Abstract
Alcohol consumption with psychostimulants is very common among drug addicts. There is little known about the possible pharmacological interactions between alcohol and psychostimulants. Among most commonly co-abused psychostimulants with alcohol are methamphetamine, cocaine, 3,4-methylenedioxymethamphetaminen, and nicotine. Co-abuse of alcohol with psychostimulants can lead to several neurophysiological dysfunctions such as decrease in brain antioxidant enzymes, disruption of learning and memory processes, cerebral hypo-perfusion, neurotransmitters depletion as well as potentiation of drug seeking behaviour. Moreover, co-abuse of alcohol and psychostimulants can lead to increase in heart rate, blood pressure, myocardial oxygen consumption and cellular stress, and the risk of developing different types of cancer. Co-abuse of alcohol with psychostimulants during pregnancy can lead to fetal brain abnormalities. Further studies are needed to investigate the pharmacokinetics, pharmacodynamics, and neurochemical changes on co-abuse of alcohol and psychostimulants.
Keywords: Alcohol; Ethanol; Methamphetamine; Nicotine; Cocaine; Alcohol interaction; 3,4-Methylenedioxymethamphetainen
Introduction
Alcohol dependence is considered a major public health problem worldwide [1–6]. Alcohol can contribute to a significant number of disabilities due to psychological, medical, injury, or other detrimental effects [7]. These effects can be dramatically severe when alcohol is consumed with other drugs of abuse. Alcohol consumption with other drugs of abuse is very common among drug users. Different pharmacological mechanisms of interactions may occur when alcohol and other psychostimulants are co-abused. It is noteworthy that drugs of abuse have been shown to alter central brain reward circuitry, which can lead addicts to increase their alcohol intake for reward effects [8,9]. Alcohol use with other drugs of abuse has been reported to hinder decision making, thinking, and neurocognitive capabilities [10–15]. Moreover, recent studies confirmed that alcohol and other drugs of abuse are usually found in the blood of deceased or seriously injured drivers involved in traffic accidents caused by psychomotor function impairment [16–20].
We discussed here several findings related to alcohol interactions with psychostimulants. According to previous reports, alcohol is commonly abused with methamphetamine (METH), cocaine and marijuana [21]. Men have higher prevalence of co-abuse of alcohol and other drugs compared to women [22]. The prevalence of drugs of abuse has been shown to have a positive correlation with the level of alcohol intake [22]. We reviewed here the available literature regarding alcohol interactions with certain psychostimulants, including METH, cocaine, nicotine, and 3,4-methylenedioxymethamphetaminen (MDMA), according to animal experimental and clinical studies.
Alcohol and METH co-abuse
METH abuse is an increasing health problem worldwide. According to the available data from national surveys between the years of 2002 and 2004, more than 16 million Americans over the age of 12 have used METH [23]. METH is a derivative of amphetamine with increased CNS activities and effects. METH can be abused by different routes such as inhalation, ingestion, or intravenous injection, with acute effects that can last for up to 24 h [24,25]. It is well known that METH can stimulate the release of monoamines such as dopamine and norepinephrine to produce euphoria and to increase alertness and libido [26–28]. METH abusers frequently use alcohol and have a higher risk of reaching alcohol intoxication level [29]. The prevalence of alcohol use disorder was found 75% higher among amphetamine dependent patients [30]. For example, a study reported that more than 60% of METH users in New York City reported abusing METH in combination with alcohol [31]. Recent study conducted on regular METH users showed that alcohol drinking increased the chances of METH use in same day by more than 4 folds [32]. Despite this evidence of high prevalence of METH and alcohol co-abuse, very few studies have investigated the effects of their co-abuse. A summary of possible effects of concurrent exposure to alcohol and METH is presented in Table 1.
Table 1.
Aspects and effects of alcohol and psychostimulants interactions.
| Drug of Abuse | Aspect of interaction | Effects of interactions |
|---|---|---|
| METH | METH metabolism | Alcohol decreased p-hydroxylated metabolites of METH in the urine of METH abusers [33]. |
| Alcohol increased the levels of METH and its active metabolite, amphetamine, in rats and rabbits [34,35]. | ||
| Performance and sleep | Lower detrimental effects on performance and sleep compared to each drug alone [36]. |
|
| Euphoria | Increased euphoria in alcohol and methamphetamine co-abuse [36]. |
|
| Cardiac effects | Increased myocardial oxygen consumption and cardiac rate [37]. | |
| Prenatal exposure | Damage to striatal region of the brain [41]. | |
| Oxidative stress | Combination caused more impairment of antioxidant enzymes in rats hippocampus and oxidative stress than either drug alone [39]. |
|
| Cocaine | Cocaine metabolism | Alcohol decreased metabolism of cocaine [68]. Alcohol decreased benzoylecgonine renal excretion, and increased in cocaine and cocaethylene blood concentrations [69]. |
| Cardiovascular and endocrine systems | Exposure to cocaine and alcohol increased heart rate, systolic blood pressure, cortisol, and prolactin levels [64,69]. |
|
| Cerebral blood perfusion | Cerebral hypo-perfusion occurred more in individuals taking cocaine and alcohol than in individuals taking cocaine or alcohol alone [72,73] |
|
| Neurobehavioral performances | Negatively affected by concurrent intake of cocaine and alcohol compared to either drug alone [74,75]. |
|
| Mesocorticolimbic dopamine system | Increased extracellular dopamine concentration than either drug alone in nucleus accumbens in rats [90] |
|
| Sense of pleasure and euphoria were found to be improved [71]. | ||
| Nicotine | Drug reinforcement | Rats have established self-administration and place preference to combination of alcohol and cocaine in concentrations that did not provoke reinforcement to either drug alone [56,78]. Cocaine potentiated alcohol seeking [59,79] |
| Mesocorticolimbic dopamine system | Increased in dopaminergic neuron firings and dopamine release in an additive mechanism [111–116]. |
|
| Pleasure and drug seeking | Increased in the pleasurable effects of each drug [119]. Rats self-administered nicotine more than rats received chronic exposure to either drug alone [108]. |
|
| Cardiovascular system | Additive effect on heart rate and blood pressure was found in healthy human volunteers [130,131]. Synergistic increase in left ventricular pressure in dogs [132]. |
|
| Cancer | Increase in the risk of developing esophageal cancer [120–123]. | |
| Prenatal exposure | Showed a multiplicative effect in increasing the risk of head and neck cancer in human [124]. |
|
| Increased the risk of fetal growth restrictions in human [133–135]. Offspring developed rapid nicotine self-administration and at a higher level in rats [136]. | ||
| MDMA | Cardiovascular system | Exacerbated cardiac cellular stress and toxicity through augmented activation of cardiac sympathetic system in adolescent mice [139]. |
| Blood level | MDMA plasma concentration increased following alcohol intake [149]. |
|
| Drug reinforcement | MDMA and alcohol induce a longer duration of euphoria [149]. | |
| Exposure to alcohol during adolescent age in mice increased the reinforcing effects of MDMA [156]. | ||
| Sedation | MDMA reversed the sedative effect induced by alcohol consumption [149]. |
|
| Learning and memory | Administration of alcohol and MDMA exhibited learning and memory impairments [159] |
|
| Dopamine reward effect | MDMA impaired dopaminergic reward pathway, leading to increase alcohol consumption [154]. |
|
| Psychopathological effect | Long term consumption of MDMA and alcohol can lead to serotonin depletion and cause psychopathological changes [155]. |
|
| Prenatal exposure | Impaired working memory, exploratory activity, and neurogenesis in rats offspring [163]. |
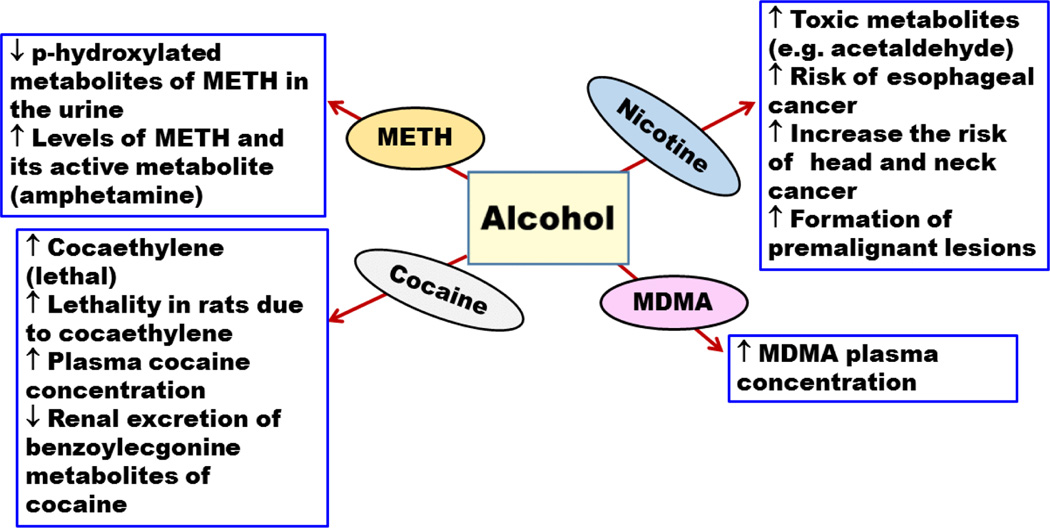
Previous findings demonstrated that alcohol can decrease p-hydroxylated metabolites of METH in the urine of METH abusers, suggesting that alcohol may inhibit METH metabolism [33] (Table 1). This may lead to higher METH blood concentration, with an increase in its stimulating effects on brain and heart. Moreover, recent findings showed that alcohol increased the absorption and distribution of METH and its active metabolite, amphetamine, in several organs, including brain in rats and rabbits (Figure 1) [34,35]. A recent study compared the acute effects of alcohol, METH, and their combination on mood, performance, and physiological behaviours of nine adult males [36]. This study showed that when alcohol and METH were co-self-administered, a greater increase in heart rate, euphoria, and lower detrimental effects on sleep and performance were observed compared to each drug self-administered alone. This may explain why METH abusers tend to consume high level of alcohol [36]. These findings raise an alarming concern of METH and alcohol co-abuse because METH might mask the signs of alcohol intoxication, such as sedation and compensated performance, allowing abusers to consume more alcohol with risk of developing alcohol toxicity.
Figure 1.
Effects of alcohol on the pharmacokinetics of methamphetamine(METH), 3,4-methylenedioxymethamphetaminen (MDMA), cocaine, and nicotine. (↑ increase or enhancement; ↓ decrease or deterioration).
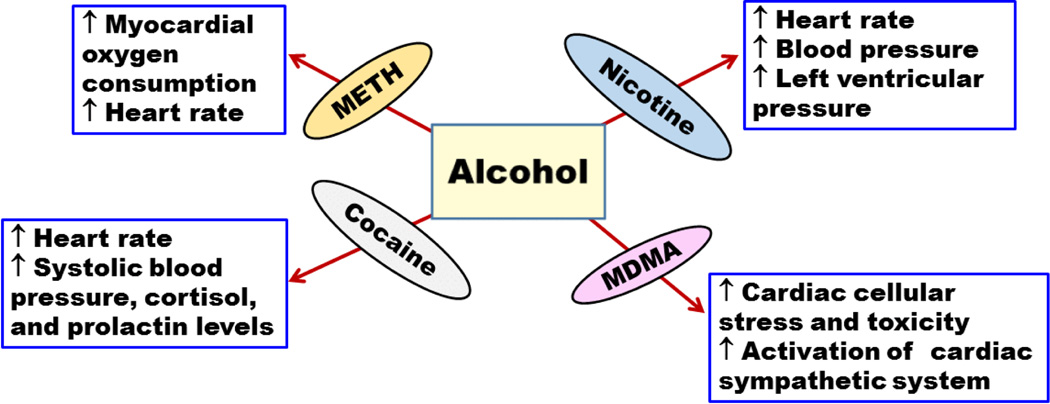
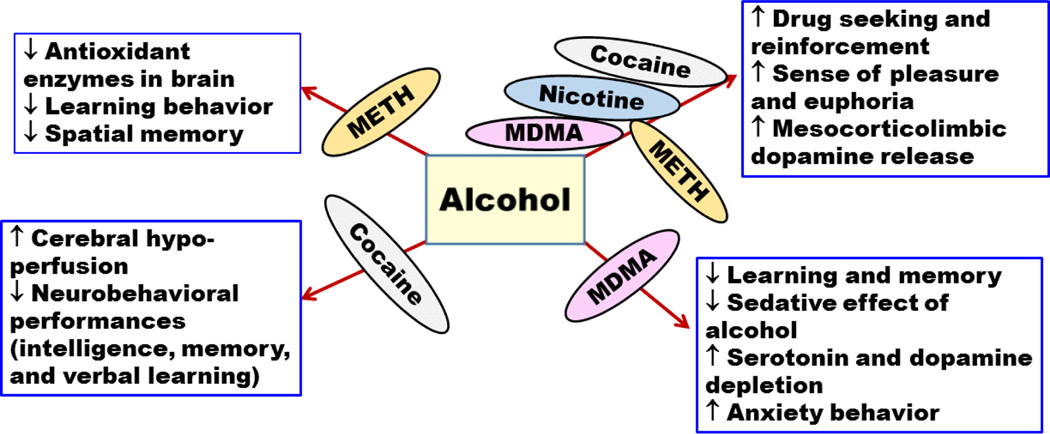
A double blind study was conducted on eight alcohol and METH abusers [37]. The abusers were found to have high myocardial oxygen consumption and increased heart rate (Figure 2). In this study, the pharmacokinetics of METH did not change significantly, which is possibly due to the limited number of subjects recruited in this study. However, further testing should be done on more subjects to reach more conclusive evidence of the effect of alcohol on METH pharmacokinetics [37]. A recent clinical study conducted on nine volunteers showed an increase in heart rate [36]. Furthermore, findings showed that concurrent consumption of METH and alcohol disrupted learning and discriminating behaviour compared to METH self-administered alone in rats [38]. However, this study did not focus on the effects of alcohol alone which may hinder the conclusion that METH and alcohol co-abuse may disturb the performance compared to METH administered alone. A recent study conducted on rats revealed that concurrent intake of METH and alcohol can lead to synergistic effect in impairment of spatial memory compared to METH administered alone [39]. Interestingly, alcohol administered alone did not cause any changes in spatial memory suggesting the synergistic effects of both drugs on memory. Moreover, this study showed that although alcohol or METH administered alone can induce oxidative stress and impairments in antioxidant enzymes in rats hippocampus, co-abuse of METH and alcohol can cause synergistic effect in impairment and oxidative stress compared to drug administered alone (Figure 3) [39].
Figure 2.
Effects of alcohol interactions with methamphetamine (METH),3,4-methylenedioxymethamphetaminen (MDMA), cocaine and nicotine on cardiovascular system, (↑ increase or enhancement; ↓ decrease or deterioration).
Figure 3.
Effects of alcohol interactions with methamphetamine (METH),3,4-methylenedioxymethamphetaminen (MDMA), cocaine, and nicotine on central nervous system. (↑ increase or enhancement; ↓ decrease or deterioration).
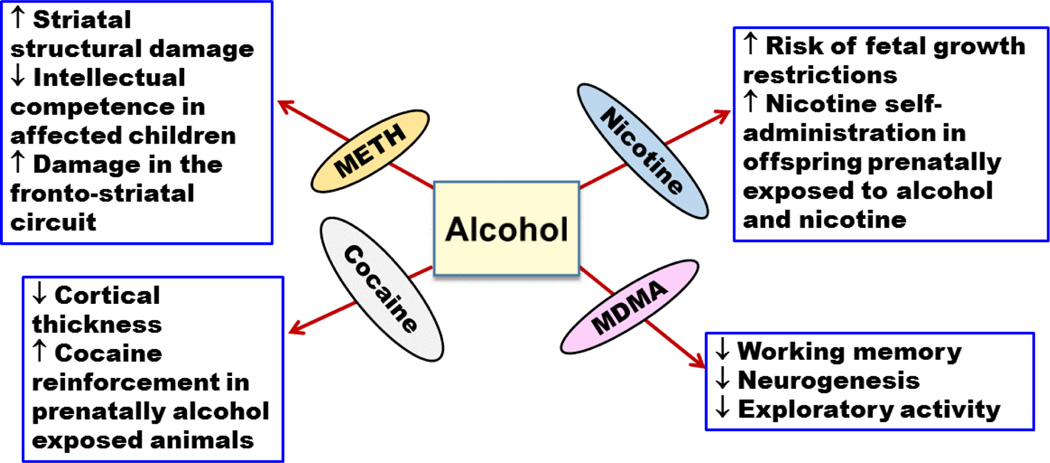
Concurrent exposure to METH and alcohol has also been observed in pregnant women. Indeed, a study demonstrated that more than 40% of pregnant women who abused METH reported using alcohol during their pregnancy [40]. Another study was conducted on 61 participants; 13 of them were exposed prenatally to alcohol, 21 were exposed prenatally to METH, and the remaining 27 control participants were not prenatally exposed to either alcohol or METH [41]. Of the 21 participants in METH group, 18 children were also exposed to alcohol during their fetal life. The results of this study suggest that prenatal exposure to METH and alcohol can cause synergistic striatal structural damage than prenatal exposure to alcohol alone. Damage to the striatal brain region hinders the overall intellectual competence of the affected children [41]. In addition, in a study that found common damage in the fronto-striatal circuit of the prenatally METH exposed group, 15 out of 19 children were exposed to alcohol and METH prenatally (Figure 4) [42]. It is important to note, however, that these studies could not precisely predict the dosage, frequency, and duration of METH or alcohol exposure during pregnancy, which may hamper our understanding of the pharmacological and neuropathological basis of drugs exposure and their interactions. Preclinical studies are warranted to show the risk of concurrent exposure of METH and alcohol during different stages of pregnancy, which may provide information about the deteriorating effects of prenatal exposure of METH and alcohol.
Figure 4.
Effects of prenatal exposure to alcohol and methamphetamine(METH),3,4-methylenedioxymethamphetaminen (MDMA), cocaine, and nicotine. (↑ increase or enhancement; ↓ decrease or deterioration).
Alcohol and cocaine co-abuse
Cocaine can produce different effects on the human body; these effects can last from minutes to hours, based on the route through which cocaine was administered into the body [43]. In the brain, cocaine can affect the reward circuitry by modulating dopamine neurotransmission [44], and acts by preventing the reuptake of dopamine from the synaptic cleft, which leads to prolongation of the pleasurable effects of dopamine [44,45]. Cocaine can produce euphoria, alertness, dependence and tolerance as well as cardiovascular changes [46–50]. Tolerance makes cocaine users increase dosage each time to reach the same level of euphoria that was reached on the first instance of taking the drug. Increasing the doses of cocaine can lead to its side effects and toxicity [51–54]. It is important to note that the prevalence of alcohol use was found 89% higher among cocaine dependents [55]. This might be due to higher increase of reward effects when alcohol and cocaine co-abused compared to either drug self-administered alone, which have been shown in preclinical studies [56–58]. In a study conducted on rats, intravenous injections of cocaine increased alcohol drinking suggesting that cocaine potentiated alcohol seeking [59]. Interestingly, a preclinical study showed a higher genetic susceptibility of the reinforcing effects of cocaine in selectively bred alcohol preferring (P) rats compared to its outbred Wistar rats, which suggests a higher sensitivity of alcoholics to the reinforcing effects of cocaine [60]. Similarly, it has been revealed that genetically predisposed subjects for alcohol dependence have a higher rate to be cocaine dependents [61].
Cocaine co-administered with alcohol can lead to production of cocaethylene, which is more lethal than cocaine itself [62,63]. This cocaethylene can also produce most of the effects that are associated with cocaine [64,65]. Concurrent exposure of alcohol and cocaine may cause more lethality in rats than either drug administered alone, which probably due to the formation of cocaethylene [66]. Interestingly, cocaethylene detection in wastewater has been utilized in recent study as an evidence of co-abuse of cocaine and alcohol in different cities [67]. Cocaethylene levels were found to be significantly higher during weekends compared to weekdays suggesting a higher co-abuse of cocaine and alcohol during weekends [67].
Alcohol has been shown to increase the plasma concentration of cocaine [68]. This is probably mediated through a decrease in the metabolism of cocaine by carboxylesterases, which hydrolyze it to benzoylecgonine and ecgonine methyl ester metabolites [68] (Table 1). Furthermore, it has been demonstrated that alcohol administered with cocaine can lead to increase in cocaethylene concentration in plasma and decrease benzoylecgonine renal excretion (Figure 1) [69]. It is noteworthy that different routes of drug exposure may produce different peak levels of cocaethylene [70]. For example, oral administration is considered the highest in raising cocaethylene concentration in blood as compared to intravenous route [70]. The inhalation route (i.e., smoking) showed the lowest effect on cocaethylene blood concentration compared to oral and IV routes [70]. Furthermore, cocaine and cocaethylene blood concentrations were obtained following concurrent use of cocaine and alcohol [71]. This study revealed that the concentration of cocaine in plasma was found to be increased by 15% after cocaine and alcohol co-exposure. Moreover, 22% of the absorbed cocaine was converted to cocaethylene. Although, cocaine half-life was not altered significantly by ingestion of alcohol, cocaethylene’s half-life was increased in comparison to cocaine’s [71]. Increasing the half-life of cocaethylene might impose serious health problem due to increasing body exposure to its deteriorating toxic effects.
Concurrent exposure to cocaine and alcohol has deleterious effects on cardiovascular and endocrine systems. Co-abuse of cocaine and alcohol was found to increase heart rate, systolic blood pressure, cortisol, and prolactin concentrations (Figure 2) [64,69]. In addition, cerebral blood perfusion was found to be affected by co-exposure to cocaine and alcohol [72,73] (Table 1). It has been shown that cerebral hypo-perfusion was more common among individuals taking cocaine and alcohol compared to individuals taking cocaine or alcohol alone [72,73]. These findings show the significant deleterious effects of the co-abuse of alcohol and cocaine on cardiovascular system that might result in debilitating conditions.
Several tests were performed on intelligence, memory, verbal learning and other aspects of neuropsychological performances to explore the effects of co-abuse of alcohol and cocaine [74,75] (Table 1). The resulting neuropsychological performances were found to be negatively affected by the concurrent intake of cocaine and alcohol compared to either drug administered alone (Figure 3) [74,75]. It has been shown that the sense of pleasure and euphoria increased in co-abuse of alcohol and cocaine and consequently elevated the risk of dependence and toxicity [71,76]. In addition, alcohol was found to significantly potentiate the effect of cocaine in conditioned place preference in rat and invertebrate animal models [56,77]. Moreover, study showed that there is a synergistic effect in self-administration of both alcohol and cocaine in concentrations that did not provoke self-administration to either drug alone [78]. Similarly, a recent study has shown the potentiating effect of cocaine on alcohol seeking and relapse-like alcohol intake in P rats [79]. This might indicate a cross reactivity between alcohol and cocaine on common drug seeking behaviour.
Several studies have shown the involvement of mesolimbic dopaminergic system in reinforcing effects of cocaine [80–83] and alcohol [84,85]. In fact, alcohol and cocaine co-exposure increased extracellular dopamine concentration in the nucleus accumbens, well known brain region involved in the rewarding and reinforcing effects of drugs of abuse [86–89], than either drug administered alone in rats [90] (Table 1). Furthermore, recent findings have demonstrated the critical role of glutamate and its uptake in central brain reward regions in the seeking and reinforcing effects of cocaine [91–93] and alcohol [94–97]. Further studies are needed for investigating the role of glutamatergic system in alcohol and cocaine co-abuse in brain regions involved in rewarding and reinforcing effects of these drugs.
Alternatively, studies have shown the detrimental effects of prenatal exposure to cocaine such as low birth weight, preterm delivery, and decrease in head circumstance [98–101]. However, prenatal co-exposure to cocaine and alcohol has not been well studied despite the findings that more than 85% of women who reported using cocaine during pregnancy, also reported concurrent alcohol use [98]. One recent study, however, has demonstrated a significant interaction in prenatal co-exposure of cocaine and alcohol on cortical thickness in youths prenatally exposed to these drugs [102]. Furthermore, it has been shown that prenatal exposure to alcohol increased the rewarding and reinforcing effects of cocaine in rats (Figure 4) [103].
Alcohol and nicotine co-abuse
Alcohol and nicotine have serious global health problems. Table 1 summarizes different studies of the effects of alcohol and nicotine co-abuse. Nicotine dependents may have high tendency to be alcohol dependents [104]. It has been reported that more than 80% of chronic alcohol users are also smokers [105–107]. In a preclinical study, rats chronically co-exposed to alcohol and nicotine showed higher nicotine self-administration as compared to drug self-administered alone [108]. Although, it has been suggested that nicotine or alcohol consumed alone may have some beneficial effect at low doses, it is clear that co-abuse of these drugs may have negative effects on human health [109].
Nicotine and alcohol activate the mesocorticolimbic dopaminergic system; there is potential synergistic effect in the increase of dopamine release when the drugs are consumed concurrently [110]. Furthermore, studies showed that alcohol and nicotine co-abuse can lead to increase dopaminergic neuronal firings and dopamine release [111–116] (Table 1). It is suggested that the synergistic effect of these drugs may influence drug reinforcement to each other and predispose smokers to become alcoholics and vice versa [110,117]. Interestingly, an additive effect on dopamine release in the nucleus accumbens shell was found between alcohol and nicotine in rats [116]. This additive effect on dopamine release was inhibited by mecamylamine pre-treatment, a nicotinic receptor antagonist, suggesting the involvement of nicotinic receptors in the reinforcing effects of alcohol. Importantly, alcohol-induced dopaminergic neurons firing in ventral tegmental area were inhibited in mice lacking nicotinic acetylcholine receptors that contain α6 subunit [118]. Moreover, it has been shown that alcohol and nicotine co-abuse can increase the pleasurable effects of each drug [119] (Table 1). This may explain some of the pharmacological mechanisms of action involving the co-abuse of nicotine and alcohol in the modulation of dopamine release (Figure 3).
The risk of developing cancer in general is higher in heavy tobacco smokers and alcohol drinkers [120–123]. In case-controlled clinical studies that were conducted on European and American subjects, alcohol and tobacco smoking elevated the risk of head and neck cancer in patients addicted to both drugs [124]. The exact mechanism of alcohol and nicotine interaction that results in the development of cancer is not well known and remains controversial. Studies have suggested that alcohol and nicotine co-abuse may produce toxic metabolites such as acetaldehyde, which may contribute to cancer development [125,126]. Other studies have suggested that alcohol and nicotine co-abuse promotes the formation of premalignant lesions (Figure 1) [127–129].
The effects of alcohol and nicotine co-abuse on cardiovascular system have been also investigated. Synergistic effects on heart rate and blood pressure were found in healthy human volunteers following alcohol and nicotine exposure [130,131]. Interestingly, the order of self-administering alcohol and nicotine plays a role in their negative interactive effect on cardiovascular system. When self-administration of alcohol was followed by nicotine, a synergistic effect on the increase in left ventricular pressure was revealed, which was alleviated when self-administration of nicotine was followed by alcohol in dogs (Figure 2) [132].
In a study investigated the link between alcohol and nicotine use during pregnancy in more than 14000 previous pregnant mothers, it was found that more than 55% of pregnant alcohol users reported smoking [133]. Alcohol and nicotine exposure during gestational period increased the risk of fetal growth abnormalities more than the exposure to alcohol alone [133–135]. Interestingly, in a study conducted on rats, alcohol and nicotine were co-administered to pregnant rats throughout the gestational period [136]. This study showed that offspring prenatally co-exposed to nicotine and alcohol developed rapid increase in nicotine self-administration as compared to controls (Figure 4) [136].
Alcohol and MDMA co-abuse
According to 2001–2002 national epidemiologic survey in United States, the prevalence of alcohol use in MDMA users is more than 95% [137]. MDMA and alcohol exposure in adolescent mice induced physiological and behavioural alteration than either drug administered alone [138]. In fact, recent study has shown that the co-abuse of MDMA and alcohol exacerbated cardiac cellular stress and toxicity through augmented activation of cardiac sympathetic system in adolescent mice (Figure 2) [139]. MDMA can induce a rapid release of dopamine and serotonin [140]. High consumption of MDMA may result in depletion of serotonin in the brain, resulting in serious psychological consequences [141–148]. MDMA is usually consumed with many drugs such as amphetamine, cocaine, cannabis, and alcohol. Alcohol and MDMA co-abuse is considered the most popular form of MDMA co-abuse [149–153]. Importantly, mice pre-treated with MDMA were found to consume high amount of alcohol compared to control mice [154] (Table 1). Therefore, mice consumed higher amounts of alcohol to reach to the same reward effect that was normally reached at lower doses of alcohol. This study also found that MDMA impaired the dopaminergic pathway. Furthermore, findings revealed that presynaptic modulation of serotonin release in the hippocampus is affected by exposure to both MDMA and alcohol [155]. This study also showed that long term consumption of MDMA and alcohol caused serotonin depletion. The alteration in the serotonergic system might be associated with the psychopathological disturbances observed in MDMA and alcohol co-abusers [155] (Table 1).
It has been found, in a double blind study conducted on nine healthy human volunteers, that MDMA and alcohol co-abuse induced a longer duration of euphoria and feeling well as compared to drug use alone [149]. Therefore, MDMA and alcohol use together can increase the abuse potential more than abusing alcohol or MDMA alone. In a preclinical study, exposure to alcohol during adolescent age in mice increased the reinforcing effects of MDMA [156]. Moreover, exposure to MDMA and alcohol during adolescence potentiated anxiety measures, impaired learning and memory, and decreased striatal dopamine contents during adult life in mice (Figure 3) [157,158]. Studies have demonstrated an increase in the MDMA plasma concentration by 13% following alcohol intake and a decrease in blood alcohol concentration of about 12% compared to either drug administered alone (Figure 1) [149]. In addition, these studies found that MDMA reversed the subjective sedative effect, which was induced by alcohol consumption.
A recent study aimed to find the effect of MDMA and alcohol co-abuse on learning and memory [159]. In this study, alcohol and MDMA were administered either together or alone to measure their effects on learning and memory in adult mice. Both drugs caused impairment of learning and memory, as the affected mice displayed an imbalance in the interaction of dopamine and serotonin. These findings suggest that the brain in adulthood is very sensitive to MDMA and alcohol damage [159]. However, other study did not demonstrate any additive effect of combining alcohol and MDMA on declarative memory in mice [160] (Figure 3). This might be due to several factors, including the doses used for alcohol and MDMA.
Prenatal exposure to alcohol and MDMA is understudied topic, although pregnant women who reported MDMA use during pregnancy also reported higher alcohol use compared to non MDMA users [161,162]. Importantly, a preclinical study found that prenatal exposure to both alcohol and MDMA impaired working memory, exploratory activity, and neurogenesis in rat’s offspring (Figure 4) [163].
Conclusion
Alcohol interaction with drugs of abuse is currently not well understood, however, there are studies that demonstrated numerous side effects, which have occurred with drugs co-abuse. The prevalence of concurrent abuse of alcohol with psychostimulants such as METH, cocaine, nicotine, or MDMA is extremely high. This increase in prevalence of co-abuse of alcohol with psychostimulants is most likely due to potentiated effects on euphoria and pleasure as well as decrease detrimental subjective effects of either alcohol or other drugs of abuse. Co-abuse of alcohol with psychostimulants can lead to serious negative consequences on the brain such as decreasing antioxidant enzymes, disrupting learning and memory processes, cerebral hypo-perfusion, neurotransmitters depletion as well as potentiating drug seeking behaviour. Moreover, co-abuse of alcohol and psychostimulants can lead to increase in heart rate, blood pressure, myocardial oxygen consumption and cellular stress as well as increase in the risk of developing different types of cancer.
Alcohol has been shown to increase the blood concentration of different psychostimulants and its active metabolites. It is suggested that the pharmacokinetics of METH, MDMA, cocaine, and nicotine, might be altered when alcohol is consumed concurrently with these drugs. We suggest here that alcohol metabolism and its metabolites may increase the blood concentration of these drugs of abuse, and consequently elevate the risk of toxicity. Importantly, alcohol co-abuse with psychostimulants during pregnancy can impose critical structural and functional damages in the fetal brains. Further studies are needed to investigate possible pharmacodynamics, pharmacokinetics, and neurochemical basis of co-abuse of alcohol and psychostimulants as well as possible therapeutic interventions.
Acknowledgments
The authors would like to thank the National Institutes on Alcohol Abuse and Alcoholism (R01AA019458 to Y.S.) for their continuous support. The authors would like also to thank Fahad Alshehri, Atiah Almalki, and Hashem Alsaab for their help in the literature.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 2.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacology and therapeutics. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock. 2008;29:377. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: Commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Experimental neurology. 2008;213:10–17. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Li TK. Drugs and alcohol: Treating and preventing abuse, addiction and their medical consequences. Pharmacology and therapeutics. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 8.Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13(Suppl 1):213–223. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 10.George S, Rogers RD, Duka T. The acute effect of alcohol on decision making in social drinkers. Psychopharmacology (Berl) 2005;182:160–169. doi: 10.1007/s00213-005-0057-9. [DOI] [PubMed] [Google Scholar]
- 11.Arasteh K, Des Jarlais DC, Perlis TE. Alcohol and HIV sexual risk behaviors among injection drug users. Drug Alcohol Depend. 2008;95:54–61. doi: 10.1016/j.drugalcdep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasch RF, Weisen CA, MacDonald B, Wechsberg WM, Perritt R, et al. Patterns of HIV risk and alcohol use among African-American crack abusers. Drug Alcohol Depend. 2000;58:259–266. doi: 10.1016/s0376-8716(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: A systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–863. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 14.Van Tieu H, Koblin BA. HIV, alcohol, and non-injection drug use. Curr Opin HIV AIDS. 2009;4:314–318. doi: 10.1097/COH.0b013e32832aa902. [DOI] [PubMed] [Google Scholar]
- 15.Tapert SF, Caldwell L, Burke C. Alcohol and the adolescent brain: Human studies. Alcohol Research and Health. 2004 [Google Scholar]
- 16.Legrand SA, Isalberti C, der Linden TV, Bernhoft IM, Hels T, et al. Alcohol and drugs in seriously injured drivers in six European countries. Drug Test Anal. 2013;5:156–165. doi: 10.1002/dta.1393. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JM, Flegel R, Atkins R, Cangianelli LA, Cooper C, et al. Drug and alcohol use among drivers admitted to a Level-1 trauma center. Accident; Analysis and Prevention. 2005;37:894–901. doi: 10.1016/j.aap.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen H, Moar R, Troncoso C. The incidence of alcohol and other drugs in drivers killed in New Zealand road crashes 2004–2009. Forensic Sci Int. 2012;223:364–370. doi: 10.1016/j.forsciint.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JM, Flegel R, Cangianelli LA, Atkins R, Soderstrom CA, et al. Epidemiology of alcohol and other drug use among motor vehicle crash victims admitted to a trauma center. Traffic injury prevention. 2004;5:254–260. doi: 10.1080/15389580490465319. [DOI] [PubMed] [Google Scholar]
- 20.Legrand SA, Houwing S, Hagenzieker M, Verstraete AG. Prevalence of alcohol and other psychoactive substances in injured drivers: Comparison between Belgium and The Netherlands. Forensic science international. 2012;220:224–231. doi: 10.1016/j.forsciint.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Caetano R, Weisner C. The association between DSM-III-R alcohol dependence, psychological distress and drug use. Addiction. 1995;90:351–359. doi: 10.1046/j.1360-0443.1995.9033515.x. [DOI] [PubMed] [Google Scholar]
- 22.Falk D, Yi H-y, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and drug use and disorders. Alcohol research and health: The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2008;31:100–110. [PMC free article] [PubMed] [Google Scholar]
- 23.Colliver JD. Misuse of prescription drugs: Data from the 2002, 2003 and 2004 National Survey on Drug Use and Health: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies. 2006. [Google Scholar]
- 24.Cunningham JK, Liu LM, Muramoto M. Methamphetamine suppression and route of administration: Precursor regulation impacts on snorting, smoking, swallowing and injecting. Addiction. 2008;103:1174–1186. doi: 10.1111/j.1360-0443.2008.02208.x. [DOI] [PubMed] [Google Scholar]
- 25.Domier CP, Simon SL, Rawson RA, Huber A, Ling W. A comparison of injecting and non-injecting methamphetamine users. J Psychoactive Drugs. 2000;32:229–232. doi: 10.1080/02791072.2000.10400233. [DOI] [PubMed] [Google Scholar]
- 26.Gibson DR, Leamon MH, Flynn N. Epidemiology and public health Consequences of methamphetamine use in California’s Central Valley. J Psychoactive Drugs. 2002;34:313–319. doi: 10.1080/02791072.2002.10399969. [DOI] [PubMed] [Google Scholar]
- 27.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32:137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- 28.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Furr CD, Delva J, Anthony JC. The suspected association between methamphetamine (‘ice’) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug and alcohol dependence. 2000;59:89–93. doi: 10.1016/s0376-8716(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 30.Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol dependence. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Halkitis PN, Green KA, Mourgues P. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: Findings from Project BUMPS. J Urban Health. 2005;82:i18–i25. doi: 10.1093/jurban/jti020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, et al. The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug and alcohol dependence. 2014;142:127–132. doi: 10.1016/j.drugalcdep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimosato K. Urinary excretion of p-hydroxylated methamphetamine metabolites in man. II. Effect of alcohol intake on methamphetamine metabolism. Pharmacology, Biochemistry and Behavior. 1988;29:733. doi: 10.1016/0091-3057(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 34.Liang M, Liu Y, Zheng N, Ananda S, Liu L. Distribution of methamphetamine and its metabolite amphetamine in acute and sub-acute ethanol-methamphetamine combination abuse model rats. Journal of analytical toxicology. 2012;36:30–35. doi: 10.1093/jat/bkr007. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Wang Y, Zhang Y, Liu M. Effects of ethanol on the toxicokinetics of methamphetamine in rabbits. Iran J Pharm Res. 2014;13:329–336. [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 2012;219:191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendelson J, Jones RT, Upton R, Jacob P., 3rd Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. doi: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 38.Yamamura T, Hishida S, Hatake K, Taniguchi T, Ouchi H. Effects of methamphetamine and ethanol on learning and brain neurotransmitters in rats. Pharmacol Biochem Behav. 1992;42:389–400. doi: 10.1016/0091-3057(92)90131-x. [DOI] [PubMed] [Google Scholar]
- 39.Vaghef L, Babri S, Vahed M. The effect of escalating dose, multiple binge methamphetamine regimen and alcohol combination on spatial memory and oxidative stress markers in rat brain. J Alcohol Drug Depend. 2014;2:2. [Google Scholar]
- 40.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, et al. The infant development, environment and lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 41.Sowell ER, Leow AD, Bookheimer SY, Smith LM, O’Connor MJ, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, et al. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: The effects of methamphetamine, alcohol and polydrug exposure. Neurolmage. 2011;54:3067–3075. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life sciences. 2000;67:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- 44.McElvain JS, Schenk JO. A multisubstrate mechanism of striatal dopamine uptake and its inhibition by cocaine. Biochem Pharmacol. 1992;43:2189–2199. doi: 10.1016/0006-2952(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 45.Missale C, Castelletti L, Govoni S, Spano PF, Trabucchi M, et al. Dopamine uptake is differentially regulated in rat striatum and nucleus accumbens. J Neurochem. 1985;45:51–56. doi: 10.1111/j.1471-4159.1985.tb05473.x. [DOI] [PubMed] [Google Scholar]
- 46.Kloner RA, Hale S, Alker K, Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407–419. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- 47.Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 48.Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther. 1985;235:677–682. [PubMed] [Google Scholar]
- 49.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, et al. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 50.Frank RA, Manderscheid PZ, Panicker S, Williams HP, Kokoris D. Cocaine euphoria, dysphoria and tolerance assessed using drug-induced changes in brain-stimulation reward. Pharmacology Biochemistry and Behavior. 1992;42:771–779. doi: 10.1016/0091-3057(92)90028-e. [DOI] [PubMed] [Google Scholar]
- 51.Petersen RC. Cocaine: An overview. Cocaine. 1977;77:5–15. [PubMed] [Google Scholar]
- 52.Abuse NIoD, America USo. Cocaine Abuse and Addiction. 1999. [Google Scholar]
- 53.Ambre JJ, Belknap SM, Nelson J, Ruo TI, Shin SG, et al. Acute tolerance to cocaine in humans. Clin Pharmacol Ther. 1988;44:1–8. doi: 10.1038/clpt.1988.104. [DOI] [PubMed] [Google Scholar]
- 54.Emmett-Oglesby MW, Lane JD. Tolerance to the reinforcing effects of cocaine. Behav Pharmacol. 1992;3:193–200. [PubMed] [Google Scholar]
- 55.Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States. Alcohol Research and Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29:94–106. [Google Scholar]
- 56.Busse GD, Lawrence ET, Riley AL. The modulation of cocaine-induced conditioned place preferences by alcohol: Effects of cocaine dose. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:149–155. doi: 10.1016/j.pnpbp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Lewis MJ, June HL. Synergistic effects of ethanol and cocaine on brain stimulation reward. J Exp Anal Behav. 1994;61:223–229. doi: 10.1901/jeab.1994.61-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moolten M, Kornetsky C. Cocaine potentiates ethanols threshold lowering effect on brain-stimulation. Abstr Soc Neurosci. 1990 [Google Scholar]
- 59.Knackstedt LA, Ben-Shahar O, Ettenberg A. Alcohol consumption is preferred to water in rats pre-treated with intravenous cocaine. Pharmacol Biochem Behav. 2006;85:281–286. doi: 10.1016/j.pbb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Katner SN, Oster SM, Ding ZM, Deehan GA, Toalston JE, et al. Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administered directly into the nucleus accumbens shell. Pharmacology Biochemistry and Behavior. 2011;99:688–695. doi: 10.1016/j.pbb.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, et al. A family study of alcohol dependence: Co-aggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- 62.Hearn W, Rose S, Wagner J, Ciarleglio A, Mash D. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacology Biochemistry and Behavior. 1991;39:531–533. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- 63.Jatlow P, Elsworth J, Bradberry C, Winger G, Taylor J, et al. Cocaethylene: A neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sciences. 1991;48:1787–1794. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- 64.Farré M, De La Torre R, González ML, Terán MT, Roset PN, et al. Cocaine and alcohol interactions in humans: Neuroendocrine effects and cocaethylene metabolism. Journal of Pharmacology and Experimental Therapeutics. 1997;283:164–176. [PubMed] [Google Scholar]
- 65.Perez-Reyes M, Jeffcoat AR, Myers M, Sihler K, Cook CE. Comparison in humans of the potency and pharmacokinetics of intravenously injected cocaethylene and cocaine. Psychopharmacology (Berl) 1994;116:428–432. doi: 10.1007/BF02247473. [DOI] [PubMed] [Google Scholar]
- 66.Busse GD, Riley AL. Effects of alcohol on cocaine lethality in rats: Acute and chronic assessments. Neurotoxicol Teratol. 2003;25:361–364. doi: 10.1016/s0892-0362(02)00351-3. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez-Álvarez T, Racamonde I, González-Mariño I, Borsotti A, Rodil R, et al. Alcohol and cocaine co-consumption in two European cities assessed by wastewater analysis. Science of the Total Environment. 2015;536:91–98. doi: 10.1016/j.scitotenv.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Boyer CS, Petersen D. Enzymatic basis for the transesterification of cocaine in the presence of ethanol: Evidence for the participation of microsomal carboxylesterases. Journal of Pharmacology and Experimental Therapeutics. 1992;260:939–946. [PubMed] [Google Scholar]
- 69.Harris DS, Everhart ET, Mendelson J, Jones RT. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug Alcohol Depend. 2003;72:169–182. doi: 10.1016/s0376-8716(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 70.Herbst ED, Harris DS, Everhart ET, Mendelson J, Jacob P, et al. Cocaethylene formation following ethanol and cocaine administration by different routes. Experimental and clinical psychopharmacology. 2011;19:95. doi: 10.1037/a0022950. [DOI] [PubMed] [Google Scholar]
- 71.McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biol Psychiatry. 1998;44:250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- 72.Gottschalk PC, Kosten TR. Cerebral perfusion defects in combined cocaine and alcohol dependence. Drug Alcohol Depend. 2002;68:95–104. doi: 10.1016/s0376-8716(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 73.Robinson JE, Heaton RK, O’Malley SS. Neuropsychological functioning in cocaine abusers with and without alcohol dependence. Journal of the International Neuropsychological Society. 1999;5:10–19. doi: 10.1017/s1355617799511028. [DOI] [PubMed] [Google Scholar]
- 74.Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- 75.Verdejo-García A, Pérez-García M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology. 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- 76.Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 77.Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, et al. Ethanol and cocaine: environmental place conditioning, stereotypy, and synergism in planarians. Alcohol. 2014;48:579–586. doi: 10.1016/j.alcohol.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, et al. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: Involvement of serotonin-3 receptors. Journal of Pharmacology and Experimental Therapeutics. 2012;340:202–209. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hauser SR, Wilden JA, Deehan GA, McBride WJ, Rodd ZA. Cocaine influences alcohol-seeking behavior and relapse drinking in alcohol-preferring (p) rats. Alcoholism: Clinical and Experimental Research. 2014;38:2678–2686. doi: 10.1111/acer.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, et al. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: Evidence for involvement of serotonin-3 receptors and dopamine neurons. Journal of Pharmacology and Experimental Therapeutics. 2005;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- 81.Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- 82.de Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Canadian Journal of Psychology/Revue Canadienne de Psychologie. 1977;31:195. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- 83.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 84.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- 85.Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: Involvement of dopamine and serotonin. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2005;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- 86.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 87.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 88.Bardo MT. Neuropharmacological mechanisms of drug reward: Beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 89.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 90.Lindholm S, Rosin Å, Dahlin I, Georgieva J, Franck J. Ethanol administration potentiates cocaine-induced dopamine levels in the rat nucleus accumbens. Brain research. 2001;915:176–184. doi: 10.1016/s0006-8993(01)02847-5. [DOI] [PubMed] [Google Scholar]
- 91.Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, et al. Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2014;39:499–506. doi: 10.1038/npp.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen HW, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2014;39:1169–1177. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, et al. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addiction Biology. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Das SC, Althobaiti YS, Alshehri FS, Sari Y. Binge ethanol withdrawal: Effects on post-withdrawal ethanol intake, glutamate-glutamine cycle and monoamine tissue content in P rat model. Behavioural Brain Research. 2016;303:120–125. doi: 10.1016/j.bbr.2016.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, et al. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicology and teratology. 2002;24:127–135. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 99.Chouteau M, Namerow PB, Leppert P. The effect of cocaine abuse on birth weight and gestational age. Obstet Gynecol. 1988;72:351–354. [PubMed] [Google Scholar]
- 100.Bateman DA, Ng SK, Hansen CA, Heagarty MC. The effects of intrauterine cocaine exposure in new-borns. Am J Public Health. 1993;83:190–193. doi: 10.2105/ajph.83.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singer LT, Arendt R, Minnes S, Salvator A, Siegel AC, et al. Developing language skills of cocaine-exposed infants. Pediatrics. 2001;107:1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gautam P, Warner TD, Kan EC, Sowell ER. Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Developmental cognitive neuroscience. 2015 doi: 10.1016/j.dcn.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, et al. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 104.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 105.DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- 106.Batel P, Pessione F, Maître C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- 107.Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13:185–190. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- 108.Deehan GA, Jr, Hauser SR, Waeiss RA, Knight CP, Toalston JE, et al. Co-administration of ethanol and nicotine: The enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats. Psychopharmacology. 2015;232:4293–4302. doi: 10.1007/s00213-015-4056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hurley LL, Taylor RE, Tizabi Y. Positive and negative effects of alcohol and nicotine and their interactions: A mechanistic review. Neurotoxicity research. 2012;21:57–69. doi: 10.1007/s12640-011-9275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: A focus on the mesocorticolimbic dopamine system. Biochemical Pharmacology. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larsson A, Edström L, Svensson L, Söderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol and Alcoholism. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- 112.Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: A dual-probe microdialysis study. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2003 doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- 113.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature neuroscience. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 114.Schier CJ, Dilly GA, Gonzales RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the long-evans rat. Alcoholism: Clinical and Experimental Research. 2013 doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tizabi Y, Copeland RL, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2002;26:394–399. [PubMed] [Google Scholar]
- 116.Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- 117.Collins AC, Wilkins LH, Slobe BS, Cao JZ, Bullock AE. Long-term ethanol and nicotine treatment elicit tolerance to ethanol. Alcoholism: Clinical and Experimental Research. 1996;20:990–999. doi: 10.1111/j.1530-0277.1996.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 118.Liu L, Zhao-Shea R, McIntosh JM, Tapper AR. Nicotinic acetylcholine receptors containing the a6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons. Biochemical pharmacology. 2013;86:1194–1200. doi: 10.1016/j.bcp.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, et al. Psychopharmacological interactions between nicotine and ethanol. Nicotine and Tobacco Research. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- 120.Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: Interview study from the Third National Cancer Survey. Journal of the National Cancer Institute. 1977;58:525–547. doi: 10.1093/jnci/58.3.525. [DOI] [PubMed] [Google Scholar]
- 121.Mashberg A, Boffetta P, Winkelman R, Garfinkel L. Tobacco smoking, alcohol drinking and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72:1369–1375. doi: 10.1002/1097-0142(19930815)72:4<1369::aid-cncr2820720436>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 122.Wynder EL, Mushinski MH, Spivak JC. Tobacco and alcohol consumption in relation to the development of multiple primary cancers. Cancer. 1977;40:1872–1878. doi: 10.1002/1097-0142(197710)40:4+<1872::aid-cncr2820400817>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 123.Wynder EL, Covey LS, Mabuchi K, Mushinski M. Environmental factors in cancer of the larynx: A second look. Cancer. 1976;38:1591–1601. doi: 10.1002/1097-0142(197610)38:4<1591::aid-cncr2820380425>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 124.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiology, Biomarkers and Prevention: A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Homann N, Tillonen J, Meurman JH, Rintamäki H, Lindqvist C, et al. Increased salivary acetaldehyde levels in heavy drinkers and smokers: A microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21:663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]
- 126.Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. International journal of Cancer. 2004;111:480–483. doi: 10.1002/ijc.20293. [DOI] [PubMed] [Google Scholar]
- 127.Maserejian NN, Joshipura KJ, Rosner BA, Giovannucci E, Zavras AI. Prospective study of alcohol consumption and risk of oral premalignant lesions in men. Cancer Epidemiol Biomarkers Prev. 2006;15:774–781. doi: 10.1158/1055-9965.EPI-05-0842. [DOI] [PubMed] [Google Scholar]
- 128.Lee CH, Ko YC, Huang HL, Chao YY, Tsai CC, et al. The pre-cancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br J Cancer. 2003;88:366–372. doi: 10.1038/sj.bjc.6600727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang HW, Ling GS, Wei WI, Yuen APW. Smoking and drinking can induce pl5 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer. 2004;101:125–132. doi: 10.1002/cncr.20323. [DOI] [PubMed] [Google Scholar]
- 130.Benowitz NL, Jones RT, Jacob P., 3rd Additive cardiovascular effects of nicotine and ethanol. Clinical Pharmacology and Therapeutics. 1986;40:420–424. doi: 10.1038/clpt.1986.200. [DOI] [PubMed] [Google Scholar]
- 131.Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, et al. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119:205–212. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- 132.Mehta MC, Jain AC, Billie M. Combined effects of alcohol and nicotine on cardiovascular performance in a canine model. J Cardiovasc Pharmacol. 1998;31:930–936. doi: 10.1097/00005344-199806000-00018. [DOI] [PubMed] [Google Scholar]
- 133.Aliyu MH, Wilson RE, Zoorob R, Brown K, Alio AP, et al. Prenatal alcohol consumption and fetal growth restriction: potentiation effect by concomitant smoking. Nicotine and Tobacco Research. 2009;11:36–43. doi: 10.1093/ntr/ntn014. [DOI] [PubMed] [Google Scholar]
- 134.Haste F, Anderson H, Brooke O, Bland J, Peacock J. The effects of smoking and drinking on the anthropometric measurements of neonates. Paediatric and Perinatal Epidemiology. 1991;5:83–92. doi: 10.1111/j.1365-3016.1991.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 135.Olsen J, Pereira Ada C, Olsen SF. Does maternal tobacco smoking modify the effect of alcohol on fetal growth? Am J Public Health. 1991;81:69–73. doi: 10.2105/ajph.81.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matta SG, Elberger AJ. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology. 2007;193:199–213. doi: 10.1007/s00213-007-0767-2. [DOI] [PubMed] [Google Scholar]
- 137.Keyes KM, Martins SS, Hasin DS. Past 12 month and lifetime comorbidity and poly-drug use of ecstasy users among young adults in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2008;97:139–149. doi: 10.1016/j.drugalcdep.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ros-Simó C, Ruiz-Medina J, Valverde O. Behavioural and neuroinflammatory effects of the combination of binge ethanol and MDMA in mice. Psychopharmacology (Berl) 2012;221:511–525. doi: 10.1007/s00213-011-2598-4. [DOI] [PubMed] [Google Scholar]
- 139.Navarro-Zaragoza J, Ros-Simó C, Milanés MV, Valverde O, Laorden ML. Binge ethanol and MDMA combination exacerbates toxic cardiac effects by inducing cellular stress. PloS one. 2015;10:e0141502. doi: 10.1371/journal.pone.0141502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3, 4-methylenedioxymethamphetamine: Implications for serotonin-dopamine interactions. Journal of Neurochemistry. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- 141.Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 142.Steele TD, McCann UD, Ricaurte GA. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): Pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–551. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 143.Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 144.Gudelsky GA, Yamamoto BK, Nash JF. Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT 2 receptor agonists. European Journal of Pharmacology. 1994;264:325–330. doi: 10.1016/0014-2999(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 145.Battaglia G, Yeh S, O’Hearn E, Molliver ME, Kuhar MJ, et al. 3,4-Methylenedioxymethamphetamine and 3, 4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H] paroxetine-labeled serotonin uptake sites. Journal of Pharmacology and Experimental Therapeutics. 1987;242:911–916. [PubMed] [Google Scholar]
- 146.Chu T, Kumagai Y, DiStefano EW, Cho AK. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochemical Pharmacology. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- 147.Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3, 4-methylenedioxymethamphetamine: association with serotonin depletion. European Journal of Pharmacology. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- 148.Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, et al. ± 3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA: The Journal of the American Medical Association. 1988;260:51–55. [PubMed] [Google Scholar]
- 149.Hernández-López C, Farré M, Roset PN, Menoyo E, Pizarro N, et al. 3,4-Methylenedioxymethamphetamine (ecstasy) and alcohol interactions in humans: Psychomotor performance, subjective effects and pharmacokinetics. Journal of Pharmacology and Experimental Therapeutics. 2002;300:236–244. doi: 10.1124/jpet.300.1.236. [DOI] [PubMed] [Google Scholar]
- 150.Lora-Tamayo C, Tena T, Rodríguez A, Moreno D, Sancho JR, et al. The designer drug situation in Ibiza. Forensic Sci Int. 2004;140:195–206. doi: 10.1016/j.forsciint.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 151.Schifano F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology (Berl) 2004;173:242–248. doi: 10.1007/s00213-003-1730-5. [DOI] [PubMed] [Google Scholar]
- 152.Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug Alcohol Depend. 1999;55:105–115. doi: 10.1016/s0376-8716(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 153.Gamella J, Alvarez-Roldan A, Romo N. The content of ecstasy in Spain. 7th annual conference on drug use and drug policy; Amsterdam. 1997. [Google Scholar]
- 154.Izco M, Marchant I, Escobedo I, Peraile I, Delgado M, et al. Mice with decreased cerebral dopamine function following a neurotoxic dose of MDMA (3,4-methylenedioxymethamphetamine,”Ecstasy”) exhibit increased ethanol consumption and preference. Journal of Pharmacology and Experimental Therapeutics. 2007;322:1003–1012. doi: 10.1124/jpet.107.120600. [DOI] [PubMed] [Google Scholar]
- 155.Cassel JC, Riegert C, Rutz S, Koenig J, Rothmaier K, et al. Ethanol, 3,4-methylenedioxymethamphetamine (ecstasy) and their combination: Long-term behavioral, neurochemical and neuropharmacological effects in the rat. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30:1870–1882. doi: 10.1038/sj.npp.1300714. [DOI] [PubMed] [Google Scholar]
- 156.Ribeiro Do Couto B, Daza-Losada M, Rodríguez-Arias M, Nadal R, Guerri C, et al. Adolescent pre-exposure to ethanol and 3, 4-methylenedioxymethylamphetamine (MDMA) increases conditioned rewarding effects of MDMA and drug-induced reinstatement. Addiction Biology. 2012;17:588–600. doi: 10.1111/j.1369-1600.2011.00382.x. [DOI] [PubMed] [Google Scholar]
- 157.Rodríguez-Arias M, Maldonado C, Vidal-Infer A, Guerri C, Aguilar MA, et al. Intermittent ethanol exposure increases long-lasting behavioral and neurochemical effects of MDMA in adolescent mice. Psychopharmacology. 2011;218:429–442. doi: 10.1007/s00213-011-2329-x. [DOI] [PubMed] [Google Scholar]
- 158.Vidal-Infer A, Aguilar MA, Miñarro J, Rodríguez-Arias M. Effect of intermittent exposure to ethanol and MDMA during adolescence on learning and memory in adult mice. Behavioral and Brain Functions. 2012;8:1. doi: 10.1186/1744-9081-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vidal-Infer A, Aguilar MA, Miñarro J, Rodríguez-Arias M. Effect of intermittent exposure to ethanol and MDMA during adolescence on learning and memory in adult mice. Behav Brain Funct. 2012;8:32. doi: 10.1186/1744-9081-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ros-Simó C, Moscoso-Castro M, Ruiz-Medina J, Ros J, Valverde O. Memory impairment and hippocampus specific protein oxidation induced by ethanol intake and 3, 4-Methylenedioxymethamphetamine (MDMA) in mice. Journal of neurochemistry. 2013;125:736–746. doi: 10.1111/jnc.12247. [DOI] [PubMed] [Google Scholar]
- 161.Singer LT, Moore DG, Fulton S, Goodwin J, Turner JJ, et al. Neurobehavioral outcomes of infants exposed to MDMA (Ecstasy) and other recreational drugs during pregnancy. Neurotoxicology and teratology. 2012;34:303–310. doi: 10.1016/j.ntt.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singer LT, Moore DG, Min MO, Goodwin J, Turner JJ, et al. One-year outcomes of prenatal exposure to MDMA and other recreational drugs. Pediatrics: Peds. 2012 doi: 10.1542/peds.2012-0666. 2012-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Canales JJ, Ferrer-Donato A. Prenatal exposure to alcohol and 3, 4-methylenedioxymethamphetamine (ecstasy) alters adult hippocampal neurogenesis and causes enduring memory deficits. Developmental neuroscience. 2014;36:10–17. doi: 10.1159/000356820. [DOI] [PubMed] [Google Scholar]