Abstract
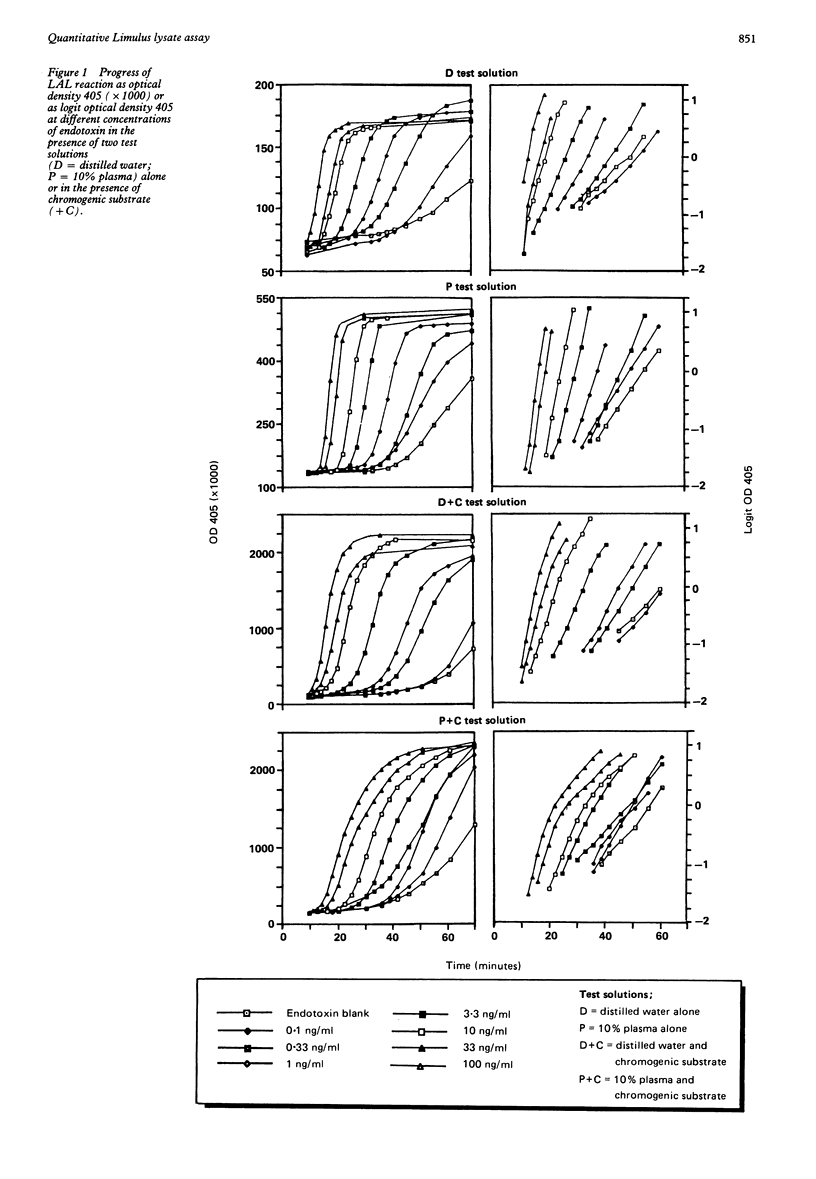
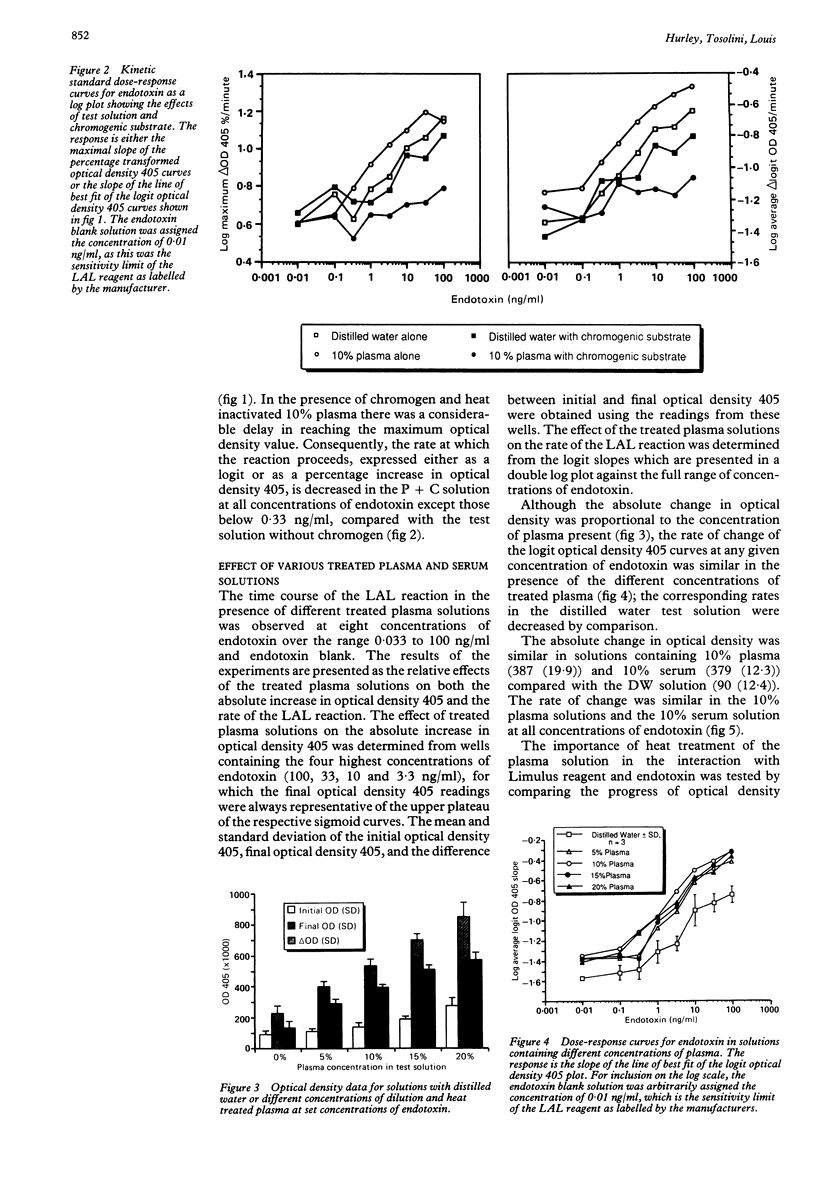
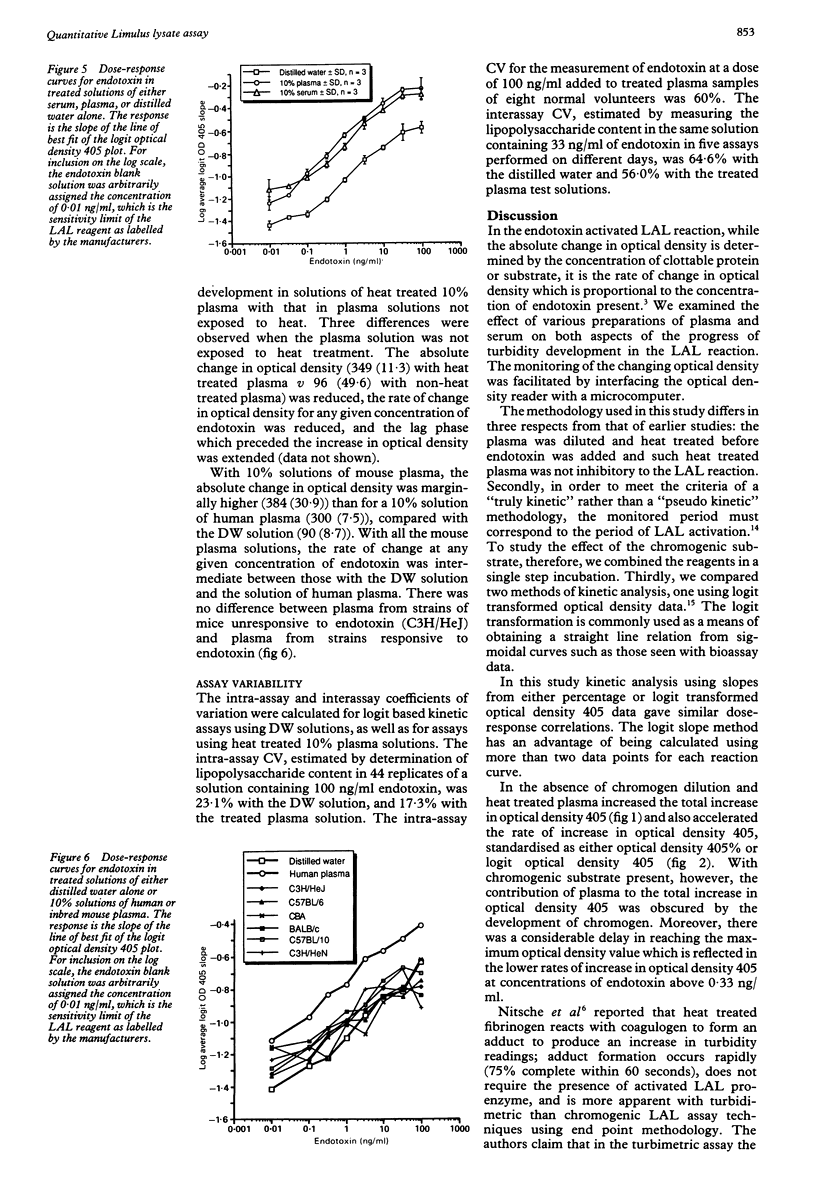
The effects of plasma and chromogenic substrate on the kinetics of the endotoxin-activated Limulus amoebocyte lysate (LAL) assay were determined. A linear correlation was observed between the rate of development of turbidity (optical density 405) with the LAL reagent and the concentration of endotoxin over a four log ten-fold range. Like chromogenic substrate, the addition of dilution and heat treated plasma to the reaction resulted in an increase in optical density proportional to the concentration of plasma present. The presence of the treated plasma also resulted in an accelerated increase in optical density with comparable results when testing plasma at different concentrations and, additionally, serum. This accelerated increase in optical density may not be recognised in assays that monitor the progress of the reaction at a single time point and may confound assays of plasma samples that use chromogenic substrate. Plasma obtained from endotoxin sensitive and resistant strains of mice showed similar effects. The use of kinetic methodology means that a quantitative assay for endotoxin in plasma can be achieved, its variability comparable with that seen with semiquantitative serial dilution but with greater economy of the LAL reagent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen J., McConnell J. S. Observations on the measurement and evaluation of endotoxemia by a quantitative limulus lysate microassay. J Infect Dis. 1984 Dec;150(6):916–924. doi: 10.1093/infdis/150.6.916. [DOI] [PubMed] [Google Scholar]
- Ditter B., Becker K. P., Urbaschek R., Urbaschek B. Detection of endotoxin in blood and other specimens by evaluation of photometrically registered LAL-reaction-kinetics in microtiter plates. Prog Clin Biol Res. 1982;93:385–392. [PubMed] [Google Scholar]
- DuBose D. A., Lemaire M., Basamania K., Rowlands J. Comparison of plasma extraction techniques in preparation of samples for endotoxin testing by the Limulus amoebocyte lysate test. J Clin Microbiol. 1980 Jan;11(1):68–72. doi: 10.1128/jcm.11.1.68-72.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Hosseini J. Clinical utility of the Limulus amebocyte lysate (LAL) test. Prog Clin Biol Res. 1985;189:307–327. [PubMed] [Google Scholar]
- Gardi A., Arpagaus G. R. Improved microtechnique for endotoxin assay by the Limulus amebocyte lysate test. Anal Biochem. 1980 Dec;109(2):382–385. doi: 10.1016/0003-2697(80)90664-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. K. Serum from LPS nonresponder C3H/HeJ mice does not support the formation of functional EAC reagents. J Immunol. 1978 Aug;121(2):619–621. [PubMed] [Google Scholar]
- Iwanaga S., Morita T., Harada T., Nakamura S., Niwa M., Takada K., Kimura T., Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7(2-3):183–188. doi: 10.1159/000214260. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Goralnick S., Osborn M. J. Isolation from human serum of an inactivator of bacterial lipopolysaccharide. Am J Pathol. 1977 Sep;88(3):559–574. [PMC free article] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Nandan R., Brown D. R. An improved in vitro pyrogen test: to detect picograms of endotoxin contamination in intravenous fluids using limulus amoebocyte lysate. J Lab Clin Med. 1977 Apr;89(4):910–918. [PubMed] [Google Scholar]
- Nitsche D., Kriewitz M., Seifert J., Hamelmann H. Investigations on an essential plasma factor disturbing the photometric determination of endotoxin in plasma samples with the Limulus test. Prog Clin Biol Res. 1987;231:331–340. [PubMed] [Google Scholar]
- Novitsky T. J., Roslansky P. F., Siber G. R., Warren H. S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985 Feb;21(2):211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Kawai T. Endotoxin-inactivating activity in normal and pathological human blood samples. Infect Immun. 1986 Aug;53(2):294–297. doi: 10.1128/iai.53.2.294-297.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson P., Olofsson C., Nylander G., Olsson P. Endotoxin inactivation in plasma from septic patients: an in vitro study. World J Surg. 1986 Apr;10(2):318–323. doi: 10.1007/BF01658154. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Flynn P. M., Barrett F. F., Stidham G. L., Westenkirchner D. F. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988 Mar;157(3):565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Mogan K. A. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984 Sep;150(3):380–388. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- Zhang G. H., Baek L., Koch C. New microassay for quantitation of endotoxin using Limulus amebocyte lysate combined with enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Aug;26(8):1464–1470. doi: 10.1128/jcm.26.8.1464-1470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



