Abstract
The genome of influenza viruses consists of multiple segments of single stranded negative-sense RNA. Each of these segments is bound by the heterotrimeric viral RNA-dependent RNA polymerase and multiple copies of nucleoprotein, forming viral ribonucleoprotein (vRNP) complexes. It is in the context of these vRNPs that the viral RNA polymerase carries out transcription of viral genes and replication of the viral RNA genome. In this Review, we discuss our current knowledge of the structure of the influenza virus RNA polymerase, how it carries out transcription and replication, and how its activities are modulated by viral and host factors. Furthermore, we discuss how advances in our understanding of polymerase function could help identifying new antiviral targets.
Influenza viruses belong to the family of Orthomyxoviridae and are the causative agents of influenza, a respiratory disease in humans. Influenza A and B viruses cause substantial morbidity and mortality in humans and a considerable financial burden worldwide, whereas influenza C viruses cause sporadic outbreaks of mild respiratory disease, mainly in children1, 2. Although vaccines against influenza A and B viruses are available, the protection that they offer is limited by antigenic variation in the haemaggluntinin (HA) and neuraminidase (NA) envelope glycoproteins of influenza virus strains3. Additionally, influenza A viruses have a reservoir in birds and pigs from which pandemic viruses with novel HA and NA proteins can emerge against which there is little pre-existing immunity in the human population. Available vaccines are unlikely to protect against such strains. Although antiviral drugs can be a first line of defense in the case of an emerging pandemic virus, the number of antiviral drugs that can be used for prophylaxis and therapeutic treatment of severe infections is limited and emerging antiviral resistance is a continuing problem4, 5. Therefore, understanding of the molecular mechanisms of influenza virus replication is critical for the development of new antiviral drugs.
Influenza viruses contain a single-stranded negative-sense RNA genome that consists of eight segments in influenza A and B viruses and seven segments in influenza C viruses6. In all three species, the viral RNA (vRNA) genome segments are bound by a heterotrimeric RNA-dependent RNA polymerase, forming a viral ribonucleoprotein (vRNP) complex7, 8. In the vRNP, the 5′ and 3′ termini of vRNA are bound to a polymerase heterotrimer, while the rest of the vRNA associates with oligomeric nucleoprotein (NP) (FIG. 1a). Structural models based on cryo-EM imaging of native vRNPs show that the vRNP is an anti-parallel double helix of NP-coated vRNA that contains a polymerase at one end and an NP loop at the other end9, 10. The influenza virus RNA polymerase consists of three subunits: polymerase basic 1 (PB1), PB2 and polymerase acidic (PA) in influenza A and B virus or polymerase 3 (P3) in influenza C virus7, 8. Upon viral infection, the vRNPs are transported into the nucleus of the host cell, where the RNA polymerase carries out transcription of viral genes and replication of the viral RNA genome in the context of the vRNP11 (BOX 1). Transcription is a primer-dependent process that produces mRNAs with a 5′ cap structure and a 3′ poly(A) tail. The influenza virus polymerase does not have inherent capping activity and it relies on host capped RNAs as cap-donors12. In a process called ‘cap-snatching’, the viral polymerase uses its PB2 cap-binding domain to capture the 5′ cap of nascent host capped RNAs and its PA/P3 endonuclease domain to cleave the capped RNA about 8-14 nucleotides downstream of the cap structure13–15. It then uses these capped RNA fragments as primers to initiate transcription. In contrast to transcription, replication is primer-independent and proceeds via a complementary RNA (cRNA) replicative intermediate. Progeny vRNPs are exported from the nucleus and traffic to the cell membrane to be incorporated into new virions (BOX 1).
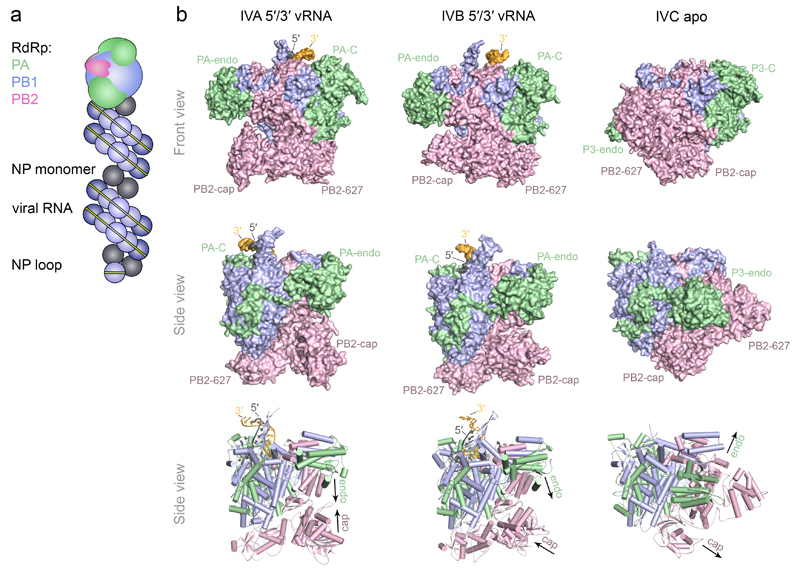
Figure 1. Model of the vRNP complex and structures of the influenza A, B and C virus RNA polymerases.
a Model of the vRNP complex. In the vRNP, the 5′ and 3′ termini of vRNA are bound by a heterotrimeric polymerase complex and the rest of the vRNA is coated by nucleoprotein. The complex is twisted into an anti-parallel double helix, the structure of which is maintained by contacts between NP monomers. The RNA forms a loop at the end opposite the polymerase-bound end9, 10. b Front and side views of the influenza A (PDB: 4WSB), B (PDB: 4WSA) and C (PDB: 5D98) virus RNA polymerase structures shown in surface (top and middle row) or cartoon model (bottom row) representation. PB1, PB2 and PA/P3 subunits are coloured in blue, pink and light green, respectively. The PB2 cap-binding (PB2-cap), PB2 627-domain (PB2-627), PA/P3 endonuclease (PA-endo) and PA/P3 C-terminal (PA-C) domains are indicated. The 5′ and 3′ termini of vRNA in the influenza A and B virus polymerase structures are shown in dark grey and yellow, respectively. In the influenza A virus polymerase the cap-binding and endonuclease domains face each other, configured for cap-snatching (left, bottom row). In the influenza B virus polymerase structure the cap-binding domain is rotated to face the product exit channel consistent with insertion of the capped primer into the active site via the product exit channel (middle, bottom row). In the influenza C virus polymerase structure the cap-binding and endonuclease domains face opposite directions, incompatible with cap-snatching (right, bottom row).
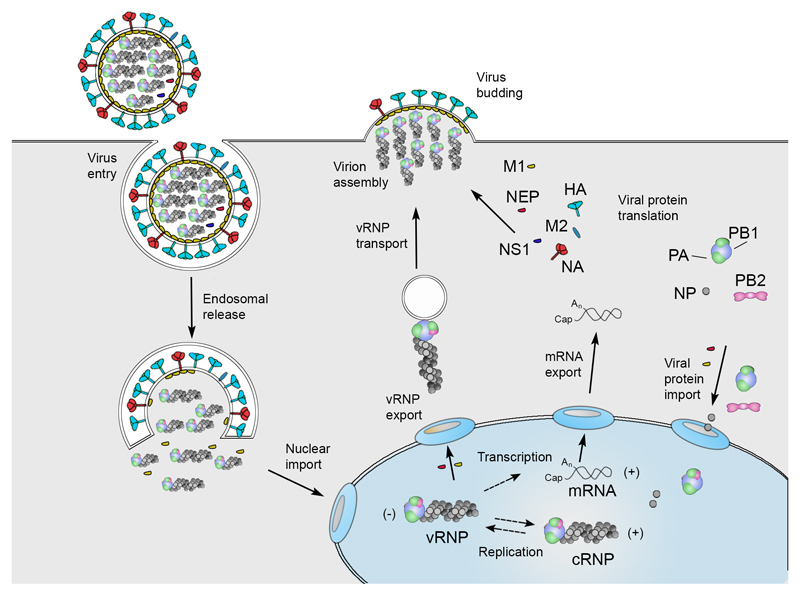
Box 1. Influenza A virus life cycle.
Viral infection initiates with the binding of a virion to cell surface receptors containing sialic acid, followed by the endocytosis of the virion. After fusion of the viral and endosomal membranes, the vRNPs are released into the cytoplasm and then transported into the nucleus. In the nucleus the viral RNA polymerase transcribes the vRNA segments into mRNAs, which are 5′ capped and 3′ polyadenylated. Viral mRNA is exported to the cytoplasm for translation by cellular mechanisms. The viral RNA polymerase also performs replication of vRNA by copying it into complementary RNA (cRNA), which serves as a template for the production of more vRNA. Newly synthesised viral polymerase and nucleoprotein are imported into the nucleus and bind to cRNA and vRNA to assemble vRNPs and cRNPs, respectively. Following nuclear export, progeny vRNPs are transported across the cytoplasm on recycling endosomes in a Rab11-dependent manner to the cell membrane, where assembly of progeny virions takes place. Mature virions incorporate a substantial amount of host proteins79 and are released by budding. Auxiliary viral proteins, PB1-F2, PB1-N40, PB2-S1, PA-X, PA-N155, PA-N182, M42, and NS3 that have been detected in infected cells, but are not known to be incorporated into progeny virions, are not shown139.
Until recently, lack of high-resolution structural information on the influenza virus RNA polymerase greatly hampered our understanding of influenza virus transcription and genome replication at the molecular level. The RNA polymerases of negative-strand RNA viruses have been refractory to structural analyses, primarily due to their large size and low expression levels in recombinant systems and, in the case of influenza virus, multi-subunit nature16, 17. Recent developments in the expression and purification of large proteins and macromolecular complexes, combined with improved methods for the structural analysis of proteins, resulted in high resolution RNA polymerase structures for influenza A, B, and C viruses as well as for other negative-strand RNA viruses, such as La Crosse virus (a bunyavirus) and Vesicular Stomatitis Indiana Virus (VSIV; a rhabdovirus)18–22. These novel structures provide unprecedented insights into their molecular mechanisms. This Review focuses on these novel structural insights and their potential to advance our understanding of viral pathogenicity and host adaptation as well as to facilitate the development of new prophylactic antivirals or treatments for influenza virus infections.
The RNA synthesis machine of influenza viruses
Viral polymerase structure
The past decade witnessed the emergence of high-resolution structural information on fragments of the three subunits of the influenza virus polymerase13–15, 23–27. Moreover, electron microscopy (EM) of the polymerase heterotrimer in isolation or as part of vRNP revealed that the polymerase has a globular structure9, 10, 28, 29. However, these structures provided limited information on the arrangement of the subunits in the polymerase heterotrimer. This changed dramatically when high-resolution x-ray crystal structures of the complete polymerase heterotrimers of the bat influenza A and human influenza B and C viruses became available (FIG. 1b)19, 21, 22. In addition, a 4.3Å resolution structure of an H5N1 avian influenza virus polymerase complex composed of the full-length PB1, PA and an N-terminal fragment of PB2 has been obtained by cryo-EM30.
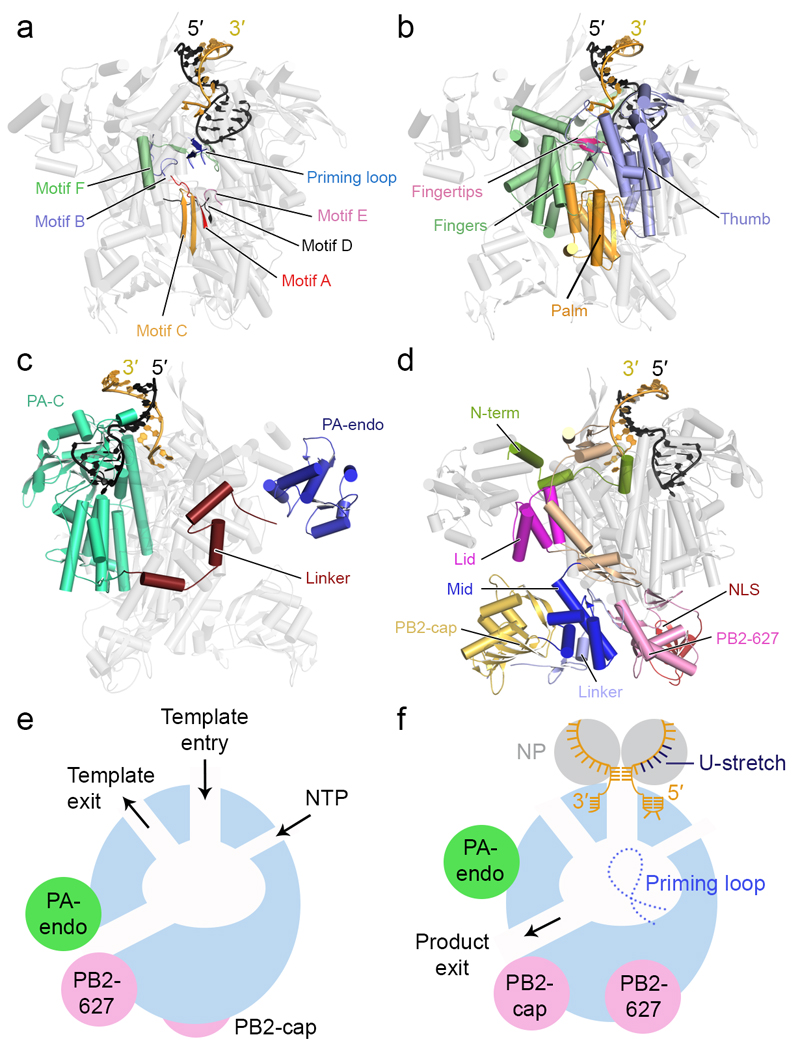
The x-ray crystallography structures of the complete polymerase heterotrimer revealed that the PB1 subunit is the centre of the polymerase (FIG. 1b and FIG. 2a,b). PB1 contains the pre-A (also known as F) and A-E motifs which are characteristic of RNA-dependent RNA polymerases (FIG. 2a)31. Accordingly, PB1 has the canonical right-hand-like fold, possessing fingers, fingertips, palm, and thumb, but with additional N- and C-terminal extensions that facilitate interactions with the PA/P3 and PB2 subunits, respectively (FIG. 2b; see also BOX2 panel b). PB1 is sandwiched by the N-terminal endonuclease domain and the large C-terminal domain of the PA/P3 subunit (FIG. 1b and FIG. 2c). A long linker connects the two domains and wraps around the external side of the PB1 fingers and palm subdomains. PB2 consists of multiple domains (FIG 1b and FIG. 2d). The N-terminal third of PB2 interacts with PB1, making contacts mostly with the PB1 thumb and C-terminal extension. The arrangement of the domains within the remaining two thirds of PB2 differs dramatically in the influenza A and B virus polymerase structures compared to that of influenza C virus, indicating that these form flexible domains (Supplementary Video 1). These include the mid-domain, the cap-binding domain, the cap-627 linker, the so called 627-domain, named after amino acid residue 627, a host range determinant of influenza viruses32, and the C-terminal nuclear localization signal (NLS) domain (FIG. 2d). The mid and cap-627 linker domains form one rigid unit referred to as the ‘mid-link’33. Overall the polymerase can be viewed as a complex made up of a polymerase core consisting of PB1, the C-terminal domain of PA/P3 and the N-terminal region of PB2, and flexible peripheral appendices that are formed by the PA/P3 endonuclease and the PB2 cap-binding, mid-link, 627, and NLS domains (FIG. 2e,f).
Figure 2. Polymerase architecture and channels.
a-d Cartoon models of the influenza A virus polymerase (PDB: 4WSB), in different orientations, showing PB1 polymerase motifs A to F and the priming loop (a), the right handed arrangement of the PB1 fingers, palm and thumb subdomains and the fingertips (b), the PA (c) and PB2 (d) domain structures. The 5′ and 3′ termini of vRNA are coloured as in FIG. 1. e, f Models of the RNA polymerase in the ‘inactive’ (e) and ‘transcription pre-initiation’ states (f) showing the conformational re-arrangement of the flexible peripheral cap-binding, 627 and endonuclease (Endo) domains. The temple entry and exit, NTP entry and product exit channels, the priming loop near the active site as well as the binding of the 5′ and 3′ vRNA termini and the positions of nucleoproteins (NP) in the ‘transcription pre-initiation’ model are shown. The U-stretch near the 5′ end of the vRNA that acts as a poly(A) signal is indicated.
Box 2. Therapeutic applications.
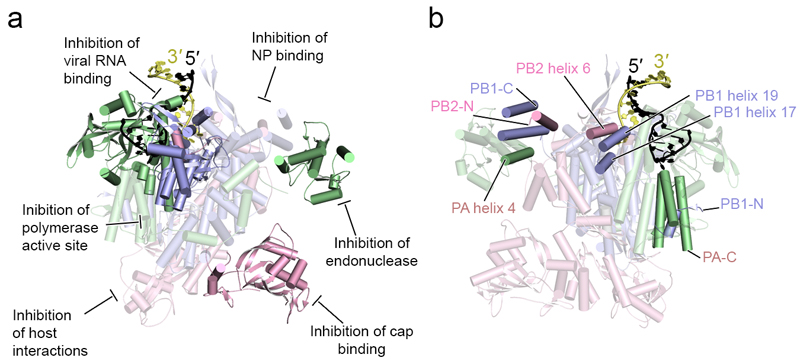
Most attempts to develop antivirals targeting the influenza virus RNA polymerase have so far focused on the PB1 active site, the PB2 cap-binding and PA endonuclease domains140. These attempts lead to the discovery of compounds that act as RNA chain terminators, mutagens or as inhibitors of the cap-snatching activity of the polymerase 141–150. Structural studies of some of these compounds in complex with either the PB2 cap-binding or PA endonuclease domain have produced valuable data that are currently being used to improve the pharmacokinetic parameters of these compounds. The novel structural data on the polymerase heterotrimer will be a source for new drug discoveries. The structures have revealed the conformation of the active site, the presence of a priming loop, the sites of viral RNA binding, and channels for template, NTP and capped primer entry as well as template and product exit. All these features of the influenza virus RNA polymerase are putative targets for small molecule inhibitors (see figure, part a; cartoon model of the influenza A virus polymerase structure (PDB: 4WSB)). Disruption of heterotrimer assembly is an alternative strategy for the development of antivirals targeting the influenza virus polymerase (see figure, part b; cartoon model of the influenza A virus polymerase structure (PDB: 4WSB) with the interaction between the PB1 N-terminal helix and three helices of the C-terminal PA domain highlighted; additional interfaces revealed by the structures of the heterotrimeric polymerase complex are also highlighted). Studies have so far focused on the interaction of the PA C-terminal domain with the PB1 N-terminus, and the interaction between the PB1 C-terminus and PB2 N-terminus as revealed by previous structural analyses of isolated domains23–25. These interactions have been targeted by antiviral peptides, peptidomimetics or small molecules151–158. The structures of the complete polymerase heterotrimer reveal numerous interaction interfaces, such as the interface between PB2 helix 6 and PB1 helices 17 and 19 of the PB1 thumb subdomain, that could be targeted using similar strategies (see figure, part b). In addition, interfaces may be targeted that are only present in one of the various conformational states that have been observed in the crystal structures. Examples are the interaction between the penultimate helix of PB1 and helix 4 of the PA endonuclease, which is only evident in the vRNA promoter-bound polymerase, and the various inter-domain interactions in the C-terminal two thirds of PB2. ‘Locking’ the polymerase in a particular conformation using a small molecule might be yet another viable strategy for the development of antivirals. Structural studies of the influenza virus polymerase in complex with other viral, e.g. NP, or host factors are likely to reveal additional interaction interfaces that could be targeted. For example, the site of the interaction with the CTD of Pol II is currently unknown but it could represent an attractive target for an antiviral. Similarly, ANP32A has emerged as an interactor of the influenza virus polymerase and a critical factor for the replication activity linked to the PB2 627-domain129, 130. Uncovering the mode of interaction of ANP32A with the viral polymerase could reveal yet another site in the polymerase that could be targeted. The availability of techniques to express and purify large quantities of the heterotrimeric polymerase will further facilitate these studies in the context of the polymerase complex and RNPs.
Promoter binding
The highly conserved sequences present at the termini of each vRNA segment have the potential to form a partially double-stranded ‘panhandle’ structure34. The polymerase needs to associate with the 5′ end of the vRNA template in order to bind and cleave host capped RNA, as well as to initiate transcription at the 3′ end of the vRNA template35–39. These observations lead to the proposal that the vRNA promoter is formed by the 5′ and 3′ terminal sequences of the vRNA segments36.
The structures of the influenza A and B virus RNA polymerases were obtained in the presence of short RNAs mimicking the 5′ and 3′ terminal vRNA sequences, which revealed how the vRNA promoter is bound to the polymerase. The crystal structures show that nucleotides 1-10 of the 5′ end of the vRNA form a compact stem-loop (hook) structure that inserts into a deep pocket at the interface of the PB1 fingers and the PA C-terminal domain (FIG. 1b and 2a, b). Multiple amino acid residues from both PB1 and PA create a highly specific and strong interaction16, 21, 22, 40. Nucleotides 1-9 of the 3′ end of the vRNA bind in a nearby binding site on the surface of the polymerase with amino acid residues from all three polymerase subunits contributing to the association. These findings are consistent with UV-cross-linking experiments, which showed that all three subunits intimately interact with the vRNA termini36, 41. The distal part of the vRNA promoter forms a double-stranded structure involving nucleotides 11-16 of the 5′ end and 10-15 of the 3′ end, which in the polymerase crystal structures projects away from the body of the polymerase. The overall arrangement of the vRNA promoter is consistent with vRNA promoter models based on mutagenic studies that predicted a 5′ hairpin-loop and base-pairing in the distal region36, 37, 42, 43. An RNA secondary structure similar to the vRNA promoter has been predicted for the cRNA replicative intermediate and is called the cRNA promoter44, 45. Although the 5′ end of the cRNA differs in sequence from the vRNA 5′ end (being the complement of the vRNA 3′ end), it forms a similar hook structure and is bound by the influenza B virus polymerase at the same site as the vRNA 5′ end33.
Conformational rearrangements of the viral polymerase
Different conformations of the polymerase can be observed in the crystal structures of the RNA-free (apo form) influenza C virus polymerase and the vRNA promoter-bound influenza A and B virus polymerases (FIG. 1b). Whereas the polymerase core changes little when vRNA is bound, the peripheral C-terminal two-thirds of PB2 (including the cap-binding, mid-link, 627, and NLS domains) and the N-terminal PA/P3 endonuclease domains undergo major rearrangements (Supplementary Video 1). In the apo structure of the influenza C virus polymerase, the PB2 cap-binding domain is immobilized by several inter-domain interactions that involve the PB1 palm subdomain. The cap-binding pocket is occluded by a part of PB2. The P3 endonuclease is located on the side of the fingers subdomain of PB1 with the PB2 NLS domain packing against the endonuclease. This arrangement of the cap-binding and endonuclease domains is incompatible with cap-snatching activity19, 33. By contrast, in the vRNA promoter-bound influenza A and B virus polymerase structures, the PB2 cap-binding and PA endonuclease domains are oriented in a way that is compatible with cap-snatching (FIG. 1b), consistent with previous observations that cap-snatching is activated by the binding of the polymerase to vRNA35, 39. In this arrangement, the cap-binding domain makes little contact with the other domains, so it can rotate freely in situ, while the endonuclease is stabilised by the PB1 C-terminus and PB2 N-terminus interaction motif. In addition, the NLS of PB2 no longer contacts the endonuclease and has instead moved with the PB2 627-domain to a site that is occupied by the PB2 cap-binding domain in the apo form (Supplementary Video 1).
The conformations captured by X-ray crystallography most likely represent the polymerase in two different functional states. The apo form of the influenza C virus polymerase corresponds to a transcriptionally inactive state and may represent the structure of the newly assembled polymerase. However, a structure of the influenza B virus polymerase in complex with 5′ cRNA shows a similar, though not identical arrangement of its peripheral domains as the apo structure of the influenza C virus polymerase33. Therefore this conformation may also be relevant for a polymerase engaged in primer-independent cRNA to vRNA replication, which does not require cap-snatching. By contrast, the structures observed in the presence of vRNA represent the polymerase in a transcription pre-initiation state, with the cap-binding and endonuclease domains aligned such that the polymerase could bind and cleave host capped RNA (FIG. 1b). Structural and biophysical characterisation of the polymerase in solution, with and without RNA, suggests that multiple conformations exist in equilibrium19, 33. Thus, the influenza virus RNA polymerase is inherently flexible. The nature of RNA present influences the equilibrium and a particular conformation may further be stabilised through interactions with other viral or host factors (see below), which may preferentially promote a particular function of the polymerase19, 33. Therefore it is possible that vRNA-bound polymerase could also exist in a transcriptionally inactive state, for example in vRNPs packaged into virions and vRNPs that are in transit between the host cell nucleus and the plasma membrane.
Polymerase active site
The polymerase active site is located at the edge of a large central cavity that is formed by PB1 and the N-terminus of PB219, 21, 22. The active site can be accessed via several channels (FIG. 2e). The putative template entry channel that leads from the site of 3′ vRNA binding into the active site is lined by conserved amino acid residues from all three subunits and the NTP entry channel is made up by highly conserved basic amino acids from PB1. In contrast to positive stand RNA viruses, influenza viruses lack a helicase, and the template and product RNA strands are separated before they exit the polymerase. Potentially, an element of PB2 called the lid-domain (FIG. 2d) could be responsible for separating the two strands which, when modelled into the available crystal structures, appear to clash with this element22. The putative template exit channel is located on the same side of the polymerase as the template entry channel and it has been identified by comparing the influenza virus RNA polymerase structure to that of the La Crosse bunyavirus, which shows a more obvious channel for template exit18. The close proximity of the template entry and exit channels on the same side of the polymerase could facilitate the copying of the RNA genome with minimal disruption of the vRNP complex (see below). The product exit channel is likely to be defined by the path that the capped RNA primer takes when its 3′ end is inserted into the active site (FIG. 2f). An important feature of the central cavity is the priming loop, a β-hairpin in the case of the influenza virus polymerase, that protrudes from the PB1 thumb subdomain towards the active site (FIG. 2a,f) and may support the sugar-base of the first NTP during de novo initiation as observed in other RNA-dependent RNA polymerases22, 46–48. A recent study has shown that the priming loop is important for primer-independent replication initiation on the vRNA template49 (see below).
Models for transcription and replication
Transcription
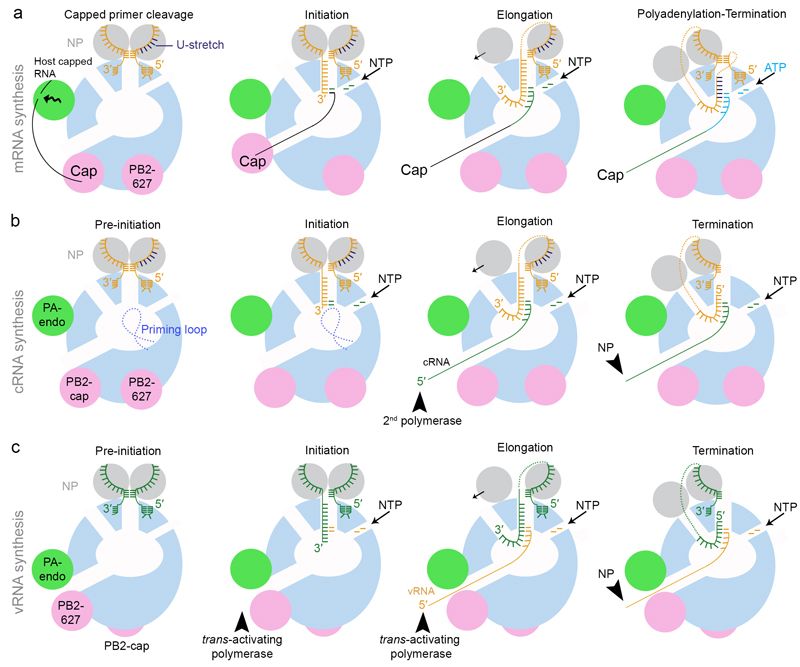
After vRNPs reach the nucleus, the resident polymerase, that is the polymerase that is bound to the vRNA termini in the vRNP, synthesizes 5′ capped and 3′ polyadenylated viral mRNA (FIG. 3a). For this process the viral polymerase requires primers which are 5′ capped RNA fragments derived from nascent host capped RNAs. Accordingly, viral transcription is sensitive to actinomycin-D and 𝛼-amanitin, an inhibitor of cellular DNA-dependent RNA polymerase II (Pol II). Host snRNAs, snoRNAs and promoter-associated capped small (cs)RNAs, which are generated by “paused” Pol II during transcription initiation, are the preferred substrates for cap-snatching over mRNAs or pre-mRNAs50–52. Cap-snatching is carried out by the viral polymerase that is in a transcription pre-activation state (as illustrated by the influenza A and B virus polymerase structures) with the PB2 cap-binding and PA endonuclease domains aligned such that the polymerase can bind and cleave host capped RNA. After cleavage of the capped RNA, the 3′ end of the capped primer must transfer from the endonuclease active site into the polymerase active site via the product exit channel. This transfer is likely facilitated by the rotation of the cap-binding domain as observed in one of the influenza B virus polymerase structures (FIG. 1b)22. Concomitantly, the 3′ end of the vRNA template must move from its binding site near the surface of the polymerase into the polymerase active site via the template entry channel (FIG. 3a). Currently, it is not clear how this is triggered, but an equilibrium might exist between conformations with the 3′ end at the surface or in the active site. Interactions between the capped RNA primer and the 3′ end of the vRNA template in the active site or binding of an initiating nucleotide might stabilise the vRNA 3′ end in the active site. Transcription initiation takes place by the addition of a G or a C residue to the 3′ end of the capped primer, directed by the penultimate C residue (C2) or the G residue at position 3 (G3) in the vRNA template. In infected cells there is a preference for transcription initiation with primers with a “CA” 3′ end, but the structural basis for this sequence specificity remains unknown53, 54. The four conserved residues at the tip of the priming loop in the polymerase active site are redundant for this process as they can be deleted without affecting transcription initiation49.
Figure 3. Models of viral transcription (mRNA synthesis) and replication (cRNA and vRNA synthesis).
a Model for mRNA synthesis. The polymerase is depicted as in FIG. 2f in the ‘transcription pre-initiation’ state. Host capped RNA is bound by the PB2 cap-binding domain and cleaved by the PA/P3 endonuclease domain. The cap-binding domain rotates to allow the insertion of the 3′ end of the capped RNA primer into the active site via the product exit channel22. The 3′ end of the vRNA template also inserts into the active site and NTPs enter via the NTP entry channel. Transcription is initiated by the addition of a GTP to the 3′ end of the capped primer that is templated by the second residue in the vRNA template. During elongation, product and template are separated and they exit through their respective exit channels. As the template is pulled into the active site, NP detaches from the entering vRNA, translocates via the surface of the polymerase and joins the vRNA template as it emerges through the template exit channel. The 5′ cap is released from the PB2 cap-binding domain as the polymerase enters elongation57. Termination is achieved through polyadenylation that occurs as the result of repeated copying of the U-stretch of the vRNA template56. The 5′ end of the vRNA remains bound to its binding site and is stabilized by base-pairing with the 3′ end of the vRNA that re-binds at its binding site near the surface of the polymerase during elongation. b Model for cRNA synthesis. The polymerase is shown in the same conformation as for mRNA synthesis. The 3′ of the vRNA inserts into the active site and NTPs enter via the NTP entry channel. De novo initiation occurs at the first residue of the vRNA template (terminal initiation) and the initiating nucleotide is stabilized by the priming loop22, 49, 62. During elongation, product and template are separated and they exit through their respective exit channels. NP translocates via the surface of the polymerase to join the vRNA template as it emerges through the template exit channel. The 5′ end of the cRNA product is bound by a second polymerase as it emerges from the product exit channel and the rest of the RNA associates with NP to assemble cRNPs. For termination, the 5′ end of the vRNA template is released from its binding site and is pulled through the polymerase active site while the 3′ end re-binds at the surface of the polymerase. c Model for vRNA synthesis. The polymerase is depicted as in FIG. 2e in the ‘inactive’ state consistent with the conformation of the polymerase bound to the 5′ end of cRNA33. For replication initiation to take place the polymerase needs to interact with a trans-activating polymerase61. The 3′ of the cRNA inserts into the active site and NTPs enter via the NTP entry channel. De novo initiation occurs at the fourth and fifth residue of the cRNA template (internal initiation) without the participation of the priming loop22, 49, 62. The resulting pppApG dinucleotide re-locates to the 3′ end of the cRNA template (not shown) and is elongated by the polymerase. Elongation and termination proceed as described above for cRNA synthesis. The 5′ end of the vRNA product is bound by the trans-activating polymerase and the rest of the vRNA associates with NP to assemble vRNPs. Note that an alternative model, involving a trans-acting polymerase, has also been proposed for cRNA to vRNA replication (not shown)10, 71.
Currently it is unknown how the polymerase copies the vRNA template in the context of the vRNP. It is likely that only local restructuring of the NP helix takes place when the polymerase ‘pulls’ the template vRNA from the NP helix into its active site. The proximity of the template entrance and exit is compatible with a model in which template vRNA is progressively dissociated from NP, translocated via the entrance channel into the polymerase active site and then re-associated with NP at the exit channel (FIG. 3a). Elongation continues in a template-dependent manner by pulling the vRNA template through the polymerase active site until a 5-7 nucleotide long U-stretch, 16 nucleotides from the 5′ end of the vRNA template, reaches the active site. Stuttering of the polymerase on the U-stretch leads to the repeated incorporation of AMP and the production of a poly(A) tail (FIG. 3a). Evidence for the stuttering mechanism has been provided by replacement of the U-stretch with an A-stretch, which resulted in the generation of mRNAs with a poly(U) tail55, 56. Although the exact mechanism that triggers stuttering is unclear, it has been proposed that steric hindrance caused by the 5′ end of the vRNA template remaining associated with the transcribing polymerase plays a role36, 38, 43. Return of the already copied 3′ end of the vRNA template to its original position near the surface of the polymerase and its base-paring with the 5′ end potentially helps to ‘lock’ the 5′ end in its binding site, thereby preventing its release during polyadenylation (FIG. 3a).
At the point of transcription initiation the 5′ cap is associated with the PB2 cap-binding domain. However, as the polymerase enters elongation the cap is released from the PB2 cap-binding domain57. Subsequently, the 5′ cap is likely bound by the nuclear cap-binding complex, triggering the assembly of the viral mRNA into a host mRNP-like structure58, 59.
Replication
Replication is a two-step, primer-independent process. In the first step of replication, incoming vRNPs carry out the synthesis of cRNA, a replicative intermediate (FIG. 3b). Biochemical data show that the resident polymerase is capable of catalyzing cRNA synthesis: vRNPs isolated both from purified virions as well as vRNPs isolated from infected cells can catalyze the generation of cRNA in a primer-independent manner in vitro60, 61. Similar to transcription initiation, replication initiation requires the translocation of the 3′ terminus of vRNA into the active site. Next, a pppApG dinucleotide is formed on residues U1 and C2 of the vRNA 3′ end (terminal initiation)62. As in other de novo initiating RNA polymerases, the formation of the dinucleotide on the terminus of the RNA template is dependent on structural support by a priming loop22, 49. Elongation presumably proceeds in a manner similar to that described for mRNA synthesis with minimal disruption of the vRNP structure. However, in contrast to transcription, during the termination of cRNA synthesis the 5′ end of the vRNA template must be released from its binding pocket to be copied (FIG. 3b). Currently it is unclear how this difference between mRNA and cRNA termination is achieved.
The cRNA product assembles into complementary ribonucleoprotein (cRNP) complex, a vRNP-like structure, with polymerase bound at the 5′ and 3′ termini and NP associating with the body of the cRNA. Negative stain EM of isolated cRNPs revealed a twisted, double helical arrangement of the cRNA-NP complex very similar to that of the vRNP61. Assembly of cRNA into cRNP is likely started as soon as its 5′ end emerges from the product exit channel and is bound by a second, newly synthesized polymerase. The second polymerase then recruits the first NP through polymerase-NP interactions. However, subsequent NPs are recruited via groove and tail-loop interactions between NP monomers in solution and cRNA-bound NPs63–66. Multiple mechanisms that include phosphorylation and interaction with host factors, i.e. importins, have been proposed to maintain NP in a monomeric form prior to being incorporated into an oligomer of NPs within the RNP67–70. A similar detailed mechanism, involving binding of polymerase to the 5′ end of emerging nascent replication product and subsequent binding of NP, has been proposed for bunyavirus replication18.
In the second step of replication, cRNA acts as template for vRNA synthesis (FIG. 3c). In contrast to replication on a vRNA template, replication initiation on a cRNA template occurs on residues U4 and C5 (internal initiation)62. The pppApG dinucleotide product is then aligned to residues U1 and C2 of the cRNA 3′ terminus to allow elongation into a full-length copy of the cRNA. Recent data show that the full-length priming loop is not required for internal initiation on a cRNA template, possibly because the 3′ UCG overhang contributes to the stabilization of the template-dinucleotide complex49. However, replication from the cRNA template is critically dependent on the presence of a second polymerase additional to the resident polymerase. In particular, cRNPs isolated from infected cell lysates were inactive in vitro unless ‘extra’ polymerase was added61. Interestingly, this second polymerase does not need to be catalytically active, suggesting that it fulfills a role that does not involve RNA synthesis and that it is trans-activating rather than trans-acting61. It is tempting to speculate that the trans-activating polymerase might induce a conformational rearrangement in the resident polymerase that favours the transfer of the cRNA 3′ terminus to the active site and/or internal initiation on the cRNA 3′ terminus. Although it is not known where and how the cRNA 3′ terminus binds the polymerase, it can be assumed based on the binding of the 5′ cRNA end to the influenza B virus polymerase33, which is similar to the binding of the vRNA 5′ end, that in a resting state the 3′ end of cRNA occupies the same site as the 3′ end of the vRNA template. In this model of vRNA synthesis, the trans-activating polymerase would also fulfill the role of the polymerase that binds to the 5′ end of the emerging nascent vRNA and recruits the first NP to start the assembly of vRNP that likely proceeds in a manner similar to that described for cRNP (see above).
An alternative model for the second step of replication, cRNA to vRNA synthesis, involves a catalytically active, trans-acting polymerase. In this model, a new polymerase, possibly associated with small viral RNA (see below), is recruited to the resident polymerase and gains access to the 3′ terminus of the cRNA template. The 3′ terminus enters the active site of the trans-acting polymerase that performs replication initiation internally on the cRNA template as described above for the trans-activating polymerase model. However, in this model, a third polymerase is proposed to bind to the 5′ end of the nascent vRNA to initiate the assembly of a vRNP. Genetic complementation experiments as well as the observation of branched RNPs in cryo-EM support this alternative model10, 71. Further studies are required to confirm whether vRNA synthesis is performed by the resident or a trans-acting polymerase.
Regulation of transcription and replication
Regulation by viral factors
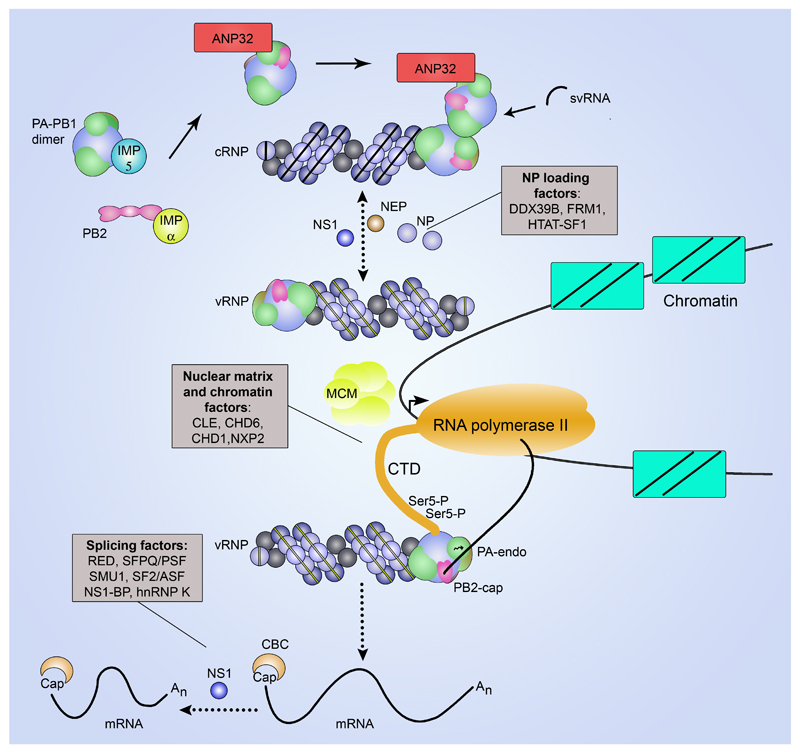
Several viral proteins have been implicated in the regulation of viral transcription and replication (FIG. 4). In particular, NP has long been considered a factor that switches the polymerase from a transcriptase to a replicase72, 73. However, more recent studies challenge this view by proposing that the initiation of mRNA or cRNA synthesis is stochastic, with no switch regulating the initiation of RNA synthesis74. In this model, newly synthesized viral polymerase and NP are required to stabilize nascent cRNA, which would otherwise be degraded by host cell nucleases. Furthermore, short genome segments (up to 76 nucleotides), with large internal deletions but preserved 5′ and 3′ terminal sequences, were found to be efficiently transcribed and replicated in the absence of NP in cell-based transcription/replication assays66, 75. NP had no effect on the ratio of transcription and replication products under these conditions. However, NP has been observed to stimulate polymerase activity and it is required for the processivity of the polymerase on long RNA templates76–78. Two other viral proteins, non-structural protein 1 (NS1) and nuclear export protein (NEP; formerly known as non-structural protein 2 (NS2)) have also been implicated in the regulation of transcription and replication (FIG. 4). NS1, recently identified as a component of the influenza virion79, was found to interact with NP and several studies suggest a role for NS1 in viral RNA synthesis80–83 and the splicing of viral mRNAs84, 85. NEP is primarily considered to play a major role in mediating the nuclear export of progeny vRNPs86. However, there is also evidence for a regulatory role of NEP in viral RNA synthesis, with NEP expression leading to an increase in replication87, 88. This function has been linked to the requirement of NEP for the generation of 22-27 nt long short viral RNAs (svRNAs) that correspond to the 5′ terminus of vRNAs and are highly expressed in infected cells89, 90. svRNAs have been implicated as viral factors involved in the replication of cRNA into vRNA91. svRNAs might associate with the trans-acting polymerase through binding to the vRNA 5′ end binding pocket.
Figure 4. Regulation of transcription and replication.
Viral RNA synthesis is performed in association with the chromatin/nuclear matrix close to sites of host Pol II transcription. This association is mediated by nuclear matrix and chromatin factors. For transcription, vRNPs bind to the serine-5 phopshorylated CTD of the large subunit of Pol II to access nascent capped host RNA for cap-snatching118, 119. Polyadenylation of viral mRNA is stimulated by the host splicing factor SFPQ/PSF; viral NS1 and host RED, SMU1, SF2/ASF, NS1-BP, hnRNP K participate in mRNA processing84, 85, 123–126. The host cap-binding complex (CBC) binds to the 5′ cap once it is released by PB258. vRNPs in close proximity to Pol II but not bound to the CTD of Pol II carry out cRNA synthesis. cRNA serves as template for vRNA synthesis. Free viral polymerase and NP are required for the assembly of cRNA and vRNA into cRNP and vRNP, respectively. DDX39B, HTAT-SF1 and FMR1 stimulate viral RNA replication by promoting NP interactions with the viral RNA polymerase or NP recruitment to nascent viral RNA during cRNP and vRNP assembly77, 96, 131. Viral NS1 regulates viral RNA replication by interacting with NP80–83 and viral NEP promotes viral RNA replication, possibly via the stimulation of svRNA synthesis87–89. svRNAs are implicated as viral co-factors in genome replication91. The cellular DNA helicase MCM as well as proteins of the ANP32 family are implicated in genome replication128, 129. ANP32A may possibly contribute by recruiting a trans-activating polymerase to the resident cRNP-bound polymerase in a host- and PB2 627-domain-specific manner to facilitate vRNA synthesis although the exact mechanism of its involvement remains unknown88, 129, 130. Importin-α, which acts as import factor for PB2, is proposed to have a role in transcription and replication independent of its role as a nuclear import receptor26, 132. Importin-5 (also known as RanBP5) acts as a co-factor for the nuclear import of the PB1-PA dimer16, 107, 109.
Regulation by host factors
A wide range of methods, including proteomics and genome-wide RNAi-based screens, have been applied to search for host factors involved in viral replication5, 92–100. Targeted interaction screens using proteomics to identify host interacting partners of the viral polymerase, NP, and RNPs have also been performed and revealed hundreds of candidate proteins that could affect viral RNA synthesis96, 99, 101–109. However, the role of very few of these host factors has been investigated in detail.
Viral transcription and replication are carried out in the nucleus of the infected cell. vRNPs are targeted to the nuclear matrix or to chromatin components, and viral transcription and replication are proposed to take place in DNase-insensitive nuclear fractions that include chromatin and the nuclear matrix81, 110–113. Specific interactions between the influenza virus polymerase and chromatin remodelers CHD1 and CHD6, nuclear matrix protein NXP2/MORC3, and Pol II function modulator CLE/C14orf166 may play a role in this targeting114–117. Viral mRNA synthesis is proposed to take place in close association with host Pol II. It has been demonstrated that the viral polymerase interacts directly with the serine-5 phosphorylated form of the C-terminal domain (CTD) of the large subunit of Pol II118, 119. This form of the CTD is known to recruit cellular capping enzymes to cap nascent Pol II transcripts near the transcription start site120. The interaction of the viral polymerase with the same form of the CTD presumably facilitates access of the viral polymerase to nascent host capped RNAs (FIG. 4). However, this interaction also brings viral mRNA synthesis close to sites of high concentration of splicing factors and host factors involved in the assembly of host mRNPs and it may therefore facilitate not only cap-snatching but also RNA processing and viral mRNP assembly59. Viral M1, NS1 and PB2 mRNAs can be spliced121, a function carried out by the cellular splicing machinery122. Cellular factors related to splicing, including RED, SMU1, SF2/ASF, SFPQ/PSF, hnRNP K and NS1-BP have been identified as important for influenza virus replication123–126. Splicing factor SFPQ/PSF that interacts with vRNPs has been shown to be important for viral mRNA synthesis by increasing the efficiency of viral mRNA polyadenylation124.
The cellular transcriptional machinery may provide the viral polymerase with a platform inside the host cell nucleus around which not only viral mRNA synthesis and processing but also viral RNA genome replication is organized. The minichromosome maintenance (MCM) complex, a cellular helicase involved in DNA replication but also participating in Pol II elongation via its interaction with the Pol II CTD127, was found to stimulate viral RNA replication. MCM interacts with the PA subunit of the viral RNA polymerase and it appears to stimulate the transition from de novo initiation to elongation on the vRNA template128. Members of the ANP32 family of proteins, implicated in multiple cellular pathways, including transcriptional regulation by chromatin remodelling, mRNA export and cell death, were found to promote the second step of replication, i.e. vRNA synthesis from a cRNA template. In particular, human ANP32A (also known as pp32) and ANP32B (also known as APRIL) were shown to interact with the viral RNA polymerase102, 104, 129, up-regulate vRNA synthesis from cRNA in vitro129, and be required for viral RNA synthesis in infected cells99. Importantly, ANP32A was found to underlie PB2 position 627-mediated polymerase host restriction. More specifically, a species-specific difference in ANP32A accounts for the suboptimal function of avian influenza virus polymerase with PB2 containing glutamic acid at position 627 (627E) in mammalian cells130. In comparison with its human counterpart, avian ANP32A possesses 33 additional amino acids between its leucine-rich repeats and the C-terminal low-complexity acidic region. This insertion enables ANP32A to stimulate the activity of avian influenza virus polymerase to levels similar to mammalian-adapted polymerase in mammalian cells. Thus, ANP32A represents a host-specific factor of the influenza virus polymerase. The requirement of ANP32 proteins for cRNA to vRNA replication is consistent with previous findings that linked PB2 627-mediated host restriction to a block in avian influenza virus cRNP activity in mammalian cells88. Although the molecular mechanisms behind the action of the ANP32 proteins remain obscure, it is tempting to speculate that they might facilitate the recruitment of the trans-activating polymerase to the cRNP-resident polymerase and promote its initiation of vRNA synthesis.
Several other host factors, including the RNA helicase DDX39B (also known as Bat1/UAP56), HTAT-SF1 (also known as Tat-SF1), and the mRNA export and translation stimulator FMR1 have been described to stimulate viral RNA replication by promoting either NP interactions with the viral RNA polymerase or NP recruitment to nascent viral RNA during RNP assembly77, 96, 131. Host importin-α isoforms have been reported to fulfill a role in influenza virus RNA replication, beyond their role as nuclear import factors, by interacting with the PB2 polymerase subunit in a host-dependent manner132.
Interactions between the viral and host transcriptional machineries on one hand provide the viral polymerase with a platform to co-ordinate cap-snatching, transcription, replication, RNA processing, as well as mRNA and vRNA nuclear export59, 86, 110, but on the other these interactions might also provide the virus with an opportunity to interfere with host gene expression, inhibiting the expression of antiviral host genes. The viral NS1 protein is well documented to interfere with the 3′ end formation of host mRNA via its interaction with the cellular polyadenylation machinery133, 134. Additionally, interaction of the viral RNA polymerase with Pol II and cleavage of nascent capped host RNA is presumably significant for the inhibition of host gene expression, including antiviral host genes118, 135. Moreover, degradation of the large subunit of Pol II has also been observed in influenza virus infected cells and been associated with increased pathogenicity of influenza virus in mice136, 137.
Outlook and conclusions
The recently published high-resolution structures of the influenza virus RNA polymerase provide unprecedented insights into the mechanisms of this molecular machine. The PB1 subunit together with the C-terminal domain of PA/P3 and the N-terminal one third of PB2 form the core of the polymerase. The architecture of this core is similar to that of other viral RNA-dependent RNA polymerases. The core encompasses the polymerase active site, which is connected to the exterior by several channels, including channels for template and NTP entry as well as template and product exit. Near the active site, the priming loop is a feature that is essential for primer-independent terminal initiation on the vRNA template, but that is not required for primer-dependent transcription and, surprisingly, also not for primer-independent internal initiation on the cRNA template. The core of the polymerase is decorated by several flexible domains that include the PB2 cap-binding and PA/P3 endonuclease domains. These domains can be arranged in two different conformations that either enable cap-snatching or are incompatible with it, suggesting that the polymerase exists in multiple functional states. These functional states are controlled by the association of the polymerase with viral RNA and possibly other viral and host factors.
Although unprecedented progress has been made in understanding the structure and function of the influenza virus RNA polymerase, many long-standing questions remain unanswered. The available crystal structures have provided insights into subunit and domain organisation. However, this is only a starting point for further investigations into how this complex machine works. For example, neither of the available structures shows the polymerase in an active conformation with template RNA in the active site and we cannot rule out that the polymerase may adopt different conformations during initiation and elongation. Likewise, the conformation of the terminating polymerase might be different. Further structural analysis by crystallography and new powerful cryo-EM technologies, will be needed to visualize other functional states of the influenza virus polymerase; i.e. in complex with RNA templates, RNA primers, and incoming nucleotides during initiation, elongation and termination.
The trans-acting and trans-activating replication models discussed above indicate that multiple polymerases are required and although oligomerisation of polymerase heterotrimers and sub-complexes has been observed30, 138, there is virtually no information available on how these polymerases might interact. Single-molecule techniques could greatly facilitate our understanding of the mechanics and kinetics of the polymerase during transcription and replication using fluorescently labelled polymerase and RNA templates40.
Another avenue of interest concerns the location, host interactions and dynamics of RNPs in the nucleus. Importantly, it remains unclear whether RNPs associate with specific nuclear sites for transcription and genome replication. Do these two processes occur at the same site or different sites? It will be important to determine how host factors, such as ANP32A contribute to polymerase function and host adaptation. It will be equally important to study what cellular factors participate in the assembly of vRNPs and viral mRNPs and their nuclear export. Ultimately, understanding these molecular mechanisms will not only improve our basic scientific knowledge of the workings of an important human and animal pathogen, it could also contribute to our understanding of virulence and adaptation mechanisms of influenza viruses. Furthermore, understanding how the influenza virus RNA synthesis machine works at the molecular level and its interaction with viral and host factors could lead to the identification of targets that might be exploited for the development of drugs that can prevent or treat influenza virus infections (BOX 2).
Supplementary Material
Supplementary Video 1 Conformational flexibility of the influenza virus RNA polymerase. Morph between the structure of the RNA-free influenza C virus polymerase (PDB: 5D98) and that of vRNA promoter-bound influenza A virus polymerase (PDB: 4WSB). PB1 and PA are coloured orange and blue, respectively. PB2 is coloured according to its domain structure with the N-terminal one third shown in green, the cap-binding domain in dark yellow, the mid-link in light yellow, the 627-domain in red and the C-terminal NLS in brown. Promoter RNA is shown in red. Modified from Hengrung et al19.
Acknowledgements
The authors are grateful to F. Vreede for his comments on the manuscript. This work was supported by Medical Research Council (MRC) grant MR/K000241/1 (to EF), Wellcome Trust grant 098721/Z/12/Z (to AT), Netherlands Organization for Scientific Research (NWO) grant 825.11.029 (to AT), and a Kemp postdoctoral fellowship from Lincoln College Oxford (to AT).
Glossary terms
- Haemagglutinin
Type I integral membrane glycoprotein that binds to cell-surface receptors and facilitates fusion between the viral envelope and endosomal membrane. It is the main target antigen of the humoral immune response of the host.
- Neuraminidase
Type II integral membrane glycoprotein, which facilitates viral release from cells by removing sialic acid from sialyloligosaccharides on the cell and viral surface.
- Cap-snatching
A process in which a cellular capped RNA is cleaved a few nucleotides downstream of the 5′ cap by an endonuclease activity encompassed within a viral RNA-dependent RNA polymerase.
- Host range determinant
Characteristic of a pathogen that allows it to replicate in a particular host.
- Panhandle structure
A double-stranded RNA structure formed by the conserved 5' and 3' RNA termini of negative-sense viral RNA genomes.
- Apo structure
A structure of a protein with no ligand bound.
- Helicase
An enzyme that catalyzes the unwinding and separation of double-stranded DNA or RNA using energy from ATP hydrolysis.
- De novo initiation
Initiation step of nucleic acid synthesis by means of a primer-independent mechanism.
- Carboxyl-terminal domain (CTD) of Pol II
An unstructured, evolutionarily conserved domain at the C-terminus of the largest Pol II subunit that comprises tandem copies of the consensus heptapeptide YSPTSPS. Phosphorylation of these repeats is crucial for the regulation of Pol II function.
- Pol II pausing
A control step in gene transcription by which RNA Pol II pauses at certain sites and requires specific stimuli and elongation factors to overcome the pausing block to enter productive elongation.
Footnotes
Competing interests statement
The authors declare no competing interests.
Author information
Aartjan te Velthuis received his Ph.D. in medicine from the University of Leiden, Netherlands, and is currently a Sir Henry Wellcome Postdoctoral Fellow in the laboratory of Ervin Fodor in the Sir William Dunn School of Pathology, University of Oxford, Oxford, UK. His research focuses on understanding the molecular mechanisms of viral RNA-dependent RNA polymerases.
Ervin Fodor received his D.Phil in pathology from the University of Oxford, UK, and is currently Professor of Virology at the Sir William Dunn School of Pathology, University of Oxford, Oxford, UK. His research group seeks to understand fundamental aspects of the structure and function of influenza viruses, as well as of virus-host interactions, including host responses to viral infection. Ervin Fodor’s homepage (http://www.path.ox.ac.uk/content/ervin-fodor).
Highlighted references
9. Arranz et al 2012 This paper describes the three-dimensional structure of native RNPs derived from influenza virions.
10. Moeller et al 2012 This paper describes the three-dimensional structure of native RNPs derived from cells expressing influenza virus RNP complex components.
19. Hengrung et al 2015 This paper presents the crystal structure of the RNA-free influenza C virus polymerase that reveals a radically different configuration of the PB2 C-terminal domains compared to the influenza A and B virus polymerase structures obtained in the presence of vRNA. SAXS studies show that the polymerase can take up a variety of conformations.
21. Pflug et al 2014 This paper presents the crystal structure of bat influenza A virus polymerase which shows the architecture of the heterotrimeric complex and the structural basis for binding the vRNA promoter which comprises the conserved 5′ and 3′ termini of the vRNA.
22. Reich et al 2015 This paper presents the crystal structure of the influenza B virus polymerase and shows the rotation of the PB2 cap-binding domain guiding the 3′ end of the capped RNA primer into the polymerase active site.
33. Thierry et al 2016 This paper presents structures of the influenza B virus polymerase showing a similar arrangement of the PB2 C-terminal domains as observed for the influenza C virus polymerase. Solution studies show the polymerase can take up a variety of conformations.
49. te Velthuis et al 2016 This paper shows that the priming loop of the influenza A virus polymerase is required for terminal de novo replication initiation on the vRNA template but it is not required for internal de novo replication initiation on the cRNA template and for capped RNA primer-dependent transcription initiation.
61. York et al 2013 This paper reports the isolation, structural and functional characterization of the influenza A virus cRNP replicative intermediate. It proposes that the cRNP associated polymerase requires a trans-activating polymerase to be able to carry out vRNA synthesis.
71. Jorba et al 2009 This paper describes a model for influenza virus transcription and genome replication. It proposes that transcription is carried out by the resident polymerase bound to the vRNA termini in the vRNP, whereas cRNA to vRNA genome replication is carried out by a trans-acting polymerase that is not part of the cRNP.
119. Engelhardt et al 2005 This is the first paper to identify the interaction between the influenza virus RNA polymerase and the host RNA polymerase II transcriptional machinery. It proposes that cap-snatching is carried out by the viral polymerase bound to the serine-5 phosphorylated form of the C-terminal domain of Pol II.
129. Sugiyama et al 2015 This paper identifies pp32 (ANP32A) and APRIL (ANP32A) as host factors required for the replication of the influenza A virus cRNA replicative intermediate into vRNA.
130. Long et al 2016 This paper identifies ANP32A as the host factor underlying influenza A virus polymerase restriction mediated by residue 627 of the PB2 subunit.
References
- 1.Molinari NA, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Muraki Y, Hongo S. The molecular virology and reverse genetics of influenza C virus. Jpn J Infect Dis. 2010;63:157–65. [PubMed] [Google Scholar]
- 3.Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–82. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 4.Das K. Antivirals targeting influenza A virus. J Med Chem. 2012;55:6263–77. doi: 10.1021/jm300455c. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Kawaoka Y. Influenza virus-host interactomes as a basis for antiviral drug development. Curr Opin Virol. 2015;14:71–8. doi: 10.1016/j.coviro.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krug R, Fodor E. In: Textbook of Influenza. Webster R, Monto A, Braciale T, Lamb R, editors. Wiley-Blackwell; 2013. pp. 57–66. [Google Scholar]
- 7.Fodor E. The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication. Acta Virol. 2013;57:113–22. doi: 10.4149/av_2013_02_113. [DOI] [PubMed] [Google Scholar]
- 8.Resa-Infante P, Jorba N, Coloma R, Ortin J. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 2011;8:207–15. doi: 10.4161/rna.8.2.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arranz R, et al. The structure of native influenza virion ribonucleoproteins. Science. 2012;338:1634–7. doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- 10.Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA. Organization of the influenza virus replication machinery. Science. 2012;338:1631–4. doi: 10.1126/science.1227270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson EC, Fodor E. Transport of the influenza virus genome from nucleus to nucleus. Viruses. 2013;5:2424–46. doi: 10.3390/v5102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotch SJ, Bouloy M, Ulmanen I, Krug RM. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–58. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 13.Dias A, et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–8. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 14.Guilligay D, et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol. 2008;15:500–6. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- 15.Yuan P, et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–13. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 16.Swale C, et al. Structural characterization of recombinant IAV polymerase reveals a stable complex between viral PA-PB1 heterodimer and host RanBP5. Sci Rep. 2016;6:24727. doi: 10.1038/srep24727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin B, Kranzusch PJ, Rahmeh AA, Whelan SPJ. The polymerase of negative-stranded RNA viruses. Curr Opin Virol. 2013;3:103–110. doi: 10.1016/j.coviro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlach P, Malet H, Cusack S, Reguera J. Structural Insights into Bunyavirus Replication and Its Regulation by the vRNA Promoter. Cell. 2015;161:1267–79. doi: 10.1016/j.cell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengrung N, et al. Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature. 2015;527:114–7. doi: 10.1038/nature15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang B, et al. Structure of the L Protein of Vesicular Stomatitis Virus from Electron Cryomicroscopy. Cell. 2015;162:314–27. doi: 10.1016/j.cell.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pflug A, Guilligay D, Reich S, Cusack S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature. 2014;516:355–60. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- 22.Reich S, et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature. 2014;516:361–6. doi: 10.1038/nature14009. [DOI] [PubMed] [Google Scholar]
- 23.He X, et al. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature. 2008;454:1123–6. doi: 10.1038/nature07120. [DOI] [PubMed] [Google Scholar]
- 24.Obayashi E, et al. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature. 2008;454:1127–31. doi: 10.1038/nature07225. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama K, et al. Structural insight into the essential PB1-PB2 subunit contact of the influenza virus RNA polymerase. Embo Journal. 2009;28:1803–1811. doi: 10.1038/emboj.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarendeau F, et al. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat Struct Mol Biol. 2007;14:229–33. doi: 10.1038/nsmb1212. [DOI] [PubMed] [Google Scholar]
- 27.Tarendeau F, et al. Host determinant residue lysine 627 lies on the surface of a discrete, folded domain of influenza virus polymerase PB2 subunit. PLoS Pathog. 2008;4:e1000136. doi: 10.1371/journal.ppat.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Area E, et al. 3D structure of the influenza virus polymerase complex: Localization of subunit domains. Proc Natl Acad Sci U S A. 2004;101:308–313. doi: 10.1073/pnas.0307127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coloma R, et al. The Structure of a Biologically Active Influenza Virus Ribonucleoprotein Complex. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang S, et al. Cryo-EM structure of influenza virus RNA polymerase complex at 4.3 A resolution. Mol Cell. 2015;57:925–35. doi: 10.1016/j.molcel.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 31.te Velthuis AJ. Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci. 2014;71:4403–20. doi: 10.1007/s00018-014-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–4. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thierry E, et al. Influenza Polymerase Can Adopt an Alternative Configuration Involving a Radical Repacking of PB2 Domains. Mol Cell. 2016;61:125–37. doi: 10.1016/j.molcel.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci U S A. 1987;84:8140–4. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cianci C, Tiley L, Krystal M. Differential activation of the influenza virus polymerase via template RNA binding. J Virol. 1995;69:3995–9. doi: 10.1128/jvi.69.7.3995-3999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fodor E, Pritlove DC, Brownlee GG. The influenza virus panhandle is involved in the initiation of transcription. J Virol. 1994;68:4092–6. doi: 10.1128/jvi.68.6.4092-4096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fodor E, Pritlove DC, Brownlee GG. Characterization of the Rna-Fork Model of Virion Rna in the Initiation of Transcription in Influenza-a Virus. J Virol. 1995;69:4012–4019. doi: 10.1128/jvi.69.7.4012-4019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiley LS, Hagen M, Matthews JT, Krystal M. Sequence-specific binding of the influenza virus RNA polymerase to sequences located at the 5' ends of the viral RNAs. J Virol. 1994;68:5108–16. doi: 10.1128/jvi.68.8.5108-5116.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao P, Yuan W, Krug RM. Crucial role of CA cleavage sites in the cap-snatching mechanism for initiating viral mRNA synthesis. EMBO J. 2003;22:1188–98. doi: 10.1093/emboj/cdg109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomescu AI, Robb NC, Hengrung N, Fodor E, Kapanidis AN. Single-molecule FRET reveals a corkscrew RNA structure for the polymerase-bound influenza virus promoter. Proc Natl Acad Sci U S A. 2014;111:E3335–42. doi: 10.1073/pnas.1406056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fodor E, Seong BL, Brownlee GG. Photochemical Cross-Linking of Influenza-a Polymerase to Its Virion Rna Promoter Defines a Polymerase Binding-Site at Residue-9 to Residue-12 of the Promoter. Journal of General Virology. 1993;74:1327–1333. doi: 10.1099/0022-1317-74-7-1327. [DOI] [PubMed] [Google Scholar]
- 42.Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–57. [PMC free article] [PubMed] [Google Scholar]
- 43.Pritlove DC, Poon LL, Devenish LJ, Leahy MB, Brownlee GG. A hairpin loop at the 5' end of influenza A virus virion RNA is required for synthesis of poly(A)+ mRNA in vitro. J Virol. 1999;73:2109–14. doi: 10.1128/jvi.73.3.2109-2114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pritlove DC, Fodor E, Seong BL, Brownlee GG. In vitro transcription and polymerase binding studies of the termini of influenza A virus cRNA: evidence for a cRNA panhandle. J Gen Virol. 1995;76(Pt 9):2205–13. doi: 10.1099/0022-1317-76-9-2205. [DOI] [PubMed] [Google Scholar]
- 45.Crow M, Deng T, Addley M, Brownlee GG. Mutational analysis of the influenza virus cRNA promoter and identification of nucleotides critical for replication. J Virol. 2004;78:6263–70. doi: 10.1128/JVI.78.12.6263-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appleby TC, et al. Viral replication. Structural basis for RNA replication by the hepatitis C virus polymerase. Science. 2015;347:771–5. doi: 10.1126/science.1259210. [DOI] [PubMed] [Google Scholar]
- 47.Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DL. A mechanism for initiating RNA-dependent RNA polymerization. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 48.Tao YZ, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage - Structural studies of reovirus polymerase lambda 3. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 49.te Velthuis AJW, Robb NC, Kapanidis AN, Fodor E. The role of the priming loop in influenza A virus RNA synthesis. Nature Microbiology. 2016;1:16029. doi: 10.1038/nmicrobiol.2016.29. [DOI] [PubMed] [Google Scholar]
- 50.Gu W, et al. Influenza A virus preferentially snatches noncoding RNA caps. RNA. 2015;21:2067–75. doi: 10.1261/rna.054221.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koppstein D, Ashour J, Bartel DP. Sequencing the cap-snatching repertoire of H1N1 influenza provides insight into the mechanism of viral transcription initiation. Nucleic Acids Res. 2015;43:5052–64. doi: 10.1093/nar/gkv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikora D, Rocheleau L, Brown EG, Pelchat M. Deep sequencing reveals the eight facets of the influenza A/HongKong/1/1968 (H3N2) virus cap-snatching process. Sci Rep. 2014;4:6181. doi: 10.1038/srep06181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaton AR, Krug RM. Selected host cell capped RNA fragments prime influenza viral RNA transcription in vivo. Nucleic Acids Res. 1981;9:4423–36. doi: 10.1093/nar/9.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw MW, Lamb RA. A specific sub-set of host-cell mRNAs prime influenza virus mRNA synthesis. Virus Res. 1984;1:455–67. doi: 10.1016/0168-1702(84)90003-0. [DOI] [PubMed] [Google Scholar]
- 55.Poon LL, Fodor E, Brownlee GG. Polyuridylated mRNA synthesized by a recombinant influenza virus is defective in nuclear export. J Virol. 2000;74:418–27. doi: 10.1128/jvi.74.1.418-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poon LL, Pritlove DC, Fodor E, Brownlee GG. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J Virol. 1999;73:3473–6. doi: 10.1128/jvi.73.4.3473-3476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braam J, Ulmanen I, Krug RM. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983;34:609–18. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- 58.Bier K, York A, Fodor E. Cellular cap-binding proteins associate with influenza virus mRNAs. J Gen Virol. 2011;92:1627–34. doi: 10.1099/vir.0.029231-0. [DOI] [PubMed] [Google Scholar]
- 59.York A, Fodor E. Biogenesis, assembly, and export of viral messenger ribonucleoproteins in the influenza A virus infected cell. RNA Biol. 2013;10:1274–82. doi: 10.4161/rna.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vreede FT, Brownlee GG. Influenza virion-derived viral ribonucleoproteins synthesize both mRNA and cRNA in vitro. J Virol. 2007;81:2196–204. doi: 10.1128/JVI.02187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.York A, Hengrung N, Vreede FT, Huiskonen JT, Fodor E. Isolation and characterization of the positive-sense replicative intermediate of a negative-strand RNA virus. Proc Natl Acad Sci U S A. 2013;110:E4238–E4245. doi: 10.1073/pnas.1315068110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng T, Vreede FT, Brownlee GG. Different de novo initiation strategies are used by influenza virus RNA polymerase on its cRNA and viral RNA promoters during viral RNA replication. J Virol. 2006;80:2337–48. doi: 10.1128/JVI.80.5.2337-2348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan WH, et al. Functional analysis of the influenza virus H5N1 nucleoprotein tail loop reveals amino acids that are crucial for oligomerization and ribonucleoprotein activities. J Virol. 2010;84:7337–45. doi: 10.1128/JVI.02474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng AK, et al. Structure of the influenza virus A H5N1 nucleoprotein: implications for RNA binding, oligomerization, and vaccine design. FASEB J. 2008;22:3638–47. doi: 10.1096/fj.08-112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye Q, Krug RM, Tao YJ. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature. 2006;444:1078–82. doi: 10.1038/nature05379. [DOI] [PubMed] [Google Scholar]
- 66.Turrell L, Lyall JW, Tiley LS, Fodor E, Vreede FT. The role and assembly mechanism of nucleoprotein in influenza A virus ribonucleoprotein complexes. Nat Commun. 2013;4:1591. doi: 10.1038/ncomms2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boulo S, et al. Human importin alpha and RNA do not compete for binding to influenza A virus nucleoprotein. Virology. 2011;409:84–90. doi: 10.1016/j.virol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Chenavas S, et al. Monomeric nucleoprotein of influenza A virus. PLoS Pathog. 2013;9:e1003275. doi: 10.1371/journal.ppat.1003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mondal A, Potts GK, Dawson AR, Coon JJ, Mehle A. Phosphorylation at the homotypic interface regulates nucleoprotein oligomerization and assembly of the influenza virus replication machinery. PLoS Pathog. 2015;11:e1004826. doi: 10.1371/journal.ppat.1004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turrell L, Hutchinson EC, Vreede FT, Fodor E. Regulation of influenza A virus nucleoprotein oligomerization by phosphorylation. J Virol. 2015;89:1452–5. doi: 10.1128/JVI.02332-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jorba N, Coloma R, Ortin J. Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLoS Pathog. 2009;5:e1000462. doi: 10.1371/journal.ppat.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaton AR, Krug RM. Transcription antitermination during influenza viral template RNA synthesis requires the nucleocapsid protein and the absence of a 5' capped end. Proc Natl Acad Sci U S A. 1986;83:6282–6. doi: 10.1073/pnas.83.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shapiro GI, Krug RM. Influenza virus RNA replication in vitro: synthesis of viral template RNAs and virion RNAs in the absence of an added primer. J Virol. 1988;62:2285–90. doi: 10.1128/jvi.62.7.2285-2290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vreede FT, Jung TE, Brownlee GG. Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J Virol. 2004;78:9568–72. doi: 10.1128/JVI.78.17.9568-9572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Resa-Infante P, Recuero-Checa MA, Zamarreno N, Llorca O, Ortin J. Structural and Functional Characterization of an Influenza Virus RNA Polymerase-Genomic RNA Complex. J Virol. 2010;84:10477–10487. doi: 10.1128/JVI.01115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honda A, Ueda K, Nagata K, Ishihama A. RNA polymerase of influenza virus: role of NP in RNA chain elongation. J Biochem. 1988;104:1021–6. doi: 10.1093/oxfordjournals.jbchem.a122569. [DOI] [PubMed] [Google Scholar]
- 77.Kawaguchi A, Momose F, Nagata K. Replication-coupled and host factor-mediated encapsidation of the influenza virus genome by viral nucleoprotein. J Virol. 2011;85:6197–204. doi: 10.1128/JVI.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newcomb LL, et al. Interaction of the influenza a virus nucleocapsid protein with the viral RNA polymerase potentiates unprimed viral RNA replication. J Virol. 2009;83:29–36. doi: 10.1128/JVI.02293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutchinson EC, et al. Conserved and host-specific features of influenza virion architecture. Nat Commun. 2014;5:4816. doi: 10.1038/ncomms5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min JY, Li S, Sen GC, Krug RM. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology. 2007;363:236–43. doi: 10.1016/j.virol.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 81.Robb NC, et al. The influenza A virus NS1 protein interacts with the nucleoprotein of viral ribonucleoprotein complexes. J Virol. 2011;85:5228–31. doi: 10.1128/JVI.02562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marion RM, Zurcher T, de la Luna S, Ortin J. Influenza virus NS1 protein interacts with viral transcription-replication complexes in vivo. J Gen Virol. 1997;78(Pt 10):2447–51. doi: 10.1099/0022-1317-78-10-2447. [DOI] [PubMed] [Google Scholar]
- 83.Falcon AM, et al. Defective RNA replication and late gene expression in temperature-sensitive influenza viruses expressing deleted forms of the NS1 protein. J Virol. 2004;78:3880–8. doi: 10.1128/JVI.78.8.3880-3888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garaigorta U, Ortin J. Mutation analysis of a recombinant NS replicon shows that influenza virus NS1 protein blocks the splicing and nucleo-cytoplasmic transport of its own viral mRNA. Nucleic Acids Res. 2007;35:4573–82. doi: 10.1093/nar/gkm230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robb NC, Fodor E. The accumulation of influenza A virus segment 7 spliced mRNAs is regulated by the NS1 protein. J Gen Virol. 2012;93:113–8. doi: 10.1099/vir.0.035485-0. [DOI] [PubMed] [Google Scholar]
- 86.Paterson D, Fodor E. Emerging roles for the influenza A virus nuclear export protein (NEP) PLoS Pathog. 2012;8:e1003019. doi: 10.1371/journal.ppat.1003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robb NC, Smith M, Vreede FT, Fodor E. NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J Gen Virol. 2009;90:1398–407. doi: 10.1099/vir.0.009639-0. [DOI] [PubMed] [Google Scholar]
- 88.Manz B, Brunotte L, Reuther P, Schwemmle ML. Adaptive mutations in NEP compensate for defective H5N1 RNA replication in cultured human cells. Nat Commun. 2012;3:802. doi: 10.1038/ncomms1804. [DOI] [PubMed] [Google Scholar]
- 89.Perez JT, et al. Influenza A virus-generated small RNAs regulate the switch from transcription to replication. Proc Natl Acad Sci U S A. 2010;107:11525–30. doi: 10.1073/pnas.1001984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umbach JL, Yen HL, Poon LL, Cullen BR. Influenza A virus expresses high levels of an unusual class of small viral leader RNAs in infected cells. MBio. 2010;1 doi: 10.1128/mBio.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perez JT, et al. A Small-RNA Enhancer of Viral Polymerase Activity. J Virol. 2012;86:13475–13485. doi: 10.1128/JVI.02295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brass AL, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–54. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hao L, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Natur. 2008;454:890–3. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karlas A, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–22. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 95.Shapira SD, et al. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–67. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naito T, et al. An influenza virus replicon system in yeast identified Tat-SF1 as a stimulatory host factor for viral RNA synthesis. Proc Natl Acad Sci U S A. 2007;104:18235–40. doi: 10.1073/pnas.0705856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sui B, et al. The use of Random Homozygous Gene Perturbation to identify novel host-oriented targets for influenza. Virology. 2009;387:473–81. doi: 10.1016/j.virol.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tripathi S, et al. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe. 2015;18:723–35. doi: 10.1016/j.chom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe T, et al. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe. 2014;16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konig R, et al. Human host factors required for influenza virus replication. Nature. 2010;463:813–7. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jorba N, et al. Analysis of the interaction of influenza virus polymerase complex with human cell factors. Proteomics. 2008;8:2077–2088. doi: 10.1002/pmic.200700508. [DOI] [PubMed] [Google Scholar]
- 102.York A, Hutchinson EC, Fodor E. Interactome analysis of the influenza A virus transcription/replication machinery identifies protein phosphatase 6 as a cellular factor required for efficient virus replication. J Virol. 2014;88:13284–99. doi: 10.1128/JVI.01813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mayer D, et al. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res. 2007;6:672–82. doi: 10.1021/pr060432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bradel-Tretheway BG, et al. Comprehensive proteomic analysis of influenza virus polymerase complex reveals a novel association with mitochondrial proteins and RNA polymerase accessory factors. J Virol. 2011;85:8569–81. doi: 10.1128/JVI.00496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bortz E, et al. Host- and strain-specific regulation of influenza virus polymerase activity by interacting cellular proteins. MBio. 2011;2 doi: 10.1128/mBio.00151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao M, et al. DnaJA1/Hsp40 is co-opted by influenza A virus to enhance its viral RNA polymerase activity. J Virol. 2014;88:14078–89. doi: 10.1128/JVI.02475-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng T, et al. Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex. J Virol. 2006;80:11911–9. doi: 10.1128/JVI.01565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fislova T, Thomas B, Graef KM, Fodor E. Association of the influenza virus RNA polymerase subunit PB2 with the host chaperonin CCT. J Virol. 2010;84:8691–9. doi: 10.1128/JVI.00813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hutchinson EC, Orr OE, Man Liu S, Engelhardt OG, Fodor E. Characterization of the interaction between the influenza A virus polymerase subunit PB1 and the host nuclear import factor Ran-binding protein 5. J Gen Virol. 2011;92:1859–69. doi: 10.1099/vir.0.032813-0. [DOI] [PubMed] [Google Scholar]
- 110.Chase GP, et al. Influenza virus ribonucleoprotein complexes gain preferential access to cellular export machinery through chromatin targeting. PLoS Pathog. 2011;7:e1002187. doi: 10.1371/journal.ppat.1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jackson DA, Caton AJ, McCready SJ, Cook PR. Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature. 1982;296:366–8. doi: 10.1038/296366a0. [DOI] [PubMed] [Google Scholar]
- 112.Lopez-Turiso JA, Martinez C, Tanaka T, Ortin J. The synthesis of influenza virus negative-strand RNA takes place in insoluble complexes present in the nuclear matrix fraction. Virus Res. 1990;16:325–37. doi: 10.1016/0168-1702(90)90056-h. [DOI] [PubMed] [Google Scholar]
- 113.Takizawa N, Watanabe K, Nouno K, Kobayashi N, Nagata K. Association of functional influenza viral proteins and RNAs with nuclear chromatin and sub-chromatin structure. Microbes Infect. 2006;8:823–33. doi: 10.1016/j.micinf.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Alfonso R, et al. CHD6 chromatin remodeler is a negative modulator of influenza virus replication that relocates to inactive chromatin upon infection. Cell Microbiol. 2011;13:1894–906. doi: 10.1111/j.1462-5822.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- 115.Marcos-Villar L, Pazo A, Nieto A. Influenza Virus and Chromatin: Role of the CHD1 Chromatin Remodeler in the Virus Life Cycle. J Virol. 2016;90:3694–707. doi: 10.1128/JVI.00053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez A, Perez-Gonzalez A, Nieto A. Cellular human CLE/C14orf166 protein interacts with influenza virus polymerase and is required for viral replication. J Virol. 2011;85:12062–6. doi: 10.1128/JVI.00684-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ver LS, Marcos-Villar L, Landeras-Bueno S, Nieto A, Ortin J. The Cellular Factor NXP2/MORC3 Is a Positive Regulator of Influenza Virus Multiplication. J Virol. 2015;89:10023–30. doi: 10.1128/JVI.01530-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez-Alonso M, Hengrung N, Fodor E. RNA-free and ribonucleoprotein-associated influenza virus polymerases directly bind the serine-5 phosphorylated carboxyl-terminal domain of host RNA polymerase II. J Virol. 2016 doi: 10.1128/JVI.00494-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engelhardt OG, Smith M, Fodor E. Association of the influenza A virus RNA-dependent RNA polymerase with cellular RNA polymerase II. J Virol. 2005;79:5812–8. doi: 10.1128/JVI.79.9.5812-5818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet. 2014;15:163–75. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamayoshi S, Watanabe M, Goto H, Kawaoka Y. Identification of a Novel Viral Protein Expressed from the PB2 Segment of Influenza A Virus. J Virol. 2015;90:444–56. doi: 10.1128/JVI.02175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubois J, Terrier O, Rosa-Calatrava M. Influenza viruses and mRNA splicing: doing more with less. MBio. 2014;5:e00070–14. doi: 10.1128/mBio.00070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fournier G, et al. Recruitment of RED-SMU1 complex by Influenza A Virus RNA polymerase to control Viral mRNA splicing. PLoS Pathog. 2014;10:e1004164. doi: 10.1371/journal.ppat.1004164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Landeras-Bueno S, Jorba N, Perez-Cidoncha M, Ortin J. The splicing factor proline-glutamine rich (SFPQ/PSF) is involved in influenza virus transcription. PLoS Pathog. 2011;7:e1002397. doi: 10.1371/journal.ppat.1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shih SR, Krug RM. Novel exploitation of a nuclear function by influenza virus: the cellular SF2/ASF splicing factor controls the amount of the essential viral M2 ion channel protein in infected cells. EMBO J. 1996;15:5415–27. [PMC free article] [PubMed] [Google Scholar]
- 126.Tsai PL, et al. Cellular RNA binding proteins NS1-BP and hnRNP K regulate influenza A virus RNA splicing. PLoS Pathog. 2013;9:e1003460. doi: 10.1371/journal.ppat.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Snyder M, Huang XY, Zhang JJ. The minichromosome maintenance proteins 2-7 (MCM2-7) are necessary for RNA polymerase II (Pol II)-mediated transcription. J Biol Chem. 2009;284:13466–72. doi: 10.1074/jbc.M809471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawaguchi A, Nagata K. De novo replication of the influenza virus RNA genome is regulated by DNA replicative helicase, MCM. EMBO J. 2007;26:4566–75. doi: 10.1038/sj.emboj.7601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sugiyama K, Kawaguchi A, Okuwaki M, Nagata K. pp32 and APRIL are host cell-derived regulators of influenza virus RNA synthesis from cRNA. Elife. 2015;4 doi: 10.7554/eLife.08939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Long JS, et al. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature. 2016;529:101–4. doi: 10.1038/nature16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou Z, et al. Fragile X mental retardation protein stimulates ribonucleoprotein assembly of influenza A virus. Nat Commun. 2014;5:3259. doi: 10.1038/ncomms4259. [DOI] [PubMed] [Google Scholar]
- 132.Resa-Infante P, et al. The host-dependent interaction of alpha-importins with influenza PB2 polymerase subunit is required for virus RNA replication. PLoS One. 2008;3:e3904. doi: 10.1371/journal.pone.0003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3'-end processing machinery. EMBO J. 1999;18:2273–83. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 135.Vreede FT, Fodor E. The role of the influenza virus RNA polymerase in host shut-off. Virulence. 2010;1:436–9. doi: 10.4161/viru.1.5.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vreede FT, Chan AY, Sharps J, Fodor E. Mechanisms and functional implications of the degradation of host RNA polymerase II in influenza virus infected cells. Virology. 2010;396:125–34. doi: 10.1016/j.virol.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Llompart CM, Nieto A, Rodriguez-Frandsen A. Specific residues of PB2 and PA influenza virus polymerase subunits confer the ability for RNA polymerase II degradation and virus pathogenicity in mice. J Virol. 2014;88:3455–63. doi: 10.1128/JVI.02263-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jorba N, Area E, Ortin J. Oligomerization of the influenza virus polymerase complex in vivo. J Gen Virol. 2008;89:520–4. doi: 10.1099/vir.0.83387-0. [DOI] [PubMed] [Google Scholar]
- 139.Vasin AV, et al. Molecular mechanisms enhancing the proteome of influenza A viruses: an overview of recently discovered proteins. Virus Res. 2014;185:53–63. doi: 10.1016/j.virusres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 140.Monod A, et al. Learning from structure-based drug design and new antivirals targeting the ribonucleoprotein complex for the treatment of influenza. Expert Opin Drug Discov. 2015;10:345–71. doi: 10.1517/17460441.2015.1019859. [DOI] [PubMed] [Google Scholar]
- 141.Byrn RA, et al. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Chemother. 2015;59:1569–82. doi: 10.1128/AAC.04623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clark MP, et al. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem. 2014;57:6668–78. doi: 10.1021/jm5007275. [DOI] [PubMed] [Google Scholar]
- 143.DuBois RM, et al. Structural and biochemical basis for development of influenza virus inhibitors targeting the PA endonuclease. PLoS Pathog. 2012;8:e1002830. doi: 10.1371/journal.ppat.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kowalinski E, et al. Structural analysis of specific metal chelating inhibitor binding to the endonuclease domain of influenza pH1N1 (2009) polymerase. PLoS Pathog. 2012;8:e1002831. doi: 10.1371/journal.ppat.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pautus S, et al. New 7-methylguanine derivatives targeting the influenza polymerase PB2 cap-binding domain. J Med Chem. 2013;56:8915–30. doi: 10.1021/jm401369y. [DOI] [PubMed] [Google Scholar]
- 146.Sagong HY, et al. Phenyl substituted 4-hydroxypyridazin-3(2H)-ones and 5-hydroxypyrimidin-4(3H)-ones: inhibitors of influenza A endonuclease. J Med Chem. 2014;57:8086–98. doi: 10.1021/jm500958x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yuan S, et al. A novel small-molecule inhibitor of influenza A virus acts by suppressing PA endonuclease activity of the viral polymerase. Sci Rep. 2016;6:22880. doi: 10.1038/srep22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sangawa H, et al. Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother. 2013;57:5202–8. doi: 10.1128/AAC.00649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sleeman K, et al. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54:2517–24. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tisdale M, et al. Inhibition of Influenza A and B Viruses by 2′-Deoxy-2′-Fluororibosides. Antiviral Chemistry and Chemotherapy. 1993;4:281–287. [Google Scholar]
- 151.Ghanem A, et al. Peptide-mediated interference with influenza A virus polymerase. J Virol. 2007;81:7801–4. doi: 10.1128/JVI.00724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Massari S, et al. A Broad Anti-influenza Hybrid Small Molecule That Potently Disrupts the Interaction of Polymerase Acidic Protein-Basic Protein 1 (PA-PB1) Subunits. J Med Chem. 2015;58:3830–42. doi: 10.1021/acs.jmedchem.5b00012. [DOI] [PubMed] [Google Scholar]
- 153.Wunderlich K, et al. Identification of high-affinity PB1-derived peptides with enhanced affinity to the PA protein of influenza A virus polymerase. Antimicrob Agents Chemother. 2011;55:696–702. doi: 10.1128/AAC.01419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yuan S, et al. Identification of a small-molecule inhibitor of influenza virus via disrupting the subunits interaction of the viral polymerase. Antiviral Res. 2016;125:34–42. doi: 10.1016/j.antiviral.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 155.Manz B, et al. Disruption of the viral polymerase complex assembly as a novel approach to attenuate influenza A virus. J Biol Chem. 2011;286:8414–24. doi: 10.1074/jbc.M110.205534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muratore G, et al. Small molecule inhibitors of influenza A and B viruses that act by disrupting subunit interactions of the viral polymerase. Proc Natl Acad Sci U S A. 2012;109:6247–52. doi: 10.1073/pnas.1119817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tintori C, et al. High-throughput docking for the identification of new influenza A virus polymerase inhibitors targeting the PA-PB1 protein-protein interaction. Bioorg Med Chem Lett. 2014;24:280–2. doi: 10.1016/j.bmcl.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 158.Li C, et al. A peptide derived from the C-terminus of PB1 inhibits influenza virus replication by interfering with viral polymerase assembly. FEBS J. 2013;280:1139–49. doi: 10.1111/febs.12107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.