Abstract
Language functional magnetic resonance imaging for neurosurgical planning is a useful but nuanced technique. Consideration of primary and secondary language anatomy, task selection, and data analysis choices all impact interpretation. In the following chapter, we consider practical considerations and nuances alike for language functional magnetic resonance imaging in the support of and comparison with the neurosurgical gold standard, direct cortical stimulation. Pitfalls and limitations are discussed.
Keywords: language fMRI, language mapping, neurosurgical planning
Language mapping using functional magnetic resonance imaging (fMRI) and direct cortical stimulation (DCS) during awake craniotomy is a common therapeutic strategy for patients with brain tumors in the dominant hemisphere. The combination of presurgical planning using fMRI maps and intraoperative cortical stimulation during awake craniotomy to validate fMRI localizations has become a powerful tool in risk assessment, patient counseling, and reducing time for intraoperative cortical mapping.1,2 However, the techniques not only assay speech differently (activation for fMRI vs deactivation for DCS) but they also have distinct methods of failure that in some cases can make concordance testing meaningless. The goals of this chapter are 3-fold. First, we aim to summarize the state of the field of language mapping for presurgical planning including common practices at Memorial Sloan-Kettering Cancer Center and within the literature. Second, we will review what can and cannot be learned about language organization and reorganization in brain tumor patients using these techniques. Lastly, we will discuss the issues with both fMRI and DCS that limit the use of these maps during tumor resection.
COMMON CLINICAL SCENARIOS
Frontal Dysfunction
Frontal lesions that involve the inferior frontal gyrus (classically defined Broca area) or the middle frontal gyrus often cause semantic and phonemic paraphasias, facial droop, speech arrest, agrammatism, dysarthria, speech apraxia, or other productive aphasia that hinders speech production.3 Although both dysarthria and speech apraxia can be caused by frontal lesions, only the presence of true speech apraxia can lend information about a patient’s dominant hemisphere. Dysarthria is a motor disorder that is very broadly seen as slurring of speech and can be caused by lesions in a variety of places in the motor system both peripheral and central, dominant and nondominant hemispheres. It is a disorder of executing planned motor speech programs. Speech apraxia is a motor speech disorder specifically affecting the formation of the motor speech programs themselves. Lesions are most often in the dominant hemisphere unlike in the case of dysarthria. Patients with speech apraxia, such as dysarthrics, have similar trouble in articulation, but linguistic components such as overlearned sequences and vowels (in contrast to consonants) can be spoken clearly by the patient. Speech is more often consistently affected in dysarthria regardless of the task.4 The aforementioned is a perfect example of how a general sign can only occasionally lend a hypothesis about whether a lesion is in the dominant or nondominant hemisphere and only after systematic testing that the routine neurosurgical workup often does not include. As a result, language mapping of some form is often indicated in both cohorts.
Temporo-parietal Dysfunction
Patients with lesions near the posterior superior temporal gyrus (classically defined Wernicke area), middle temporal gyrus, supramarginal gyrus, and or angular gyrus often present with more varied deficits. For example, they may have word-finding difficulty, receptive aphasia (inability to answer questions, or understand commands), agraphia (difficulty with writing), alexia (difficulty with reading), perseveration (naming a second picture the same as the first), or circumlocuitous speech where they specifically avoid the most direct or appropriate word in favor of one they can find more easily. This mixed bag of deficits seen with lesions in this area is recapitulated by an increased variability in the localization of speech function using DCS in the temporo-parietal region. In an early study of 11 adult epileptic patients, although frontal speech was consistently localized in the same inferior frontal region, naming, a commonly attributed temporo-parietal function, was only localized in 50% to 80% of the temporo-parietal sites assayed.5 A contemporary of this classic study characterized the distribution of 5 types of paraphasic-naming errors upon cortical stimulation of the temporo-parietal region in 110 patients. This study similarly found a widely distributed network subserving temporo-parietal speech. For example, semantic paraphasias were found upon stimulation of 18 separate cortical regions in the temporo-parietal area.6 Later, we will see that this diffuse control of function translates to more variable functional MRI mapping of the same region.
Lateralization and Handedness
Patients are generally referred for language mapping to localize function in relation to a lesion or lateralize speech function to a hemisphere. Handedness is positively correlated with hemispheric lateralization for language. Although the estimated percentages are of some debate, language is the purview of the left hemisphere in approximately 95% of right-handed people and 70% of left-handed people.7 The degree of language laterality has been shown to correlate with the degree of handedness. That is, the degree of atypical right hemisphere dominance increases linearly with the degree of left-handedness.8,9 As a result, tools that quantify a patient’s handedness such as the Edinburgh Handedness Inventory are often used as a surrogate to language mapping.10 At MSKCC, language lateralization mapping is most often requested in right-handed patients with left hemispheric lesions, left-handed patients with left or right hemispheric lesions, or right-handed patients with right hemispheric lesions and signs or symptoms of aphasia. This last patient cohort will report speech symptoms during seizures or fatigue and the fMRI is ordered to rule out atypical right hemisphere dominance.
Localization
Once a patient’s hemispheric risk is considered, they are then further subdivided into more specific categories on the basis of the location of their lesion. With some exceptions, most patients who are referred for language mapping fall into 3 categories: patients with frontal language at risk, patients with temporal language at risk, and patients for whom the question is hemispheric laterality. In this context, we will review some common fMRI paradigms used to map the language system. It is important to note that although fMRI paradigms are designed to target either frontal or temporal speech (Broca area or Wernicke area, respectively), many fMRI paradigms, as a result of significant interconnectivity between language centers, will measure both. However, we will review the presurgical fMRI paradigms in terms of their targets particularly because the intraoperative signs during DCS are fairly well segregated.
THE ANATOMY AND ITS FUNCTIONALITY
A Note of Caution
It should be noted that inferences drawn from the multitude of structure/function fMRI studies on language often draw their conclusions from group analyses. That is, the resultant map of function asks the question “which of these structures is conserved in activation across all of my subjects.” This should conceptually be a sensitive, conservative approach and particularly relevant to neurosurgery given that any structure that is commonly activated is more likely to be a necessary rather than sufficient structure. However, there are a variety of technical limitations to this approach that limit the use of group results to individual patient treatment planning particularly using fMRI.
The most basic form of group fMRI analysis requires that each subject’s brain be normalized to a template space. This kind of nonlinear transformation is necessary in order to have precise anatomical localization of a function that can be generalized to the population. Because there is significant variability in subject anatomy, the composite “fitted” brain is a best fit. This means that there is necessarily error in the anatomical registration between subjects. This error can be as large as 1 cm.11 Any error in spatial registration necessarily limits the sensitivity of the measurement of any cognitive process across subjects that may reside in similar but different regions. This mismatch can result in low reliability and weak sensitivity in group studies (particularly in weakly activated regions such as secondary language areas) and in turn difficult usability in patient treatment planning.12 In fact, even normal control studies of language can show significant variability in localization of function. In a Positron Emission Tomography (PET) study of 20 healthy volunteers performing a verb generation task, as much as 9.9 mm of variability was measured in the location of cross-referenced loci of PET activation.
Thus, it follows that statements about language function at the level of pathological cohorts (seizure patients, tumor patients, etc) should exhibit so much variability in structure–function relationships as to make the predictability of secondary language organization certainly, but primary language organization occasionally very difficult.13 Lastly, group results in any cohort do not account for lesional modifications in function. This can be in the form of true translocation of function14,15 to the more common reorganization of function that has mixed clinical significance.16 It is in this context that we review the current thinking in the organization language organization in the clinical setting of neurosurgical planning. Note that the structure–function relationships reported here are limited and are necessarily a mix of single patient, patient cohorts, and control group studies and should be used as a guide.
FRONTAL LANGUAGE MAPPING
Broca Area
Mapping considerations for frontal language areas primarily involve localizing putative Broca area and its surrounds mostly as a result of the dramatic deficits (including mutism) seen as a result of iatrogenic damage to this area. Classically defined Broca area includes the pars triangularis and pars opercularis portions of the inferior frontal gyrus.17,18 There have been many attempts to localize and lateralize Broca area purely using anatomical morphometry, but significant enough variability between subjects has rendered this approach unreliable.19 One study attempted to localize Broca area by identifying 3 variants of sulcal topography. The predicted sites were then confirmed using DCS. The authors in part conclude that for the 70% of patients who fell into the “Type I category” where Broca area was distributed around the inferior precentral sulcus, speech arrest was located at a mean of 2.4 cm from the antero-inferior aspect of the pars opercularis. Although these types of classifications may be more useful in patients with nonspace-occupying lesions, patients with brain tumors in the frontal operculum often have distorted anatomy escaping morphometric classification alone. Further, anatomical rules say nothing about the function of the cortex, which can be altered particularly in children with seizure disorders and patients with brain tumors.20,21
In addition to subserving frank motor coordination and execution of speech, Broca area is now thought to support a wide variety of additional language subcomponents. It has been shown to either drive or participate in the syntactic component of speech22,23 in addition to the phonological processing and manipulation of sound patterns in language24,25 and it has been implicated in language expression including sentence construction and grammar (rather than word content and meaning).26,27 Although the aforementioned functions fit in the context of Broca area as a production, coordination, and execution entity, there is increasing evidence that this region also plays a role in some cognitive aspects of speech. The inferior frontal gyrus has accordingly been shown to be involved in implicit selection and determination of the meaning of a word within a given context.26,28 Although this degree of specificity is beyond the scope of most language mapping for neurosurgical planning, one can imagine the gradual incorporation of more sensitive measures of speech localization.
Secondary Frontal Language
Modern models of speech production also suggest a significant role for the insula, the middle frontal gyrus, the supplementary motor area (SMA), and the inferior aspect of the primary motor gyrus in the frontal lobe. Although their participation in speech is well documented, the extent to which these areas support essential speech and should be avoided during a neurosurgical procedure varies significantly between the patients. It is for this reason that patient-specific mapping such as presurgical fMRI plays a role in treatment planning.
The Insula
The insula is a phylogentically old region of the brain tucked deep to the frontal operculum. Damage to the precentral gyrus of the insula has been shown to be correlated with speech apraxia,29 though a causal relationship between insular damage and speech apraxia is of some debate. The main argument is that patients with speech apraxia always have insular damage, but patients with insular damage do not always have speech apraxia.30 The insula has been posited to contribute to aspects of naming, word finding, and articulation31 and is also commonly thought to control ventilatory needs during articulation.32 Ackermann and Riecker,32 in a comprehensive review of insular function, note that lesions restricted to the insula are rare and putative speech areas and their connections are close such that conclusions about the role of the insula in speech production are unreliable.
DCS of the insula, while uncommon due to its deep location, has achieved speech interruption in some patients.33,34 A recent electrical stimulation study of 25 patients with drug refractory focal epilepsy showed speech arrest or hypophonia in the left middle short gyrus of the insula in 5 patients.35
fMRI activation in the insula during speech tasks is not uncommon36–38 and the range of function attributed to this region is wide including but not nearly limited to vowel production, bilingual language attainment, and the phonetic-linguistic structure of verbal utterances.39 To date, functional imaging paradigms do not target the insula for neurosurgical planning, but in our experience, this area appears frequently during fMRI language mapping particularly in vocalized speech tasks.
Middle Frontal Gyrus/DLPFC
The middle frontal gyrus is a large area that is partly composed of the premotor area and the dorso-lateral prefrontal cortex (DLPFC). This area, in its location just superior to Broca area (Brodmann areas 46 and 9), contributes to aspects of verbal working memory.40 As a result, it commonly activates during speech fMRI examinations. Further, it has been implicated in a variety of higher cognitive functions, including top-down control of working memory capacity,41 selective attention, and task management.42 Its role in essential speech production and hence its relevance to neurosurgical planning is of some debate. Pure dysarthria as a result of an isolated stroke to the middle frontal gyrus has been reported.43 A recent surgical study showed that of 151 patients who had electrocortical stimulation testing in the portion of the DLFPC just superior to Broca area showed anomia in anywhere between 3.6% and 10.8% of patients stimulated depending on the location within the middle frontal gyrus and region of the inferior frontal sulcus.44 However, the same study also shows 10 regions within the middle frontal gyrus where 100% of patients stimulated did not show interruptions of speech. In our own experience of over 1000 patient studies at MSKCC, fMRI activates the middle frontal gyrus often in speech studies. However, only a small proportion of those cases have shown DCS interruption at these fMRI localizations (anecdotal data).
Supplementary Motor Area
The SMA is another secondary language area in the medial frontal lobe of interest in neurosurgical planning. Although its precise anatomical boundaries are not well-defined on the anterior margin, the posterior margin of the SMA is functionally delimited by the foot motor region (Fig. 1) The SMA is functionally divided into 2 regions; the anterior portion of the SMA (pre-SMA) subserves language planning and the posterior portion is responsible for planning motor execution.45 This functional subdivision while likely variable in its precise localization has been delineated by the VCA line (vertical commisure anterior line) drawn vertically from the anterior commisure.46 It has been suggested that there is a graded functionality separating the pre-SMA and the SMA proper. Peck et al45 showed an area of common activation during both motor and language tasks, suggesting that there may be a region of the SMA that might carry a high probability of deficit if damaged. However, operative deficits relative to this region were not examined in this study.45
FIGURE 1.
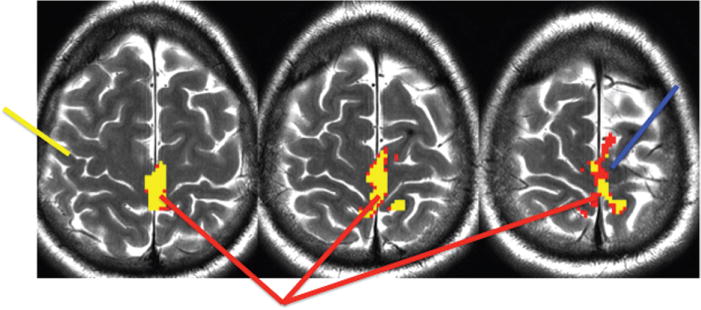
fMRI foot motor fMRI (red arrow), motor supplementary motor area (SMA) (blue arrow), position of the central sulcus (yellow arrow). Although not precise anatomical marker for the position of the motor component of the SMA exists, in our experience, it is often found medial to the precentral sulcus.
Damage to the SMA results in an ataxic syndrome that can have both motor and/or speech components. The language component of SMA syndrome is characterized by reduced spontaneous speech, but in severe cases, iatrogenic lesions can produce mutism.47 Interestingly, these deficits usually recover on the order of weeks to months. Using fMRI to predict whether patients are at risk for motor deficits, speech deficits or both is a topic of debate. A recent study suggested that the presence of preoperative fMRI activation in the contralateral, healthy SMA was correlated with postoperative SMA syndrome.47 Furthermore, most studies agree that although fMRI activation of the SMA during speech tasks commonly activates both hemispheres, iatrogenic lesions to the SMA in the language-dominant hemisphere are most likely to cause deficits. A neurosurgical study determined that the risk of postoperative speech deficit was 100% when the distance between the SMA fMRI centroid of activation was 5 mm or less to the border of a tumor.48 In addition, there is evidence in the motor system that contralateral SMA plasticity (as indicated by increased fMRI signal) following surgery may shorten recovery time.49 Of course, motor recovery and language recovery are likely to involve different mechanisms.
TEMPORO-PARIETAL LANGUAGE MAPPING
Superior Temporal Gyrus
Wernicke area is located in the posterior aspect of the superior temporal lobe just posterior to Heschle gyrus extending to the posterior most aspect of the sylvian fissure. Its posterior margin is poorly delineated and the end of Wernicke area and the beginning of the angular gyrus is not well defined. It is broadly responsible for speech reception and comprehension,50,51 but recent studies have refined and broadened its function. Many of the studies that characterize speech reception and comprehension focus on distinguishing between speech and nonspeech sounds. For example, Wise et al52 delineated perceptual subsystems within Wernicke area. Portions of the supratemporal cortical plane responded to speech and nonspeech sounds; the most posterior and medial aspect of the superior temporal gyrus at the parietal junction responded to speech production rather than perception and the superior temporal sulcus by contrast responded to external sources of speech as well as lexical recall. Although this level of subdivision is beyond the scope of neurosurgical planning, it is worth noting that Wernicke area and its surrounds have both perceptual and integrative components that will both be relevant in the interpretation of temporo-parietal speech fMRI studies and the unraveling of iatrogenic deficits in this region.
Language mapping with aural stimuli often elicits bilateral temporal fMRI activations. Without an understanding of Wernicke area role in speech perception and phonological processing,53 these bilateral activations can confound judgments of hemispheric laterality of language. fMRI of aurally delivered speech not only elicits the expected bilateral primary auditory activations in Heschle gyrus but bilateral superior temporal activations as well. A recent study looked at the anatomical specificity for speech perception and rhythm. It concluded that intonation or prosody is not only the purview of the right homologue of Broca area in the frontal lobe but also the right posterior superior temporal sulcus. Intonation also activated the bilateral superior temporal gyrus and sulcus.54
It has also been suggested that lesions to Wernicke area and surrounds can produce not only the typical fluent aphasia but also category-specific deficits. Campanella et al55 showed deficits in naming manipulable objects in patients with lesions in the left posterior temporal lobe. These lesions, however, encompassed large regions of the posterior temporal lobe. In an analysis of the literature on category-specific disorders, Gainotti56 attributed the impairment of living things to the bilateral antero-mesial and inferior portions of the temporal lobes. Lexical impairments in “plants” were found secondary to a lesion in infero-mesial parts of the temporo-occipital region in the left hemisphere.56
A variety of studies have implicated Wernicke area in verbal memory. In a study of 210 stoke patients, investigators showed that the integrity of the superior temporal gyrus and sulcus predicted not only the ability to comprehend sentences but also auditory memory capacity.51 Given its role in acoustic integration and comprehension, it is no surprise that the posterior superior temporal region is heavily involved in lexical memory. It is intuitive that word mimicry and phonetic sequencing are necessary processes for long-term potentiation of words.52
SECONDARY TEMPORO-PARIETAL LANGUAGE AREAS
Middle Temporal Gyrus
Speech requires that meaning or semantics get queried with phonological input. The middle temporal gyrus situated just inferior to Wernicke area has been implicated in many aspects of speech from phonemic discrimination57 to lexical-semantic retrieval58 on the word level. Whitney et al59 used Transcranial Magnetic Stimulation (TMS) to determine to what degree the posterior middle temporal gyrus (and inferior frontal gyrus) acted as a semantic store and/or whether these areas have executive mechanisms that can direct semantic knowledge instead. In this experiment, TMS did not interrupt decisions with automatic semantic associations (salt:pepper) only those with weak associations (salt:grain). From this, the authors concluded that the posterior middle temporal lobe does not likely act as a semantic store, but rather as a semantic control/execution center. More broadly, other studies have implicated the middle temporal gyrus in semantic decision making as well.60,61 (eg, Is this an animal and is it used by humans?) Given its integrative function, the middle temporal gyrus would be expected to act as a semantic relay station. Turken and Dronkers,50 in fact in a functional connectivity analysis, show that the left middle temporal gyrus is functionally connected to many subregions within the left temporal, right temporal, left frontal, right frontal, and left parietal regions. Given its semantically heavy responsibility, it has been shown that the middle temporal gyrus also participates in speech comprehension at the word (rather than the sentence) level.62
Inferior Temporal Gyrus
The inferior temporal gyrus located, as the name implies, in the inferior aspect of the temporal lobe mediates a variety of cognitive processes best summarized as functions of recognition. Portions of the anterior fusiform gyrus situated in part in the inferior temporal lobe have been suggested to be functionally specialized for word strings63 and has since been termed the visual word form area.64 However, the specificity of this area as such has been challenged.65 Studies have also implicated the area in lexico-semantic judgments.66,67
ANTERIOR TEMPORAL LOBE
The anterior temporal lobe activates variably in functional imaging studies of language for presurgical planning. Two dominant hypotheses for this region consider the anterior temporal lobe responsible for driving either the semantic properties of words or lexical retrieval. A recent case report of a patient with an anterior temporal low-grade glioma revealed that upon detailed neuropsychological testing, the patient had deficits in name retrieval for proper names, tools, but no difficulty retrieving animate object names, suggesting that the anterior temporal lobe may subserve category-specific naming.68 Of course, a single patient with a lesion known to alter functional connectivity makes it difficult to draw broad conclusions. Grabowski et al69 in a PET study dissociated the effects of conceptual/semantic processing from word retrieval by having subjects’ name famous landmarks and famous faces. There was no main effect of category in that both activated the same region in the left temporal pole. This led the authors to also conclude that the temporal pole supports word retrieval rather than semantic processing. Many others have attributed the anterior temporal lobe to word retrieval and naming.70,71 Interestingly, it has been suggested that the age of acquisition of the noun may affect its vulnerability to temporal lobe resection.72 This region has also been implicated in face naming.73,74
Supramarginal and Angular Gyri
The supramarginal and angular gyri are situated just anterior to and surrounding the posterior aspect of the lateral sulcus, respectively. The supramarginal gyrus broadly supports the phonological aspects of word processing75 (both left and right supramarginal gyri), whereas the angular gyrus contributes the semantic aspects particularly in reading.76 In fact, the left angular gyrus has been shown to play a particularly important role in learning to read and may be able to distinguish poor readers from good readers.77–79 Mapping these parietal regions for neurosurgical planning, however, has historically applied a broad strategy for localizing reading-associated cortices at risk during a surgical procedure and has not achieved the level of sensitivity that would be required to predict reading deficits from fMRI patterns of activation.
fMRI PARADIGMS
Frontal Speech Mapping fMRI Paradigms
There are a variety of productive speech paradigms that are used during fMRI mapping to target frontal speech areas. Fluency tasks such as phonemic and semantic fluency wherein patients are asked to generate (often silently) words to a given letter or words that fit a category (fruits, countries), respectively, are widely used. The simplest version of this task is contrasted with rest in the baseline (Fig. 2). Another common productive paradigm is verb generation. During verb generation, a patient is presented with a noun either visually or aurally (such as baby or pilot) and is asked to covertly generate verbs. Verb generation is a commonly used task for a variety of reasons. Overall, it shows more specificity than fluency tasks80 and has also shown less variability in measuring hemispheric dominance both across different language tasks and across different groups of patients.81 The reason for this may be a result of a more difficult level of processing than similar productive tasks. The common factor in all of these paradigms is fluency where patients are required to generate lexical responses. Overall, word generation tasks have been shown to reliably localize frontal speech areas.82 In fact, in a comparative study between 6 different language mapping fMRI paradigms, silent word generation was shown to reliably lateralize language even in patients with lesions in putative language areas.83
FIGURE 2.
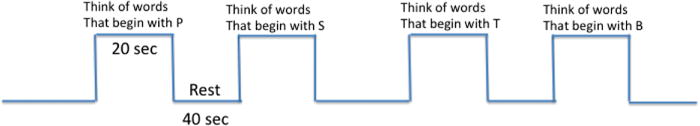
A typical block designed paradigm maximizes detection of the fMRI signal and is ideal for clinical fMRI scanning. Time spent in each condition varies. Twenty seconds in activation and 20 seconds in rest is also common. Forty seconds allow the hemodynamic response maximal time to recover hence maximizing contrast between conditions.
Although the targets of these paradigms are frontal productive speech areas, the responses are most often generated silently during fMRI mapping. Historically, speech fMRI paradigms have been performed silently to avoid head motion. It is well known that head motion can cause both Type I and Type II statistical errors in a functional MRI examination.84,85 However, our group has shown that silent speech fMRI paradigms may not adequately predict the location of frontal speech arrest using electrocortical stimulation during awake craniotomy. Although the clinician performing the fMRI examination must balance choosing the most appropriate behavioral task with maximizing the quality of the images, the surgeon’s goal is to preserve vocalized speech while maximizing lesion resection. In this way, speech arrest during awake craniotomy is used as a primary measure of frontal speech localization. However, silent speech biases the fMRI map anteriorly toward the inferior frontal gyrus in many cases and may underestimate the spatial location where the surgeon can expect to find speech arrest. For this reason, we suggest the inclusion of a vocalized speech task for frontal language mapping. (see Huang et al86 for a technical discussion of how to acquire vocalized speech fMRI while minimizing the effects of head motion) It should be noted however that vocalized tasks may elicit a more bi-hemispheric speech network (prosodic components of speech, motor areas, and auditory perceptual processing, for example) independent of hemispheric dominance. As a result, at this point, vocalized speech tasks should only be used for localization studies and not speech lateralization. The silent/vocalized speech discrepancy between what is typically being measured during the fMRI examination and what is maximally predictive in the context of neurosurgical planning is a good example of the disconnect between mapping procedures. We will highlight these areas of discordance throughout this chapter in an effort to bridge the gap between methodologies.
TEMPORO-PARIETAL LANGUAGE MAPPING
Temporal Language Mapping paradigms
Temporal language is more difficult to measure than frontal language areas.87,88 This may be in part a result of a more distributed posterior language system. Typical language mapping paradigms in fMRI aim to localize receptive regions such as Wernicke area. Less commonly, posterior language mapping must also consider reading and writing (supramarginal and angular) areas in the mapping workup.
Common receptive language paradigms (for Wernicke activation), unlike frontal speech fMRI mapping, come in a variety of forms. The auditory responsive naming paradigm in which patients are given simple questions (eg, What color is grass?) is a popular variation.89,90 This task can be performed with either visually or aurally cued questions and patients are instructed to answer the questions silently. Another fMRI paradigm used to elicit activation in Wernicke area is sentence completion.91 This task is often performed visually and patients have to fill in a sentence with a variety of on-screen choices. (Bill gives haircuts and shampoos. He is a _________________ (1) butcher (2) barber (3) batch (4) beer) Using a visually cued receptive paradigm like sentence completion confers the added advantage of assaying both receptive speech and reading simultaneously. Passive listening to aurally generated sentences has also been used to localize posterior language areas, but it has been suggested that productive paradigms such as silent word generation and rhyming may in fact provide stronger posterior language lateralization.92 In keeping with this idea, verb generation, while broadly considered a productive paradigm, has both in our experience and in the literature been reliable in activating posterior language areas.92
MAPPING LANGUAGE USING fMRI: GENERAL CONSIDERATIONS
In many of the language areas covered, it is noteworthy that all of them have a wide and varied list of functions attributed to them. This is a fundamental problem with using fMRI to predict speech localization or speech interruption during DCS for presurgical planning. An fMRI activation can represent many things. It can mean that the area is excitatory and essential to the function (and would be expected to be amenable to interruption during DCS). This is the clearest scenario for surgical planning because in the best case scenario, there is a one-to-one correlation between what fMRI predicts to be active and what areas show good concordance with DCS. However, the activation may also represent excitatory activation that is a result of having been recruited by an upstream or downstream area to support the function. This area would not necessarily be reliably interrupted during DCS. The SMA is a good example of such an area. Many language tasks will reliably elicit SMA activation, as there is little doubt that it is involved in speech planning. Further, if the dominant SMA is resected, it can produce language dysfunction as severe as mutism. However, because it is thought to be an intermediary or secondary planning and motor execution area, it can be difficult to interrupt using DCS of the SMA during speech tasks. This is especially evidenced by patients in whom DCS fails to elicit speech disruption but demonstrated post-op speech deficits.93 This is likely because it requires a significant amount of current to a secondary area to shut down the primary effector that it supports, in this case presumably Broca area. The last possibility is that the fMRI activation represents an inhibitory activation. In this way, fMRI activations during speech tasks can be difficult to interpret in the context of risk management and planning. It is assumed that if a major language area such as Broca or Wernicke area activates in its expected anatomical location, then that area is essential. However, because fMRI is best used as a screening tool for atypical functional organization, it becomes increasingly difficult for a surgeon to use atypical information that also does not test out during DCS or does so unreliably.
fMRI maps of language function for neurosurgical planning most often serve to assess for atypical hemispheric dominance or to localize fMRI activations in relation to a lesion. Although the behavioral paradigms generally do not differ for lateralization versus localization studies, paradigms wherein patients vocalize their responses during the fMRI examination (in contrast to the more widely performed covert response paradigm used to avoid head motion) have special considerations. There are 2 main considerations for vocalized speech fMRI paradigms for the purpose of neurosurgical planning. First, there has been some question about the predictive value of silent speech fMRI as a guide to intraoperative cortical stimulation.94 Our group has shown that localizations from silent speech underestimate the spatial extent of Broca area in the context of intraoperative speech arrest. For this reason, we recommend the inclusion of a vocalized speech paradigm in the fMRI examination. New techniques have been devised to minimize the effects of head motion on the vocalized speech map. In this technique, the delay in the hemodynamic response function is used to detect and remove the first image where motion artifact is present from the vocalization. The subsequent image wherein the hemodynamic response predominates can then be used to create the fMRI map without the affiliated head motion.86
However, even when motion artifacts are considered when a patient vocalizes their responses during an fMRI examination, they activate a variety of bihemispheric primary sensory systems that are both irrelevant and problematic to the measure of hemisphere dominance. For example, it has been shown that auditory feedback is primarily a right hemispheric system.95 Given the preponderance of left hemispheric language lateralization in right (and left) handed people, these right hemispheric systems would artifactually skew the laterality index. The laterality index is calculated as a ratio measure of the number of voxels in the right and left hemispheres.
Of course, the resultant fMRI map of speech function (or any function for that matter) is directly dependent upon the behavioral paradigm that is used. Further, evaluating an fMRI task can be difficult. There are many parameters that can be extracted from a BOLD fMRI map, including things such as time to peak of the hemodynamic response function, magnitude of the fMRI response, and reliability of the fMRI localizations. With this said, it should be kept in mind that the mandate of the fMRI map for neurosurgical planning is a conservative one, that is, fMRI should ideally measure any cortical area that participates in an essential way in the language system.
MEASURING LANGUAGE LATERALITY USING fMRI
One main goal for presurgical speech mapping with fMRI is lateralization or predicting which hemisphere primarily controls speech. Predicting hemispheric dominance for language using fMRI is a nuanced topic with an extensive literature. We will summarize the main considerations that facilitate application of fMRI for language lateralization in the context of neurosurgical planning.
CONCORDANCE WITH GOLD STANDARDS
The Wada Test
The intracarotid amytal procedure, also known as the Wada test, has served the neurosurgical community since roughly the early 1950s in the measurement of hemispheric dominance for language. During a Wada test, a barbituate is injected into 1 internal carotid artery causing the temporary inactivation of a single hemisphere. Typically, upon infusion into the left carotid artery, the patient will have trouble naming items or responding. However, there are risks associated with the invasive angiography procedure and using fMRI as a noninvasive alternative was a welcome possibility. The Wada test measures inactivations of language areas, fMRI measures activation of language areas, and accordingly, there remains discordance between the techniques that is not altogether unexpected. Further, determining concordance between Wada and fMRI in tumor patients encounters an added complexity of tumor-induced decoupling wherein a lesion’s abnormal neovasculature imparts false negatives on the language map and artifactual determinations of language dominance.96 Despite this and other limitations, fMRI has gained considerable traction as a surrogate to Wada testing and a replacement in many cases. A recent study compared language lateralization measures in 100 epilepsy patients who had both fMRI and Wada testing. It concluded that there was 91% concordance between techniques with fMRI incorrectly categorizing language dominance in 9% of cases.97
Using a combined task analysis wherein the dominance is calculated on a composite output of multiple language tasks (to identify essential ra-her than task dependent activations) is one technique that has increased confidence in the fMRI lateralization measure and concordance between techniques in turn.98 Of note, the single verb generation task has been shown to have similar concordance with WADA as Combined Task Analysis, which is worth noting in practices wherein numerous tasks cannot be performed during fMRI.99 Further, it is felt that concordance between the techniques is quite high in well lateralized patients, but that Wada may be less sensitive in the case of codominance for language where language errors can be more subtle and less consistent.99,100
Codominance is the single most difficult clinical scenario in language mapping adult brain tumor patients and arguably across clinical disciplines. Detecting atypical language dominance is the mandate of the fMRI examination and yet the clinical significance of codominance on Wada and/or fMRI is yet unknown. That is, it is unknown whether codominance for language affords any cognitive protection to a unilateral (often left hemispheric) lesion. One study showed that bilaterality assessed by dichotic listening and fMRI has been correlated with higher performance on many language tests.101 Yet, Lazar and Antoniello102 show that codominant patients still show signs of significant aphasia. Lastly, knowing whether fMRI measures of codominance mean essential, supportive, or other function can be difficult with right inferior frontal, middle frontal, and medial temporal regions activating in left dominant individuals.103
DIRECT CORTICAL STIMULATION
fMRI language maps for neurosurgical planning (speech localization particularly) are often evaluated in the context of concordance with intraoperative cortical stimulation testing. DCS testing is the gold standard by which neurosurgeons plan safe surgical margins during tumor resection and other pathologies. Further, it is the method by which most speech fMRI maps will be validated. In this regard, it is essential that the cortical stimulation procedure be reviewed for a full circle understanding of the end-stage users’ perspective. A comprehensive discussion of the procedure can be found in the study by Szelenyi et al.104
During DCS, the patient is lightly anesthetized and positioned such that the surgical draping does not obscure the patient’s face. An electrode is placed at the median nerve and communicates with a strip electrode on the exposed cortex. Often, language mapping begins by measuring somatosensory-evoked potentials. In this technique, the median nerve is stimulated and the response is detected from a strip electrode array draped over the Rolandic region.105 A phase reversal between 2 of the recording cortical electrodes indicates a likely position of the central sulcus. Bipolar stimulation will then begin at this location.
Because the impedance of the brain varies from patient to patient, bipolar stimulation of the motor cortex often precedes language mapping in order to establish a threshold of action. Depending on the exposure of the craniotomy, facial muscle fasciculations, hand grasp, or arm muscle fasiculations are recorded. Using the threshold that affected a motor response, language mapping commences. Frontal language mapping is mapped by asking the patient to count, recite the days of the week, months of the year, or name pictures. Arrests of speech, paraphasias (errors), or hesitations are noted as they coincide with the electrical stimulation. If no effect is seen, current is escalated until either a response is seen or after discharges are noted on the Electroencephalography. Note that bipolar stimulation of speech areas causes a negative sign, while electrical stimulation of the motor areas causes a positive sign. A good review of the neurophysiology accounting for this effect can be found by Borchers et al.106
CONCLUSION
We have discussed the most common clinical scenarios for language mapping. The goal was to also discuss ways in which the language fMRI map should be interpreted with caution. fMRI for language mapping is gaining in its ubiquitous application to presurgical planning, nuanced acquisition and analysis. As more centers work out the efficacy of language fMRI in their own hands, it is anticipated that language fMRI will continue to contribute to the presurgical toolbox.
Footnotes
The authors report no conflicts of interest.
References
- 1.Petrella JR, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006;240:793–802. doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- 2.Medina LS, et al. Seizure disorders: functional MR imaging for diagnostic evaluation and surgical treatment–prospective study. Radiology. 2005;236:247–253. doi: 10.1148/radiol.2361040690. [DOI] [PubMed] [Google Scholar]
- 3.Amunts K, et al. Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8:e1000489. doi: 10.1371/journal.pbio.1000489. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eickhoff SB, et al. A systems perspective on the effective connectivity of overt speech production. Philos Transact A Math Phys Eng Sci. 2009;367:2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang. 1978;6:239–260. doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- 6.Corina DP, et al. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain Lang. 2010;115:101–112. doi: 10.1016/j.bandl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav Brain Sci. 2003;26:199–208. doi: 10.1017/s0140525x03000062. discussion 208–60. [DOI] [PubMed] [Google Scholar]
- 8.Knecht S, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs KL, et al. Degree of handedness and cerebral dominance. Neurology. 2006;66:1855–1858. doi: 10.1212/01.wnl.0000219623.28769.74. [DOI] [PubMed] [Google Scholar]
- 10.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 11.Thirion B, Pinel P, Poline JB. Finding landmarks in the functional brain: detection and use for group characterization. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):476–483. doi: 10.1007/11566489_59. [DOI] [PubMed] [Google Scholar]
- 12.Thirion B, et al. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Giussani C, et al. Anatomical correlates for category-specific naming of living and non-living things. Neuroimage. 2011;56:323–329. doi: 10.1016/j.neuroimage.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 14.Holodny AI, et al. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr. 2002;26:941–943. doi: 10.1097/00004728-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Jacola LM, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Oers CA, et al. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49:885–893. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Dronkers NF, et al. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 18.Pearce JM. Broca’s aphasiacs. Eur Neurol. 2009;61:183–189. doi: 10.1159/000189272. [DOI] [PubMed] [Google Scholar]
- 19.Keller SS, et al. Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Petrovich NM, et al. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am J Neuroradiol. 2004;25:130–133. [PMC free article] [PubMed] [Google Scholar]
- 21.Saltzman J, Smith ML, Scott K. The impact of age at seizure onset on the likelihood of atypical language representation in children with intractable epilepsy. Brain Cogn. 2002;48:517–520. [PubMed] [Google Scholar]
- 22.Petersson KM, Folia V, Hagoort P. What artificial grammar learning reveals about the neurobiology of syntax. Brain Lang. 2012;120:83–95. doi: 10.1016/j.bandl.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Tyler LK, et al. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134(Pt 2):415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahin NT, et al. Sequential processing of lexical, grammatical, and phonological information within Broca’s area. Science. 2009;326:445–449. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaden KI, Jr, Piquado T, Hickok G. Sublexical properties of spoken words modulate activity in Broca’s area but not superior temporal cortex: implications for models of speech recognition. J Cogn Neurosci. 2011;23:2665–2674. doi: 10.1162/jocn.2011.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grindrod CM, et al. The role of the left inferior frontal gyrus in implicit semantic competition and selection: an event-related fMRI study. Brain Res. 2008;1229:167–178. doi: 10.1016/j.brainres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C, et al. Speech and language functions that require a functioning Broca’s area. Brain Lang. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Blumstein SE. Auditory word recognition: evidence from aphasia and functional neuroimaging. Lang Linguist Compass. 2009;3:824–838. doi: 10.1111/j.1749-818X.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- 30.Hillis AE, et al. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- 31.Shafto MA, et al. On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. J Cogn Neurosci. 2007;19:2060–2070. doi: 10.1162/jocn.2007.19.12.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2010;214:419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- 33.Duffau H, et al. The insular lobe: physiopathological and surgical considerations. Neurosurgery. 2000;47:801–810. doi: 10.1097/00006123-200010000-00001. discussion 810–811. [DOI] [PubMed] [Google Scholar]
- 34.Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg. 2009;110:696–708. doi: 10.3171/2008.8.JNS08741. [DOI] [PubMed] [Google Scholar]
- 35.Afif A, et al. Middle short gyrus of the insula implicated in speech production: intracerebral electric stimulation of patients with epilepsy. Epilepsia. 2010;51:206–213. doi: 10.1111/j.1528-1167.2009.02271.x. [DOI] [PubMed] [Google Scholar]
- 36.Chee MW, et al. Left insula activation: a marker for language attainment in bilinguals. Proc Natl Acad Sci U S A. 2004;101:15265–15270. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H, Iverson GK, Park HJ. Neural correlates in the processing of phoneme-level complexity in vowel production. Brain Lang. 2011;119:158–166. doi: 10.1016/j.bandl.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.de Guibert C, et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(Pt 10):3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riecker A, et al. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang. 2008;107:102–113. doi: 10.1016/j.bandl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 41.Edin F, et al. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 43.Uzawa A, Hiraga A, Kamitsukasa I. Pure dysarthria resulting from a small cortical infarction located at the left middle frontal gyrus. Intern Med. 2009;48:75–76. doi: 10.2169/internalmedicine.48.1540. [DOI] [PubMed] [Google Scholar]
- 44.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 45.Peck KK, et al. Joint activation of the supplementary motor area and presupplementary motor area during simultaneous motor and language functional MRI. Neuroreport. 2009;20:487–491. doi: 10.1097/WNR.0b013e3283297d71. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, et al. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krainik A, et al. Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area. Neurology. 2003;60:587–594. doi: 10.1212/01.wnl.0000048206.07837.59. [DOI] [PubMed] [Google Scholar]
- 48.Nelson L, et al. Preoperative mapping of the supplementary motor area in patients harboring tumors in the medial frontal lobe. J Neurosurg. 2002;97:1108–1114. doi: 10.3171/jns.2002.97.5.1108. [DOI] [PubMed] [Google Scholar]
- 49.Krainik A, et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323–1332. doi: 10.1212/01.wnl.0000120547.83482.b1. [DOI] [PubMed] [Google Scholar]
- 50.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leff AP, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132(Pt 12):3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wise RJ, et al. Separate neural subsystems within “Wernicke’s area”. Brain. 2001;124(Pt 1):83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- 53.Chang EF, et al. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13:1428–1432. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, et al. Common and distinct neural substrates for the perception of speech rhythm and intonation. Hum Brain Mapp. 2010;31:1106–1116. doi: 10.1002/hbm.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campanella F, et al. Naming manipulable objects: anatomy of a category specific effect in left temporal tumours. Neuropsychologia. 2010;48:1583–1597. doi: 10.1016/j.neuropsychologia.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Gainotti G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: a review. Cortex. 2000;36:539–559. doi: 10.1016/s0010-9452(08)70537-9. [DOI] [PubMed] [Google Scholar]
- 57.Ashtari M, et al. Left middle temporal gyrus activation during a phonemic discrimination task. Neuroreport. 2004;15:389–393. doi: 10.1097/00001756-200403010-00001. [DOI] [PubMed] [Google Scholar]
- 58.Acheson DJ, et al. A common neural substrate for language production and verbal working memory. J Cogn Neurosci. 2011;23:1358–1367. doi: 10.1162/jocn.2010.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitney C, et al. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex. 2011;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Binder JR, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Visser M, et al. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J Cogn Neurosci. 2012;24:1766–1778. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- 62.Dronkers NF, et al. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- 64.Cohen L, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 65.Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 66.Demonet JF, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115(Pt 6):1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 67.Kuperberg GR, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12:321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- 68.Bi Y, et al. The role of the left anterior temporal lobe in language processing revisited: evidence from an individual with ATL resection. Cortex. 2011;47:575–587. doi: 10.1016/j.cortex.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Grabowski TJ, et al. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- 71.Semenza C. Retrieval pathways for common and proper names. Cortex. 2006;42:884–891. doi: 10.1016/s0010-9452(08)70432-5. [DOI] [PubMed] [Google Scholar]
- 72.Kay J, Hanley JR, Miles R. Exploring the relationship between proper name anomia and word retrieval: a single case study. Cortex. 2001;37:501–517. doi: 10.1016/s0010-9452(08)70590-2. [DOI] [PubMed] [Google Scholar]
- 73.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 74.Ives-Deliperi VL, Butler JT. Naming outcomes of anterior temporal lobectomy in epilepsy patients: a systematic review of the literature. Epilepsy Behav. 2012;24:194–198. doi: 10.1016/j.yebeh.2012.04.115. [DOI] [PubMed] [Google Scholar]
- 75.Hartwigsen G, et al. Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci U S A. 2010;107:16494–16499. doi: 10.1073/pnas.1008121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoeckel C, et al. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45:1091–1096. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meyler A, et al. Brain activation during sentence comprehension among good and poor readers. Cereb Cortex. 2007;17:2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyler A, et al. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welcome SE, Joanisse MF. Individual differences in skilled adult readers reveal dissociable patterns of neural activity associated with component processes of reading. Brain Lang. 2012;120:360–371. doi: 10.1016/j.bandl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Sanjuan A, et al. Comparison of two fMRI tasks for the evaluation of the expressive language function. Neuroradiology. 2010;52:407–415. doi: 10.1007/s00234-010-0667-8. [DOI] [PubMed] [Google Scholar]
- 81.Ruff IM, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol. 2008;29:528–535. doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brannen JH, et al. Reliability of functional MR imaging with word-generation tasks for mapping Broca’s area. AJNR Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 83.Pillai JJ, Zaca D. Relative utility for hemispheric lateralization of different clinical fMRI activation tasks within a comprehensive language paradigm battery in brain tumor patients as assessed by both threshold-dependent and threshold-independent analysis methods. Neuroimage. 2011;54(suppl 1):S136–S145. doi: 10.1016/j.neuroimage.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 84.Boegle R, Maclaren J, Zaitsev M. Combining prospective motion correction and distortion correction for EPI: towards a comprehensive correction of motion and susceptibility-induced artifacts. MAGMA. 2010;23:263–273. doi: 10.1007/s10334-010-0225-8. [DOI] [PubMed] [Google Scholar]
- 85.Jezzard P, Clare S. Sources of distortion in functional MRI data. Hum Brain Mapp. 1999;8:80–85. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<80::AID-HBM2>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 2002;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrington GS, Buonocore MH, Farias ST. Intrasubject reproducibility of functional MR imaging activation in language tasks. AJNR Am J Neuroradiol. 2006;27:938–944. [PMC free article] [PubMed] [Google Scholar]
- 88.Lehericy S, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 89.Pouratian N, et al. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. Neurosurg Focus. 2002;13:e4. [PubMed] [Google Scholar]
- 90.Niskanen E, et al. The effect of fMRI task combinations on determining the hemispheric dominance of language functions. Neuroradiology. 2012;54:393–405. doi: 10.1007/s00234-011-0959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaca D, et al. Effectiveness of four different clinical fMRI paradigms for preoperative regional determination of language lateralization in patients with brain tumors. Neuroradiology. 2012;54:1015–1025. doi: 10.1007/s00234-012-1056-2. [DOI] [PubMed] [Google Scholar]
- 92.Pillai JJ, Zaca D. Relative utility for hemispheric lateralization of different clinical fMRI activation tasks within a comprehensive language paradigm battery in brain tumor patients as assessed by both threshold-dependent and threshold-independent analysis methods. Neuroimage. 2011;54(Suppl 1):S136–S145. doi: 10.1016/j.neuroimage.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg K, et al. Prediction of neurological deficits and recovery after surgery in the supplementary motor area: a prospective study in 26 patients. J Neurosurg. 2010;113:1152–1163. doi: 10.3171/2010.6.JNS1090. [DOI] [PubMed] [Google Scholar]
- 94.Petrovich N, et al. Discordance between functional magnetic resonance imaging during silent speech tasks and intraoperative speech arrest. J Neurosurg. 2005;103:267–274. doi: 10.3171/jns.2005.103.2.0267. [DOI] [PubMed] [Google Scholar]
- 95.Toyomura A, et al. Neural correlates of auditory feedback control in human. Neuroscience. 2007;146:499–503. doi: 10.1016/j.neuroscience.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 96.Ulmer JL, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–579. doi: 10.1227/01.neu.0000134384.94749.b2. discussion 580–581. [DOI] [PubMed] [Google Scholar]
- 97.Woermann FG, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- 98.Ramsey NF, et al. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage. 2001;13:719–733. doi: 10.1006/nimg.2000.0722. [DOI] [PubMed] [Google Scholar]
- 99.Rutten GJ, et al. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17:447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- 100.Benke T, et al. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada test. Epilepsia. 2006;47:1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 101.van Ettinger-Veenstra HM, et al. Right-hemispheric brain activation correlates to language performance. Neuroimage. 2010;49:3481–3488. doi: 10.1016/j.neuroimage.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 102.Lazar RM, Antoniello D. Variability in recovery from aphasia. Curr Neurol Neurosci Rep. 2008;8:497–502. doi: 10.1007/s11910-008-0079-x. [DOI] [PubMed] [Google Scholar]
- 103.Van Ettinger-Veenstra H, et al. Right-hemispheric cortical contributions to language ability in healthy adults. Brain Lang. 2012;120:395–400. doi: 10.1016/j.bandl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Szelenyi A, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- 105.Ulbert I, et al. Multiple microelectrode-recording system for human intracortical applications. J Neurosci Methods. 2001;106:69–79. doi: 10.1016/s0165-0270(01)00330-2. [DOI] [PubMed] [Google Scholar]
- 106.Borchers S, et al. Direct electrical stimulation of human cortex: the gold standard for mapping brain functions? Nat Rev Neurosci. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]


