Abstract
Some individuals with noise-induced hearing loss (NIHL) also report balance problems. These accompanying vestibular complaints are not well understood. The present study used a rat model to examine the effects of noise exposure on the vestibular system. Rats were exposed to continuous broadband white noise (0–24kHz) at an intensity of 116dB sound pressure level (SPL) via insert ear phones in one ear for three hours under isoflurane anesthesia. Seven days after the exposure, a significant increase in ABR threshold (43.3±1.9dB) was observed in the noise-exposed ears, indicating hearing loss. Effects of noise exposure on vestibular function were assessed by three approaches. First, fluorescein-conjugated phalloidin staining was used to assess vestibular stereocilia following noise exposure. This analysis revealed substantial sensory stereocilia bundle loss in the saccular and utricular maculae as well as in the anterior and horizontal semicircular canal cristae, but not in the posterior semicircular canal cristae. Second, single unit recording of vestibular afferent activity was performed under pentobarbital anesthesia. A total of 548 afferents were recorded from 10 noise-treated rats and 12 control rats. Noise exposure produced a moderate reduction in baseline firing rates of regular otolith afferents and anterior semicircular canal afferents. Also a moderate change was noted in the gain and phase of the horizontal and anterior semicircular canal afferent’s response to sinusoidal head rotation (1 and 2Hz, 45 degrees/s peak velocity). Third, noise exposure did not result in significant changes in gain or phase of the horizontal rotational and translational vestibular-ocular reflex (VOR). These results suggest that noise exposure not only causes hearing loss, but also causes substantial damage in the peripheral vestibular system in the absence of immediate clinically measurable vestibular signs. These peripheral deficits, however, may lead to vestibular disorders over time.
Keywords: noise, vestibular, hair cell, vestibular afferent, vestibule-ocular reflex (VOR), rat
1. Introduction
Hearing loss as a result of high intensity noise exposure is an unavoidable aspect of many occupations, particularly those associated with industry and the military services. It has been reported that some individuals with noise-induced hearing loss (NIHL) also suffer from balance disorders (Oosterveld et al., 1982; Juntunen et al, 1987; Golz et al., 2001). Reduced vestibular caloric response (Manabe et al., 1995; Golz et al., 2001), reduced vestibular-evoked myogenic potentials (VEMP) (Wang et al., 2006; Wang & Young, 2007; Kumar et al., 2010; Akin et al., 2012; Zuniga et al., 2012), nystagmus (Man et al., 1980; Shupak et al., 1994; Oosterveld et al., 1982; Golz et al., 2001), and increased body sway (Ylikoski 1988; Kilburn et al., 1992) have been reported. Despite our understanding of the effect of noise on auditory function, the mechanisms underlying noise-induced vestibular deficiency remain to be elucidated.
The vestibular system is exquisitely sensitive to head rotation, translation, and changes in orientation with respect to gravity (for review, Goldberg et al., 2012). However, because vestibular end organs share the same fluid environment with the auditory end organ, they are also impacted by intense acoustic waves. Acoustic activation of the vestibular system occurs not only in pathological conditions where the bony canal is compromised by fenestration or by canal dehiscence (Tullio, 1929; Minor et al., 1998), but also occurs in healthy human subjects (Parker et al., 1978) and in animal models with intact labyrinths (Young et al. 1977, Xu et al., 2007, monkeys; Wit et al., 1984, pigeons; McCue and Guinan 1994a, 1994b, 1995b, 1997, cats; Murofushi et al. 1995, Murofushi and Curthoys, 1997, Curthoys et al., 2006; Curthoys and Vulovic, 2011, Curthoys et al., 2012, guinea pig; Carey et al., 2004, chinchilla; Zhu et al., 2011, 2014, rats). Electrophysiological and anatomical studies indicate that both the otolith organs and the semicircular canals are activated by loud sound (80 dB above ABR threshold), although the strongest excitations are from otolith organ organs (Zhu et al., 2011, 2014). Nevertheless, it is unclear whether high-intensity noise exposure produces damage in the five vestibular end organs. The current study employed three approaches to examine effects of exposure to high intensity broadband noise on the vestibular system of rodents, i.e., analysis of vestibular hair cell morphology, singe unit recording of vestibular afferents and testing of the rotational and translational vestibulo-ocular reflex (VOR). Our results show that a single high-intensity noise exposure results in substantial damage to the peripheral vestibular end organs, in the absence of immediate signs of vestibular dysfunction.
2. Methods
2.1. Animals
Adult male Sprague-Dawley (SD) rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 250–350 grams were used in the morphological and neurophysiological studies. Pigmented female Long-Evans rats (Harlan Labs, Indianapolis, IN) weighing 175–225 grams were used for VOR testing. All procedures were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
2.2. High intensity noise exposure
Rats were held under isoflurane anesthesia and exposed to broadband white noise [0–24 kHz, 116 dB sound pressure level (SPL)] that was delivered continuously to the left external ear canal for three hours. The contralateral external ear canal was blocked with an ear plug. During the noise exposure, body temperature was maintained at 36–37°C with a heating pad (Frederick Haer, Bowdoinham, ME, USA). Noise was generated through a MA3 stereo microphone amplifier (Tucker-Davis Technologies, Alachua, FL) and delivered to the external ear canal via sound-conducting tubing, connected to an insert ear phone (ER-3A, Etymotic Research, Inc., Elk Grove Village, IL) with a 3.5mm infant tip adapter. Control rats received a sham noise exposure under isoflurane anesthesia for three hours while connected to an inset ear phone without noise exposure. Before the ear phone was inserted, the ear canal was checked with an otoscope to ensure patency. Noise intensity was calibrated by a sound level meter (Brüel and Kjoer, Copenhagen, Denmark).
2.3. Auditory brainstem response
Auditory brainstem response (ABR) was measured under isoflurane anesthesia before and seven days after noise exposure. Stainless steel subdermal electrodes were placed at the vertex (active), behind the stimulated ear (reference), and in the hind leg (ground) (Simpson et al., 1985). The sound stimulus was a 0.1ms broad band click of alternating polarity. Clicks were generated by a MA3 stereo microphone amplifier and delivered via an insert ear phone (ER-3A) at a rate of 25.1clicks/s. EEG signals were amplified (×100,000), filtered (100 Hz–3kHz), averaged over 2000 trials and digitized at 20kHz over a 15-ms epoch (ICS Chartr EP 200 evoked potential assessment device; GN Otometrics, Taastrup, Denmark). Click intensity began at 110 dB peak sound pressure level (pSPL) and was lowered by 5 dB until ABR threshold was obtained. ABR threshold was determined as the lowest intensity at which clicks generate well-defined and reproducible wave I. Animals with elevated ABR thresholds (>70 dB pSPL before noise or sham exposure) were excluded due to possible hearing loss.
2.4. Vestibular afferent recording
Seven days after noise or sham exposure, vestibular afferent activity was recorded under pentobarbital sedation (50 mg/kg, i.p.) as described previously (Zhu et al., 2011, 2014). Sedation was maintained by injection of a 5 mg/kg dose of pentobarbital as needed. The rat body temperature was monitored and maintained at 36–37°C with a heating pad. Surgical procedures were conducted aseptically. Each rat’s head was secured on a stereotaxic frame (David Kopf Instruments, Tujunga, CA) via a surgically implanted head holder. The left side occipital bone was opened and the cerebellum was exposed. With the assistance of a surgical microscope, the left cerebellar hemisphere, flocculus, and paraflocculus were removed by aspiration (Model 130 Schuco-Vac, Allied Healthcare Products Inc., St Louis, MO) to access the 8th nerve (Zhu et al., 2011 and 2014).
Single unit recording of vestibular afferent activity was performed on the side ipsilateral to the noise or sham-exposed ear. Rats were secured on a gimbaled structure that allowed roll and pitch tilts. The structure was mounted on the platform of a rotator for small animals, which was servo-controlled to deliver angular and linear accelerations (Neuro Kinetic, Inc., Pittsburgh, PA, USA). A quartz microelectrode (Sutter Instruments, Novato, CA, USA), filled with 3 M sodium chloride (10–20 MΩ) was positioned over the superior branch of the vestibular nerve with the assistance of a surgical microscope. Every spontaneously active nerve fiber encountered was tested. First, at least 30 seconds of background discharge activity was recorded for calculating the regularity and baseline firing rate of the afferent. Next, each semicircular canal was brought into the plane of earth-horizontal rotation and whether the isolated afferent’s firing rate was modulated by sinusoidal earth-horizontal rotations or by sinusoidal pitch rotations (dynamic vertical head tilt) was tested. Extracellular recording was obtained using a MNAP system (Plexon Inc., Dallas, TX, USA). The rotational stimuli were delivered at 0.5, 1, and 2 Hz, at a peak velocity of 45 degrees/s. Single-unit data along with horizontal and vertical head-position signals were recorded for 30 cycles.
Extracellular voltage signals were sampled by a CED Power 1401 system (Cambridge Electronics Devices, Cambridge, UK) at 20 kHz with 16-bit resolution and a temporal resolution of 0.01ms. Head position signals were sampled at 1 kHz. Offline data analysis was performed on PC workstations using Spike 2 (Cambridge Electronics Devices, Cambridge, UK), MATLAB (MathWorks, Inc., MA, USA) and SigmaPlot (Systat Software Inc., CA, USA) software. Regularity of vestibular afferents was determined by calculating their normalized coefficient of variation of interspike intervals, i.e., CV*s, using the methods described in Lasker et al. (2008). Vestibular afferents were classified as regular (CV*<0.1) or irregular (CV*>0.2) units based on their CV* (Goldberg et al., 1984; Young et al., 1977). Permutation analyses were carried out, as described by Liu and Angelaki (2009), to determine whether units were significantly modulated by rotational stimuli. To determine an afferent’s sensitivity to head rotation, the fundamental response was extracted from the averaged data using a fast Fourier transform (FFT) analysis and was then corrected trigonometrically using the methods described in Hullar and Minor (1999). Gains and phases relative to head velocity were calculated at 0.5, 1, and 2 Hz. Vestibular afferents were classified as horizontal canal afferents (HC), anterior canal afferents (AC), and superior branch otolith organ afferents (SO) based on previously established criteria (Estes et al., 1975; Daunicht and Pellionisz 1987; Blanks and Torigoe 1989; Goldberg and Fernandez 1975; Young et al., 1977). The SO afferents consisted of both saccular and utricular afferents. In the present study, we did not further classify SO afferents into saccular afferents and utricular afferents.
2.5. Tissue collection and phalloidin staining
Seven days after treatment, noise-exposed and control rats were euthanized with an overdose of sodium pentobarbital (100 mg/kg body weight, i.p.), transcardially perfused with 4% paraformaldehyde (PFA, pH=7.4) in phosphate-buffered saline (PBS, 0.01M), and decapitated. Petrous temporal bones were collected and post fixed in 4% PFA at 4°C overnight. On the following day, the petrous temporal bones were cleaned and partially dissected down to the bony labyrinth and decalcified in a PBS solution containing 5% ethylenediaminetetraacetic acid (EDTA) and 4% PFA (pH=4.8). The decalcified inner ear was dissected and the semicircular canal cristae and otolith organ maculae were removed under the guidance of a dissection microscope and transferred to PBS solution (0.01M, pH=7.4) for overnight storage at 4° C. On the following day, the cristae and maculae were incubated with a 1:500 solution of Alexa-488-conjugated phalloidin (Life Technologies, Carlsbad, CA) which binds with the filamentous actin in sensory cilium and cuticular plate of hair cells. Phalloidin incubation was carried out in a dark container for 40 minutes at room temperature. The specimens were washed three times in PBS, whole-mounted on glass slides, and cover-slipped. The maculae and cristae were examined with a Nikon C1 confocal scanning microscope (Nikon Corporation, Yurakucho, Tokyo, Japan). Two-dimensional z-stack collapsed images were produced with EZ-C1 software (v3.90, Nikon Corporation, Yurakucho, Tokyo, Japan) and saved for analysis at a PC workstation.
Stereocilia bundle quantification methods were adapted from previous studies (Zhou et al., 2009; Higgs et al., 2001; Sun et al., 2011). For the saccule and utricle, hair cell stereocilia bundle density was assessed by counting each stereocilia bundle in 18 non-overlapping 50 μm2 square grids that sample the striolar and marginal regions (Figure 4, A and B) using Corel Draw X7 (Corel Corporation, Ottawa, Ontario, Canada). The striolar regions and marginal regions were sampled based on previous anatomical studies in rodents (Desai et al., 2005a and 2005b). For the canal cristae, the entire central region was quantified and a final average density was determined by dividing the total count by the sampled area. Although structural damage was observed in the remaining stereocilia bundles, no attempt was made to assess the level of damage in stereocilia bundles, therefore, all bundles were counted, regardless of integrity of individual cilia. Stereocilia bundle counts were averaged by area in the control and noise-exposure groups and were presented as mean counts per 50 μm2.
Figure 4.
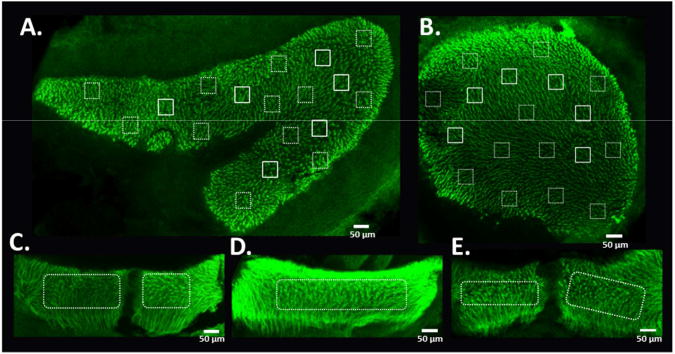
Methods for quantification of stereocilia bundle density in the utricular and saccular maculae and the semicircular canal cristae. Stereocilia density was assessed by counting a 50 μm2 grid in each of the specified regions from utricle (A) and saccule (B). Solid line boxes are for striolar regions and dotted line boxes are for extra-striolar regions. For the semicircular canal cristae (C, AC; D, HC; E, PC), stereocilia bundle densities are assessed in the central regions.
2.6. Vestibulo-ocular reflex (VOR)
Our research has shown that noise-induced vestibular hair cell damage and hearing loss were similar between SD rats and female Long-Evans (LE) rats (Harlan, 175–225 grams) (data not shown). LE rats were used to test the effects of noise exposure on the VOR since their pupils are better tracked for eye movement recording and they have been shown to tolerate the restraint method well (Quinn et al., 1998). The rats were briefly anesthetized with isoflurane and restrained in a custom-made acrylic tube which prevented body from moving while the head was stabilized with a surgically implanted stainless steel head holder. Following one week of recovery from the head holder surgery, rats were given thirty minutes per day for a minimum of three days to adapt to the head and body restraint.
Horizontal rotational and translational VOR responses were recorded in complete darkness using a video-based ISCAN ETS-200 eye tracking system (ISCAN, Burlington, MA). Rats were secured on a gimbaled structure. The head was positioned 40 degrees nose down to activate the horizontal canal by earth horizontal rotation. The gimbaled structure was mounted on a servo-controlled rotator/sled. An infrared camera equipped with a zoom lens (Computar TV Zoom Lens, Computar Optics Group, Japan) was attached to the platform of the rotator/sled and was focused on the eye. The eye of the rat was illuminated by a standard ISCAN multiple infrared LED illuminator attached to the camera mount on a flexible arm to produce a reference corneal reflection (CR) during calibration and illumination during VOR testing. The ISCAN system tracks the centers of the pupil and reference CR and provides real-time signals related to pupil position and reference CR position, which were digitized and sampled at 1 kHz with head position signals by a CED Power 1401 system (Cambridge Electronics Devices, Cambridge, UK). Calibration was achieved by rotating the camera by +/−10 degrees (i.e., 20 degrees peak to peak) around the vertical axis of the turntable. The tracked pupil and the CR recorded in the extreme positions of the camera rotation were used to calculate the radius of rotation of the pupil. Angular position of the eye was calculated using the methods described by De Jeu and De Zeeuw (2012). Following the calibration, a series of rotational and translational accelerations were delivered. Responses of the eye to horizontal angular rotation at 0.1, 0.2, 0.5 and 1Hz (60 degrees/s peak velocity, semicircular canal function) and translations at 0.2 Hz (0.1g peak acceleration) along 45 degrees from nasal-occipital axis (utricular function) were recorded. Eye position signals were recorded for 12 cycles per stimulation condition.
Baseline VOR responses were measured for seven consecutive days. Rats were then anesthetized with isoflurane and the left ear was noise- or sham-exposed under the same conditions as described in the noise exposure section (2.2.). VOR responses were measured for seven consecutive days after noise or sham exposure.
Offline data analysis was performed on PC workstations using Spike 2 (Cambridge Electronics Devices, Cambridge, UK), MATLAB (MathWorks, Inc., MA, USA) and SigmaPlot (Systat Software Inc., CA, USA) software. For the rotary VOR, saccades were removed from eye velocity traces. Gains and phases relative to head velocity were calculated by fitting the eye velocity trace with a sine function. The gain was computed as the ratio of peak eye velocity to peak head velocity, and the phase was computed as the difference (in degrees) between peak eye velocity and peak head velocity. Negative phase values indicate that eye velocity leads head velocity. For the translational VOR, saccades and DC were removed from eye position traces. Gains and phases relative to head position were calculated by fitting the eye position trace with a sine function. The gain was computed as the ratio of peak eye position to peak head position, and the phase was computed as the difference (in degrees) between peak eye position and peak head position.
2.7. Statistical analysis
Statistical analyses were performed using Excel (Microsoft, Seattle, WA) and SigmaPlot. Differences between or among the groups were analyzed by t-test or one-way ANOVA. Comparisons of averaged data before and after the treatment were performed using paired t-test or one-way repeated measurement ANOVA. P values of less than 0.05 were considered statistically significant. All p-values were provided in the figure legends. Error bars were the SEM.
3. Results
3.1. Noise-induced hearing loss
Seven days after unilateral noise exposure, ABR threshold to click stimuli increased 43.3 (±1.9) dB over baseline measurements taken prior to exposure (P<0.001, paired t-test, t=−22.7, df=23; Figure 1), indicating a noise-induced hearing loss. Sham exposure did not significantly alter the ABR threshold (P=1.0, paired t-test, t=0.00, df=4). No static symptoms such as spontaneous nystagmus, spinning or body posture change were observed within 7 days after the noise exposure.
Figure 1.
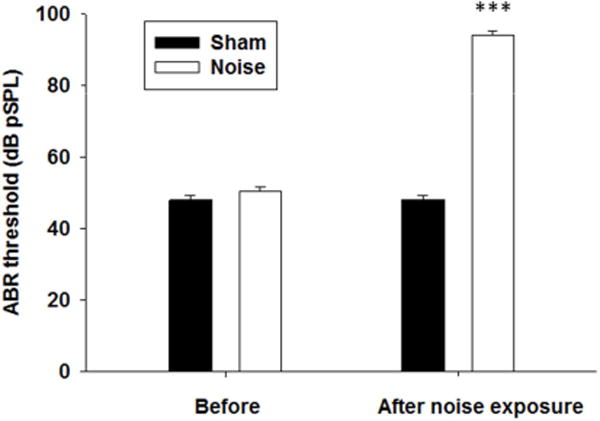
Changes in ABR thresholds after noise exposure. Rats were exposed to 116 dB (pSPL) white noise for 3 hours under isoflurane anesthesia. Seven days after exposure, a ~43 dB ABR threshold shift was observed in the noise-exposed ears, indicating hearing loss (***P <0.001). Sham exposure did not cause significant ABR threshold shift.
3.2. Effects of noise exposure on spontaneous firing rate of the vestibular afferents
Single unit recording of vestibular afferent activity was conducted 7 days after noise or sham exposure. A total of 311 vestibular afferents were recorded from 10 noise-exposed rats, including 120 HC afferents, 77 AC afferents and 114 SO afferents. A total of 237 vestibular afferents were recorded from 12 control rats, including 95 HC afferents, 69 AC afferents and 73 SO afferents. Figure 2 compares averaged spontaneous firing rates of vestibular afferents from different end organs in noise-treated and control conditions. The proportions of irregular and regular afferents were similar in the two conditions (Chi-square=0.036, P=0.85). Noise exposure resulted in a decrease in spontaneous firing rate in the regular afferents of AC (t=−2.24, df=94, P< 0.02, t-test) and SO (t=−1.90, df=46, P<0.05, t –test), but not HC (t=0.74, df=113, P = 0.46). Noise exposure has no effect on the irregular afferents of HC (t = 1.04, df=88, P=0.30, t-test), AC (t = −1.02, df=39, P=0.31, t-test) and SO (t = −1.32, df=130, P=0.19, t-test).
Figure 2.
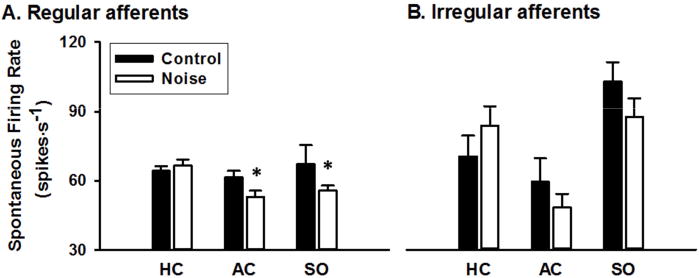
Average spontaneous firing rates of the HC, AC, and SO regular (A) and irregular (B) afferents in the control and noise exposed rats. Noise exposure resulted in a decrease in spontaneous firing rate in regular AC (P< 0.02) and SO afferents (*P<0.05). AC: anterior canal units; HC: horizontal canal units; SO: otolith units in the superior branch.
3.3. Effects of noise exposure on vestibular afferent sensitivity to head rotation
Responses to head rotation were measured for 548 vestibular afferents. Figure 3A shows the representative responses of a regular HC afferent to 2Hz sinusoidal head rotation. The afferent firing rate increased with leftward head rotation with a gain of 0.27 spikes/s/degree/s and a phase lag of 14.2 degrees with respect to head velocity. Noise exposure induced moderate gain changes in regular AC and HC vestibular afferents (Figure 3B and D, left panels), but no gain changes in irregular AC and HC afferents (Figure 3C and E, left panels). For AC afferents, noise exposure resulted in a decrease in regular AC afferent gains at 2 Hz (0.19±0.10 vs 0.13±0.08 spikes/s/degree/s, t=2.97, df=93, P=0.0037, t-test) but not at 0.5 Hz (t = 0.45, df= 42, P=0.65) and 1 Hz (t = −1.58, df=73, P=0.12) (Figure 3B). For HC afferents, noise exposure resulted in an increase in regular HC afferent gains at 1 Hz (0.12±0.02 vs. 0.17±0.01 spikes/s/degree/s, t = 2.06, df=67, P=0.043) and at 2 Hz (0.13±0.01 vs. 0.17±0.01 spikes/s/degree/s, t = 2.91, df=111, P=0.004), but not at 0.5Hz (t = 1.22, df=37, P=0.23) (Figure 3D). Noise exposure resulted in a significant decrease in phase lag in regular HC afferents at 2 Hz (−28.21±5.76 vs. −7.03±2.85 degrees, t=3.47, df=111, P=0.00073, Figure 3D right panel) and a decrease in phase lead in irregular HC afferents at 1 Hz (15.62±2.62 vs. −0.29±6.0 degrees; t=−1.69, df=55; P=0.048; Figure 3E, right panel). No significant changes in phase were observed in AC afferents (Figure 3B and C, right panels).
Figure 3.
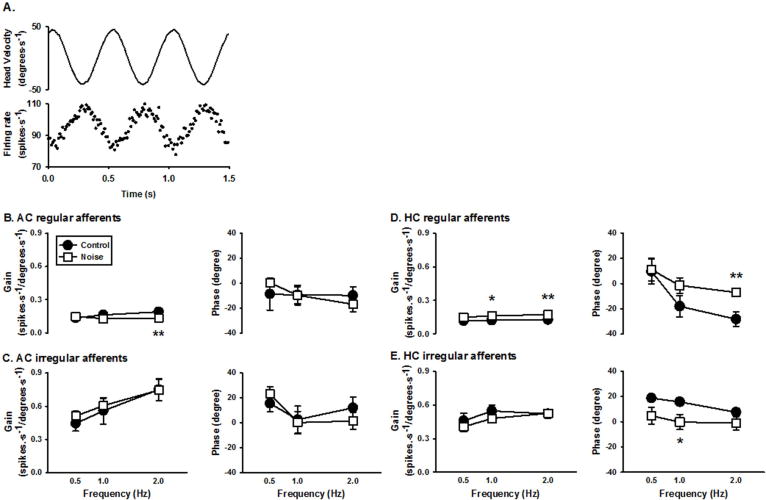
Changes in vestibular afferent sensitivity to head rotation following noise exposure. A. Representative responses of a regular horizontal canal afferent to horizontal head rotation. Top trace: head velocity. Bottom trace: discharge activity of the afferent during 2 Hz earth-horizontal rotation. This regular HC afferent had a gain of 0.27 spikes/s/degree/s and a phase lag of 14.20 degrees with respect to ipsilateral head velocity. B–E. Average gains and phases of the regular (B) and irregular (C) AC afferents, and regular (D) and irregular (E) HC afferents at 3 rotational frequencies (0.5, 1, and 2 Hz) in the control and noise exposed rats. Noise exposure significantly affected the gains of regular HC and AC afferents and the phases of regular and irregular HC afferents (*P<0.05; ** P<0.01). AC: anterior canal units; HC: horizontal canal units.
3.4. Noise-induced decrease in stereocilia bundle density
Vestibular stereocilia bundles were examined 7 days after noise exposure. Six striolar areas and twelve marginal areas were sampled from the saccular and utricular maculae (Figure 4A and 4B). The entire central portion of the AC, HC and posterior semicircular canal (PC) cristae were sampled (Figure 4C, 4D and 4E). Noise exposure resulted in a significant decrease in stereocilia bundle density in both the saccular and utricular maculae as well as the semicircular canal cristae (Figures 5 and 6). In the saccular maculae, noise exposure resulted in a reduction of 35% (t-test, t=3.53, df=8, P<0.01) and 47% (t-test, t=6.22, df=8, P<0.001) in stereocilia bundle density in the striolar and extra-striolar regions, respectively. In the utricular maculae, noise exposure resulted in a reduction of 48% (t-test, t=5.09, df=10, P<0.005) and 43% (t-test, t=3.83, df=10, p<0.005) in stereocilia bundle density in the striolar and extra-striolar regions, respectively. For both of the utricle and saccule, there was no significant difference in hair cell bundle loss between the striolar and extra-striolar regions (P=0.114, P=0.57, Chi-square). Noise exposure resulted in a reduction of 61% (t=5.57, df=11, p<0.001) and 50% (t=3.17, df=7, P<0.02) in stereocilia bundle density in the central regions of the AC and HC cristae, respectively, but had no effect on stereocilia bundle density in the PC cristae (t=0.22, df=6, P=0.83).
Figure 5.
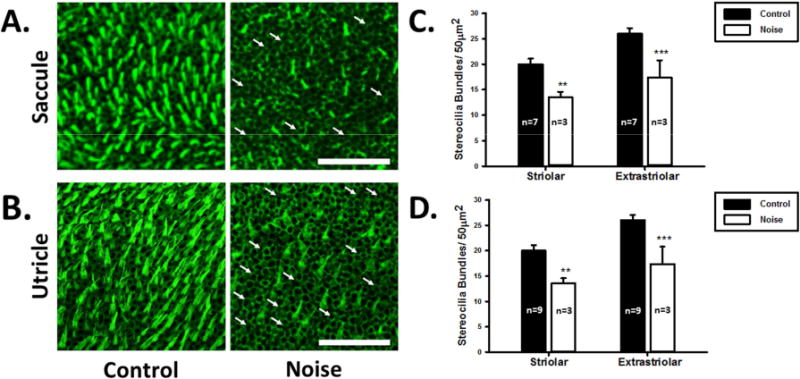
Effects of noise exposure on hair cell stereocilia bundle density in the saccular and utricular maculae. A and B. Representative images of the saccule macula and the utricle macula stained with phalloidin from the control (left panels) and noise-exposed rats (right panels). Arrows indicate intact cuticular plates with missing stereocilia bundles. Scale bar is 50 μm. C and D. Stereocilia bundle density in the striolar and marginal regions of the maculae of the control and noise-treated rats. Noise exposure decreases stereocilia bundle density in both the striolar and extra-striolar regions of the saccules and the utricles (**P<0.01; ***P<0.0005).
Figure 6.
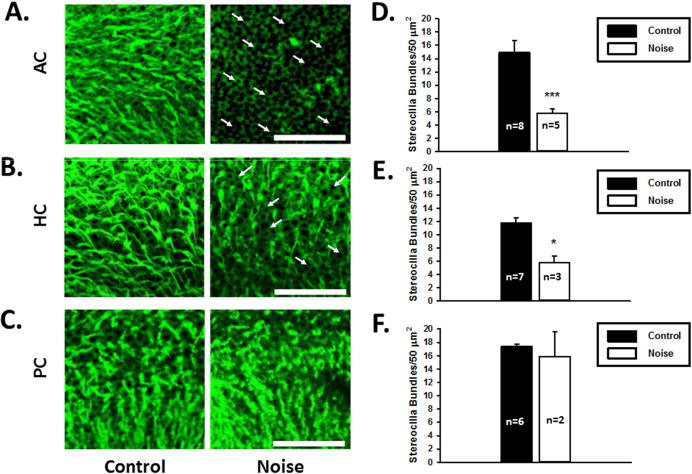
Effects of noise exposure on hair cell stereocilia bundle density in the semicircular canal cristae. A, B and C. Representative images of the anterior (AC), horizontal (HC), and posterior (PC) semicircular canal cristae stained with phalloidin in normal (left panels) and noise exposure rats (right panels). Arrows indicate missing stereocilia bundles. Scale bar is 50 μm. D, E and F. Stereocilia bundle densities of the semicircular canal cristae of the control and noise-treated rats. (*P<0.05; ***P<0.0005).
3.5. Effects of noise-exposure on the VOR
The effects of noise exposure on vestibular function was also examined by measuring the gain and phase of the horizontal rotational and translational VOR in awake young female LE rats. Our previous research has shown similar ABR threshold shift and vestibular hair bundle loss in SD and LE rats after noise exposure (data not shown). Compensatory eye movement during sinusoidal head rotation (0.25, 0.5, and 1 Hz, 60 degrees/s peak velocity) and translation (0.2 Hz, 0.1 g peak acceleration) was recorded from 7 noise-treated rats and 4 control rats. Figure 7 shows representative eye movement responses during 0.25 Hz horizontal rotation and 0.2 Hz horizontal translation. Gain and phase of the rotational and translational VOR were measured for 7 consecutive days after noise or sham exposure (Figure 8). Noise exposure did not result in significant changes in the gain and phase of either the rotational (Gain: 0.25Hz: P=0.15; 0.5Hz: P=0.70; 1Hz: P=0.22; Phase: 0.25 Hz: P=0.05; 0.5Hz: P=0.69; 1Hz: P=0.06, one way repeated measure ANOVA) or translational VOR (Gain: P=0.63; Phase: P=0.16, one way repeated measure ANOVA).
Figure 7.
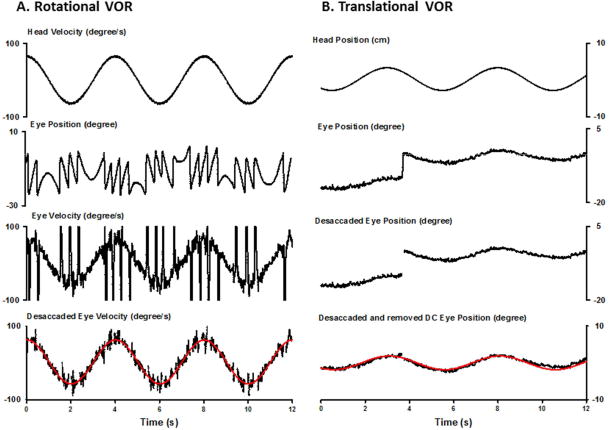
Representative horizontal eye movement responses of a noised treated rat during head rotation (A) and translation (B). A. From top to bottom, the traces are head velocity, eye position, eye velocity and de-saccaded eye velocity fitted with a sinewave function. B. From top to bottom, the traces are for head position, eye position, de-saccaded eye position and DC removed eye position fitted with a sinewave function (angular VOR at 0.25Hz: gain 0.99, phase 2.99 degrees; translational VOR at 0.2 Hz, gain 0.0625 degrees/cm, phase −19.21 degrees).
Figure 8.
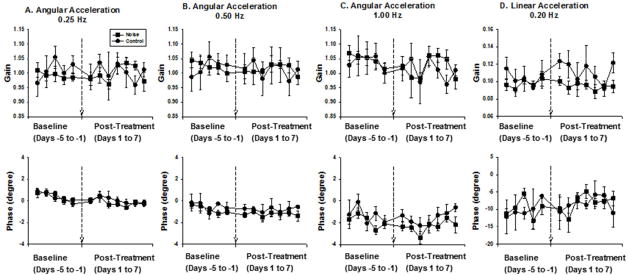
Effects of noise exposure on the VOR. A, B and C. Gains and phases of the angular VOR at 0.25 Hz (±40 degrees); 0.5 Hz (± 20 degrees) and 1Hz (±10 degrees). D. Gains and phases of translational VOR at 0.2Hz (±20 cm, 45 degrees with respect to nasal-occipital direction). Noise exposure does not result in significant changes in gains and phases of the angular and translational VOR.
4. Discussion
In the present study, we employed morphological, electrophysiological and behavioral approaches to quantitatively examine the effects of high intensity noise exposure on the vestibular system (Table 1). The results of these experiments indicate that a single 3-hour unilateral noise exposure not only results in an ABR threshold shift (~43 dB), consistent with hearing loss, but also results in a substantial decrease (30–60%) in stereocilia bundle density in all the vestibular end organs with the exception of the posterior semicircular canal. We further found that noise exposure results in moderate changes in regular vestibular afferent baseline firing rates and in the gain and phase of response to head rotation. Despite these observed changes in vestibular hair cell and vestibular afferent activity, we found no significant changes in the gain and phase of the rotational and translational VOR. This suggests that the noise-induced damage to the vestibular periphery may not be sufficient to result in observable changes in behavior. To the best of our knowledge, this is the first systematic and quantitative analysis of the effects of noise-exposure on the vestibular system. The results provide a foundation for future studies to investigate the underlying mechanisms of the damage and for development of effective protocols for prevention, diagnosis, and treatment of noise-induced vestibular deficits.
Table 1.
Summary of the effects of noise exposure on vestibular hair cell stereocilia bundle density, vestibular afferent activity and VOR.
| End organs | Stereocilia bundle loss | Vestibular afferent baseline activity | Vestibular afferent sensitivity to rotation Gain | Vestibular afferent sensitivity to rotation Phase | VOR | |||
|---|---|---|---|---|---|---|---|---|
| Regular | Irregular | Regular | Irregular | Regular | Irregular | |||
| HC | 50% | No change | No change | 34% increase | No change | Decreased phase lag | Decreased phase lead | No change |
| AC | 61% | 14% decrease | No change | 31% decrease | No change | No change | No change | – |
| PC | No change | – | No change | – | – | – | – | – |
| Saccule | 35–47% | 17% decrease (SO) | No change | – | – | – | – | – |
| Utricle | 43–48% | No change | ||||||
The anesthetized noise model was adopted for two reasons. First, compared to the awake model, it allowed us to focus on the mechanical effects of noise exposure on the vestibular system. Second, it allowed us to effectively provide noise exposure to one labyrinth. Despite protective effect of anesthesia (Kim et al., 2005), the current model consistently induced significant hearing loss (~ 43dB ABR threshold shift, 7 days following the noise exposure), which is comparable to that found in earlier animal studies (Hsu et al., 2008; Tamir et al., 2010). Direct comparison of the effects of noise on vestibular end organs between the anesthetized model and awake model may provide new insights into the neural mechanisms underlying noise-induced vestibular deficits.
4.1. Previous studies of the effects of noise-exposure on the vestibular system
Over the past three decades, several clinical studies have examined noise-induced vestibular deficits, but have reported inconsistent, and at times conflicting, results. For examples, on the one hand, in 2001 Golz et al. reported on 258 military subjects who had been heavily exposed to noise. Of this group 11% of the individuals with symmetrical hearing loss and 21% with asymmetrical hearing impairment had vestibular symptoms, and nearly 12% of the individuals showed reduced caloric responses. On the other hand, other studies of individuals with noise exposure history did not find abnormal posturography (Farkkila et al., 1988; Pyykko et al., 1989) or found only marginal effects on eye movement responses to head rotation and caloric irrigation (Shupak et al., 1994). Correlation between the severity of hearing loss and vestibular symptomatology and pathology has been found in some reports (Ylikoski et al., 1988, Shupak et al., 1994) but not in others (Golz et al., 2001). While it is difficult to account for the discrepancies among these studies, it is likely that multiple factors, including different exposure regimen and testing methods, are involved.
Animal models have also been employed to examine the effects of exposure to impulse noise or chronic noise on the vestibular end organs (Ylikoski, 1987; Hsu et al., 2008). Almost three decades ago, Ylikoski (1987) showed in guinea pigs that exposure to rifle shots damages both the semicircular canal cristae as well as utricular and saccular maculae. Studies from the same group also showed that exposure to low frequency noise results in delayed endolymphatic hydrops in chinchillas (Ylikoski, 1988). Hsu et al (2008) showed that long term exposure to white noise results in loss and disruption of hair cells in the saccular macula. However, no previous studies have quantitatively examined the noise-induced damage to the vestibular end organs and have associated such findings with changes in vestibular function, as we have done in the current study.
4.2. Effects of noise exposure on vestibular end organs, vestibular afferent activity and VORs
By counting stereocilia bundles in otolithic maculae and semicircular canal cristae, we quantitatively analyzed the mechanical effects of noise exposure on each of the five vestibular end organs. We found that a three hour exposure to 116 dB (pSPL) white noise results in substantial reductions in stereocilia bundle density in the utricular and saccular maculae, as well as the HC and AC cristae, but not in the PC crista. While these results demonstrate the damaging effects of noise exposure on vestibular end organs, they also provide insights into how acoustic energy propagates within the labyrinth. Interestingly, the results are consistent with our neurophysiology findings showing that PC afferents are less sensitive to sound stimulation (Zhu et al., 2011 and 2014). In these studies, we showed that loud clicks or tone bursts activate vestibular afferents innervating both the semicircular canals and the otoliths of rats (Zhu et al., 2011, 2014). The extent to which different end organs are activated by sound is stimulus-dependent and the order of vestibular end organ sound sensitivity is otolith organs>AC>HC>PC.
By employing a single unit recording approach, we examined the effects of noise exposure on discharge activity of vestibular afferents. In spite of nearly a 50% reduction in stereocilia bundle density in four of the five vestibular end organs, we only found marginal or moderate changes in vestibular afferent baseline firing rates (AC and SO units) and responses to head rotation (HC and AC units). The resiliency of vestibular afferents to noise exposure is likely due to the redundancy of the vestibular peripheral system, e.g., each vestibular afferent innervates multiple hair cells. While the finding that noise exposure reduced vestibular afferent baseline firing rates and sensitivity to head rotation were expected, it was unexpected to find that HC units exhibited a moderate increase in gain to head rotation at 1 Hz and 2 Hz (Figure 3). One explanation for this finding comes from a study by Holstein et al. (2004) which showed that convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses. Future studies will further examine how noise exposure results in increases in vestibular afferent responses to head rotation.
By measuring eye movement responses to head rotation and translation, we assessed the effects of noise exposure on angular and linear VORs. In spite of substantial loss of stereocilia bundles and moderate changes in vestibular afferent activity, we found no changes in the VORs 7 days after noise exposure. Three mechanisms may contribute to the intact VORs in the presence of substantial loss of vestibular hair cell bundles and moderate changes of vestibular afferent activity. One is the convergence connection of the VOR pathways. For example, a single vestibular afferent receives inputs from multiple vestibular hair cells and a single vestibular nucleus neuron receives inputs from multiple vestibular afferents. Thus, peripheral vestibular damage may not lead to dysfunction of the VOR pathway. The second is that the noise exposure was unilateral and the contribution of the other labyrinth to the VORs remained intact. The third is the robust central compensation mechanisms that can restore vestibular nucleus neuron baseline firing rates and sensitivities to head rotation (for reviews, Darlinton and Smith, 2002; Straka et al., 2005). These results call into question the effectiveness of using head rotation tests to assess vestibular function shortly after noise exposure. Indeed, clinical studies have reported a low incidence of clinical signs of vestibular malfunction in patients with NIHL. Thus, vestibular malfunction associated with NIHL can be overlooked by physicians and patients. Nevertheless, because the compensatory mechanisms begins to fail during the process of aging, vestibular dysfunctions may be more common in the NIHL population than in the general population. This view is supported by clinical studies which have demonstrated a strong association between hearing loss and vestibular deficits in the elderly (Zuniga et al., 2012). Vestibular dysfunction, a risk factor for falling, may contribute to increased incidents of falls in the elderly with hearing loss (Viljanen et al., 2009; Lopez et al., 2011; Lin and Ferrucci, 2012).
One limitation of this study is that it only examined vestibular canal afferent responses to head rotation, but did not test otolithic afferent responses to head translation. Future studies are needed to address this limitation in order to further elucidate the mechanisms underlying noise-exposure induced vestibular deficits.
Highlights.
The current study examines effects of high intensity noise exposure on the vestibular system in rats.
Exposure to high intensity noise causes substantial reductions in density of vestibular hair cell stereocilia bundles.
Noise exposure results in moderate changes in vestibular afferent discharge activity.
Noise exposure does not result in measureable changes in gains and phases of the horizontal rotatory and translational vestibular-ocular reflex (VOR).
Acknowledgments
This work was supported by NIH R01DC012060 (HZ). We thank Dr. Douglas Vetter for his comments on the manuscript.
Abbreviations
- ABR
Auditory brainstem response
- AC
anterior canal afferents
- CR
corneal reflection
- CV*
normalized coefficient of variation of interspike intervals
- dB
decibel
- FFT
fast Fourier transform
- HC
horizontal canal
- LE
Long-Evans
- NIHL
noise-induced hearing loss
- PC
posterior semicircular canal
- SO
superior branch otolith organ afferents
- PFA
paraformaldehyde
- PBS
phosphate-buffered saline
- pSPL
peak Sound Pressure Level
- SD
Sprague-Dawley
- VOR
vestibulo-ocular reflex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akin FW, Murnane OD, Tampas JW, Clinard C, Byrd S, Kelly JK. The effect of noise exposure on the cervical vestibular evoked myogenic potential. Ear Hear. 2012;33:458–465. doi: 10.1097/AUD.0b013e3182498c5f. [DOI] [PubMed] [Google Scholar]
- Blanks RHI, Torigoe Y. Orientation of the semicircular canals in rat. Brain Res. 1989;487:278–287. doi: 10.1016/0006-8993(89)90832-9. [DOI] [PubMed] [Google Scholar]
- Carey JP, Hirvonen TP, Hullar TE, Minor LB. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol. 2004;25:345–352. doi: 10.1097/00129492-200405000-00024. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. 2011;210:347–352. doi: 10.1007/s00221-010-2499-5. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Vulovic V, Sokolic L, Pogson J, Burgess AM. Irregular primary otolith afferent from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res Bull. 2012;89:16–21. doi: 10.1016/j.brainresbull.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Darlingtona CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Progress in Neurobiology. 2000;62:313–325. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Daunicht WJ, Pellionisz AJ. Spatial arrangement of the vestibular and the oculomotor system in the rat. Brain Res. 1987;435:48–56. doi: 10.1016/0006-8993(87)91585-x. [DOI] [PubMed] [Google Scholar]
- De Jeu M, De Zeeuw CI. Video-oculography in Mice. JoVE. 2012;65:e3971. doi: 10.3791/3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005a;93:251–266. doi: 10.1152/jn.00746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005b;93:267–280. doi: 10.1152/jn.00747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MS, Blanks RHI, Markham CH. Physiologic characteristics of vestibular first-order canal neurons in the cat. I. Response plane determination and resting discharge characteristics. J Neurophysiol. 1975;38:1232–1249. doi: 10.1152/jn.1975.38.5.1232. [DOI] [PubMed] [Google Scholar]
- Farkkila M, Pyykko I, Jantti V, Aatola S, Starck J, Korhonen O. Forestry workers exposed to vibration: Neurological study. Br J Ind Med. 1988;45:188–192. doi: 10.1136/oem.45.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Responses of peripheral vestibular neurons to angular and linear accelerations in the squirrel monkey. Acta Otolaryngol. 1975;80:101–110. doi: 10.3109/00016487509121307. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Broussard DM, Buttner-Ennever JA, Fukushima K, Minor LB. The Vestibular System: A Sixth Sense. Oxford University Press; 2012. Clinical manifestation of peripheral vestibular dysfunction. [Google Scholar]
- Golz A, Westerman ST, Westerman LM, Goldenberg D, Netzer A, Wiedmyer T, Fradis M, Joachims HZ. The effects of noise on the vestibular system. Am J Otolaryngol. 2001;22:190–196. doi: 10.1053/ajot.2001.23428. [DOI] [PubMed] [Google Scholar]
- Higgs DM, Souza MJ, Wilkins HR, Presson JC, Popper AN. Age- and size-related changes in the inner ear and hearing ability of the adult zebrafish (Danio rerio) JARO. 2001;3:174–184. doi: 10.1007/s101620020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein GR, Rabbitt RD, Martinelli GP, Friedrich VL, Jr, Boyle RD, Highstein SM. Convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses. PNAS USA. 2004;101:15766–15771. doi: 10.1073/pnas.0402824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WC, Wang JD, Lue JH, Day AS, Young YH. Physiological and morphological assessment of the saccule in guinea pigs after noise exposure. Arch Otolaryngol Head Neck Surg. 2008;134:1099–1106. doi: 10.1001/archotol.134.10.1099. [DOI] [PubMed] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Juntunen J, Ylikoski J, Ojala M, Matikainen E, Ylikoski M, Vaheri E. Postural body sway and exposure to high-energy impulse noise. Lancet. 1987;330:261–264. doi: 10.1016/s0140-6736(87)90840-3. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH, Hanscom B. Are hearing loss and balance dysfunction linked in construction iron workers? Br J Ind Med. 1992;49:138–141. doi: 10.1136/oem.49.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JU, Lee HJ, Kang HH, Shin JW, Ku SW, Ahn JH, Kim YJ, Chung JW. Protective effect of isoflurane anesthesia on noise-induced hearing loss in mice. Laryngoscope. 2005;115:1996–1999. doi: 10.1097/01.mlg.0000180173.81034.4d. [DOI] [PubMed] [Google Scholar]
- Kumar K, Vivarthini CJ, Bhat JS. Vestibular evoked myogenic potential in noise-induced hearing loss. Noise & Health. 2010;12:191–194. doi: 10.4103/1463-1741.64973. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Han GC, Park HJ, Minor LB. Rotational responses of the vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. JARO. 2008;9:334–348. doi: 10.1007/s10162-008-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172:369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Angelaki DE. Vestibular signals in macaque extrastriate visual cortex are functionally appropriate for heading perception. J Neurosci. 2009;29:8936–8945. doi: 10.1523/JNEUROSCI.1607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, McCaul KA, Hankey GJ, Norman PE, Almeida OP, Dobson AJ, Byles JE, Yeap BB, Flicker L. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment–is there a gender difference? Maturitas. 2011;69(4):359–364. doi: 10.1016/j.maturitas.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Man A, Segal S, Naggan L. Vestibular involvement in acoustic trauma (an electronystagmographic study) J Laryngol Otol. 1980;94:1395–1400. doi: 10.1017/s0022215100090228. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Kurokawa T, Saito T, Saito H. Vestibular dysfunction in noise induced hearing loss. Acta Otolaryngol Suppl. 1995;519:262–264. doi: 10.3109/00016489509121919. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. J Neurosci. 1994a;14:6071–6083. doi: 10.1523/JNEUROSCI.14-10-06071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994b;14:6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat. J Neurophysiol. 1995;74:1563–1572. doi: 10.1152/jn.1995.74.4.1563. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18:355–360. [PubMed] [Google Scholar]
- Minor LB, Solomon D, Zinreich J, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995;103:174–178. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol. 1997;117:66–72. doi: 10.3109/00016489709117994. [DOI] [PubMed] [Google Scholar]
- Oosterveld WJ, Polman AR, Schoonheyt J. Vestibular implications of noise-induced hearing loss. Br J Audiol. 1982;16:227–232. doi: 10.3109/03005368209081467. [DOI] [PubMed] [Google Scholar]
- Parker DE, Tubbs RL, Littlefield VM. Visual field displacements in human beings evoked by acoustical transients. J Acoust Soc Am. 1978;63:1912–1918. doi: 10.1121/1.381894. [DOI] [PubMed] [Google Scholar]
- Pyykko I, Aalto H, Ylikoski J. Does impulse noise induce vestibular disturbances? Acta Otolaryngol (Stockh) 1989;468:211–216. doi: 10.3109/00016488909139048. [DOI] [PubMed] [Google Scholar]
- Quinn KJ, Rude SA, Brettler SC, Baker JF. Chronic recording of the vestibulo-ocular reflex in the restrained rat using a permanently implanted scleral search coil. J Neurosci Methods. 1998;80:201–208. doi: 10.1016/s0165-0270(98)00005-3. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Knight RT, Brailowsky S, Prospero-Garcia O, Scabini D. Altered peripheral and brainstem auditory function in aged rats. Brain Res. 1985;348:28–35. doi: 10.1016/0006-8993(85)90355-5. [DOI] [PubMed] [Google Scholar]
- Shupak A, Bar-El E, Podoshin L, Spitzer O, Gordon CR, Ben-David J. Vestibular findings associated with chronic noise induced hearing impairment. Acta Otolaryngol. 1994;114:579–585. doi: 10.3109/00016489409126109. [DOI] [PubMed] [Google Scholar]
- Stahl JS, van Alpen AM, De Zeeuw CI. A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Methods. 2000;99:101–110. doi: 10.1016/s0165-0270(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: Function, development and plasticity. Progress in Neurobiology. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sun H, Lin CH, Smith ME. Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma. PLoS ONE. 2011;6:e28372. doi: 10.1371/journal.pone.0028372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26–32. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio P. Das ohr und die Entstehung der Sprache und Schrift. Berlin: Urban & Schearzenberg; 1929. [Google Scholar]
- Xu Y, Simpson I, Tang X, Zhou W. Acoustic clicks activate both the canal and otolith vestibulo-ocular reflex pathways in behaving monkeys. JARO. 2009;10:569–577. doi: 10.1007/s10162-009-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen A, Kaprio J, Pyykko I, Sorri M, Koskenvuo M, Rantanen T. Hearing acuity as a predictor of walking difficulties in older women. J Am Geriatr Soc. 2009;57:2282–2286. doi: 10.1111/j.1532-5415.2009.02553.x. [DOI] [PubMed] [Google Scholar]
- Wang YP, Hsu WC, Young YH. Vestibular evoked myogenic potentials in acute acoustic trauma. Otol Neurotol. 2006;27:956–961. doi: 10.1097/01.mao.0000231590.57348.4b. [DOI] [PubMed] [Google Scholar]
- Wang YP, Young YH. Vestibular-evoked myogenic potentials in chronic noise-induced hearing loss. Otolaryngol Head Neck Surg. 2007;137:607–611. doi: 10.1016/j.otohns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wit HP, Bleeker JD, Mulder HH. Responses of pigeon vestibular nerve fibers to sound and vibration with audiofrequencies. J Acoust Soc Am. 1984;75:202–208. doi: 10.1121/1.390396. [DOI] [PubMed] [Google Scholar]
- Ylikoski J. Impulse noise induced damage in the vestibular end organs of the guinea pig. Acta Otolaryngol (Stockh) 1987;103:415–421. [PubMed] [Google Scholar]
- Ylikoski J. Delayed endolymphatic hydrops syndrome after heavy exposure to impulse noise. Am J Otolaryngol. 1988;9:282–285. [PubMed] [Google Scholar]
- Ylikoski J, Juntunen J, Matikainen E, Ylikoski M, Ojala M. Subclinical vestibular pathology in patients with noise-induced hearing loss from intense impulse noise. Acta Otolaryngol. 1988;105:558–563. doi: 10.3109/00016488809119520. [DOI] [PubMed] [Google Scholar]
- Young ED, Fernandez C, Goldberg JM. Responses of squirrel monkey vestibular neuron to audio-frequency sound and head vibration. Acta Otolaryngol. 1977;84:352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ding D, Kraus KS, Yu D, Salvi RJ. Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin. Aud Med. 2009;7:189–199. doi: 10.3109/16513860903335795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Tang X, Wei W, Mustain W, Xu Y, Zhou W. Click-evoked responses in vestibular afferents in rats. J Neurophysiol. 2011;106:754–763. doi: 10.1152/jn.00003.2011. [DOI] [PubMed] [Google Scholar]
- Zhu H, Tang X, Wei W, Maklad A, Mustain W, Rabbitt R, Highstein S, Allison J, Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. JARO. 2014;15:73–86. doi: 10.1007/s10162-013-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga MG, Dinkes RE, Davalos-Bichara M, Carey JP, Schubert MC, King WM, Walston J, Agrawal Y. Association between hearing loss and saccular dysfunction in older individuals. Otol Neurotol. 2012;33:1586–1592. doi: 10.1097/MAO.0b013e31826bedbc. [DOI] [PMC free article] [PubMed] [Google Scholar]


