Summary
Genetic abnormalities are present in all tumor types, although the frequency and type can vary. Chromosome abnormalities include highly aberrant structures, particularly chromothriptic chromosomes. The generation of massive sequencing data has illuminated the scope of the mutational burden in cancer genomes, identifying patterns of mutations (mutation signatures), which have the potential to shed light on the relatedness and etiologies of cancers and impact therapy response. Some mutation patterns are clearly attributable to disruptions in pathways that maintain genomic integrity. Here we review recent advances in our understanding of genetic changes occurring in cancers and the roles of genome maintenance pathways.
Keywords: Cancer genomes, chromothripsis, kataegis, mutation signature, homologous recombination, BRCA1, BRCA2
Cancer builds on a foundation of germline variation and constant or even explosive damage to somatic genomes. Throughout the life of an individual, DNA is damaged as a result of ongoing endogenous processes and from environmental mutagens. The sum of genomic alterations is dependent on the repair processes our cells enact to manage perturbations, the landscape of which is beginning to be illuminated by whole genome and exome sequencing. Whole genome sequencing also allows a more complete assessement of chromosome structural variation and a wide-angle view of the genomic landscape. Sequencing predictions have often been confirmed by molecular and cell biological approaches, which are also uncovering novel cellular mechanisms. The pace of discovery in the last few years has been remarkable and provides promise that cancer will yield to our collective advances and novel biological insights.
DNA damage: a range of sources
Sources and types of DNA damage are numerous (see (Ciccia and Elledge, 2010) and references therein). Sun-exposure to skin is one of the best known sources of exogenous damage, often leading to pyrimidine dimers, as is cigarette smoke, which commonly results in DNA adducts. Radiation incurred from medical scans, radiotherapy, and other sources, or, in the extreme case, radioactive fallout, can result in double-strand breaks (DSBs). DNA damage arising through endogenous processes is as or more common as that from exogenous agents, including cytosine deamination, depurination, and base oxidation and methylation. Reactive oxygen species are well-known sources of DNA damage. More recently, endogenous aldehydes, including acetaldehyde and formaldehyde, which are also byproducts of cellular metabolism, have been appreciated as an important source of endogenous DNA damage in animals, such that their inadequate repair is associated with cancer predisposition and other disease states (Langevin et al., 2011; Pontel et al., 2015).
DNA replication can lead to DNA breaks, especially at structures that are difficult to replicate, and base mismatches. Base mismatches are typically kept in check by the proofreading activities of DNA polymerases; however, missense mutations in the proofreading domains of the leading (POLE) and lagging (POLD1) strand polymerases are found in some cancers with ultramutated genomes (Cancer Genome Atlas Research et al., 2013; Palles et al., 2013; Seshagiri, 2013). Repair of the various lesions encountered in DNA involves specialized, often well-characterized pathways, including nucleotide and base excision repair, mismatch repair (MMR), nonhomologous end-joining (NHEJ), and homologous recombination (HR).
Because both strands of the DNA helix are disrupted, DSBs are considered particularly dangerous lesions that can jeopardize the stability of the genome. However, DSBs are intermediates in certain developmental programs, in particular, during antigen receptor rearrangement and class switch recombination (Alt et al., 2013; Casellas et al., 2016). Astoundingly, during meiosis, which is key to the transmission of the genome, a couple hundred DSBs are introduced genome-wide (Cole et al., 2010), indicating that cells are able to repair high DNA damage loads with accuracy at least under some circumstances. Accurate repair may rely on particular aspects of the pathways involved in the repair of programmed DSBs, such as tight binding of the RAG recombinase to DNA ends during antigen receptor rearrangement; however, errors in the repair processes can give rise to oncogenic lesions (Alt et al., 2013; Casellas et al., 2016).
More recently, DSBs have been suggested to occur during another developmental program, i.e., during the rapid expression of immediate early genes in response to neuronal activity (Madabhushi et al., 2015). DSBs in the promoters of these activity-induced genes, which are likely generated by topoisomerase IIβ, have been hypothesized to relieve torsional stress within topological domains to promote a rapid transcriptional response. Topoisomerase IIβ-generated DSBs in another context–nuclear receptor-induced genes–have been implicated in oncogenic rearrangements in prostate cells (Haffner et al., 2010; Lin et al., 2009). Within neuronal cells, recurrent DSBs have also been observed in genes rearranged in some cancers (Lyu et al., 2006; Wei et al., 2016).
A surprising source of exogenously induced DSBs is from microbial invaders (Nougayrede et al., 2006), such as from Helicobacter pylori (Toller et al., 2011), which is associated with gastric cancer. In this case, DSBs appear to arise through the nucleotide excision repair pathway (Hartung et al., 2015). Microbial invaders can also lead to other types of DNA damage. Members of the APOBEC family of cytidine deaminases, which is involved in the defense against retroelements, can be induced by viral infection (Chan and Gordenin, 2015), for example, by HPV infection, which is associated with head and neck and cervical cancers. These tumors exhibit an overall high rate of mutations expected by APOBEC induction as well as specific mutations in genes linked to tumorigenesis, including PIK3CA (Henderson et al., 2014; Vieira et al., 2014).
Explosive events that alter the genome: Chromothripsis and kataegis
A long-standing tenet in cancer etiology has been that mutations accumulate gradually over an extended period of time (see e.g., (Jones et al., 2008)). However, the advent of more advanced genome sequencing technologies has provided evidence that the relatively constant mutation rate may be interrupted by squalls of instability. Chromothripsis is a recently identified mutational process in which specific chromosomal regions undergo catastrophic shattering characterized by extensive genomic rearrangements (Stephens et al., 2011). Chromothriptic chromosomes can have dozens or hundreds of chromosome segments from one or a few chromosomes stitched together in random order and orientation with oscillating copy numbers (Korbel and Campbell, 2013). They have been observed in multiple tumor types and, surprisingly, even constitutionally in rare individuals (Kloosterman et al., 2012; Weckselblatt et al., 2015). Estimates are that up to 5% of tumors show evidence of chromothripsis, although some tumor types have higher frequencies (Kloosterman et al., 2014; Malhotra et al., 2013; Stephens et al., 2011). Chromothripsis can lead to disruption of tumor suppressor genes, oncogenic gene fusions, and oncogene amplification (Kloosterman et al., 2014; Leibowitz et al., 2015). Massive amplification associated with chromothripsis may involve double minute formation from excised fragments and subsequent reintegration as homogeneously staining regions (e.g., including the MYC locus) (Rausch et al., 2012; Stephens et al., 2011).
Two recent studies have provided possible mechanisms that could give rise to chromothripsis. Pellman and colleagues hypothesized that one route may involve DNA micronucleus formation, when the nuclear envelope reforms around chromosomes or chromosome fragments that become separated from the main chromosome complement during mitotic exit (Crasta et al., 2012). An attractive feature of this model is the physical isolation of DNA in micronuclei from bulk genomic DNA. Further, DNA in micronuclei undergoes breakage and extensive fragmentation (“pulverization”) likely due to asynchronous replication and collapse of the nuclear envelope (Crasta et al., 2012; Hatch et al., 2013). However, eventually micronuclear DNA can return to the nucleus for subsequent transmission to daughter cells. More recently, the complex genomic rearrangements consistent with chromothripsis have been confirmed by single-cell sequencing, providing evidence that chromothripsis occurs within a single-cell cycle and so is an episodic mutational phenomenon (Zhang et al., 2015). Of note, evidence for double minute formation during chromothriptic events was also provided.
Another new study from de Lange and colleagues has suggested that chromothripsis occurs as a consequence of telomere crisis resolution in the early stages of tumorigenesis (Maciejowski et al., 2015). Dicentric chromosomes that arise due to dysfunctional telomeres can form long-lived chromatin bridges that lead to nuclear envelope rupture during interphase (“NERDI”). Dicentrics have traditionally been thought to break due to forces pulling the chromosomes to opposite poles; however, Maciejowski et al. demonstrate that the cytoplasmic nuclease TREX1 localizes to chromatin bridges and gives rise to RPA-coated single-stranded DNA. Following telomere crisis resolution, clusters of genomic rearrangements were observed consistent with chromothripsis.
Disease causality or disease progression attributed to chromothripsis is difficult to determine with certainty. Attribution has been inferred in glioblastoma multiforme based on short latency, aggressive tumor biology and high prevalence of chromothripsis (Malhotra et al., 2013). In childhood retinoblastoma, chromothripsis was identified as the mechanism of RB1 loss, due to complex structural variation on chromosome 13 missed by conventional analysis (McEvoy et al., 2014), suggesting causality. In prostate cancer, the incidence of chromothripsis has been reported to be high, but it was of similar prevalence in both low grade tumors that do not progress and aggressive high grade tumors (Kovtun et al., 2015). This study identified no difference in clinical outcome that associated with the presence or absence of chromothripsis, and chromothriptic events did not involve the most common genetically altered drivers associated with prostate cancer. Thus, the data in prostate cancer suggest that chromothripsis is not related to cancer progression but may be related to cancer initiation given its relatively common occurrence in low grade tumors. Chromothripsis is associated with poor prognosis in some reports, but evidence that its presence is an independent prognosticator is limited (Molenaar et al., 2012; Rausch et al., 2012).
A second catastrophic process is clustered mutagenesis, sometimes termed kataegis, which is typically associated with chromosomal rearrangements (Nik-Zainal et al., 2012; Roberts et al., 2012). Up to 50% of some tumor types show evidence of kataegis (Chen et al., 2014; Nik-Zainal et al., 2012). The mutations typically involve C to T transitions in TpC dinucleotides which arise from APOBEC3A/B acting on single-stranded DNA; in B cell lymphomas, a related APOBEC family member, AID, is also implicated in mutagenesis but at a distinct motif (Casellas et al., 2016; Chan and Gordenin, 2015) (Figure 1A). Single-stranded DNA can arise in cells through several cellular processes, including DNA replication, especially lagging strand synthesis, and end resection during DSB repair, to become a target for APOBECs (Chan and Gordenin, 2015; Haradhvala et al., 2016; Hoopes et al., 2016; Kazanov et al., 2015; Seplyarskiy et al., 2016). Further, the chromothriptic chromosomes described above that arose from telomere fusions show the characteristic hypermutation pattern of kataegis, suggesting that the single-stranded DNA in chromatin bridges is processed by APOBEC-mediated cytosine deamination (Maciejowski et al., 2015).
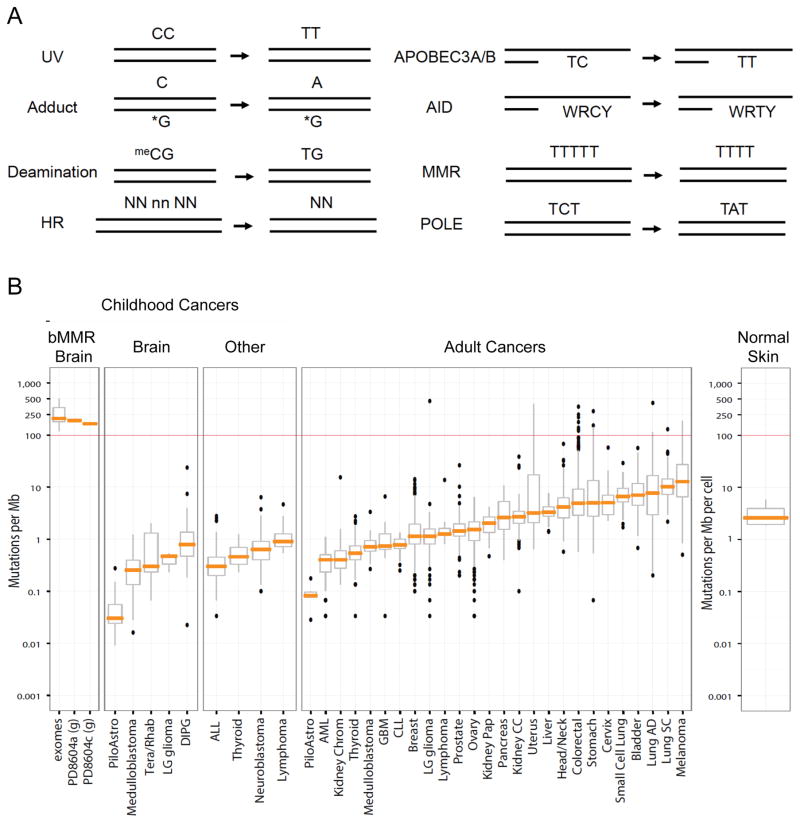
Figure 1. Mutation signatures and frequencies found in cancer genomes.
A. Examples of mutation signatures. See http://cancer.sanger.ac.uk/cosmic/signatures for more examples and detail. Note that for APOBEC3A/B and AID the mutation at C is equally to T (as shown) as to G (not shown). WRCY: W = A/T, R = purine, Y = pyrimidine; N,n: random nucleotides. The HR signature example represents a deletion with a 2 bp microhomology at the breakpoint junction (NN).
B. Somatic mutation frequencies in multiple tumor types, adapted from Shlien et al., 2015 and including additional data from non-malignant skin (Martincorena et al., 2015). Mutation frequencies in bMMR-deficient (bMMRD) ultra-hypermutated malignant brain tumors (mean = 249 mutations/Mb) are high compared to that in other childhood brain cancers (<1 mutation/Mb), childhood cancers (<1 mutation/Mb), and adult cancers (<10 mutations/Mb). bMMRD data are from exome sequencing except in the two cases with whole genome sequencing, denoted with a “g”. In these latter two cases, genomic sequences are derived from the primary and relapsed glioblastoma multiforme (GBM) from the same patient. The box plots represent the interquartile range with the median indicated by the orange line. PiloAstro, Pilocytic Astrocytoma; Tera/Rhab = Atypical Teratoid/Rhabdoid Tumor; LG glioma, Low Grade Glioma; DIPG, Diffuse Intrinsic Pontine Glioma; ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia; Kidney Chrom, Kidney Chromophobe; CLL, Chronic Lymphocytic Leukemia; Kidney Pap, Kidney Renal Papillary Cell Carcinoma; Kidney CC, Kidney Clear Cell Carcinoma; Lung AD, Lung Adenocarcinoma; Lung SC, Lung Squamous Cell Carcinoma.
Mutational signatures in cancer genomes
It is well established that the genomes of all cancers carry somatic mutations, although the scope is just now becoming apparent. Advances in DNA sequencing technologies have made it possible to identify thousands of individual somatic mutations in a single cancer genome (Pleasance et al., 2010). Among various adult tumor types, frequencies of 1 to 10 nonsynonymous mutations per Mb are common, although tumors with 10-fold higher or lower numbers of mutations are observed as well, even within the same tumor type (Alexandrov et al., 2013; Lawrence et al., 2013) (Figure 1B). The mutation rate is not constant throughout the genome but differs ~5-fold, and a higher mutation rate is typically seen in late replicating and low transcribing genes (see also (Morganella et al., 2016)).
Diverse mutational processes in cancer genomes produce unique patterns of mutations, referred to as mutational signatures (Alexandrov et al., 2013; Lawrence et al., 2013; Roberts and Gordenin, 2014) (Figure 1A). To date, ~30 unique mutational signatures have been identified, which are based on specific base substitutions at a pyrimidine and the immediate 5′ and 3′ nucleotides (http://cancer.sanger.ac.uk/cosmic/signatures). Certain signatures are characterized by exposure to strong mutagens; for example, exposure to UV light is associated with C to T and CpC to TpT mutations in skin, while tobacco exposure is associated with C to A mutations and is primarily found in lung, liver and head and neck cancers. Another signature, which is found in all tumor types, is associated with spontaneous deamination of 5-methylcytosine in the context of CpG; the numbers of mutations in this signature in tumors correlates well with age of diagnosis to act as a clock-like process (Alexandrov et al., 2015a). Other signatures are associated with enzymes that modify DNA, such as the APOBECs (Alexandrov et al., 2013; Roberts et al., 2013), as discussed above, defective polymerase proofreading, and defects in DNA repair pathways, such as MMR, nucleotide excision repair (Kim et al., 2016), and HR (see below). MMR defects are associated with high numbers of short insertion/deletions (< 3bp) at mono/polynucleotide repeats. The etiology of some other signatures is unclear. Interestingly, APOBEC-induced mutagenesis favors early-replicating regions of cancer genomes, which is the opposite to the genomic preference of other mutation types (Kazanov et al., 2015). Certain mutational signatures are more prevalent than others; the APOBEC3A/B signature is the second most common after the age-associated 5-methylcytosine deamination signature.
More recently, sequencing of 560 breast tumors, the largest cohort of a single tissue type to date, has led to the description of six rearrangement mutational signatures (Nik-Zainal et al., 2016). These signatures are distinguished by the prevalent type of rearrangement (i.e., deletion, tandem duplication, inversion, translocation), the length of the rearranged segment, and whether they occur within rearrangement clusters (e.g., at amplified genomic locations).
The term mutational signatures tends to imply an unchanging, identifiable character, however, the mutational signatures identified in a tumor can change. Recent work analyzing subclonal tumor populations demonstrated early and late mutational signatures and identified differences across several tumor types arising from tumor evolution (McGranahan et al., 2015). In some tumors, this was characterized by an early broad non-specific cancer signature followed by a later more defined etiologic process, whereas, in others exposure to tobacco or UV-light defined the early signature but other mutational processes occurring later in tumor development were evident. A key observation was that a large percentage of subclonal mutations in driver genes could be explained by APOBEC-mediated cytosine deamination.
Although much of the information regarding tumor heterogeneity and clonal evolution is derived from cancer gene sequencing, a focus on mutational signatures may yield strategies suitable for exploitation in the clinic. Notably, 20% of tumors from a pan-cancer analysis identified subclonal mutations in the BRCA1/2 pathway, suggesting tumor populations that may benefit from targeted therapies (McGranahan et al., 2015) (see below). How often changes occur in the mutational signatures identified within a cancer is not known. In a small number of cases (4 women with triple negative breast cancer), the mutational signature was minimally altered in residual breast cancer after neoadjuvant chemotherapy as compared to the pretreatment biopsy. The changes appeared to be small increases in the APOBEC related signatures, leading to the conclusion that there was a minimal contribution introduced to the mutational signature by chemotherapy (Yates et al., 2015). The timeframe between biopsy and surgery following neoadjuvant chemotherapy is relatively short; whether changes to the mutational signature introduced by treatment would be identified in clonal expansion of resistant tumor cells remains a possibility.
Mutations may be exploitable: High mutation burden and immunotherapy response
A recent advance in cancer immunotherapy has been the development of immune checkpoint inhibitors, including monoclonal antibodies directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 receptor (PD-1), which have demonstrated promising results in the clinical setting (Postow et al., 2015). Immune checkpoint blockade harnesses a patient’s own immune response against a tumor by blocking inhibitory signaling molecules expressed on T cells, thereby strengthening the anti-tumor response. However, not all tumors respond to treatment with immune checkpoint inhibitors, and it has been hypothesized that tumors with a large number of somatic mutations may be particularly responsive to these immunotherapies, as the larger number of mutations may make the tumor appear more foreign to a patient’s own immune system (Schumacher and Schreiber, 2015).
Cancers that have so far shown significant responses to treatment with checkpoint blocking antibodies include melanoma and non-small cell lung cancer, both of which are linked to high mutagen exposure. Within these tumor types, a wide variation in mutation burden nonetheless exists between individual tumors (Lawrence et al., 2013; Vogelstein et al., 2013), which may help predict response to immunotherapy. In studies of melanoma patients, a higher mutational load was associated with a more sustained response to anti-CTLA-4 treatment, and patients benefiting the most had a high neoantigen load that could lead to T cell activation (Snyder et al., 2014; Van Allen et al., 2015). In the case of lung cancer, tumors in smokers typically have a larger mutational load than non-smokers, and the higher nonsynonymous mutation burden was shown to associate with an increased response to anti-PD1 immunotherapy and longer progression-free survival (Rizvi et al., 2015). The association of mutational load and beneficial immune response is not exact, however, and other cellular factors may substantially alter a response (Hugo et al., 2016).
The connection between increased mutational load and immunotherapy response suggests that tumors with a large number of mutations due to defective DNA repair may also respond better to immune checkpoint inhibition. A small study recently showed that patients with colorectal and other tumors with defects in the MMR pathway were more responsive to treatment with anti-PD1 antibodies compared to MMR-proficient tumors (Le et al., 2015). Further, a lung cancer with mutations in POLD1 and MSH2, but without the typical smoking signature, had one of the highest mutation burdens in the tumor cohort, and this patient responded to immunotherapy (Rizvi et al., 2015).
Perhaps the most dramatic case of high mutation load in tumors is associated with germline, biallelic MMR (bMMR) deficiency, a syndrome in which affected children are at extremely high risk of developing malignancies at a young age, particularly glioblastoma (Shlien et al., 2015). As a result of the inherited bMMR deficiency, somatic mutations arise more frequently in POLE or POLD1, leading to “ultra-hypermutated” cancers: the combined bMMR and polymerase mutations lead to a massive increase in replication errors, estimated at ~600 mutations per cell division. This high rate leads to ~400-fold more mutations than other pediatric tumors, which generally have a low frequency of mutations compared with adult tumors (Figure 1B). Two children with bMMR deficiency and recurrent, multifocal glioblastoma multiforme have recently been treated with anti-PD1 immunotherapy with clinically significant responses, suggesting an increased effective neoantigen load associated with these ultra-hypermutated cancers (Bouffet et al., 2016).
The high mutagenesis rate in the bMMR tumors allows an estimate of the mutation burden tumor cells can cope with before becoming disadvantaged. Mutations were found to level off at ~20,000 per exome, implying that this is the threshold beyond which cancer cell survival is compromised (Shlien et al., 2015). Chromosomal instability also appears to have a threshold, since tumors with extreme instability paradoxically have a better prognosis than those with somewhat less instability (Birkbak et al., 2012).
Mutations are not always problematic: High mutation burden in non-cancerous skin
Starting from the earliest stages of development until death, cells of an individual undergo mutations. Despite robust proofreading activities of polymerases and MMR pathways, every S phase brings with it the chance of mutations arising from replication errors. Moreover, DNA breaks that arise during replication, as well as other processes, have a low but detectable frequency of causing rearrangements. DNA damage arising from other endogenous processes, as well as from environmental insults, may also not always be precisely repaired. As a result, oncogenic mutations and chromosome aberrations are observed in normal tissues from individuals without evidence of disease. Cellular context is essential; a cell limited by low self-renewal and life-span capacity would typically be eliminated prior to the growth advantaged selection process, resulting in clonal dominance and full oncogenic potential.
One of the most dramatic examples of the mutation burden in cells from a normal, cancer-free tissue comes from deep-sequencing analysis of skin (Martincorena et al., 2015). Epidermis from four older individuals undergoing blepharoplasty–a procedure involving the excision of skin from the eyelids–was positionally biopsied >200 times to detect subclonal mutations in a large number of cancer-associated genes. Several thousand mutations were identified which matched the spectrum expected for sunlight-induced DNA damage. The estimated mutation burden is ~2–6 per Mb, which is lower than that of many skin tumors, but higher than that of many other adult tumors (Figure 1B). Numerous cancer genes were found to be under positive selection in skin: more than 25% of skin cells carry cancer-causing mutations while functioning as normal epidermis. Mutations were clustered into clones from a single mutant cell, but driver mutations conferred only a modest advantage in clonal expansion, presumably because cell-cell interactions constrained growth.
The high mutation load in skin is likely to be exceptional compared with other tissues because of its high exposure to mutagen. Indeed, peripheral blood cells show a much lower mutation rate (e.g., (Genovese et al., 2014; Jaiswal et al., 2014)). The clock-like accumulation of mutations at methylated CpG dinucleotides observed in tumors from individuals at different ages will likely provide an upper estimate on the number of mutations to be found in different tissue types (Alexandrov et al., 2015a). Nonetheless, it will be interesting to determine the variation in mutation load and signature in other normal tissues to understand tissue-specific mutational processes and how often oncogenic mutations are observed to understand the processes that constrain disease.
Defective HR in cancer
HR is a critical pathway for the precise repair of DSBs that arise during normal cellular processes, including replication, and as a result of exposure to exogenous DNA damaging agents (Chapman et al., 2012; Jasin and Rothstein, 2013). As an acknowledgement of its role in DNA repair, HR is also often termed homology-directed repair, although HR proteins can also prevent DNA damage from occurring independent of repair by protecting stalled replication forks from degradation (Hashimoto et al., 2010; Schlacher et al., 2011; Schlacher et al., 2012). Defects in HR result in error-prone repair of DSBs that ultimately predisposes cells to genome instability (Moynahan and Jasin, 2010). HR utilizes an undamaged homologous sequence as a repair template, which, with its functioning preferentially in late S and G2 phases of the cell cycle, is typically the sister chromatid.
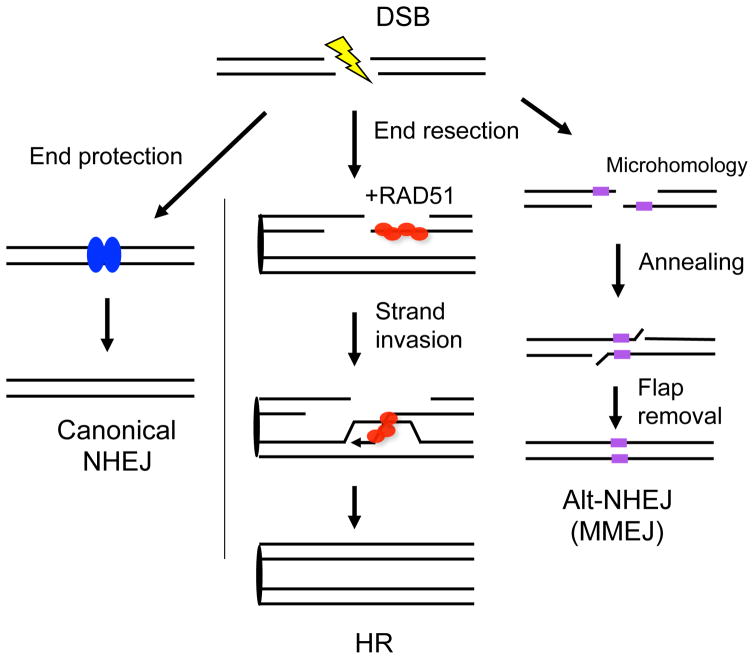
HR involves multiple steps beginning with end resection which generates 3′ single-stranded DNA from the ends of the broken chromosome (Chapman et al., 2012; Jasin and Rothstein, 2013) (Figure 2). Resection is a critical step in DSB repair pathway choice, because once resected, the DNA ends are refractory to repair by canonical NHEJ. The single-stranded DNA is bound initially by replication protein A (RPA) and subsequently by the strand exchange protein RAD51. Paradoxically, RPA both promotes RAD51 binding, by melting secondary structures in the single-stranded DNA, and occludes RAD51 binding; thus, mediator proteins are critical to promote RAD51 binding to RPA-coated single-stranded DNA. The active form of RAD51 is bound to single-stranded DNA in a nucleoprotein filament, which invades homologous DNA to prime repair synthesis using the homologous DNA as the template. HR reactions can be completed in a number of ways; however, most HR events lead to repair of the DSB without crossing-over. Even if the DNA ends are not perfectly preserved upon breakage, HR with the identical sister chromatid can restore the original sequence, whereas the canonical NHEJ pathway will lead to insertion/deletion formation.
Figure 2. Multiple pathways to repair DSBs.
Canonical NHEJ is operational throughout the cell cycle. It can protect DNA ends prior to ligation, but deletions and insertions can occur, especially if the ends are frayed. HR is a relatively precise pathway to repair DSBs, the defining step of which is strand invasion catalyzed by RAD51. RAD51 forms a nucleoprotein filament on single-stranded DNA formed by end resection. The 3′ invading end primes repair DNA synthesis from the homologous template, primarily the sister chromatid. End resection is also an intermediate step in alternative-NHEJ (alt-NHEJ) involving microhomology, i.e., a few bp of sequence identity between the DNA ends. For this reason, it is also termed microhomology-mediated endjoining (MMEJ). If single-stranded DNA forms containing longer stretches of complementarity, they can also anneal, in which case the pathway is termed single-strand annealing (SSA, not shown).
The breast cancer suppressors BRCA1 and BRCA2 play critical roles in HR at different stages of the pathway (Moynahan et al., 1999; Moynahan et al., 2001; Prakash et al., 2015; Stark et al., 2004). BRCA1 acts initially at the end resection step where it competes with 53BP1 and other proteins to promote end resection (Bouwman et al., 2010; Bunting et al., 2010; Zimmermann and de Lange, 2014), while BRCA2 is involved in loading RAD51 onto single-stranded DNA (Jensen et al., 2010). BRCA1 and BRCA2 were initially identified more than two decades ago for their roles as familial breast and ovarian cancer predisposition genes when mutated (Miki et al., 1994; Wooster et al., 1995). Germline mutations in BRCA1 and BRCA2 are also associated with other cancers, including prostate and pancreatic. Tumorigenesis is effectively suppressed in the heterozygous state, with a primary requirement for loss of the wild-type allele to provoke genomic instability due to unrepaired/misrepaired DNA damage and subsequent tumorigenesis. The decreased latency for incurring tumorigenesis with even more substantial loss of HR activity is best exemplified by inheritance of biallelic BRCA2 hypomorphic mutations where infants and children exhibit susceptibility to early onset medulloblastoma and leukemia (Meyer et al., 2014).
Along with BRCA1 and BRCA2, several other genes in the HR pathway have also been linked to tumorigenesis, although at a lower frequency, including the RAD51 paralogs RAD51C and RAD51D (Prakash et al., 2015). Particularly notable is PALB2, as the PALB2 protein seems to act as a bridge between BRCA1 and BRCA2 to promote cell cycle regulated localization of BRCA2 to chromatin (Orthwein et al., 2015; Xia et al., 2006). Thus, BRCA1 through PALB2 has a later function in HR in addition to promoting end resection.
In addition to HR and canonical NHEJ, DSBs can be repaired by two additional pathways that do not involve strand invasion: both pathways involve the annealing of complementary single-strands at DNA ends, either with minimal homology (a few bp, alternative NHEJ or microhomology-mediated NHEJ, MMEJ) (Figure 2) or more extended homology (10s–100s bp; single-strand annealing) (Jasin and Rothstein, 2013; Sfeir and Symington, 2015). The latter pathway was used to determine that BRCA1 acts upstream of BRCA2 in HR; thus, single-strand annealing is reduced in BRCA1-deficient cells but increased in BRCA2-deficient cells (Stark et al., 2004). The physiological roles of alternative NHEJ and single-strand annealing are not clear, although it is known that loss of the MMEJ pathway component polymerase theta (encoded by POLQ) is detrimental to BRCA-deficient cells (Ceccaldi et al., 2015; Mateos-Gomez et al., 2015), consistent with a role for MMEJ as a “back-up”, error-prone DSB repair pathway in HR mutants.
Most tumor suppressor genes that are mutated in cancer syndromes are also found to incur somatic loss or mutation at high prevalence (e.g., TP53 and RB1). Surprisingly, early analyses did not readily identify HR deficiencies through somatic mutation. However, somatic loss of function has been found to occur at significant frequency through epigenetic BRCA1 silencing (Cancer Genome Atlas Research, 2011), and clinically-relevant somatic mutations in BRCA1, BRCA2, and other HR genes are now being detected. Together germline and somatic mutations in HR genes have been found in a significant fraction of some tumor types (10–20%), e.g., high-grade serous ovarian carcinomas, the most malignant epithelial ovarian cancer subtype (Cancer Genome Atlas Research, 2011), other ovarian, fallopian tube, and peritoneal carcinomas (Norquist et al., 2016; Pennington et al., 2014), gastric tumors (Alexandrov et al., 2015b), and metastatic castration-resistant prostate cancers (Robinson et al., 2015). Interestingly, this latter case is a larger fraction than previously observed in primary prostate cancers (Barbieri et al., 2012; Cancer Genome Atlas Research, 2015), suggestive of tumor evolution.
However, just as it has been difficult to determine the functional consequence of BRCA1/2 sequence variants identified in the germline (Jhuraney et al., 2015), it is often with uncertainty that a DNA repair defect can be ascribed to somatic missense mutations. Additionally, haploinsufficiency phenotypes have thus far not been observed in mouse models and are equivocal in highly sensitive repair assays in vitro, and importantly human tumors from carriers almost always show loss of the wild-type allele (Roy et al., 2012). Whether more profound gene dosage deficiency results in HR defects that promote genetic instability or tumor cell hypersensitivity has not been fully evaluated, but in cultured cells, decreased expression of BRCA1 results in minor decreases in HR. Whether combinations of genetic alterations can additively diminish HR repair to incur hypersensitivity to DNA damaging agents is a particularly clinically relevant question as tumors with multiple genetic alterations are often observed by sequencing.
HR gene mutations and mutational signatures
Curiously, despite the different molecular roles of BRCA1 and BRCA2 in HR repair, their mutational signature at the nucleotide level is reported to be similar. A mutational signature associated with defective HR was first identified in BRCA1 or BRCA2 germline mutant breast cancers (Nik-Zainal et al., 2012), and later in ovarian, pancreatic, and gastric cancers (Alexandrov et al., 2015b; Alexandrov et al., 2013; Patch et al., 2015; Waddell et al., 2015). This signature consists of elevated numbers of indels (>3 bp) associated with microhomology at the breakpoint junctions (Figure 1A). Some tumors with this signature do not harbor BRCA1 or BRCA2 mutations, suggesting that they may contain mutations in other genes in the HR pathway (Alexandrov et al., 2013).
This signature has been postulated to be due to an increased reliance on MMEJ, consistent with a role for POLQ in BRCA1/2 mutant cells (Ceccaldi et al., 2015; Mateos-Gomez et al., 2015). However, a reliance on both BRCA1 and BRCA2-deficient cells on MMEJ is paradoxical, because of their different roles in HR, i.e., BRCA1-deficiency would limit end resection, an early step in MMEJ as well as in HR (Zhang and Jasin, 2011), while BRCA2 deficiency would be predicted to increase the lifespan of resected ends (Bouwman et al., 2010; Bunting et al., 2010; Stark et al., 2004). It is important to note, however, that the presence of microhomology at breakpoint junctions does not definitively distinguish whether a junction arises from MMEJ: canonical NHEJ will join DNA ends with long 5′ overhangs to give rise to junctional microhomology (Ghezraoui et al., 2014). Thus, an alternative is that the DNA end structure may contribute to microhomology use.
The recent sequencing of a large number of breast tumors, including 90 with germline (60) or somatic (14) mutation of BRCA1 or BRCA2 or BRCA1 promoter methylation (16), has led to the identification of rearrangement signatures associated with mutation of these genes (Nik-Zainal et al., 2016). BRCA1 mutation or promoter hypermethylation and BRCA2 mutation showed substantial numbers of small deletions (<100 kb). However, distinguishing these genes, BRCA1 mutation was additionally associated with small tandem duplications (<100 kb). Some breast cancers without BRCA1/2 mutations also shared these signatures, illustrating the complexity of utilizing signatures or mutation status alone for considering treatment options. Interestingly, association of these signatures with genomic features related to essential processes such as replication suggest how DSB repair pathways may be utilized in cells: Rearrangement signatures that are associated with HR defects are enriched in early replicating regions, while signatures associated with microhomology-mediated indels and base substitution are enriched in late replicating regions, perhaps suggesting a contingency route (Morganella et al., 2016). This work also illuminates how DSB repair pathways may normally be utilized in cells.
HR gene mutations and therapeutics
There has been renewed focus on the role of BRCA1 and BRCA2 as well as other HR genes in multiple cancer types since the discovery of poly(ADP-ribose) polymerase (PARP) inhibitors as a new antitumor therapy that cause synthetic lethality in cells with BRCA1/2 mutations (Bryant et al., 2005; Farmer et al., 2005). In support of these findings, a recent clinical study has shown that patients with metastatic castration-resistant prostate cancers with an HR gene mutation had a greater response rate to PARP inhibitor therapy after standard treatments had failed (Mateo et al., 2015). Besides PARP inhibitors, many standard cancer treatments cause DNA damage that requires HR for repair due to DSB intermediates, e.g., platinum-based chemotherapy drugs which crosslink both DNA strands. New strategies are also being developed, including one that targets a specific defect incurred by BRCA1/2 mutant cells when replicating through DNA structures (Zimmer et al., 2016).
Germline and somatic mutations in HR genes have been shown to predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas (Pennington et al., 2014). Interestingly, high-grade serous ovarian cancers with BRCA1/2 mutations are known to contain a higher mutational load than other tumors, and recent work has shown that these tumors have a higher neoantigen load and increased PD-1 and PD-L1 expression compared to HR-proficient tumors, suggesting that BRCA1/2-mutated ovarian cancers may be more sensitive to immune checkpoint inhibition (Strickland et al., 2016). Similarly, in a cohort of melanoma patients, tumors responsive to anti-PD1 therapy were enriched for BRCA2 mutations (Hugo et al., 2016).
However, a particularly important question is whether the presence of a mutational signature is robust enough to determine treatment strategy. In recent studies, the mutational signature attributed to defective HR was associated with therapeutic response to platinum-based therapy in a small cohort of pancreatic cancer patients with somatic or germline BRCA2 mutations (Waddell et al., 2015), but not in primary high-grade serous ovarian cancer patients without HR gene deficiencies (Patch et al., 2015). In addition to analysis of mutation signatures, there has been intense interest in developing a predictive biomarker to identify cancers with HR deficiencies, which would be expected to respond to platinum based therapy or PARP inhibition. A recent study has shown that a combined HR deficiency (HRD) score, taking into account three different measures of genomic instability, i.e. loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions (chromosome breaks >10 Mb in adjacent DNA segments) (Abkevich et al., 2012; Birkbak et al., 2012; Popova et al., 2012), could help predict which breast tumors are most likely to respond to neoadjuvant platinum-based therapy (Telli et al., 2016).
Surviving genomic chaos
Unstable genomes, whether they arise from a high constant rate of instability due to germline HR or other deficiency or a cancer with a low mutation rate interspersed with squalls of instability due to chromothripsis or other explosive events, must retain some order amidst chaos to successfully traverse the cell cycle. With an abrupt disruption, the shock to the cell would be expected to result in elimination; however in the cancer state, checkpoints that guard against genomic instability have largely been eliminated. Consistent with this is that many adult tumors with chromothripsis also have lost p53 function (Morrison et al., 2014; Rausch et al., 2012), although chromothripsis and somatic p53 loss has not been associated in pediatric tumors, unless co-occurring kataegis is also identified (reviewed in (Chen et al., 2015)). Unstable genomes that associate with HR defects were noted to activate p53 responses in animal models and mutation or loss of p53 is associated with BRCA1/2-mutated cancer (Moynahan, 2002; Patch et al., 2015). Unlike the ongoing genomic instability from defective HR where the underlying DNA repair defect itself offers a potential treatment strategy, chromothriptic or other explosive events may lead to the expression of potential neoantigens that could be targeted by immunotherapy.
The DNA repair field garnered significant recognition in 2015 through the Lasker Award and Nobel Prizes selections, where one recognized a researcher whose work began in the 1940s. Despite the significant progress over the last decades, the genomic chaos recently elucidated in cancers makes it clear that basic studies of DNA repair and translation of findings need to expand in scope to have the best chance of transforming cancer care.
Acknowledgments
We thank Adam Shlien (Toronto) for providing the graph in Figure 1B. The investigators are supported by NIH K99 CA184122 (E.M.K.), The Hecksher Foundation for Children (M.E.M.), MSK Cancer Center Support Grant/Core Grant NIH P30 CA008748, MSK Functional Genomics Initiative funded by the Cycle for Survival, and NIH R01 CA185660 (M.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, Smith-McCune K, Broaddus R, Lu KH, Chen J, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, Stratton MR. Clock-like mutational processes in human somatic cells. Nat Genet. 2015a;47:1402–1407. doi: 10.1038/ng.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Siu HC, Leung SY, Stratton MR. A mutational signature in gastric cancer suggests therapeutic strategies. Nat Commun. 2015b;6:8683. doi: 10.1038/ncomms9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, Bowman-Colin C, Li Y, Greene-Colozzi A, Iglehart JD, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–375. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, Hiddingh S, Thanasoula M, Kulkarni A, Yang Q, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas R, Basu U, Yewdell WT, Chaudhuri J, Robbiani DF, Di Noia JM. Mutations, kataegis and translocations in B cells: understanding AID promiscuous activity. Nat Rev Immunol. 2016;16:164–176. doi: 10.1038/nri.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI, O’Connor KW, Konstantinopoulos PA, Elledge SJ, Boulton SJ, et al. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature. 2015;518:258–262. doi: 10.1038/nature14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Gordenin DA. Clusters of Multiple Mutations: Incidence and Molecular Mechanisms. Annu Rev Genet. 2015;49:243–267. doi: 10.1146/annurev-genet-112414-054714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Pappo A, Dyer MA. Pediatric solid tumor genomics and developmental pliancy. Oncogene. 2015;34:5207–5215. doi: 10.1038/onc.2014.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F, Keeney S, Jasin M. Evolutionary conservation of meiotic DSB proteins: more than just Spo11. Genes Dev. 2010;24:1201–1207. doi: 10.1101/gad.1944710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–58. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezraoui H, Piganeau M, Renouf B, Renaud JB, Sallmyr A, Ruis B, Oh S, Tomkinson AE, Hendrickson EA, Giovannangeli C, et al. Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol Cell. 2014;55:829–842. doi: 10.1016/j.molcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, Isaacs WB, Bova GS, Liu W, Xu J, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haradhvala NJ, Polak P, Stojanov P, Covington KR, Shinbrot E, Hess JM, Rheinbay E, Kim J, Maruvka YE, Braunstein LZ, et al. Mutational Strand Asymmetries in Cancer Genomes Reveal Mechanisms of DNA Damage and Repair. Cell. 2016;164:538–549. doi: 10.1016/j.cell.2015.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung ML, Gruber DC, Koch KN, Gruter L, Rehrauer H, Tegtmeyer N, Backert S, Muller A. H. pylori-Induced DNA Strand Breaks Are Introduced by Nucleotide Excision Repair Endonucleases and Promote NF-kappaB Target Gene Expression. Cell Rep. 2015;13:70–79. doi: 10.1016/j.celrep.2015.08.074. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Ray Chaudhuri A, Lopes M, Costanzo V. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nat Struct Mol Biol. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S, Chakravarthy A, Su X, Boshoff C, Fenton TR. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014;7:1833–1841. doi: 10.1016/j.celrep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Hoopes JI, Cortez LM, Mertz TM, Malc EP, Mieczkowski PA, Roberts SA. APOBEC3A and APOBEC3B Preferentially Deaminate the Lagging Strand Template during DNA Replication. Cell Rep. 2016;14:1273–1282. doi: 10.1016/j.celrep.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol. 2013;5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467:678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhuraney A, Velkova A, Johnson RC, Kessing B, Carvalho RS, Whiley P, Spurdle AB, Vreeswijk MP, Caputo SM, Millot GA, et al. BRCA1 Circos: a visualisation resource for functional analysis of missense variants. J Med Genet. 2015;52:224–230. doi: 10.1136/jmedgenet-2014-102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanov MD, Roberts SA, Polak P, Stamatoyannopoulos J, Klimczak LJ, Gordenin DA, Sunyaev SR. APOBEC-Induced Cancer Mutations Are Uniquely Enriched in Early-Replicating, Gene-Dense, and Active Chromatin Regions. Cell Rep. 2015;13:1103–1109. doi: 10.1016/j.celrep.2015.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mouw KW, Polak P, Braunstein LZ, Kamburov A, Tiao G, Kwiatkowski DJ, Rosenberg JE, Van Allen EM, D’Andrea AD, et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet. 2016 doi: 10.1038/ng.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman WP, Koster J, Molenaar JJ. Prevalence and clinical implications of chromothripsis in cancer genomes. Curr Opin Oncol. 2014;26:64–72. doi: 10.1097/CCO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Tavakoli-Yaraki M, van Roosmalen MJ, van Binsbergen E, Renkens I, Duran K, Ballarati L, Vergult S, Giardino D, Hansson K, et al. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1:648–655. doi: 10.1016/j.celrep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Korbel JO, Campbell PJ. Criteria for inference of chromothripsis in cancer genomes. Cell. 2013;152:1226–1236. doi: 10.1016/j.cell.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Kovtun IV, Murphy SJ, Johnson SH, Cheville JC, Vasmatzis G. Chromosomal catastrophe is a frequent event in clinically insignificant prostate cancer. Oncotarget. 2015;6:29087–29096. doi: 10.18632/oncotarget.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz ML, Zhang CZ, Pellman D. Chromothripsis: A New Mechanism for Rapid Karyotype Evolution. Annu Rev Genet. 2015;49:183–211. doi: 10.1146/annurev-genet-120213-092228. [DOI] [PubMed] [Google Scholar]
- Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, Zhang J, Rose DW, Fu XD, Glass CK, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu YL, Lin CP, Azarova AM, Cai L, Wang JC, Liu LF. Role of topoisomerase IIbeta in the expression of developmentally regulated genes. Mol Cell Biol. 2006;26:7929–7941. doi: 10.1128/MCB.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R, Gao F, Pfenning AR, Pan L, Yamakawa S, Seo J, Rueda R, Phan TX, Yamakawa H, Pao PC, et al. Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell. 2015;161:1592–1605. doi: 10.1016/j.cell.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Lindberg M, Faust GG, Leibowitz ML, Clark RA, Layer RM, Quinlan AR, Hall IM. Breakpoint profiling of 64 cancer genomes reveals numerous complex rearrangements spawned by homology-independent mechanisms. Genome Res. 2013;23:762–776. doi: 10.1101/gr.143677.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Gomez PA, Gong F, Nair N, Miller KM, Lazzerini-Denchi E, Sfeir A. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature. 2015;518:254–257. doi: 10.1038/nature14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy J, Nagahawatte P, Finkelstein D, Richards-Yutz J, Valentine M, Ma J, Mullighan C, Song G, Chen X, Wilson M, et al. RB1 gene inactivation by chromothripsis in human retinoblastoma. Oncotarget. 2014;5:438–450. doi: 10.18632/oncotarget.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7:283ra254. doi: 10.1126/scitranslmed.aaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Tischkowitz M, Chandler K, Gillespie A, Birch JM, Evans DG. Fanconi anaemia, BRCA2 mutations and childhood cancer: a developmental perspective from clinical and epidemiological observations with implications for genetic counselling. J Med Genet. 2014;51:71–75. doi: 10.1136/jmedgenet-2013-101642. [DOI] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J, Westerman BA, van Arkel J, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- Morganella S, Alexandrov LB, Glodzik D, Zou X, Davies H, Staaf J, Sieuwerts AM, Brinkman AB, Martin S, Ramakrishna M, et al. The topography of mutational processes in breast cancer genomes. Nat Commun. 2016;7:11383. doi: 10.1038/ncomms11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Liu P, Woloszynska-Read A, Zhang J, Luo W, Qin M, Bshara W, Conroy JM, Sabatini L, Vedell P, et al. Whole-genome sequencing identifies genomic heterogeneity at a nucleotide and chromosomal level in bladder cancer. Proc Natl Acad Sci U S A. 2014;111:E672–681. doi: 10.1073/pnas.1313580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan ME. The cancer connection: BRCA1 and BRCA2 tumor suppression in mice and humans. Oncogene. 2002;21:8994–9007. doi: 10.1038/sj.onc.1206177. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016 doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Yi Q, Burger RA, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- Orthwein A, Noordermeer SM, Wilson MD, Landry S, Enchev RI, Sherker A, Munro M, Pinder J, Salsman J, Dellaire G, et al. A mechanism for the suppression of homologous recombination in G1 cells. Nature. 2015;528:422–426. doi: 10.1038/nature16142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, et al. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol Cell. 2015;60:177–188. doi: 10.1016/j.molcel.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova T, Manie E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, Delattre O, Sigal-Zafrani B, Bollet M, Longy M, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–5462. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7:a016600. doi: 10.1101/cshperspect.a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch T, Jones DT, Zapatka M, Stutz AM, Zichner T, Weischenfeldt J, Jager N, Remke M, Shih D, Northcott PA, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nat Rev Cancer. 2014;14:786–800. doi: 10.1038/nrc3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena G, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45:970–976. doi: 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Sterling J, Thompson C, Harris S, Mav D, Shah R, Klimczak LJ, Kryukov GV, Malc E, Mieczkowski PA, et al. Clustered mutations in yeast and in human cancers can arise from damaged long single-strand DNA regions. Mol Cell. 2012;46:424–435. doi: 10.1016/j.molcel.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Seplyarskiy VB, Soldatov RA, Popadin KY, Antonarakis SE, Bazykin GA, Nikolaev SI. APOBEC-induced mutations in human cancers are strongly enriched on the lagging DNA strand during replication. Genome Res. 2016;26:174–182. doi: 10.1101/gr.197046.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshagiri S. The burden of faulty proofreading in colon cancer. Nat Genet. 2013;45:121–122. doi: 10.1038/ng.2540. [DOI] [PubMed] [Google Scholar]
- Sfeir A, Symington LS. Microhomology-Mediated End Joining: A Back-up Survival Mechanism or Dedicated Pathway? Trends Biochem Sci. 2015;40:701–714. doi: 10.1016/j.tibs.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlien A, Campbell BB, de Borja R, Alexandrov LB, Merico D, Wedge D, Van Loo P, Tarpey PS, Coupland P, Behjati S, et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet. 2015;47:257–262. doi: 10.1038/ng.3202. [DOI] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JM, Pierce AJ, Oh J, Pastink A, Jasin M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol Cell Biol. 2004;24:9305–9316. doi: 10.1128/MCB.24.21.9305-9316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, Garber JE, Chowdhury D, Wu CJ, D’Andrea AD, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telli ML, Timms KM, Reid JE, Hennessy B, Mills GB, Jensen KC, Szallasi Z, Barry WT, Winer EP, Tung N, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple Negative Breast Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108:14944–14949. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MH, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VC, Leonard B, White EA, Starrett GJ, Temiz NA, Lorenz LD, Lee D, Soares MA, Lambert PF, Howley PM, et al. Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B. MBio. 2014;5 doi: 10.1128/mBio.02234-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckselblatt B, Hermetz KE, Rudd MK. Unbalanced translocations arise from diverse mutational mechanisms including chromothripsis. Genome Res. 2015;25:937–947. doi: 10.1101/gr.191247.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei PC, Chang AN, Kao J, Du Z, Meyers RM, Alt FW, Schwer B. Long Neural Genes Harbor Recurrent DNA Break Clusters in Neural Stem/Progenitor Cells. Cell. 2016;164:644–655. doi: 10.1016/j.cell.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, Aas T, Alexandrov LB, Larsimont D, Davies H, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–759. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nat Struct Mol Biol. 2011;18:80–84. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Tacconi EM, Folio C, Badie S, Porru M, Klare K, Tumiati M, Markkanen E, Halder S, Ryan A, et al. Targeting BRCA1 and BRCA2 Deficiencies with G-Quadruplex-Interacting Compounds. Mol Cell. 2016;61:449–460. doi: 10.1016/j.molcel.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, de Lange T. 53BP1: pro choice in DNA repair. Trends Cell Biol. 2014;24:108–117. doi: 10.1016/j.tcb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]