Abstract
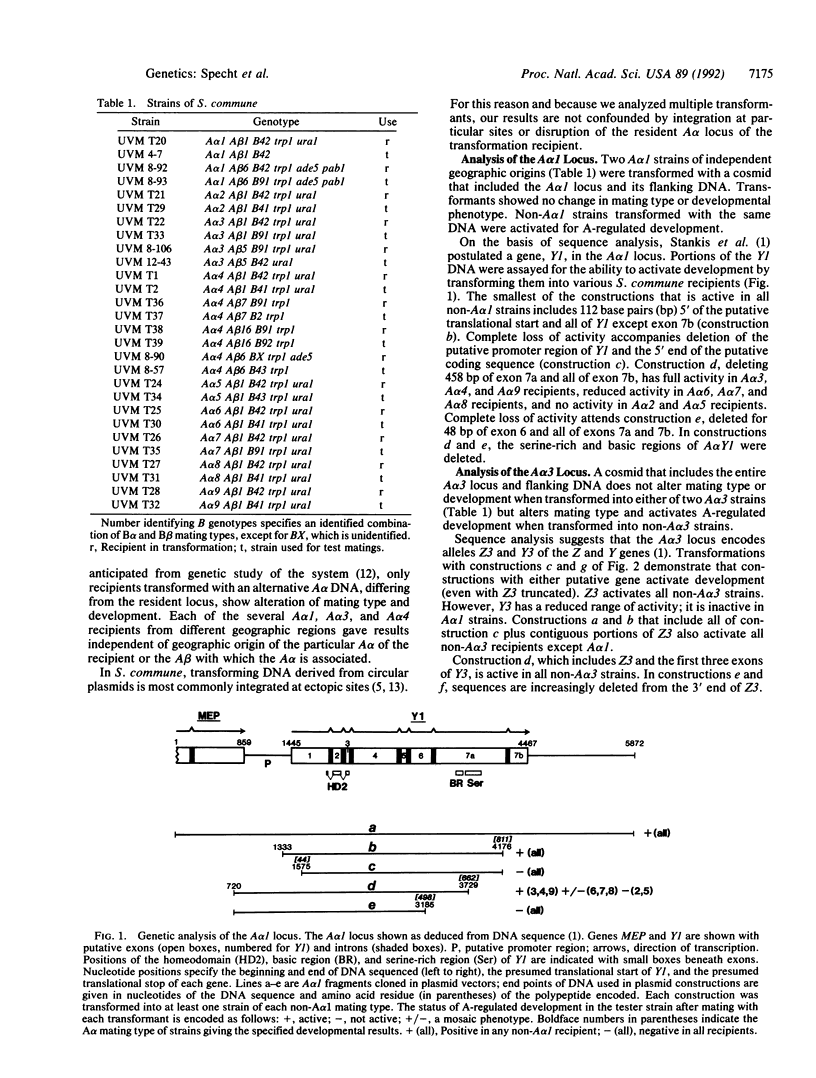
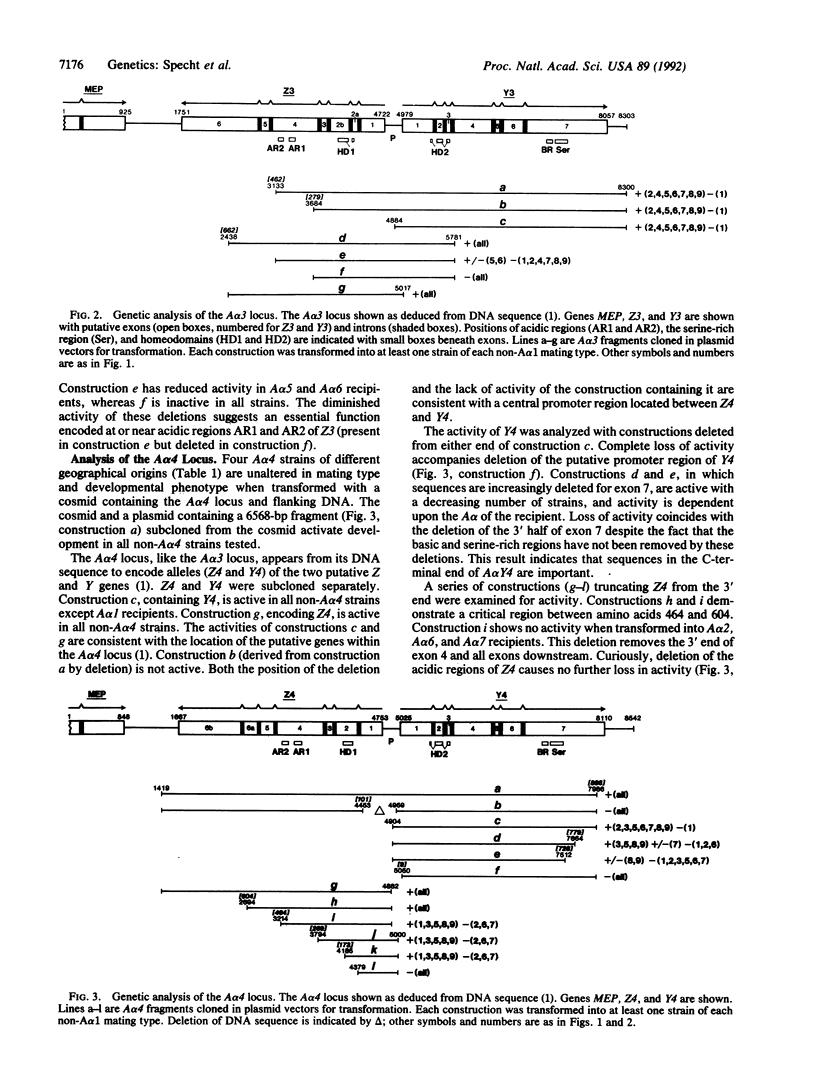
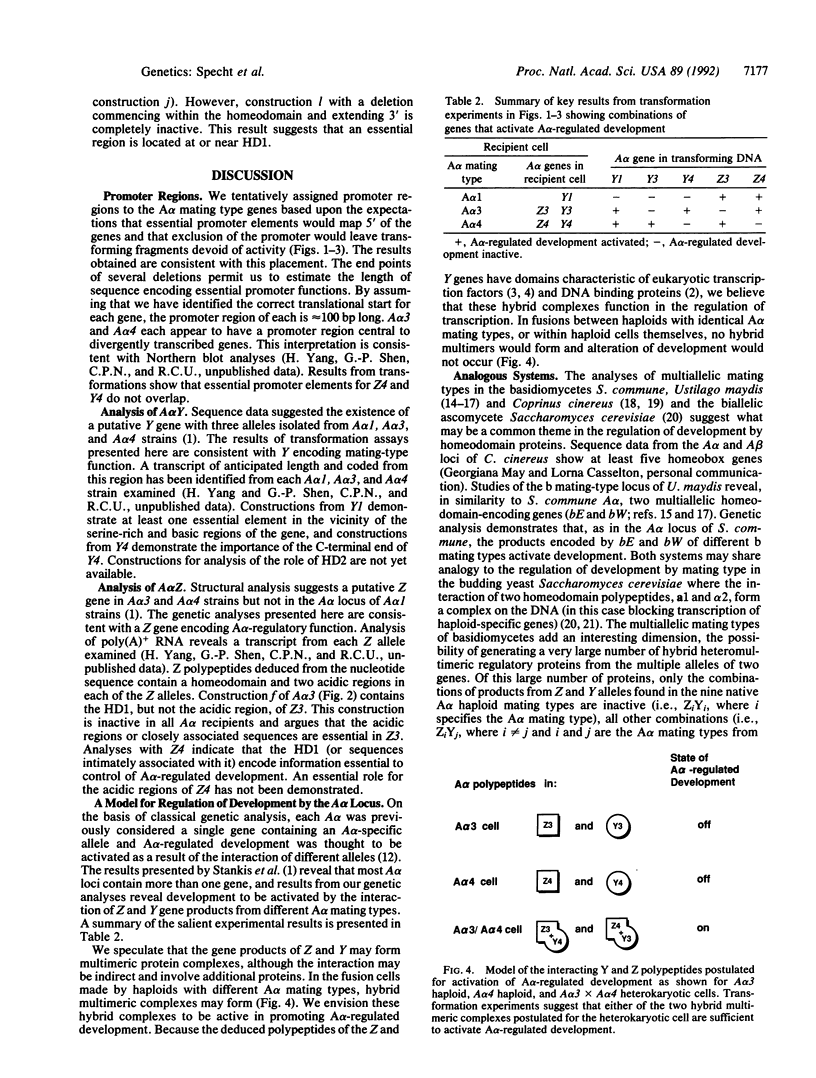
DNA-mediated transformation was used to correlate function with putative genes from three alternative A alpha mating-type loci (A alpha 1, A alpha 3, and A alpha 4) of Schizophyllum commune. Each DNA was tested in at least nine haploid strains, one for each of the nine A alpha mating types found in the world-wide population of S. commune. The Y and Z genes (tentatively identified by sequence analysis elsewhere) individually activate A alpha-regulated development when transformed into any strain with a different A alpha mating type. The only exceptions are when the Y alleles of A alpha 3 or A alpha 4 (i.e., Y3 or Y4, respectively) are introduced into an A alpha 1 strain (the A alpha 1 locus encodes Y1 but lacks a Z gene). These observations indicate that A alpha-regulated development is activated by the interaction (direct or indirect) of products from different genes (e.g., Z3 and Y1) rather than from different alleles of the same gene (e.g., Y1 and Y3). Therefore, the activating interaction is of the form ZiYj where i not equal to j and i and j are the A alpha mating types from which the Z and Y polypeptides, respectively, are derived. Transformations with truncated or mutagenized genes begin to define essential regions of the genes and their products. Activity is in some cases dependent upon the particular A alpha mating type of the recipient. A working hypothesis for the activation of A alpha-regulated development is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dranginis A. M. Binding of yeast a1 and alpha 2 as a heterodimer to the operator DNA of a haploid-specific gene. Nature. 1990 Oct 18;347(6294):682–685. doi: 10.1038/347682a0. [DOI] [PubMed] [Google Scholar]
- Froeliger E. H., Muñoz-Rivas A. M., Specht C. A., Ullrich R. C., Novotny C. P. The isolation of specific genes from the basidiomycete Schizophyllum commune. Curr Genet. 1987;12(7):547–554. doi: 10.1007/BF00419565. [DOI] [PubMed] [Google Scholar]
- Froeliger E. H., Ullrich R. C., Novotny C. P. Sequence analysis of the URA1 gene encoding orotidine-5'-monophosphate decarboxylase of Schizophyllum commune. Gene. 1989 Nov 30;83(2):387–393. doi: 10.1016/0378-1119(89)90127-3. [DOI] [PubMed] [Google Scholar]
- Giasson L., Specht C. A., Milgrim C., Novotny C. P., Ullrich R. C. Cloning and comparison of A alpha mating-type alleles of the Basidiomycete Schizophyllum commune. Mol Gen Genet. 1989 Jul;218(1):72–77. doi: 10.1007/BF00330567. [DOI] [PubMed] [Google Scholar]
- Gillissen B., Bergemann J., Sandmann C., Schroeer B., Bölker M., Kahmann R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell. 1992 Feb 21;68(4):647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Leong S. A. The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev. 1990 Aug;4(8):1384–1395. doi: 10.1101/gad.4.8.1384. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- May G., Le Chevanton L., Pukkila P. J. Molecular analysis of the Coprinus cinereus mating type A factor demonstrates an unexpectedly complex structure. Genetics. 1991 Jul;128(3):529–538. doi: 10.1093/genetics/128.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Rivas A. M., Specht C. A., Ullrich R. C., Novotny C. P. Isolation of the DNA sequence coding indole-3-glycerol phosphate synthetase and phosphoribosylanthranilate isomerase of Schizophyllum commune. Curr Genet. 1986;10(12):909–913. doi: 10.1007/BF00398288. [DOI] [PubMed] [Google Scholar]
- Schulz B., Banuett F., Dahl M., Schlesinger R., Schäfer W., Martin T., Herskowitz I., Kahmann R. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell. 1990 Jan 26;60(2):295–306. doi: 10.1016/0092-8674(90)90744-y. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Stankis M. M., Specht C. A., Yang H., Giasson L., Ullrich R. C., Novotny C. P. The A alpha mating locus of Schizophyllum commune encodes two dissimilar multiallelic homeodomain proteins. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7169–7173. doi: 10.1073/pnas.89.15.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]


