Abstract
The purpose of this study was to describe a presumed case of Epstein–Barr virus (EBV)-associated retinal vasculitis in a 42-year-old female with sudden unilateral vision loss and successful treatment with acyclovir therapy. Diagnostic vitreous biopsy of the right eye was performed to test for EBV and other known infectious causes of retinitis and evaluate vitreous cells and serological testing. Vitreous polymerase chain reaction viral DNA testing result was positive for EBV but negative for herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Serologic testing was negative for toxoplasma gondii, syphilis, tuberculosis, and HIV. Histopathologic analysis of vitreous cells revealed atypical lymphocytes. Fluorescein angiography showed disk leakage, occluded retinal artery, peripheral vascular leakage, and ischemic area of the right eye. Intravenous acyclovir, 10 mg/kg/d, was prescribed for 14 days followed by oral acyclovir for 3 months. All lesions have become quiet. EBV may be a cause of retinal disease, and intravenous acyclovir is a successful treatment choice.
Keywords: Epstein-Barr virus, retinal vasculitis, acyclovir, treatment
Case report
In 2013, a 42-year-old woman presented for the first time to Phramongkutklao Hospital, Thailand. She had suffered from floaters for 4 weeks and sudden loss of vision for a week in the right eye. She had no ocular pain or photophobia. Her left eye was not affected. The visual acuity was finger count 3’ in the right eye and was 20/25 in the left eye. Anterior segment examination revealed anterior chamber cell trace and fine keratic precipitates in the right eye and normal examination in the left eye. Intraocular pressure was 18 in both the eyes.
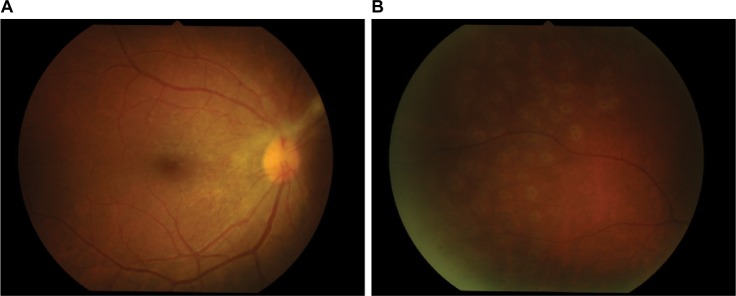
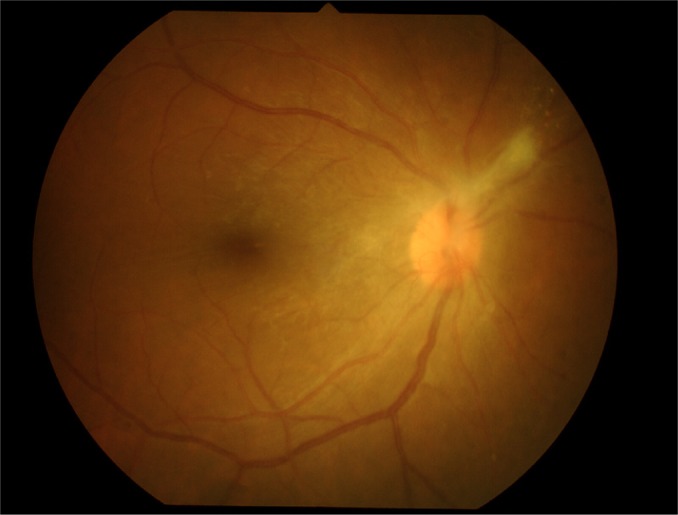
Fundus examination showed anterior vitreous cells’ grade 3+ (Figure 1), vitreous haze grade 3, snow balls, and unclear optic disk in the right eye. The left eye’s fundus was normal. Three days later, the inflammation increased; therefore, diagnostic vitrectomy was performed immediately.
Figure 1.

Dense vitritis.
Intraoperatively (Figure 2), we found dense vitritis, fibrous tissue on the superior disk with diffuse sclerotic vessels, and sheathing of both artery and vein. A vitreous sample was sent to the laboratory to perform polymerase chain reaction (PCR) for tuberculosis, herpesvirus family, and cytology for malignancy.
Figure 2.
Intraoperative findings were dense vitritis, fibrous tissue on superior disk with diffuse periphery sclerotic vessels, and sheathing of both artery and vein.
Serum CD3 was 2,327 cells/mL (67.5%), and CD4 was 1,138 cells/mL (33%). Serum Epstein–Barr virus (EBV) immunoglobulin (Ig) M was positive (borderline 0.8), and EBV IgG was also positive (9.50; normal: 0–0.8), while serum IgM and IgG for herpes simplex virus (HSV), varicella-zoster virus, and cytomegalovirus were negative. Serum tests for HIV, syphilis, toxoplasmosis, and tuberculosis were negative. Anergy skin test and chest scan for sarcoidosis were also negative.
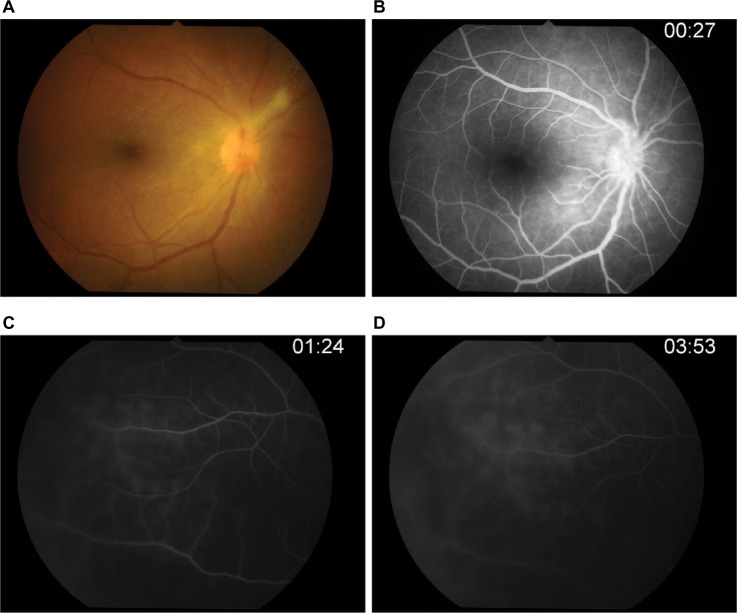
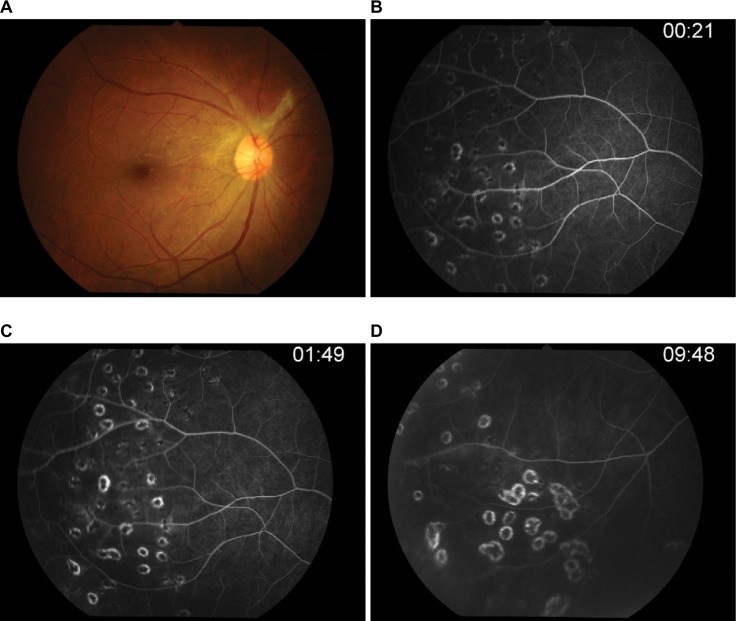
Three days after vitrectomy, the right fundus showed mild vitreous haze, fibrous tissue, and optic disk edema with normal retinal vasculature at the posterior pole in the right eye. Fluorescein angiography (Figure 3) confirmed leakage at the optic disk and occluded retinal artery and retinal vein leakage at the periphery in all quadrants of the right eye. The ischemic area was also observed at the periphery. Vitreous PCR viral DNA testing result was positive for EBV but negative for HSV-1 and -2, varicella-zoster virus, and also cytomegalovirus.
Figure 3.
Fluorescein angiography.
Notes: (A) Photo fundus showed fibrous band on hyperemia optic disk. (B) Optic disk showed leakage in early phase. (C–D) Ischemic area was observed at periphery with occluded retinal artery and leakage retinal vein in all quadrants of the right eye.
After vitrectomy, her right visual acuity improved to 20/25, intraocular pressure was 30, anterior chamber cells’ grade was 2+, and anterior vitreous cells’ grade was 2+. She was treated with intravenous acyclovir 10 mg/kg for 2 weeks; antiglaucoma drugs, topical prednisolone acetate, and antibiotics were prescribed. Panretinal photocoagulation of the right eye at the ischemic area was performed (Figure 4).
Figure 4.
Panretinal photocoagulation of the right eye at ischemic area was performed.
Notes: (A) Posterior pole. (B) Laser scar at periphery.
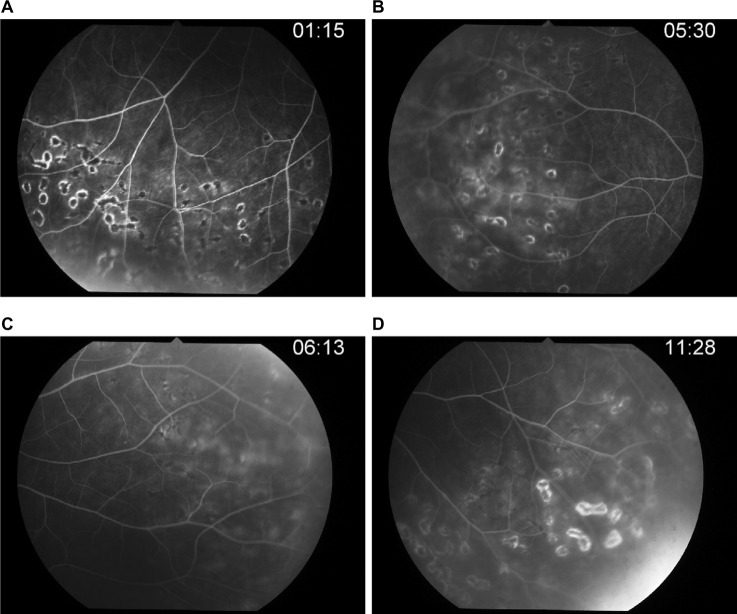
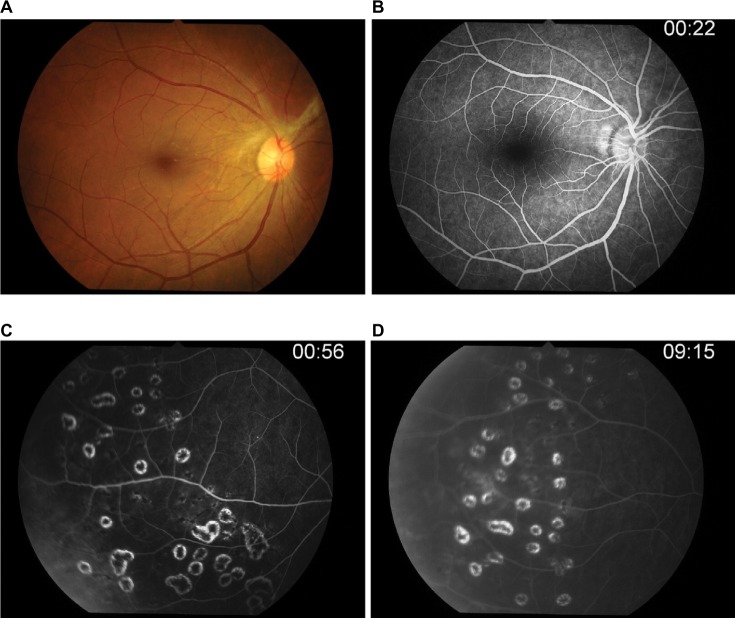
After 14-day therapy (Figure 5) with intravenous acyclovir, her anterior chamber activity in the right eye was much improved, but intraocular pressure still rose to 32. The patient was continued on oral acyclovir (800 mg) five times daily and antiglaucoma drugs.
Figure 5.
Post-IV acyclovir after 14 days of therapy.
Notes: (A) Laser scar. (B) Repeated FA, peripheral retinal vein still has leakage. (C–D) Late phase.
Abbreviation: IV, intravenous; FA, fluorescein angiography.
After 4 weeks of therapy (Figure 6), the fluorescein angiography showed that her right optic disk edema had decreased and retinal phlebitis had improved.
Figure 6.
Post-IV acyclovir after 4 weeks of therapy.
Notes: (A) Photo fundus showed optic disk edema decreased. (B–D) FA confirmed retinal phlebitis improved.
Abbreviation: IV, intravenous; FA, fluorescein angiography.
After 3 months of therapy (Figure 7), her right visual acuity increased up to 20/20, intraocular pressure was 17, and no anterior chamber cells were observed. Repeat fluorescein angiography showed neither retinal vascular nor optic disk leakage. She discontinued oral acyclovir; however, she remained on brimonidine/timolol ophthalmic solution and brinzolamide.
Figure 7.
Post-acyclovir after 3 months of therapy.
Notes: (A) Normal optic disk. (B–D) Retinal vascular leakage was not observed.
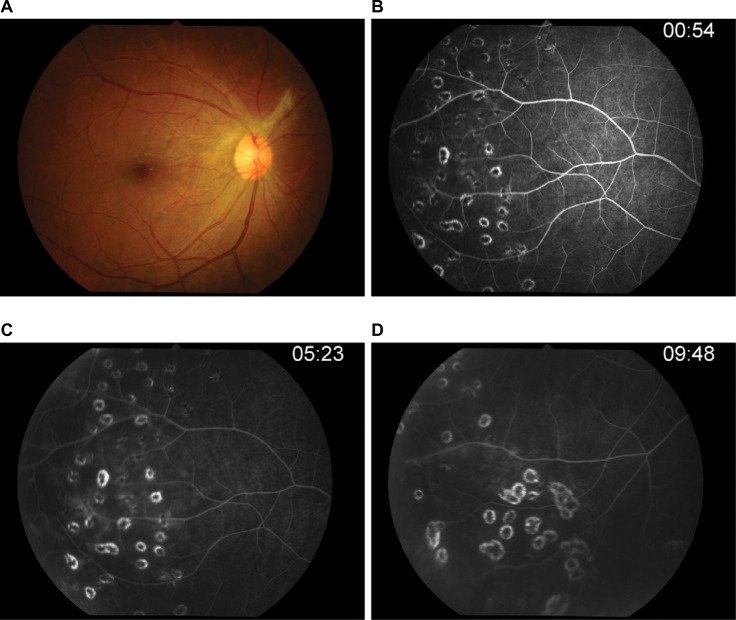
After 6 months of therapy (Figure 8), the visual acuity was 20/20 in both eyes, and intraocular pressure was controlled. Anterior chamber and vitreal cells were also quiet, and inactive retinal vasculitis was confirmed by fluorescein angiography. This case report was approved by The Institutional Review Board, Royal Thai Army Medical Department who have deemed that informed consent is not required when the patient cannot be identified, directly or through identifiers linked to the patient.
Figure 8.
Post-acyclovir after 6 months of therapy (after 3 months of discontinued oral acyclovir).
Notes: (A) There was no vitreous haze and disk edema. (B) Optic disk showed no leakage. (C–D) Periphery has become quiet.
Discussion
EBV, also known as human herpesvirus 4, is a member of the herpesvirus family and is associated with infectious mononucleosis. The Center for Disease Control estimates that 90% of adults between the ages of 35 years and 40 years have been infected; however, only a few patients have presented with ocular disease. Mitchell et al1 found that the presence of detectable HSV-1, EBV, and human herpesvirus 6 in vitreous biopsy was not associated with clinical disease, so EBV may be detected in the retina without the virus being the cause of the retinal infection.
Previously described cases of “ocular EBV infection”, such as uveitis, vitritis, and optic disk vasculitis,2–5 are not typical for viral retinitis. One study reported EBV-related typical acute retinal necrosis (ARN) and EBV detected by vitreous PCR and molecular pathology within retinal cells that were confirmed as the sole causative agent.6 In our case, EBV-associated retinal vitritis and vasculitis was proved by PCR from vitreous biopsy and also serological testing. The clinical signs were vitritis and retinal ischemia in the area of occlusive vasculitis, which was shown by fluorescein angiography. Despite the usual unilateral onset such as ARN, left eye was followed up regularly.7
Several antiviral drugs have been used to inhibit EBV replication; however, none of them are licensed for the treatment of EBV in the clinic.8 Andersson et al found a significant reduction in spontaneous outgrowth of in vivo EBV-infected B-lymphocytes after acyclovir therapy. Meanwhile, some studies suggest that acyclovir, which effectively inhibits in vitro EBV production,9–11,15 was the first-line regimen. Rafailidis et al12 reviewed the usage of antivirals for severe EBV infection studies from PubMed and Scopus from 1982 to 2009 and found that acyclovir monotherapy was the most commonly prescribed antiviral regimen.
Concerning intraocular treatment, one report has shown the intravitreal acyclovir concentration of 17.9 mM after given intravenously at 13 mg/kg three times daily,13,14 while acyclovir has an ED50 (mean effective dose) of only 0.3 mM against EBV replication in vitro.15 Accordingly, some studies showed good results of treating EBV-associated ocular involvement,16 and ARN,17 with systemic acyclovir therapy.
Intraocular EBV infection can be found in immunocompetent patients, where there are no systemic diseases. We suggest that early vitrectomy and fluorescein angiography should be performed when occluded vascular is observed, and antiviral drugs should be considered. Corticosteroids are controversial for severe inflammation. This is the first case report of EBV vasculitis in an immunocompetent patient, which was identified by fluorescein angiography and proved by vitreous PCR; then the patient was successfully treated with early vitrectomy, focal retinal photocoagulation, and acyclovir therapy.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Mitchell SM, Fox JD, Tedder RS, Gazzard BG, Lightman S. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994;43(4):336–340. doi: 10.1002/jmv.1890430404. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Barañano DE, Grossniklaus HE, Martin DF. Epstein–Barr infection of the retina: case report and review of the literature. Retin Cases Brief Rep. 2011;5(1):1–5. doi: 10.1097/ICB.0b013e3181babf1f. [DOI] [PubMed] [Google Scholar]
- 3.Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92(7):928–932. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Ohga S, Ohnishi Y, Inomata H. Optic disk vasculitis associated with chronic active Epstein–Barr virus infection. Ophthalmologica. 2002;216(3):221–225. doi: 10.1159/000059638. [DOI] [PubMed] [Google Scholar]
- 5.Matoba AY. Ocular disease associated with Epstein–Barr virus infection. Surv Ophthalmol. 1990;35(2):145–150. doi: 10.1016/0039-6257(90)90069-8. [DOI] [PubMed] [Google Scholar]
- 6.Schaal S, Kagan A, Wang Y, Chan CC, Kaplan HJ. Acute retinal necrosis associated with Epstein–Barr virus: immunohistopathologic confirmation. JAMA Ophthalmol. 2014;132(7):881–882. doi: 10.1001/jamaophthalmol.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland GN, Executive Committee of the American Uveitis Society Standard diagnostic criteria for the acute retinal necrosis syndrome. Am J Ophthalmol. 1994;117(5):663–666. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 8.Gershburg E, Joseph S. Pagano: Epstein Barr virus infections: prospect for treatment. J Antimicrob Chemother. 2005;56(2):277–281. doi: 10.1093/jac/dki240. [DOI] [PubMed] [Google Scholar]
- 9.Andersson J, Sköldenberg B, Henle W, et al. Acyclovir treatment in infectious mononucleosis: a clinical and virological study. Infection. 1987;15(suppl 1):S14–S20. doi: 10.1007/BF01650106. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J, Sköldenberg B, Ernberg I, Britton S, Henle W, Andersson U. Acyclovir treatment in primary Epstein–Barr virus infection. A double-blind placebo-controlled study. Scand J Infect Dis Suppl. 1985;47:107–115. [PubMed] [Google Scholar]
- 11.Yaro A. Epstein–Barr infection: current treatment options. Ann Trop Med Public Health. 2013;6(1):10–13. [Google Scholar]
- 12.Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J Clin Virol. 2010;49(3):151–157. doi: 10.1016/j.jcv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Schulman JA, Peyman GA, Fiscella RG, Pulido J, Sugar J. Parentally administered acyclovir for viral retinitis associated with AIDS. Arch Ophthalmol. 1984;102(12):1750. doi: 10.1001/archopht.1984.01040031416011. [DOI] [PubMed] [Google Scholar]
- 14.Patrick MK, Claire YH, Susan L. Antiviral selection in the management of acute retinal necrosis. Clin Ophthalmol. 2010;4:11–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano JS, Sixbey JW, Lin JC. Acyclovir and Epstein–Barr virus infection. J Antimicrob Chemother. 1983;12(suppl B):113–121. doi: 10.1093/jac/12.suppl_b.113. [DOI] [PubMed] [Google Scholar]
- 16.Wong KW, D’Amico DJ, Hedges TR, 3rd, Soong HK, Schooley RT, Kenyon KR. Ocular involvement associated with chronic Epstein–Barr virus disease. Arch Ophthalmol. 1987;105(6):788–792. doi: 10.1001/archopht.1987.01060060074036. [DOI] [PubMed] [Google Scholar]
- 17.Roberto G, Miquel H, Jose JG, Inmaculada S, Enrique E, Maria DP. Epstein–Barr virus and acute retinal necrosis in a 5-year-old immunocompetent child. Clin Ophthalmol. 2008;2(2):451–455. doi: 10.2147/opth.s1757. [DOI] [PMC free article] [PubMed] [Google Scholar]