Abstract
The present paper asks whether the motor cortex contributes to prediction-based guidance of target selection. This question was inspired by recent evidence that suggests (i) recurrent connections from the motor system into the attentional system may extract movement-relevant perceptual information and (ii) that the motor cortex cannot only generate predictions of the sensory consequences of movements but may also operate as predictor of perceptual events in general. To test this idea we employed a choice reaching task requiring participants to rapidly reach and touch a predictable or unpredictable colour target. Motor cortex activity was modulated via transcranial direct current stimulation (tDCS). In Experiment 1 target colour repetitions were predictable. Under such conditions anodal tDCS facilitated selection versus sham and cathodal tDCS. This improvement was apparent for trajectory curvature but not movement initiation. Conversely, where no predictability of colour was embedded reach performance was unaffected by tDCS. Finally, the results of a key-press experiment suggested that motor cortex involvement is restricted to tasks where the predictable target colour is movement-relevant. The outcomes are interpreted as evidence that the motor system contributes to the top-down guidance of selective attention to movement targets.
Keywords: Motor cortex, predictability, target selection, tDCS, attention, choice reaching task
1. Introduction1
Selective attention is a crucial mechanism for dealing with the complexities of our visual world (see Chun, Golomb & Turk-Browne, 2011; for a recent review). The deployment of selective attention is influenced by a range of bottom-up and top-down factors (e.g. Andersen, Heinke, & Humphreys, 2010). For instance, attentional deployment is more efficient when the colour of an odd-colour target is the same as on the previous trial (e.g. Maljkovic & Nakayama, 1994). This priming of pop-out (PoP) effect is often seen as the result of a bottom-up, short-term memory process (e.g. Maljkovic & Nakayama, 2000). Moreover, the PoP effect is further enhanced when the target colour is more likely to repeat than to switch over the course of a trial block (e.g. Geyer & Müller, 2009; Pascucci, Mastropasqua, & Turatto, 2012). In other words, attentional deployment is facilitated by predictability of the target colour (a top-down factor). The dorsal attentional network, including the posterior parietal cortex (PPC) and frontal eye fields (FEFs), has been shown to be crucially involved in the selection of a predictable target (see Tseng, Chang, Chiau, Liang, et al., 2013; for a review). The present paper investigates whether the motor system is also involved in the selection of a predictable target.
Positing such a role for the motor system is at odds with traditional serial stage models of the brain. These models assume that the motor system ‘simply’ has to read out previous processing in order to execute a movement (e.g., Marr, 1980; Sternberg, 1969). Recently, however, converging evidence has revealed an interaction between motor and perceptual/attentional processes consistent with functional connectivity between motor and posterior parietal regions (see Koch & Rothwell, 2009; for a review). For instance, the motor system has been implicated in cognitive operations traditionally thought to be completed ‘upstream’ (e.g., Hatsopoulos & Suminski, 2011; Resulaj, Kiani, Wolpert, & Shadlen, 2009). It has been shown that the strength of beta oscillations in primary motor cortex varies with attention to task-relevant cues (Saleh, Reimer, Penn, Ojakangas, & Hatsopoulos, 2010), and that the motor cortex accumulates perceptual evidence prior to executing a motor response (e.g., de Lange, Rahnev, Donner, & Lau, 2013; Donner, Siegel, Fries, & Engel, 2009). Importantly, de Lange et al. (2013) also demonstrated that the accumulation was biased by the choice of the participant on the previous trial. As well as perceptual information affecting activation in the motor cortex in a feedforward manner, the motor cortex has also been shown to bias perceptual judgments in a feedback fashion. For instance, learning processes in the motor system can change the psychophysical judgment of perceptual stimuli (e.g., Hecht, Vogt, & Prinz, 2001; Brown, Wilson, Goodale, & Gribble, 2007; Ostry, Darainy, Mattar, Wong, & Gribble, 2010), and visual discrimination is better at locations that form movement targets than elsewhere (e.g., Baldauf & Deubel, 2010; Deubel, Schneider, & Paprotta, 1998).
Research has also demonstrated the involvement of the motor system in generating sensory predictions (see Schubotz, 2007; for a review). For example, Schubotz and von Cramon (2002) asked participants to predict the size of a square based on a preceding size sequence. Analysis of fMRI activity showed that premotor cortex was activated by the attempt to predict the sequential perceptual pattern despite the lack of a movement component. Furthermore, the motor cortex has been shown to encode general uncertainty surrounding the presentation of a perceptual object based on the probability of a cue being a good predictor of a target over a trial block (Bestmann, Harrison, Blankenburg, Mars, Haggard, et al., 2008). Bestmann et al. showed that when a high proportion of pre-cues validly predicted the target of a key-press decision pre-stimulus motor cortex activity was biased to a greater extent towards the cued effector than when pre-cues were less predictive.
1.1. The present studies
The studies outlined above suggest that the motor cortex encodes the predictability of perceptual stimuli. In Experiment 1 ‘streaks’ of colour repetitions will be embedded within a trial block meaning that a target repeat is more likely than chance. We expect the motor system to be involved under such conditions. Conversely, we do not expect motor system involvement in Experiment 2 where target colour is unpredictable. Finally, we examine whether the predictable target needs to represent the end point of an overt movement in order to observe motor system involvement (Experiment 3).
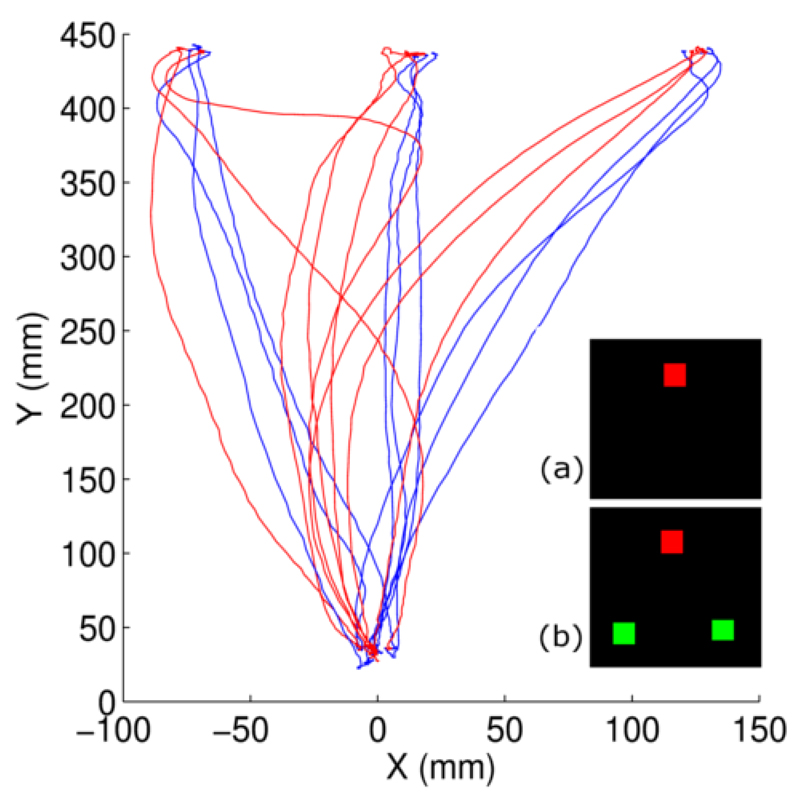
To operationalise this movement aspect we combined an odd-colour search task with the choice reaching task (CRT) where participants are asked to reach and touch a target item (see Song & Nakayama, 2009; for a review, and Strauss & Heinke, 2012; for a computational model of the CRT). Song and Nakayama (2006) have previously demonstrated how the CRT is sensitive to predictable target repetitions. As the target colour predictably repeated there were reductions in both the time taken to initiate the reach (initiation latency; IL), and the maximum deviation of the reach trajectory (MD) from the ideal path (straight line; see Fig. 6). Thus, the ongoing competition between search items ‘leaks’ into the reach trajectory providing continuous insight into the target selection process (see Song & Nakayama, 2009).
Fig. 6.
Experiment 2: Illustration of reach trajectories from a representative participant pre-AtDCS. Three example trajectories are shown for each target position per display type. X axis displays X-dimension distance (i.e. left-right), Y axis displays Y dimension distance (i.e. forward-backward). Blue trajectories indicate reaches to single targets (see insert (a)), red trajectories to odd-colour targets (see insert (b)).
Evidence that the motor cortex can form representations of perceptual information if the information is strongly related to movements (e.g., Zach, Inbar, Grinvald, Bergman, & Vaadia, 2008; Eisenberg, Shmuelof, Vaadia, & Zohary, 2011), gives additional credence to the hypothesised role of the motor system in our paradigm. For example, Zach et al. showed motor cortex neurons responded to the colour of a target when it was associated with the end point of a reaching movement. It is important to note that the present paper is not concerned with the roles of the cerebellum (e.g., Shadmehr, Smith, & Krakauer, 2010) or the superior colliculus (e.g., Song, Rafal, & McPeek, 2011) in movement control. Rather, it attempts to investigate the role of the motor system in prediction-based guidance of target selection.
In all studies transcranial direct current stimulation (tDCS) was applied to the motor cortex during task-completion. tDCS is known to increase (anodal tDCS) or decrease (cathodal tDCS) excitability of the underlying cortex (e.g., Nitsche & Paulus, 2001). In both cases, there are measurable behavioural consequences (e.g., Reis, Schambra, Cohen, Buch, Fritsch, et al., 2009). Although on the face of it, electrical stimulation over the motor cortex seems fairly non-specific, a recent study combining tDCS and electroencephalography demonstrated effects predominantly on the motor cortex and functionally-related areas (Notturno, Marzetti, Pizzella, Uncini, & Zappasodi, 2014).
2. Experiment 1: The effects of tDCS on reaching to a predictable target
In Experiment 1 target colour streaks introduced predictability into the experimental design. The proportion of target colour repeats to switches is 80%:20%, hence we expect the motor cortex to be recruited during task performance. Thus the repetition effect should be strengthened in the anodal stimulation group (AtDCS) but weakened in the cathodal stimulation group (CtDCS).
2.1. Method
2.1.1. Participants
27 University of Birmingham students were recruited in exchange for cash or course credit. Participants in all 3 experiments were right-handed and had normal or corrected-to-normal colour vision. Participants were randomly assigned to an AtDCS, CtDCS, or sham (StDCS) stimulation group. The participant information for each group was as follows: AtDCS: n=9, 5 females, aged 18-29 (mean 22.1), StDCS: n=9, 5 males, aged 20–35 (mean 25.9), CtDCS: n=9, 7 females, aged 19–23 (mean 20.22). Procedures were approved by the local ethics committee at the University of Birmingham and informed consent was gathered from all participants after the completion of a tDCS safety screening questionnaire.
2.1.2. Procedure
Participants were seated in a semi-darkened room facing a visual display (19” SyncMaster 940N, Samsung). Their right-hand rested beside a trigger switch on the table in front of them (aligned with body midline; 10cm from participant, 45cm from display). When prompted by on-screen instructions participants held down the trigger switch to commence each trial. A white fixation cross (0.9°) was presented alone in the centre of a black background for 1000ms before being joined by three squares (3.8°x3.8°) positioned at 12 o’clock, 4 o’clock or 8 o’clock around a circle (radius of 12.2°). Two of the squares (the distractors) were green (u’=0.11, v’=0.24, L=64.43) and the other square (the target) was red (u’=0.46, v’=1.03, L=22.43), or vice versa.
Participants were instructed to reach as quickly and as accurately as possible to the target square. To encourage participants to start their reaching movements as quickly as possible we included a tone at 400ms after stimulus presentation on every trial. If participants failed to initiate their reach before the tone they were instructed to concentrate on doing so on the next trial by the experimenter. An initiation period of 400ms was chosen based on previous studies within our laboratory that showed this to approximate the average latency. Trials that exceeded the 400ms threshold were still included in the analysis (unless removed as outliers; see 2.2 Results and Discussion).
As well as commencing the onscreen trial procedure, the trigger switch also activated motion capture cameras (Qualisys ProReflex MCU240, 120Hz). The cameras recorded the 3D position of a small (4mm), passive reflective marker attached to the participant’s right index fingernail from the point at which the trigger switch was depressed until 3000ms had elapsed – long enough for the participant to complete their reach.
2.1.3. tDCS Protocol
A battery-driven stimulator delivered a 1.2 mA current (NeuroConn DC-Stimulator Plus, Rogue Resolutions) to two electrodes covered by saline-soaked sponges (5cm x 5cm) to give a current density of 0.048 mA/cm2. The anode was positioned over the left motor cortex (contralateral to right-hand), at position C3’ of the 10–20 EEG system, with the reference electrode positioned above the right supraorbital ridge. Stimulation was administered for 20 minutes, ramping up and down at the start and end over 10 seconds. Sham participants received stimulation for a brief period of 20 seconds at both the beginning and end of the trial block, with the current ramping up to 1.2mA over 10 seconds, and immediately ramping down to zero again.
2.1.4. Design & Analysis
Participants in all three stimulation conditions participated in three testing sessions: Pre-, during- and post-tDCS. Each session comprised two blocks of 96 trials. Following the pre-tDCS session the tDCS equipment was set-up and stimulation activated. The 20-minute stimulation period (AtDCS) was sufficient for the participant to complete the during-tDCS session (2x96 trials). After a 30-minute break the participant completed a further 2 blocks in the post-tDCS session.
Embedded within each block were colour streaks up to a length of 6, similar to the design used by Song & Nakayama (2006). Block A comprised streaks of 2, 4 and 6 target colour repetitions (6x2, 6x4, 10x6 repetitions). For instance, in a streak of 4 followed by a streak of 2 the target colour might be RRRRGG, where the first R and first G are the ‘switch trials’ followed by colour repetitions. Block B comprised streaks of 3, 5 and 6 consecutive target colour repetitions (6x3, 6x5, 8x6 repetitions). Different length streaks were included in blocks A and B to reduce the possibility of the participant learning the length of the streaks and anticipating the switch in colour. The streaks were randomly mixed within the overall block of 96 trials and the order of block A and block B was counterbalanced across participants and collapsed across prior to analysis.
The analysis focused on two crucial dependent variables: Initiation latency (IL; ms) refers to the time between stimulus presentation and movement onset. Movement onset was defined as the first of five consecutive frames at which the index finger velocity exceeded 20mm/s. Maximum Deviation (MD; mm) is measured by taking the absolute deviation of the index finger trajectory from a straight line between the start and end of the movement (see Fig. 6 for an illustration). Firstly, a 3-way mixed ANOVA was conducted with factors stimulation (anodal vs. sham), streak (switch vs. 2 vs. 3 vs. 4 vs. 5 vs. 6), and session (pre- vs. during- vs. post-tDCS). Significant effects of session were explored with paired-samples t-tests. Significant effects of streak were subject to planned comparisons between switch and streak 6 (based on previous research, e.g. Song and Nakayama, 2006). Despite being included in the ANOVA (to provide accurate analysis of session and streak effects) we do not report the effects of stimulation. Instead, a standardised score was calculated by subtracting the pre-tDCS performance from during- and post-tDCS performance. This way any a priori stimulation group differences were excluded from the analysis. This standardised score was then subject to a simple effects analysis comparing AtDCS, CtDCS, and StDCS at the during- and post-tDCS sessions.
2.2. Results and Discussion
Data were collapsed across target colour and position (both equally split across conditions) and any error trials (incorrect responses: <0.1%, technical errors: 1.6%) and outliers (1.9%) were removed. Outliers were classified as trials where the IL was >2 standard deviations from the mean per participant.
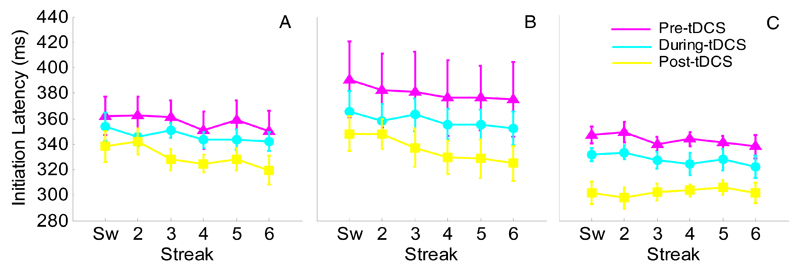
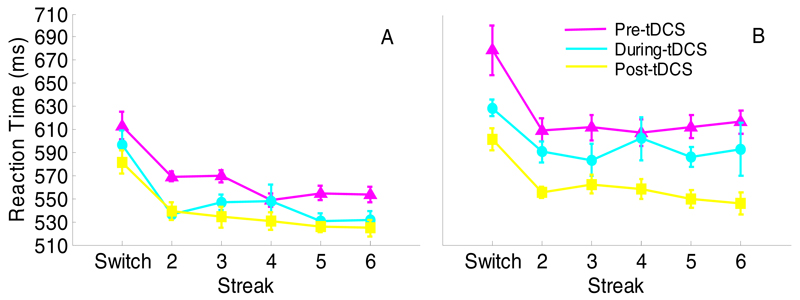
Raw IL data (Fig. 1) showed main effects of both session (F(2,48)=8.33, p=0.001, ηp2=0.26) and streak (F(5,120)=7.55, p<0.001, ηp2=0.24). Pre- and during-tDCS showed longer ILs than post-tDCS across all stimulation groups (t(26)=3.37, p=0.002, and t(26)=3.85, p=0.001, respectively). Thus, the effect of session is likely due to practice. The expected priming effect was present, as evidenced by a significant decrease from switch trial to streak 6 (344ms vs. 331ms; t(26)=4.11, p<0.001). No interactions approached significance. Importantly, the standardised scores showed no effect of stimulation on IL at either during- (Fig. 2A; F(2,24)=0.11, p=0.90, η2=0.009) or post-tDCS (Fig. 2B; F(2,24)=0.21, p=0.82, η2=0.02).
Fig. 1.
Experiment 1: Raw mean initiation latencies for AtDCS (A), StDCS (B), and CtDCS (C). Pre-tDCS performance is shown in magenta, during-tDCS in cyan and post-tDCS in yellow. Sw = switch trial, numbers refer to consecutive colour repetitions. Error bars reflect within-subjects standard error (Cousineau, 2005).
Fig. 2.
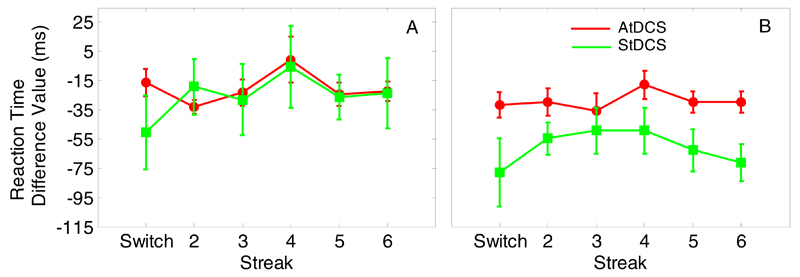
Experiment 1: Standardised initiation latency difference scores (red = AtDCS, green = StDCS, blue = CtDCS) for during-tDCS (A) and post-tDCS (B). Numbers refer to consecutive colour repetitions. Error bars reflect between-subjects S.E.M.
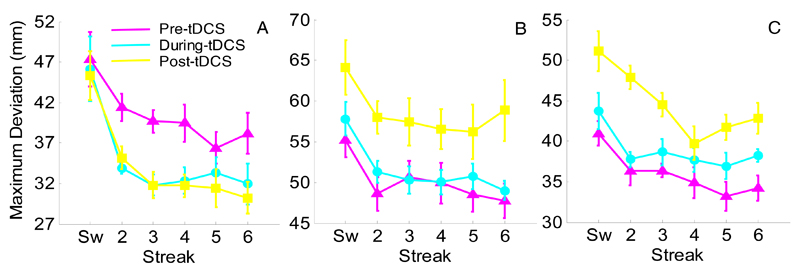
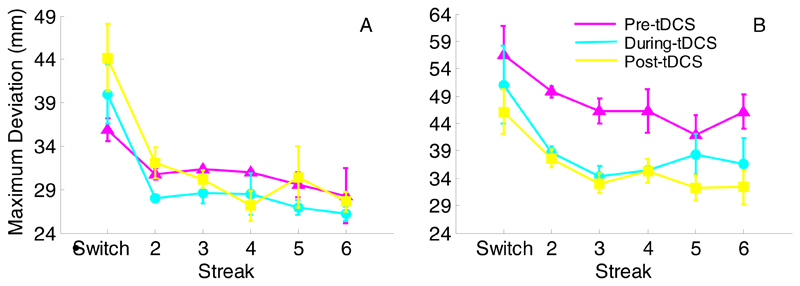
Raw MD values (Fig. 3) were affected by streak (F(5,120)=20.68, p<0.001, ηp2=0.46) and session (F(2,48)=4.99, p=0.011, ηp2=0.17). The expected priming pattern was present (switch = 50.70mm, streak 6 = 41.43mm; t(26)=5.13, p<0.001), and during-tDCS showed less deviation than post-tDCS (43.30mm vs. 46.52mm; t(26)=2.35, p=0.026) when collapsed across stimulation groups. There were no significant interactions.
Fig. 3.
Experiment 1: Raw mean maximum deviation for AtDCS (A), StDCS (B), and CtDCS (C). Pre-tDCS performance is shown in magenta, during-tDCS in cyan and post-tDCS in yellow. Sw = switch trial, numbers refer to consecutive colour repetitions. The y-axis scale varies from figure-to-figure so that the stimulation group differences do not obscure the important differences between sessions. Error bars reflect within-subjects standard error (Cousineau, 2005).
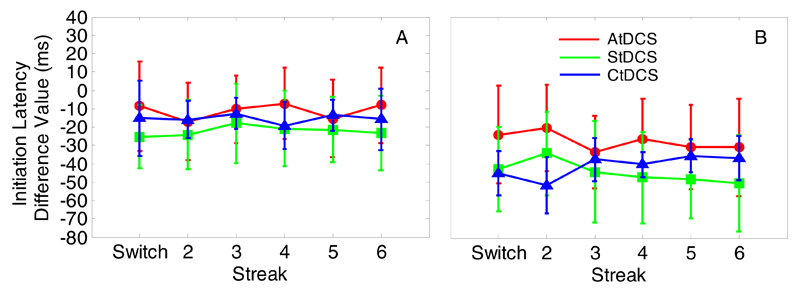
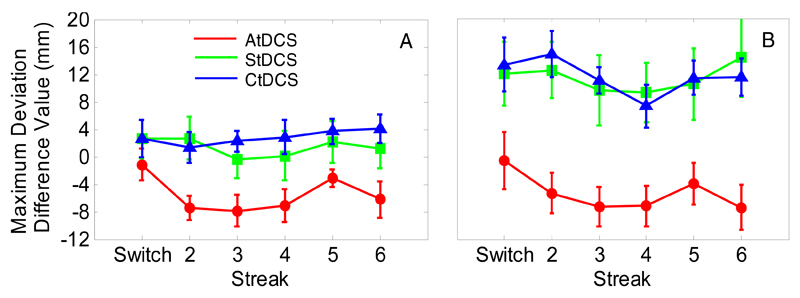
Importantly, standardised scores for MD showed a main effect of stimulation for both during- (Fig. 4A; F(2,24)=4.71, p=0.02, η2=0.28) and post-tDCS (Fig. 4B; F(2,24)=8.38, p=0.002 η2=0.41) sessions. There was a significant reduction in deviation during-AtDCS compared to during-StDCS (t(16)=2.22, p=0.04) and during-CtDCS (t(16)=4.57, p<0.001). This reduction in deviation in the AtDCS group was maintained 30 minutes later at post-tDCS (t(16)=3.13, p=0.006 and t(16)=4.32, p=0.001, versus StDCS and CtDCS, respectively). It should be noted that this maintenance effect post-tDCS is enhanced by a decay in performance post-StDCS and post-CtDCS in combination with the maintenance of improvement post-AtDCS (see Fig. 3). It is unclear why performance decayed in the StDCS and CtDCS but not in the AtDCS condition. To speculate, it may be that faster ILs post-tDCS (see Fig. 1) resulted in a speed-accuracy trade-off where the shorter IL increased the chances of distractor selection and subsequently increased MD. This affected post-tDCS MD in the sham and cathodal groups but the trade-off was diminished by anodal stimulation. No differences were observed between StDCS and CtDCS standardised scores at either during- (t(16)=1.08, p>0.250) or post-tDCS (t(16)=0.01, p>0.250). Thus, in terms of MD we see anodal stimulation of the motor cortex improving performance compared to cathodal and sham stimulation. It should be noted that weaker cathodal compared to anodal effects have been reported previously in tDCS-behavioural studies (e.g., Nitsche, Schauenburg, Lang, Liebetanz, Exner et al., 2003; Spiegel, Hansen, Byblow, & Thompson, 2012).
Fig. 4.
Experiment 1: Standardised maximum deviation difference scores (red = AtDCS, green = StDCS, blue = CtDCS) for during-tDCS (A) and post-tDCS (B). Numbers refer to consecutive colour repetitions. Error bars reflect between-subjects S.E.M.
If, as proposed, AtDCS is strengthening the PoP effect when colour repetition is predictable, then the data should show an increase in MD on the switch trials at during- and post- compared to pre-AtDCS. In other words, the increased repetition benefit should accentuate the decrease in performance when the target colour changes unexpectedly. There is a hint of this switch trial increase in the standardised MD values presented in Fig. 4 where the effect of AtDCS approaches zero on the switch trial. To further investigate this effect we split the participants into two groups, high precision and low precision, based on their mean MD at streak 6 compared to the median MD value at streak 6 across all participants (i.e. a median split). The rationale for this was that there might be a ceiling effect for those participants that naturally showed greater trajectory curvature. Thus, even when the colour does switch MD cannot rebound to a higher level. In contrast, the lower deviation intrinsic to the high precision group should allow for the expected reduction in performance on the switch trial to occur. A lack of statistical power (n=4) meant that planned t-tests examining switch trial MD between pre-, during-, and post-AtDCS sessions failed to reach significance (ps>0.08). Nevertheless, as shown in Fig. 5A, the high precision participants exhibit the expected effect: there is a reduction in deviation during-AtDCS (and to some extent post-tDCS) on target colour repeat trials, yet this performance enhancement results in a detrimental effect on the switch trial where during- and post-tDCS show considerably greater deviation than pre-tDCS. Thus, when initial performance is good it allows for both positive and negative repercussions. IL did not differ between high and low precision groups (F(1,7)<1, p>0.250, ηp2= 0.01) when entered into a 3-way ANOVA with factors precision, session, and streak. This suggests that the differences between high and low precision groups are not the result of a speed accuracy trade-off. The expected priming effect was present on IL (F(5,35)=4.04, p=0.005, ηp2=0.37) but no other main effects or interactions were observed (ps>0.1).
Fig. 5.
Experiment 1: Raw mean maximum deviation values for AtDCS participants split into high precision (A) and low precision (B) groups. Pre-AtDCS performance is shown in magenta, during-AtDCS in cyan and post-AtDCS in yellow. Numbers refer to consecutive colour repetitions. Error bars reflect within-subjects standard error (Cousineau, 2005).
Finally, additional analysis was conducted on the time at which maximum deviation occurred (as a percentage of the movement period). This was to rule out an alternative theoretical explanation based on pre-planned trajectories (see 5. General Discussion for details). There was no main effect of session (F(2,48)=0.13, p>0.250, ηp2=0.005) or stimulation (F(2,24)=0.12, p>0.250, ηp2=0.01), but a main effect of streak (F(5,120)=3.00, p=0.014, ηp2=0.11) with MD getting slightly later as target colour repeated (e.g. streak 2=42.4%, streak 6=41.5%; t(26)=3.17, p=0.004).
In summary, results showed that AtDCS reduced MD over the course of target colour repetitions. In other words, where predictability is high, modulation of motor cortex activity improves our ability to select and localise a movement target. However, the IL of the reach was immune to tDCS effects. Reasons for this are discussed in 5. General Discussion.
3. Experiment 2: The effects of tDCS on reaching to an unpredictable target
In Experiment 2 the colour of the target was equally likely to switch or repeat from trial-to-trial, producing low predictability (i.e. no colour streaks). As laid out in the introduction we did not expect a tDCS effect because research has shown the motor cortex is not recruited under such conditions. However, the motor system may still be involved when repetition of the target colour is at chance-level, i.e. based on bottom-up, short-term memory of the previous trial(s).
Moreover, a possible explanation for the AtDCS effect in Experiment 1 is that AtDCS improved general motor control (e.g., Hummel, Heise, Celnik, Floel, Gerloff, & Cohen, 2010). Such improvements would be most clear when reaching to a target in the absence of distractors. Thus, rather than presenting solely odd-colour (OC) search displays Experiment 2 also included single target (ST) trials (see also, Song & Nakayama, 2007). Note that this does not affect target colour (un)predictability which remained 50-50 regardless of display type. Thus, Experiment 2 will examine the effects of tDCS on reaching to an unpredictable target, and will use ST trials to rule out tDCS improvements in reach precision as an explanation for the results of Experiment 1.
3.1. Method
The method matched that of Experiment 1 with the following exceptions.
3.1.1. Participants
18 University of Birmingham students took part in Experiment 2. Participants were randomly assigned to an AtDCS or StDCS stimulation group. The CtDCS group was not included based on the lack of difference to StDCS in Experiment 1. The participant information for each group was as follows: AtDCS: n=9, 5 females, aged 18-22 (mean 20.2), StDCS: n=9, 6 females, aged 19-22 (mean 21.3).
3.1.2. Procedure
Rather than presenting solely OC search trials, where two distractors surround the target (as in Experiment 1), Experiment 2 also included ST trials where the target appeared alone (see insets, Fig. 6).
3.1.3. Design & Analysis
Participants in both stimulation conditions participated in the pre-, during- and post-tDCS sessions. 50% of each block were ST trials and 50% were OC trials with target colour pseudorandomly assigned to ensure a 50-50 split of red and green.
Again the analysis focused on IL and MD. A mixed ANOVA was conducted with factors stimulation (anodal vs. sham), display type (ST vs. OC), and session (pre- vs. during- vs. post-tDCS). Significant ANOVA effects were explored with paired-samples t-tests. Standardised scores (during- and post-tDCS minus pre-tDCS) were entered into simple effects analyses to examine the effect of stimulation.
3.2. Results and Discussion
Data were collapsed across target colour and position (both equally split across conditions) and outliers (2.2%) and error trials (incorrect target selection (<0.1%), motion capture errors (<1%)) were removed.
Fig. 6 shows exemplar reaches from a representative participant to ST and OC targets prior to receiving AtDCS. Table 1 presents raw IL and MD data from Experiment 2. The analysis of the raw ILs revealed a main effect of session (F(2,32)=34.40, p<0.001, ηp2=0.68) with pre-tDCS having longer ILs than during- (t(17)=5.22, p<0.001) and post-tDCS (t(17)=7.27, p<0.001) and post-tDCS having shorter ILs than during-tDCS (t(17)=3.98, p=0.001). As in Experiment 1, the presence of this pattern in AtDCS and StDCS groups suggests it is the result of practice. Results also replicated Song & Nakayama (2007) who found no difference in IL when ST and OC trial types were randomly mixed (F(1,16)=0.17, p>0.250, ηp2=0.01). A simple effects analysis, carried out separately on during- and post-tDCS standardised ILs did not show an effect of stimulation (F(1,16)=0.32, p>0.250, η2=0.02; and F(1,16)=0.01, p>0.250, η2<0.01; for during- and post-tDCS differences, respectively).
Table 1.
Experiment 2: Mean initiation latency (IL; ms) and maximum deviation (MD; mm) values (and standard deviations) according to display type (ST = single target, OC = odd colour trial), stimulation group, and session.
| Initiation Latency (IL; ms) | Maximum Deviation (MD; mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anodal | Sham | Anodal | Sham | |||||||||
| Pre- | During- | Post- | Pre- | During- | Post- | Pre- | During- | Post- | Pre- | During- | Post- | |
| ST | 302 (60) | 273 (57) | 256 (62) | 335 (49) | 302 (53) | 292 (47) | 35.68 (8.8) | 37.43 (6.9) | 36.61 (8.5) | 38.62 (8.5) | 38.46 (7.6) | 38.48 (9.6) |
| OC | 302 (83) | 286 (80) | 262 (80) | 329 (58) | 306 (64) | 289 (58) | 47.89 (7.4) | 48.02 (9.9) | 49.33 (9.7) | 52.49 (9.5) | 51.92 (10.6) | 54.01 (11.3) |
There was increased MD for OC versus ST trials (F(1,16)=62.43, p<0.001, ηp2=0.80), but there was no main effect of session (F(2,32)=0.28, p>0.250, ηp2=0.02). No interactions approached significance. Importantly, the standardised MD scores showed no differences between stimulation groups at during- or post-tDCS (F(1,16)=0.32, p>0.250, η2=0.02, and, F(1,16)=0.02, p>0.250, η2=0.001, respectively).
As stated earlier, if the chance repetition of the target is sufficient to recruit the motor system it would suggest that the activation is due to a bottom-up, short-term memory trace from the previous trial independent of the wider repetition probability. Conversely, if no tDCS effect is observed it suggests that motor system involvement is top-down in nature and restricted to situations where repeats are more likely to occur over a longer period. To shed light on this issue an additional analysis was conducted whereby occurrences of colour repetitions (across both ST and OC trials) were entered into a four-way mixed ANOVA with factors stimulation (AtDCS vs. StDCS), session (pre- vs. during- vs. post-tDCS), display type (OC vs. ST), and repetition (switch vs. repeat). Note that including both ST and OC trial types maximised the number of colour repetitions for analysis (on average, 96 trials per 192 trial stimulation session), compared to when the analysis is restricted to OC colour repetitions. IL results remained the same as in the previous analysis with the only significant main effect being exerted by session (F(2,32)=34.03, p<0.001, ηp2=0.68). There was no effect of repetition (p>0.250) and, no interaction between repetition and stimulation, session, or display type (ps>0.250).
For MD, there was again a main effect of display type (F(1,16)=62,40, p<0.001, ηp2=0.80) but there were no effects of session or stimulation (ps>0.250). A main effect of repetition was observed (switch = 44.56mm, repeat = 43.56mm; F(1,16)=13.233, p=0.002, ηp2=0.45) that interacted with display type (F(1,16)=6.35, p=0.02, ηp2=0.28) but not with session or, crucially, stimulation (ps>0.250). The repetition x display type interaction showed that, unsurprisingly, OC trials were more affected by repetitions of colour (switch = 51.01mm vs. repeat = 47.50mm; t(17)=3.29, p=0.002) than ST trials (37.72mm vs. 37.34mm; p>0.250). The main effect of repetition indicates that, when indexed by MD, bottom-up priming effects are present despite the random nature of the target repetition. However, the fact that repetition and stimulation did not interact suggests the motor system involvement is restricted to when top-down probability favours a repeat over a switch. It should be noted, however, that the size of the repetition effect for OC trials in Experiment 2 is smaller than in Experiment 1 (3.5mm vs. 9.3mm). It is possible that, in combination with the lower predictability of the target colour, the presence of the ST trials may have reduced the repetition effect and contributed to the lack of tDCS effect. However, first and foremost, it was important to rule out improvements in motor control due to tDCS that may have caused the tDCS effects in Experiment 1. In general, these results show that chance occurrence of colour repetitions were insufficient for motor cortex recruitment, hence the lack of stimulation effects or interactions between repetition and stimulation. This suggests that motor cortex involvement is restricted to cases where the repetition of target colour is predictable across trials.
In summary, the results from Experiment 2 show that both reach parameters were unaffected by motor cortex stimulation. This has two important implications. Firstly, it suggests that in the absence of any predictable colour repetition tDCS has no discernable effect on target selection, in contrast to the predictable target results of Experiment 1. Secondly, the ST trial results show that tDCS does not lead to simple improvements in reach performance meaning that the effects in Experiment 1 are unlikely to have resulted from improvements in motor control.
4. Experiment 3: The effects of tDCS in a predictable target key-press task
The design of Experiment 1 was originally based on the key-press task of Maljkovic and Nakayama (1994). Experiment 3 aims to replicate their findings and, in doing so, test whether a motor cortex tDCS effect can be found without requiring an overt reaching movement to achieve the target. Current evidence makes conflicting predictions. On the one hand a large number of neuroimaging studies (see Kristjansson & Campana, 2010; for a review) failed to report motor cortex activity during primed search tasks. On the other hand, the sequence prediction task used by Schubotz and von Cramon (2002) showed recruitment of the motor system, as did Bestmann et al. (2008) despite the target signalling an arbitrary key-press.
4.1. Method
4.1.1. Participants
18 University of Birmingham students were randomly assigned to either an AtDCS (n=9, 3 males, aged 18-23 (mean 21.9)) or StDCS (n=9, 4 males, aged 18-20 (mean 18.6)) stimulation group.
4.1.2. Behavioural Task & Data Analysis
The task was derived from that of Maljkovic and Nakayama (1994). Participants were presented with displays consisting of 3 diamonds with a ‘cut-off’ section on either the left or right. Their task was to discriminate the side of the cut-off section on the odd-colour item, pressing ‘H’ on a keyboard if the right side was cut-off and ‘B’ if the left side was cut-off. Participants completed 8 blocks of 96 trials in each session (pre-, during-, post-tDCS). Both this task and the CRT have been shown to rely on focal attention (Song and Nakayama, 2006). The same streaks of target colour repetitions were embedded as in Experiment 1. Dependent variables were raw and standardised reaction times (during- and post- minus pre-tDCS; RT) and accuracy of discrimination of the cut-off side.
4.2. Results and Discussion
Results were collapsed across target colour, position and cut-off side. There were no differences in accuracy according to stimulation (F(1,16)=0.51, p>0.250, ηp2=0.03), session (F(2,32)=0.07, p>0.250, ηp2=0.004), or streak (F(5,80)=1.45, p=0.22, ηp2=0.08), and no interactions. Error trials (3.2%) were then removed prior to RT analysis.
Main effects of session (F(2,32)=12.16, p<0.001, ηp2=0.43; pre-tDCS slowest, post-tDCS fastest and during-tDCS intermediate, ps<0.05) and streak (F(5,80)=24.61, p<0.001, ηp2=0.61; switch trial = 617ms, streak 6 = 561ms; t(17)=5.20, p<0.001)) were observed on raw RTs indicating practice and priming effects, respectively (Fig. 7). There were no significant simple effects of stimulation on standardised RTs either during- (Fig. 8A; F(1,16)=0.06, p>0.250, η2=0.004) or post-tDCS (Fig. 8B; F(1,16)=3.99, p=0.063, η2=0.20).
Fig. 7.
Raw mean RTs for AtDCS (A), and StDCS (B). Pre-tDCS performance is shown in magenta, during-tDCS in cyan and post-tDCS in yellow. Numbers refer to consecutive colour repetitions. Error bars reflect within-subjects standard error (Cousineau, 2005).
Fig. 8.
Standardised RT difference scores (red = AtDCS, green = StDCS) for during-tDCS (A) and post-tDCS (B). Numbers refer to consecutive colour repetitions. Error bars reflect between-subjects S.E.M.
In summary, stimulation of the motor cortex did not affect RTs when the participants had to make a key-press response, even when the target colour repeated predictably. This is in contrast to the CRT findings of Experiment 1 and suggests that the motor system is only recruited when the predictable perceptual information signals the target of an overt movement. This explains why previous imaging experiments have failed to show motor cortex activity during primed target selection tasks requiring key-press responses (Kristjansson & Campana, 2010), but conflicts with the findings of Bestmann et al. (2008) and Schubotz and von Cramon (2002).
5. General Discussion
In this paper we tested whether the motor system is sensitive to the predictability of target features. The results suggest motor system involvement in the selection of predictable colour-defined movement targets. Anodal tDCS was shown to facilitate localisation when target colour repeats were predictable (Experiment 1). Conversely, modulation of motor system activity did not influence behaviour when the target was unpredictable (even when some bottom-up, short-term memory effects were observed; Experiment 2), or when a key-press rather than a reaching response was required (Experiment 3). These findings are consistent with recent research highlighting the role of the motor system in tasks requiring perceptual prediction (e.g., Bestmann et al., 2008; Schubotz & von Cramon, 2002), and more generally with evidence of motor system involvement in perceptual/attentional processing (e.g., Ostry et al., 2010; Baldauf & Deubel, 2010). The present findings are particularly intriguing considering the systematic predictability pertains to the target colour rather than its location.
The fact that tDCS influenced target selection when repeats were predictable (Experiment 1), but not when they occurred by chance (Experiment 2), suggests that the underlying neural processes are sensitive to top-down modulation. There are at least two possible mechanisms for this top-down influence. Firstly, the motor cortex itself may be sensitive to the probability of target repeats. This is consistent with the findings of Bestmann et al. (2008) that demonstrated that pre-stimulus motor cortex activity is biased by the average uncertainty pertaining to a cued target over the course of a trial block. On blocks of trials where the cue reliably predicts the target (i.e. on 85% of trials) motor cortex activity is higher than on blocks where the cue is less valid (i.e. on 55% of trials). Secondly, tDCS may have modulated the strength of feedback signals from the motor system to posterior parietal regions previously implicated in the selection of a primed target (e.g. Muggleton, Kalla, Juan, & Walsh, 2011; Taylor, Muggleton, Kalla, Walsh, & Eimer, 2011). This is consistent with Baldauf and Deubel’s (2010) theory of “visual preparation”. Their theory suggests that the feedback connections from motor regions guide attention to extract movement-relevant information from visual stimuli. This guidance is assumed to occur in a top-down manner according to the weight attached to the visual inputs from potential movement targets. To speculate, in the present experiments the top-down weight attached to the visual input may have been biased according to the target colour built-up over the previous trials. More broadly, the feedback explanation is also in accordance with a recent investigation by Notturno et al. (2014) that showed M1 tDCS modulated low alpha desynchronisation in parietal regions. Such desynchronisation is typically associated with attentional processing (see Klimesch, 2012; for a review).
An open question is why M1 tDCS modulated MD but not IL in Experiment 1. This dissociation could be explained by a methodological factor. To ensure that the reach trajectories reflected ongoing rather than completed target processing participants were instructed to commence their reach before a warning tone 400ms post-stimulus onset. It is possible that this warning tone homogenised ILs across stimulation groups, as supported by ILs that are just below 400ms in each group. However, also note that the 400ms tone was present in previous experiments within our laboratory without this homogenisation occurring. Furthermore, ILs were still reduced by predictable repetition of the target colour in Experiment 1, arguing against homogenisation caused by the warning tone. Another explanation is that reach trajectories are simply more sensitive to the effects of tDCS than reach latencies. This study is not the first to show a lack of M1 tDCS effects on movement latencies but an effect on later movement parameters such as end-point error and trajectory control (e.g. Galea, Vazquez, Pasricha, Orban de Xivry, & Celnik, 2011; Hunter, Sacco, Nitsche, & Turner, 2009).
The authors have attempted to explain the IL-MD dissociation using a biologically plausible computational model (CoRLEGO; Strauss, Woodgate, & Heinke, submitted). CoRLEGO separates the detection of the odd-colour (i.e. red or green?) and the localisation of the target item into two distinct but parallel processes. Now, in order to localise the odd colour item, the odd-colour detection steers the target localisation towards the location of the odd-colour item. CoRLEGO assumes that the movement begins before the localisation is completed leading to curved reach trajectories. To replicate the colour PoP-effect CoRLEGO assumes that the colour from the previous trial is stored in PPC. This stored colour information leads to a speed up of the odd-colour detection which, in turn, leads to faster and better direction of target localisation, reducing IL and MD. To explain the IL-MD dissociation, CoRLEGO had to be extended. First, it assumes that colour information from previous trials is also stored in M1. Second, it assumes that this information contributes to steering the target localisation (consistent with Baldauf and Deubel’s (2010) suggestion that the motor system directs attention to movement-relevant information), but that the start of the movement is largely determined by the detection of the odd-colour. Consequently, IL is not affected by motor cortex tDCS since the initiation of the movement is based on the stored colour information in PPC. However, as CoRLEGO’s M1 still influences the localisation of the target, tDCS over M1 can still affect the movement trajectories.
It is important to note that our interpretation of the present results is strongly based on the assumption that reaching trajectories are not fully pre-planned. Instead, the execution of the movement is continuously influenced by the selection process (c.f. Song and Nakayama’s (2009) ”leakage” hypothesis). However, there is an alternative theoretical framework (e.g. Stewart, Baugh, Gallivan, & Flanagan, 2013) that suggests that the brain first plans the trajectory to all possible targets and begins executing an “averaged” trajectory before the final target is known to the motor system. In their paradigm participants are signalled the target after they start moving. In our design the theory of several pre-planned trajectories may play out as follows. After a few trials participants know the potential positions of target and distractors and thus can pre-plan the three possible trajectories. It is therefore possible to initiate the movement towards the centre of the display before selective attention identifies the target and the corresponding planned trajectory is executed. This offers another explanation for the lack of tDCS effect on IL: The reach is initiated to the centre of the display without considering the target identity or location. However, if participants were using this strategy we would expect to see an effect of tDCS on the time at which MD occurs and not just the magnitude of MD. MD should become earlier as a result of AtDCS since focal attention is drawn away from the average of the three positions and towards the target more quickly. Supplementary analysis rendered this unlikely since there was no effect of stimulation on the time at which maximum deviation occurred (see 2.2 Results and Discussion). This argues against the pre-planned trajectory hypothesis in favour of the continuous competition hypothesis.
The other intriguing finding is the lack of tDCS effect on RTs in Experiment 3. This suggests that M1 is only involved in the selection of a predictable target when it signals the end-point of an overt movement (see also Eisenberg et al., 2011). This is consistent with many neuroimaging studies that fail to show M1 activation in primed key-press response tasks (see Kristjansson & Campana, 2010; for a summary) but, to a degree, contradicts the findings of Bestmann et al. (2008) and Schubotz and von Cramon (2002; see section 1.1). However, M1 involvement in both Bestmann et al. (2008) and Schubotz and von Cramon (2002) followed explicit instruction to learn the predictive nature of visual stimuli whereas the current task relies on implicitly learned predictions. The role of the motor cortex in implicit learning of overt movements is well documented (e.g. the serial reaction time task (SRTT); Robertson, 2007). Hence, if learning is implicit, as in the present studies, then an overt movement may be required to see any effect of tDCS (see Sami, Robertson, & Miall, 2014; for evidence for distinct implicit and explicit motor networks underlying the SRTT). Therefore it is possible that Schubotz and von Cramon’s (2002) key-press findings rely on the explicit prediction of visual stimuli. Moreover, Schubotz and von Cramon’s (2002) task modulated activity in premotor cortex, which is important for task-related planning independent of the method of response (e.g., Baker, Rogers, Owen, Frith, Dolan, et al., 1996), rather than the primary motor cortex that was targeted in the present experiments. Finally, in light of Schubotz and von Cramon’s (2002) results it is also worth pointing out that the lack of RT effect in Experiment 3 helps to counter a criticism of tDCS based on its low spatial precision: if premotor activity was being unintentionally modulated then we would expect an effect on key-press responses.
In summary, target selection was improved when tDCS was applied over the motor cortex, but only if target colour repetitions were predictable. This supports a role for the motor cortex in prediction-based guidance of target selection. Furthermore, the results from Experiment 3 suggest that the perceptual information must be movement-relevant, in this case signalling the end-point of a reach. These conclusions are consistent with recent literature that highlights the role of the motor system in processing previously thought to be completed at an earlier neural stage.
Acknowledgements
Our thanks to Joseph Galea, Glyn Humphreys and Chris Miall for very helpful discussions and comments on earlier drafts. P.J.W.W was supported by an EPSRC-DTC grant.
Footnotes
PoP = Priming of pop-out, CRT = Choice reaching task, IL = initiation latency of reach, MD = maximum deviation of reach trajectory. ST = single target, OC = odd-colour search trial.
Contributor Information
Philip J.W. Woodgate, Email: pjw081@bham.ac.uk.
Soeren Strauss, Email: soestra@yahoo.de.
Saber A. Sami, Email: ssa42@medschl.cam.ac.uk.
Dietmar Heinke, Email: d.g.heinke@bham.ac.uk.
References
- Anderson GM, Heinke D, Humphreys GW. Featural Guidance in Conjunction Search: The Contrast Between Orientation and Color. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1108–1127. doi: 10.1037/a0017179. [DOI] [PubMed] [Google Scholar]
- Baldauf D, Deubel H. Attentional landscapes in reaching and grasping. Vision Research. 2010;50:999–1013. doi: 10.1016/j.visres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Harrison LM, Blankenburg F, Mars RB, Haggard P, Friston KJ, Rothwell JC. Influence of Uncertainty and Surprise on Human Corticospinal Excitability during Preparation for Action. Current Biology. 2008;18:775–780. doi: 10.1016/j.cub.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Goodale MA, Gribble PL. Motor Force Field Learning Influences Visual Processing of Target Motion. Journal of Neuroscience. 2007;27:9975–9983. doi: 10.1523/JNEUROSCI.1245-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A Taxonomy of External and Internal Attention. Annual Review of Psychology. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1:42–45. [Google Scholar]
- De Lange FP, Rahnev DA, Donner TH, Lau H. Prestimulus oscillatory activity over motor cortex reflects perceptual expectations. Journal of Neuroscience. 2013;33:1400–1410. doi: 10.1523/JNEUROSCI.1094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Paprotta I. Selective dorsal and ventral processing: Evidence for a common attentional mechanism in reaching and perception. Visual Cognition. 1998;5:81–107. [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of Choice-Predictive Activity in Human Motor Cortex during Perceptual Decision Making. Current Biology. 2009;19:1581–1585. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Shmuelof L, Vaadia E, Zohary E. The Representation of Visual and Motor Aspects of Reaching Movements in the Human Motor Cortex. Journal of Neuroscience. 2011;31:12377–12374. doi: 10.1523/JNEUROSCI.0824-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Orban Xivry J, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cerebral Cortex. 2011;21:1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer T, Müller HJ. Distinct, but top-down modulable color and positional priming mechanisms in visual pop-out search. Psychological Research. 2009;73:167–176. doi: 10.1007/s00426-008-0207-x. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron. 2011;72:477–487. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht H, Vogt S, Prinz W. Motor learning enhances perceptual judgment: a case for action-perception transfer. Psychological Research. 2001;65:3–14. doi: 10.1007/s004260000043. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Heise K, Celnik P, Floel A, Gerloff C, Cohen LG. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiology of Aging. 2010;31:2160–2168. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. Journal of Physiology. 2009;587:2949–2961. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behavioural Brain Research. 2009;202:147–152. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Kristjansson A, Campana C. Where perception meets memory: A review of repetition priming in visual search tasks. Attention Perception and Psychophysics. 2010;72:5–18. doi: 10.3758/APP.72.1.5. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of popout: III. A short-term implicit memory system beneficial for rapid target selection. Visual Cognition. 2000;7:571–595. [Google Scholar]
- Muggleton NG, Kalla R, Juan CH, Walsh V. Dissociating the contributions of human frontal eye fields and posterior parietal cortex to visual search. Journal of Neurophysiology. 2011;105:2891–2896. doi: 10.1152/jn.01149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of Implicit Motor Learning by Weak Transcranial Direct Current Stimulation of the Primary Motor Cortex in the Human. Journal of Cognitive Neuroscience. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Notturno F, Marzetti L, Pizzella V, Uncini A, Zappasodi F. Local and remote effects of transcranial direct current stimulation on the electrical activity of the motor cortical network. Human Brain Mapping. 2014;35:2220–2232. doi: 10.1002/hbm.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of Features. Memory and Cognition. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AG, Wong J, Gribble PL. Somatosensory Plasticity and Motor Learning. Journal of Neuroscience. 2010;30:5384–5393. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci D, Mastropasqua T, Turatto M. Permeability of priming of pop out to expectations. Journal of Vision. 2012;12(10):21, 1–13. doi: 10.1167/12.10.21. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–268. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG. Fast and Slow Oscillations in Human Primary Motor Cortex Predict Oncoming Behaviourally Relevant Cues. Neuron. 2010;65:461–471. doi: 10.1016/j.neuron.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S, Robertson EM, Miall RC. The time course of task-specific memory consolidation effects in resting state networks. Journal of Neuroscience. 2014;34:3982–3992. doi: 10.1523/JNEUROSCI.4341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends in Cognitive Science. 2007;11:211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Predicting Perceptual Events Activates Corresponding Motor Schemes in Lateral Premotor Cortex: An fMRI Study. NeuroImage. 2002;15:787–796. doi: 10.1006/nimg.2001.1043. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annual Reviews of Neuroscience. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. Journal of Vision. 2006;6:982–995. doi: 10.1167/6.9.11. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Automatic adjustment of visuomotor readiness. Journal of Vision. 2007;7(5):2, 1–9. doi: 10.1167/7.5.2. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Target selection in visual search as revealed by movement trajectories. Vision Research. 2008;48:853–861. doi: 10.1016/j.visres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Song JH, Rafal RD, McPeek RM. Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proceedings of the National Academy of Sciences of the USA. 2011;108:1433–1440. doi: 10.1073/pnas.1109656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel DP, Hansen BC, Byblow WD, Thompson B. Anodal Transcranial Direct Current Stimulation Reduces Psychophysically Measured Surround Suppression in the Human Visual Cortex. Plos One. 2012;7:e36220. doi: 10.1371/journal.pone.0036220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BM, Baugh LA, Gallivan JP, Flanagan JR. Simultaneous encoding of the direction and orientation of potential targets during reach planning: evidence of multiple competing reach plans. Journal of Neurophysiology. 2013;110:807–816. doi: 10.1152/jn.00131.2013. [DOI] [PubMed] [Google Scholar]
- Strauss S, Heinke D. A robotics-based approach to modelling of choice reaching experiments on visual attention. Frontiers in Psychology. 2012;3:105. doi: 10.3389/fpsyg.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Woodgate PJW, Heinke D. Attentional guidance by movement-relevant perceptual representations in the human motor cortex. Neural Networks, submitted. (in submission) [Google Scholar]
- Taylor PCJ, Muggleton NG, Kalla R, Walsh V, Eimer M. TMS of the right angular gyrus modulates priming of pop-out in visual search: combined TMS-ERP evidence. Journal of Neurophysiology. 2011;106:3001–3009. doi: 10.1152/jn.00121.2011. [DOI] [PubMed] [Google Scholar]
- Tseng P, Chang C-F, Chiau H-Y, Liang W-K, Liu C-L, Hsu T-Y, Hung DL, Tzeng OJL, Juan C-H. The dorsal attentional system in oculomotor learning of predictive information. Frontiers in Human Neuroscience. 2013;7:404. doi: 10.3389/fnhum.2013.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zach N, Inbar D, Grinvald Y, Bergman H, Yaadia E. Emergence of Novel Representations in Primary Motor Cortex and Premotor Neurons during Associative Learning. Journal of Neuroscience. 2008;28:9545–9556. doi: 10.1523/JNEUROSCI.1965-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]