long-lasting decrease in jejunal glucose absorption coincident with reduced expression of the sodium-linked glucose transporter. The small intestine and colon showed a modest upregulation of several different pro-inflammatory mediators such as NOS-2. The persistent inflammation in the post-TBI-BM5 period was associated with a long-lasting impairment of mucosal restitution and a reduced expression of intestinal and serum levels of alkaline phosphatase (ALP). Mucosal healing in the postirradiation period is dependent on sparing of stem cell crypts and maturation of crypt cells into appropriate phenotypes. At 30 days after 10 Gy TBI-BM5, there was a significant downregulation in the gene and protein expression of the stem cell marker Lgr5 but no change in the gene expression of enterocyte or enteroendocrine lineage markers. These data indicate that even a threshold dose of 10 Gy TBI-BM5 induces a persistent impairment of both mucosal barrier function and restitution in the GI tract and that ALP may serve as a biomarker for these events. These findings have important therapeutic implications for the design of medical countermeasures.
INTRODUCTION
A new model of chronic gastrointestinal (GI) syndrome in nonhuman primates (NHPs) after high-dose total body irradiation (TBI) has been recently reported, in which 5% of the bone marrow was spared (TBI-BM5). The goal of these TBI-BM5 studies was to maintain the sequelae of acute GI radiation syndrome (GI-ARS) associated with TBI (1, 2) while preserving sufficient bone marrow to allow survival of the subsequent hematopoietic syndrome. These studies provided unique insights into the events associated with the GI syndrome, with the recognition of a distinct “prolonged” GI syndrome (1). Important outcomes of this model were persistent weight loss and the absence of mucosal restitution along the length of the GI tract throughout the entire postirradiation period despite recovery from the hematopoietic syndrome. Since the reporting of these findings, this model has emerged as a reality-based model that allows investigation of the sequelae of organ injury and the means to evaluate potential countermeasures.
A limitation of studies in which the primary outcome is survival is that secondary outcomes are linked inexorably to Institutional Animal Care and Use Committee–defined euthanasia criteria. This status does not allow for a rigorous assessment of time-dependent changes that may provide more accurate indices of changes induced by radiation alone. A study of NHPs euthanized at predetermined time points after TBI-BM5 showed impaired mucosal restitution all along the GI tract at doses greater than 10 Gy (1). These data indicate that the “prolonged” GI syndrome may be a “chronic” condition similar to other inflammatory GI pathologies, such as inflammatory bowel disease (IBD) and celiac disease (3). In the current study we investigated the mechanisms involved in the development of acute and chronic GI syndromes. For the chronic studies, NHPs were euthanized at specified time points after 9–12.5 TBI-BM5, with the majority of studies focused on the threshold dose of 10 Gy. The goals of the current study were to: 1. Compare the effects of TBI and TBI-BM5 on GI morphology and function in the acute postirradiation period; 2. Determine the changes in gut morphology and function that may contribute to the sequelae of the chronic GI syndrome; and 3. Investigate the mechanism of these changes. Our data demonstrate that the “prolonged” GI syndrome is a chronic condition characterized by an early and persistent mucosal barrier dysfunction, modest mucosal inflammation and impaired and nonresolving mucosal restitution.
METHODS
Animals
Male rhesus macaques weighing 6–9 kg received TBI at target doses of 10.0–14.0 Gy or TBI-BM5 at target doses of 9.0–12.5 Gy. Details of animal care and medical management, provided previously (1, 2), were performed in accordance with the Animal Welfare Act (7 U.S.C. 2131 et. seq.) and the Guide for the Care and Use of Laboratory Animals. All animals received medical management according to a protocol of defined triggers to start and stop treatments, including parenteral fluids, blood transfusions, analgesics, antiemetics, antiulceratives, antidiarrheals, antibiotics, anti-inflammatory agents (e.g., corticosteroids), antipyretics, diuretics and nutritional support. NHPs were euthanized using DEA class III euthanasia solution (Euthasol®; Virbac Animal Health, Inc., Fort Worth, TX) according to a standardized set of clinical criteria, or at 15 days after TBI or by 200 days after TBI-BM5. Subsets of NHPs exposed to 10.0 Gy TBI-BM5 were euthanized on day 6 and 7, and on or about day 30, 45, 60, 100, 120 and 180 postirradiation. Necropsies of all animals were performed immediately after euthanasia.
Radiation Exposure and Dosimetry
NHPs were irradiated utilizing 6 MV LINAC-derived photons at a dose rate of approximately 0.80 Gy/min (2 MV average; Varian Medical Systems Inc., Palo Alto, CA). NHPs were sedated with ketamine, secured in a supine restraint device and then administered xylazine to ensure that proper radiation field placement would be maintained. Half of the prescribed uniform dose was delivered with an anteroposterior beam and half with a posteroanterior beam. For TBI-BM5, NHPs were positioned with their tibiae, ankles and feet outside of the beam field. Animals were observed via in-room cameras throughout the exposure procedure. After irradiation, NHPs were anesthetized, transported back to the NHP housing area, given lactated Ringer’s solution (10–15 ml kg−1 IV) and a dose of Zofran® (ondansetron) (1–2 mg kg−1 IM or IV) and returned to their home cage.
The LINAC was set up for NHP irradiations based on dose measurements taken using ion chambers and water phantoms that approximated the diameter of the experimental NHP (1, 2). During NHP irradiations, in vivo dosimetry was performed using a silicon diode system. During TBI, diodes were placed on the chest to measure the delivered dose. During TBI-BM5, one diode was placed on the chest to measure the delivered dose and another was secured on the tibiae to confirm that the tissue outside of the beam field was spared from exposure. According to these measurements, the spared areas were exposed to approximately 0.5 Gy. Quality assurance and control procedures that were performed prior to each set of NHP irradiations included LINAC energy output checks and diode calibrations.
Clinical Chemistry
NHPs were anesthetized daily with ketamine (10 ± 5 mg/kg, IM; Ketaset®, Fort Dodge, IA) from days 1–21 postirradiation to assess clinical parameters such as body weight, body temperature and hydration status. After day 21, clinical observations and supportive measures were performed weekly through day 60 and then biweekly until the end of the study, unless indicated by a change in the animal’s health status that required more frequent observation or intervention. A baseline blood sample was taken 3–4 days preirradiation and then at each observation time point postirradiation. Samples were processed for subsequent determination of electrolytes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as indices of liver function, blood urea nitrogen (BUN) and creatinine as indices of kidney function, and total protein (albumin and globulin) and albumin as indices of general organ function. Serum alkaline phosphatase (ALP) was used as an index of liver function and also as a potential marker of intestinal epithelial maturity (4). Data presented are values from NHPs beginning at day 0 and euthanized at >100 days postirradiation (n = 8).
Macroscopic and Microscopic Analyses of Full-Thickness Sections of Small Intestine and Colon
At necropsy the gut was removed en bloc from NHP and inspected along its length for evidence of macroscopic injury or abnormalities. Sections from jejunum, ileum, proximal colon and distal colon were processed for hematoxylin and eosin (H&E) staining as described previously (1). Sections of jejunum or proximal colon were prepared for staining with Masson’s trichrome to assess collagen deposition. These two regions were considered as representative of small intestine and colon and were used for subsequent analyses.
Physiological Measurements
Transepithelial electrical resistance (TEER)
The modified microsnap well system is a miniaturized version of the standard Ussing chamber that has been engineered to measure mucosal TEER (5). At necropsy, sections of jejunum, proximal colon and distal colon were stripped of the muscularis externa and mounted in microsnap wells to measure changes in TEER.
Ussing chambers
To investigate in vitro epithelial cell ion and nutrient transport, muscularis externa-free sections of small intestine were mounted in Ussing chambers as described previously (6). Potential difference was measured using agar-salt bridges and electrodes. Every 50 s, the tissue was short circuited at 1 V (DVC 1,000 voltage clamp; World Precision Instruments, Sarasota, FL), and the short circuit current (Isc) was monitored continuously. In addition, every 50 s the clamp voltage was adjusted to 1 V for 2 s to allow calculation of tissue resistance utilizing Ohm’s law (V = IR). After a second 15 min period, concentration-dependent changes in Isc were measured in response to the cumulative addition of glucose to the mucosal side of the jejunum. This is a measurement of sodium-linked glucose transport that can be attributed directly to the activity of the SGLT1, the primary glucose transporter in the small intestine. For all tissue segments taken from an individual NHP, resistance (R) and changes in Isc in response to glucose were averaged to yield a mean response per animal and then averaged to yield a mean value per group.
Preparation of Tissue, Frozen Blocks and Sectioning for Laser Capture Microdissection (LCM)
Sections of jejunum tissues were taken at necropsy and opened along the mesenteric aspect, prepared for Swiss roll and embedded in Tissue-Tek O.C.T Compound (Sakura Finetek USA Inc., Torrance, CA). The tissues were frozen immediately and then removed from the cryomolds and placed at −80°C in an airtight container until sectioned. Tissue sections (4 µm) used for LCM were obtained from frozen blocks using plain uncoated slides and a HM505E cryostat (Richard-Allan Scientific/Thermo Fisher Scientific™ Inc., Kalamazoo, MI). The slides were placed on dry ice immediately and stored at −80°C until needed. Sections of frozen tissue were H&E stained and dehydrated, then LCM was performed with a PicCell II (Arcturus Engineering, Mountain View, CA) (7). Cells were captured from well-defined crypts in small intestine and transferred to CapSure™ LCM caps (Arcturus Engineering).
RNA Extraction, cDNA Synthesis and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from sections of small intestine or colon that had been separated into muscularis externa and mucosae (which may retain some attached muscularis mucosae) and stored until analyzed as described previously (7). RNA samples (2 µg) were reverse transcribed to cDNA using random hexamer primers and RevertAid™ reverse transcriptase. Real-time qPCR was performed using an iCycler™ detection system (Bio-Rad Laboratories Inc., Hercules, CA) (7). Primers for qPCR were designed using Beacon Designer™ 7.0 software (PREMIER Biosoft International, Palo Alto, CA) and synthesized by Sigma-Aldrich® (St. Louis, MO). The primer sequences for genes are listed in Table 1. Values were corrected to a housekeeping gene (18S) and expressed as a fold change from controls (nonirradiated animals).
TABLE 1.
List of Primers
| Gene | Probe | Sequence |
|---|---|---|
| ALPi | Sense | AATATGAGATCAACCGAGAC |
| Antisense | TCCACGAAGAGGTAGAAG | |
| IL-17A | Sense | AGTGAAGGCAGGAATAGCAATC |
| Antisense | AGGTGAGGTGGATCGGTTG | |
| NOS-2 | Sense | GCAGGACGAGAAGCGGAGAC |
| Antisense | CAGCATACAGGCAAAGAGCACAG | |
| SGLT1 | Sense | AATCCTGACTGGGTTTGC |
| Antisense | TCGGAAGATGTGGAATGAG | |
| Hes-1 | Sense | AAGACGAAGAGCAAGAATA |
| Antisense | AAGAAAGAGCAACAACATTT | |
| Lgr5 | Sense | ACTAAGAACACTGACTCTGAATGG |
| Antisense | TAGCACTTGGAGATTAGGTAACTG | |
| IFN-γ | Sense | GGTTCTCTTGGCTGTTACTG |
| Antisense | TCTGTCACTCTCCTCTTTCC | |
| NGN-3 | Sense | TAAGAGCGAGTTGGCACTA |
| Antisense | GAGTTGAGGTTGTGCATT | |
| Glut2 | Sense | TGGTCCCTGTCTGTATCC |
| Antisense | AGTCCTGATATGCTTCTTCC |
Western Blot
Sections of jejunum or colon were homogenized in 1 ml of tissue protein extraction reagent (T-PER, Thermo Fisher Scientific). Samples were then centrifuged and supernatants were collected for further analysis. The Pierce® BCA protein assay kit (Thermo Fisher Scientific) was used to measure the protein concentration for each sample. Samples (60 µg) were loaded into wells of 12% Tris-Glycine gel (Invitrogen™, Carlsbad, CA) and electrophoresed. The membrane used to transfer the separated protein bands was stained with Ponceau S solution for determination of equal loading. The membrane was then blocked with 5% milk and incubated with anti-leucine-rich repeat-containing G-protein-coupled receptor-5 (Lgr5) (2 µg/ml, cat. no. 325600; Invitrogen) or ALP antibody (1:500, cat. no. PA5-22210; Invitrogen) overnight followed by an anti-rabbit IgG HRP–labeled secondary antibody (1/100,000, KPL), and the chemiluminescence signal was measured using a Fujifilm darkbox (Fujifilm, Valhalla, NY).
Statistics
Data are expressed as means ± SEM. Parametric data were analyzed using analysis of variance (ANOVA) followed by Bonferroni correction to compare individual means (Prism; Graph-Pad Software Inc., LaJolla, CA). Nonparametric data were analyzed using Kruskal-Wallis followed by Dunn’s test to compare differences among individual means. For some analyses and presentation of data obtained from 10.0 Gy TBI-BM5, NHP groups were divided into acute (6.5 days), 30–100 days survival (n = 10) and >100 days survival (n = 10).
RESULTS
TBI and TBI-BM5 Models
All TBI animals were euthanized at day 15 or before, if they met established criteria (Fig. 1A). As reported previously (1), the LD50/15 for the TBI model was 11.33 Gy, which was significantly lower than the LD50/15 for the TBI-BM5 model at 11.95 Gy. This shows the positive effects derived from sparing 5% of bone marrow on survival postirradiation (1). We previously reported that NHPs subjected to TBI-BM5 exhibited both grade 3 neutropenia [absolute neutrophil count (ANC) ≤500 µl−1] and thrombocytopenia (platelets ≤20,000 µl−1) (1), and that TBI-BM5 preserves the ability of the ANC and platelets to reconstitute. Initial TBI-BM5 cohorts exposed to 9.0 Gy all survived (n = 4), whereas TBI-BM5 cohorts exposed to 12.5 Gy did not survive beyond day 19 (n=4). Long-term sequelae in the TBI-BM5 cohorts included weight loss, peripheral edema (legs, scrotum), intermittent diarrhea and respiratory distress. These sequelae and their treatment were described previously (1). It was determined that timed euthanasia would be confined to TBI-BM5 cohorts exposed to 10 Gy (Fig. 1B, hatched box), because the number of NHPs needed to obtain a sufficient number of animals for statistical analyses for each time point at higher doses could not be justified. Targeted time points occurred at: 6–7 days (n = 2); 34–45 days (n = 6); 46–99 days (n=5); 100–119 days (n=5); and 171–191 days (n= 5) (Fig. 1B).
FIG. 1.
Survival of 10–14 Gy TBI NHPs. Panel A: Average day of survival in each group is indicated by the line. Panel B: Timed necropsies of the TBI-BM5 cohorts exposed to 9–12.5 Gy. Studies are focused on the threshold dose of 10 Gy (hatched box).
TBI and TBI-BM5 Induced Changes in Intestinal Morphology
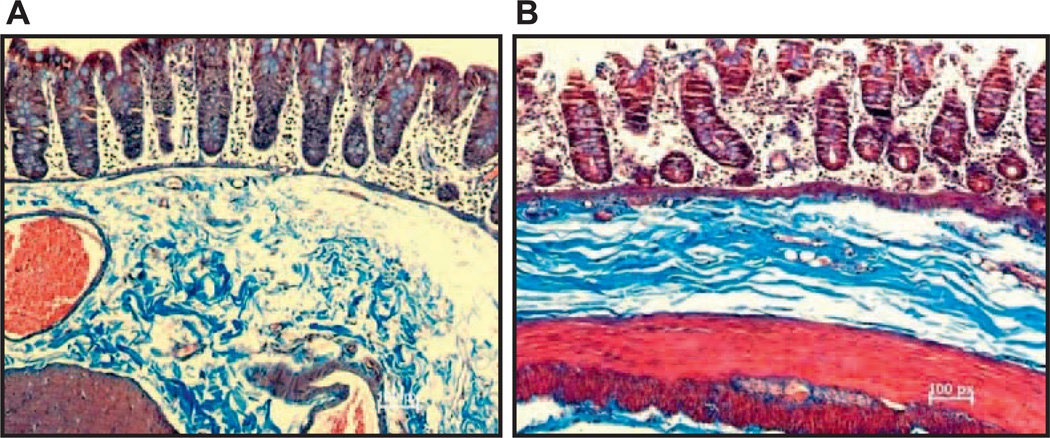
General observations for most doses after TBI and TBI-BM5 were fluid accumulation in the small intestine and semisolid/liquid stool in the colon. Longer term changes were visible petechiae along the length of the small intestine and colon and a stiffening of the colon. Previously published studies (1, 2) demonstrated dose-dependent microscopic damage along the length of the small intestine with a characteristic loss of surface epithelial cells, broadening of villi, increased crypt depth, crypt dropout, marked expansion of the submucosa (consistent with edema) and thickening of the muscularis externa (consistent with remodeling) (Fig. 2). Analysis of the control and 10 Gy TBI-BM5 colon showed a similar loss of epithelial cell continuity and crypt dropout at day 181 (Fig. 3) consistent with previous results (1).
FIG. 2.
Representative photomicrographs of jejunum after 10 Gy TBI-BM5 in control (panel A), 43 days (panel B), 119 days (panel C) and 181 days (panel D) postirradiation. Arrows show submucosal edema, and arrow heads show increased thickness of smooth muscle. All photos are at 20× magnification.
FIG. 3.
Representative photomicrographs of the proximal colon after 10 Gy TBI-BM5 in control (panel A) and 181 days (panel B) postirradiation. All photos are at 20× magnification.
Radiation Induced Changes in Clinical Chemistry
We assessed changes in clinical chemistries for the 8 NHPs euthanized between days 100–181 postirradiation. Values represent preirradiation samples taken 3–4 days before exposure and the average of samples taken during 1–10 days, 10–30 days and at 30-day intervals until day 181. Individual values for each animal were averaged to obtain a mean for that animal and then averaged with other animals to obtain a group mean for the interval. There were no changes in serum sodium, potassium, calcium concentrations or blood glucose (Table 2) throughout the entire postirradiation period. Total serum protein (Fig. 4A) and serum albumin (Fig. 4B) levels were unchanged in the first 10 days postirradiation, but were significantly decreased for the remainder of the postirradiation period. The liver enzyme, AST, was elevated only in the acute period and was unchanged for the rest of the postirradiation period (Fig. 4C and D), whereas ALT remained unaltered throughout. BUN and creatinine were unaffected during the first 90 days postirradiation, but both products were elevated significantly thereafter (Fig. 4E and F). The BUN/creatinine ratio was unchanged by irradiation, indicating that there were parallel changes in the levels of the individual products.
TABLE 2.
Effect of Irradiation on Serum Chemistries Levels
| Days postirradiation | Na+ (mEq/l) (140–152) | K+ (mEq/l) (140–152) | Ca2+ (mEq/l) (9.3–10.3) | Blood glucose (mg/dl) |
|---|---|---|---|---|
| 0 (n = 17–19) | 147 ± 1 | 4.0 ± 0.1 | 9.6 ± 0.1 | 61.2 ± 2.3 |
| <8 (n = 5) | 144 ± 1 | 3.9 ± 0.2 | 9.1 ± 0.2 | 66.6 ± 7.0 |
| 8–99 (n = 7–12) | 145 ± 1 | 4.1 ± 0.1 | 9.2 ± 0.2 | 68.5 ± 2.4 |
| >100 (n = 5–7) | 144 ± 2.5 | 4.3 ± 0.3 | 9.1 ± 0.3 | 61.4 ± 2.3 |
Note. Values represent measurements taken at necropsy.
FIG. 4.
Changes in serum chemistries over time after 10 Gy TBI-BM5. All values were taken from NHPs that survived more than 100 days. Panels A–F show total protein, albumin, AST, ALT, BUN and creatinine, respectively. P < 0.05 vs. 0 (control, solid bars). Values are means ± SEM.
TBI and TBI-BM5 Impair Mucosal Barrier Function
One of the most important functions of the gut is to maintain the integrity of the mucosal barrier. The epithelial layer is an important first line of defense and limits passage of intraluminal bacteria that trigger innate and adaptive immune responses. In the acute (days 3–15 postirradiation) period after TBI, there was a decrease in TEER, an index of mucosal permeability, at doses greater than 11 Gy in the jejunum (Fig. 5A) and at doses greater than 13 Gy in the proximal colon (Fig. 5B). As TBI-BM5 improves survival for doses from 9–12.5 Gy, the changes in TEER in Fig. 5C and D represent a range of times (see Fig. 1B) for a given radiation dose. NHPs euthanized during the chronic period after TBI-BM5 demonstrated that a reduction in TEER occurs at doses greater than 9 Gy in both the jejunum (Fig. 5C) and proximal colon (Fig. 5D).
FIG. 5.
Dose-related changes in transepithelial electrical resistance (TEER) in muscle-free sections of jejunum (panels A and C) and proximal colon (panels B and D) after TBI (panels A and B) or TBI-BM5 (panels C and D). Total-body irradiated NHPs were euthanized ≤15 days after exposure (panels A and B). The TBI-BM5 animals were euthanized at set intervals between 30 and 190 days postirradiation. *P < 0.05 and **P < 0.01 vs. 0 (control, solid bars). Values are means ± SEM.
Data from NHPs euthanized at 6.5 (acute), 30–100 and >100 days after 10 Gy TBI-BM5 show that TEER was decreased significantly in jejunum at 6.5 days and remained suppressed throughout the postirradiation period (Fig. 6A). TEER in the proximal colon was not significantly reduced until day 61 after 10 Gy TBI-BM5 but remained low thereafter (Fig. 6B). Resistance across tissues mounted in Ussing chambers is calculated using Ohm’s law (V = I/R). Resistance was significantly lower in both jejunum (Fig. 6C) and proximal colon (Fig. 6D), consistent with TEER measurements (Fig. 6A and B). These data show that radiation induced an early and persistent reduction in barrier function in the small intestine that coincided with the absence of mucosal restitution.
FIG. 6.
Time-dependent changes in transepithelial electrical resistance (TEER) in muscle-free sections of jejunum (panel A) and proximal colon (panel B) after 10 Gy TBI-BM5. Resistance was measured in Ussing chambers in muscle-free sections of jejunum (panel C) and proximal colon (panel D) after 10 Gy TBI-BM5. Resistance is calculated using V = IR where V is set at 1 and I is the current generated by the tissue in response to this challenge. *P < 0.05 and **P < 0.01 vs. 0 (control, solid bars). Values are means ± SEM.
TBI and TBI-BM5 Impair Epithelial Cell Function
Previous studies showed a persistent weight loss (percentage of initial weight) in surviving NHPs in the mid- (11–11.5 Gy) and high- (12–12.5 Gy) dose TBI-BM5 groups (1). In the current study, the rate of weight loss in the 10 Gy irradiated group was consistent between days 1–35 postirradiation (Fig. 7A). Of interest, the rate of weight loss improved after day 49, followed by a net gain in weight between days 89–134. At day 134, there was a net weight loss in the remaining NHPs that persisted until termination of the study.
FIG. 7.
Change in weight over time in surviving NHPs after 10 Gy TBI-BM5 (panel A). Glucose absorption was determined in muscle-free sections of jejunum in response to 20 mM glucose added to the mucosal side of the tissue (panel B). Fold change in gene expression of SGLT1 in jejunal mucosa. *P < 0.05 and **P < 0.01 vs. 0 (control, solid bars). Values are means ± SEM.
To determine if changes in absorptive function contributed to this weight loss we assessed changes in glucose absorption in jejunal mucosae mounted in Ussing chambers. There was a significant inhibition of glucose absorption in NHPs between days 30–100 and >100 days after 10 Gy TBI-BM5 (Fig. 7B). These data show that there is a functional correlate between the failed mucosal restitution and impaired epithelial cell function in the postirradiation period. The sodium-linked glucose transporter-1 (SGLT1) is the major transporter for glucose in the small intestine. There was a significant downregulation of SGLT1 expression in the acute (6.5 days) and chronic (>100 days) periods after 10 Gy TBI-BM5 (Fig. 7C). There were no changes in glucose transporter 2, which transports glucose out of the basolateral side of the enterocyte (data not shown). Thus, changes in glucose absorption are consistent with reduced expression of SGLT1 and reduced numbers of enterocytes and intact villi. The lack of mature enterocytes was also confirmed by protein expression of ALP in the small intestine (Fig. 7D). Of interest, the failure of the mucosa to heal was paralleled by a decrease in serum ALP (Fig. 7E).
TBI-BM5 Upregulates Pro-inflammatory Mediators
The early and persistent increase in permeability suggests that radiation induces a chronic impairment of mucosal barrier function. We investigated if barrier dysfunction was accompanied by changes in the local cytokine environment, selecting prototypic cytokines as general indices of inflammation. IFN-γ is considered a classic Th1 cytokine associated with other inflammatory GI pathologies, including IBD and celiac disease (3). IL-17A is a member of the Th17 family that plays a critical role in autoimmune-based and chronic inflammatory pathologies (8). Nitric oxide synthase-2 (NOS-2, inducible NOS) is synthesized in response to upregulation of pro-inflammatory cytokines and is a marker for classically activated macrophages (M1) (9). There was a significant upregulation of IL-17A gene expression in the jejunum throughout the postirradiation period but only a transient increase in IL-17A in the proximal colon at 30–100 days (Fig. 8A and D). NOS-2 expression was upregulated in both the small intestine and colon (Fig. 8C and F). In contrast, there was a significant increase in IFN-γ gene expression after 30 days in the jejunum (Fig. 8B), but no changes in the colon (Fig. 8E). These data show development of a modest, but significant Th1/Th17-dominant pro-inflammatory environment in the gut after irradiation, an environment consistent with the presence of classically activated macrophages.
FIG. 8.
Fold changes in mucosal gene expression of pro-inflammatory mediators. IL-17A in mucosa of jejunum (panel A) and proximal colon (panel D); IFN-γ in jejunum (panel B) and proximal colon (panel E); and NOS-2 in jejunum (panel C) and proximal colon (panel F). *P < 0.05 and **P < 0.01 vs. 0 (control, solid bars). Values are means ± SEM.
TBI-BM5 Inhibits Epithelial Regeneration
The failure to restore normal mucosal architecture and function in the acute and chronic postirradiation period indicates a defect in the ability of epithelial cells to proliferate and/or differentiate. In this study, we determined changes in the expression of stem cell, lineage and maturation markers. The mRNA of Lgr5, a stem cell marker that labels crypt basal cells, was downregulated significantly in the small intestine after 10 Gy TBI-BM5 (Fig. 9A). Lgr5 protein expression was increased transiently at 10 days but was absent after 100 days postirradiation (Fig. 9B). In contrast, there was no change in Lgr5 expression in the proximal colon postirradiation (data not shown). These data confirm that persistent loss of crypts in the postirradiation period results in reduced numbers of Lgr5 cells. In a small number of samples taken from the jejunum, we performed laser capture microdissection of crypts and surface cells and analyzed them for expression of Hes-1, the enterocyte marker and NGN-3, the enteroendocrine cell marker. There were no significant changes in Hes-1 and NGN-3 expression over time in the crypt cells (Fig. 9C and D).
FIG. 9.
Fold changes in mucosal Lgr5 gene expression (panel A) and protein (panel B) over time in jejunum. Fold change in mucosal expression of the absorptive cell lineage marker, Hes-1 (panel C), and the enteroendocrine cell lineage marker, NGN-3 (panel D), in LCM samples of intestinal crypt cells. *P < 0.05 and **P < 0.01 vs. 0 (control, solid bars). Values are means ± SEM.
DISCUSSION
The TBI-BM5 model was developed to establish a reality-based model of radiation-induced injury that would include the likely sparing of some bone marrow in a nuclear event. In terms of model development, sparing 5% of the bone marrow allowed for assessment of mortality and morbidity associated with acute GI-ARS induced by TBI, while preserving sufficient active bone marrow to ensure survival through the hematopoietic syndrome, which may result in a reduced need for blood transfusions. Some of the salient features of the TBI and TBI-BM5 NHP models were reported previously (1, 2). The current study demonstrates that the chronic GI syndrome is characterized by persistent changes in both the small intestine and colon, including impaired mucosal restitution, inflammation and reduced barrier function. There is also a reduction in Lgr5 expression, indicating a loss of crypt basal cells and impaired crypt regeneration despite recovery of the hematopoietic compartment.
There was a dose-sensitive response to TBI, with the small intestine exhibiting a greater sensitivity than the proximal colon. TBI doses of 13 and 14 Gy are above the LD50, with a mean survival time of 7.8 days (1), and these doses induced a uniform decrease in permeability in both regions. Previous studies showed that preservation of 5% of the bone marrow through shielding shifted the LD50 of lethal radiation doses in these NHPs to the right, consistent with improved survival (1). ANC and platelet recovery in these NHPs occurred between 30 and 60 days after 9–12 Gy TBI-BM5, although CD4+ and CD8+ T cells remained below baseline through 180 days postirradiation (1, 2). A more recent study showed that bone marrow transplantation improved survival in mice coincident with improved intestinal permeability after sublethal 8 Gy TBI (10). In the current study, the significant increase in permeability after 10 Gy TBI-BM5 occurred early and remained lower than in controls throughout the entire postirradiation period. These data imply that minimal sparing of bone marrow (5%) alone is not sufficient to improve the radiation-induced increase in intestinal permeability or mucosal damage. The slow immune cell reconstitution in the TBI-BM5 model (1) likely contributes to these sequelae and further suggests that transplantation may provide a benefit to mucosal restitution that is not afforded by partial bone marrow shielding. Taken together, these data suggest that impaired permeability impedes restoration of mucosal damage in the acute phase, emphasizing the importance of barrier function in mucosal repair.
Impaired barrier function facilitates passage of luminal contents to subepithelial structures including resident immune cells. In response to injury, epithelial cells and activated resident immune cells release chemokines that recruit additional immune cells to the affected area. These processes are altered by high doses of radiation as the loss of bone marrow-derived cells impacts the inflammatory process. There have been attempts to determine if plasma cytokines are useful biomarkers after TBI (11), but changes in plasma levels do not necessarily parallel changes in the specific organs. In addition, there is little information on radiation-induced changes in cytokine expression in the gut. Studies using 10 Gy TBI-BM5 allowed us to investigate changes in inflammatory mediators in the local environment over time. We found a modest, but significant, increase in the expression of IL-17A at all times postirradiation in the jejunum but only at 30–100 days in the colon. It should be noted, however, that constitutive expression of IL-17A in the colon is ~2-fold higher than in the jejunum. There was also a small upregulation of IFN-γ in the jejunum but not in the colon. The constitutive expression of IFN-γ in the colon is ~8-fold higher than the expression in the jejunum. NOS-2 was also upregulated in the colon at 6.5 days, consistent with data in mouse colon after 10 Gy TBI (12). It should be noted that these levels are low when compared to sites of active inflammation in GI pathologies such as celiac disease (13) and IBD (14). Taken together, these data indicate that there are regional changes in specific cytokines in the gut in response to radiation exposure, consistent with a subclinical inflammation that may reflect the slow recovery of CD4+ T cells after TBI-BM5 (15).
The TBI-BM5 model provided critical information on changes in clinically relevant parameters over time. Edema was present in some NHPs, more often at higher doses and generally confined to the lower extremities (legs and scrota). The lower serum protein and albumin evident at >10 days postirradiation may indicate a progression toward reduced renal function, a likelihood that is increased when combined with elevated BUN and creatinine at >90 days postirradiation. Of interest, serum electrolytes were unchanged by irradiation, and liver enzyme AST showed only transient increases in the acute phase. These data indicate that the liver is fairly resistant to the radiation doses that induce a chronic GI syndrome, but that kidney damage may be initiated early in the postirradiation period.
A prominent feature of NHPs exposed to doses higher than 9 Gy TBI-BM5 was persistent weight loss during the postirradiation period, in some cases reaching euthanasia criteria thresholds (>25% of preirradiation weight for >72 h) (1). Glucose is one of the major nutrients absorbed in the small intestine. In the current study, 10 Gy TBI-BM5 induced two periods of weight loss over time, with a transient “recovery” between 90 and 135 days. Mature enterocytes at the tip of villi have the highest expression of SGLT1, the major physiological transporter. Indeed, glucose absorption is an important developmental maturation marker during weaning (16). Radiation exposure impaired glucose absorption and also decreased expression of SGLT1. This effect is likely due to the lack of mucosal restitution and reduced numbers of enterocytes rather than a decrease in SGLT1 activity or to a downregulated expression of the transporter in individual enterocytes. Changes in glucose handling may underlie the generalized weight loss that occurs, however, the transition from net weight gain to net weight loss late in the postirradiation period suggests that other factors (e.g., appetite, hormones) could play a role. There was a significant decrease in ALP activity beginning at day 10 postirradiation, an effect not observed in other liver enzymes, ALT or AST. ALP is present in intestinal tissues, particularly in the upper portions of villi in the small intestine, where its presence is considered a marker for enterocyte maturation. The presence of ALP in enterocyte membranes is also important for maintenance of intestinal barrier function (17). In addition, ALP contributes to host defense through an ability to detoxify LPS, thereby blunting the subsequent LPS-mediated activation of pro-inflammatory cytokines (4). Taken together these data suggest that the impaired restitution of intestinal epithelium with reduced numbers of mature villi coincides with reduced protein expression of ALP in intestinal tissue. This event is paralleled by a reduction in serum ALP, indicating that this could be a biomarker for intestinal injury in chronic GI syndrome.
Mucosal healing in the postirradiation period is dependent upon a number of factors, including sparing of crypts containing the stem cells, migration of newly formed cells up the villus and maturation of these cells into appropriate phenotypes. Previous studies showed that transit of BrdU-labeled crypt cells to the villus tip takes approximately 7 days, which is longer than the 3 days migration time observed in mice (2). The current view is that a population of rapidly recycling, radiosensitive Lgr5+ crypt basal cells coexists with a more radioresistant population of stem cells, which are distinct from the Lgr5+ crypt basal cells and may serve as a reserve population (18). The general response to mucosal damage is expansion of the stem cell compartment to facilitate restoration of mucosal homeostasis. In the current study, there was a decrease in Lgr5 gene expression in the jejunum at 6.5 days that reflects the reported time of maximal loss of crypts (27% of preirradiated controls) after 10 Gy TBI-BM5 in NHPs (1). There is a partial recovery of crypts after 10 Gy TBI-BM5, reaching a maximum of 70% at day 180 (1). Lgr5 gene expression was reduced after 10 Gy TBI-BM5 and does not recover fully during the entire postirradiation period. Changes in Lgr5 protein parallel gene expression in the chronic postirradiation period. Surprisingly, there was no change in Lgr5 expression in the colon despite a similar lack of recovery of crypts in this area (1). This may be attributed, in part, to a lower constitutive level of expression in this region as Lgr5+ cells are more scattered in the colon. The results of the current study suggest that radiation impairs not only the Lgr5+ crypt basal cell population but also the reserve population that is thought to be the +4 population (18). It may be that persistent mucosal injury in chronic GI syndrome leads to intestinal stem cell exhaustion, as observed for hematopoietic stem cells (19).
To determine if radiation impacts the expression of stem cell lineage makers, we performed laser capture microdissection of crypt cells in the jejunum. There was no change in the expression of Hes-1 and NGN-3 postirradiation, suggesting that remaining crypt cells do not lose their ability to express the lineage markers for absorptive (Hes-1) or enteroendocrine (NGN-3) phenotypes. Taken together, these data suggest that radiation induces an irrevocable loss in Lgr5+ cells, resulting in an impaired ability to reconstitute normal mucosal architecture. The mechanisms responsible for the failed restitution may be related to the chronic increase in mucosal permeability and the persistent, if modest, inflammatory environment. The microenvironment impacts the function of cells that support the stem cell niche, and stem cells are considered a target of neoplastic transformation.
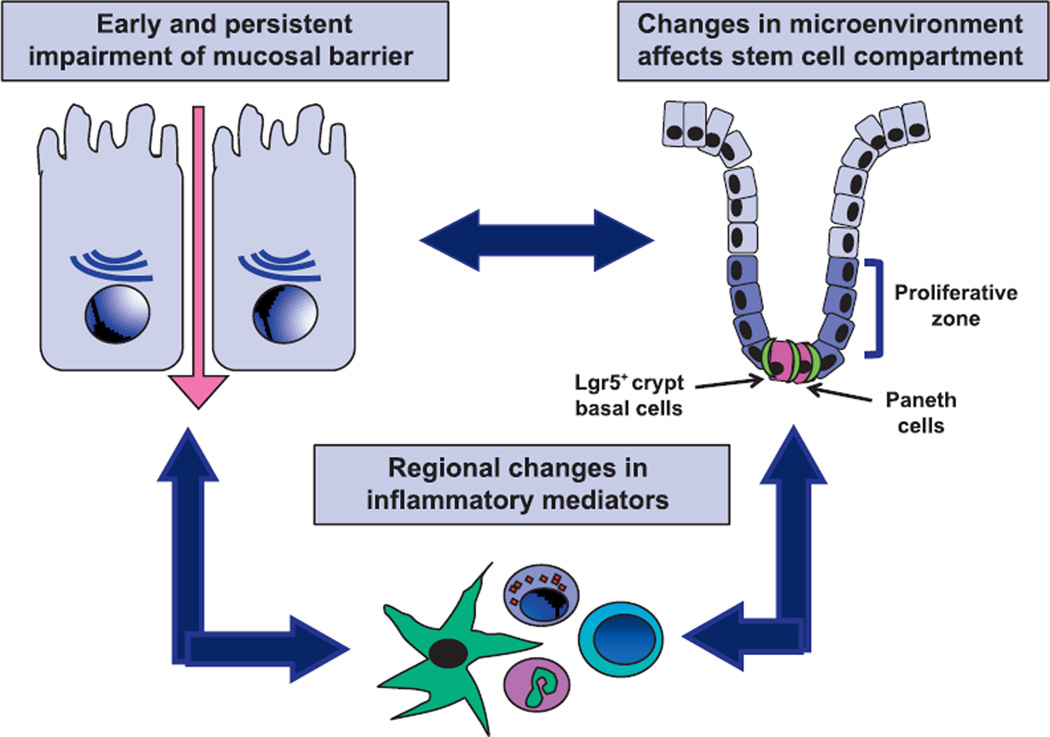
In conclusion, we propose that exposure to radiation induced changes in mucosal damage that promoted a modest inflammatory response with impaired mucosal restitution, thereby providing persistent stimuli for epithelial proliferation (Fig. 10). This microenvironment induces a remodeling of both mucosa and smooth muscle. The impaired mucosal restitution also results in a reduced number of the mature enterocytes that are critical for nutrient absorption and likely contributes to the continued weight loss observed in a number of NHPs in the postirradiation period. These findings suggest that partial bone marrow shielding does not allow full recovery from exposure to high-dose radiation, but instead allows development of a chronic GI pathology that progresses to multiple organ injury.
FIG. 10.
Schematic of interactions among mucosal barrier function, inflammation and mucosal restitution that contribute to chronic GI syndrome in NHPs after 10 Gy TBI-BM5.
Acknowledgments
This work was supported by the National Institute of Allergy and Infect ious Diseases [NIAID; contract nos. RCI AI78520, HHSN266200500043C (TSD) and HHSN272201000046C (TM)].
REFERENCES
- 1.MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, et al. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103:427–453. doi: 10.1097/HP.0b013e318266eb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- 3.Monteleone I, Pallone F, Monteleone G. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Med. 2011;9:122. doi: 10.1186/1741-7015-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 5.Asmar RE, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 6.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, et al. The Role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto M, Zhao A, Sun R, Stiltz J, Madden KB, Mentink-Kane M, et al. IL-13 receptor 2 regulates the immune and functional response to nippostrongylus brasiliensis infection. J Immunol. 2009;183:1934–1939. doi: 10.4049/jimmunol.0804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean LP, Cross RK, Shea-Donohue T. Combined blockade of IL-17A and IL-17F may prevent the development of experimental colitis. Immunotherapy. 2013;5:923–925. doi: 10.2217/imt.13.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luzina IG, Keegan AD, Heller NM, Rook GAW, Shea-Donohue T, Atamas SP. Regulation of inflammation by interleukin-4: a review of “alternatives”. J Leukoc Biol. 2012;92:753–764. doi: 10.1189/jlb.0412214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, et al. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat Res. 2014;181:229–239. doi: 10.1667/RR13548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh VK, Grace MB, Parekh VI, Whitnall MH, Landauer MR. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int Immunopharmacol. 2009;9:1401–1410. doi: 10.1016/j.intimp.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Freeman SL, MacNaughton WK. Ionizing radiation induces iNOS-mediated epithelial dysfunction in the absence of an inflammatory response. Am J Physiol Gastrointest Liver Physiol. 2000;278:G243–G250. doi: 10.1152/ajpgi.2000.278.2.G243. [DOI] [PubMed] [Google Scholar]
- 13.Monteleone I, Sarra M, Del Vecchio Blanco G, Paoluzi OA, Franze E, Fina D, et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol. 2010;184:2211–2218. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- 14.Rismo R, Olsen T, Cui G, Christiansen I, Florholmen J, Goll R. Mucosal cytokine gene expression profiles as biomarkers of response to infliximab in ulcerative colitis. Scand J Gastroenterol. 2012;47:538–547. doi: 10.3109/00365521.2012.667146. [DOI] [PubMed] [Google Scholar]
- 15.MacVittie TJ, Bennett AW, V Cohen M, Farese AM, Higgins A, Hankey KG. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys. 2014;106:84–96. doi: 10.1097/HP.0b013e3182a2a9b2. [DOI] [PubMed] [Google Scholar]
- 16.Montagne L, Boudry G, Favier C, Huërou-Luron IL, Lallès J-P, Sève B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 2007;97:45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- 17.Bowie RV, Donatello S, Lyes C, Owens MB, Babina IS, Hudson L, et al. Lipid rafts are disrupted in mildly inflamed intestinal microenvironments without overt disruption of the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2012;302:G781–G793. doi: 10.1152/ajpgi.00002.2011. [DOI] [PubMed] [Google Scholar]
- 18.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruzankina Y, Brown EJ. Relationships between stem cell exhaustion, tumour suppression and ageing. Br J Cancer. 2007;97:1189–1193. doi: 10.1038/sj.bjc.6604029. [DOI] [PMC free article] [PubMed] [Google Scholar]