Figure 1.
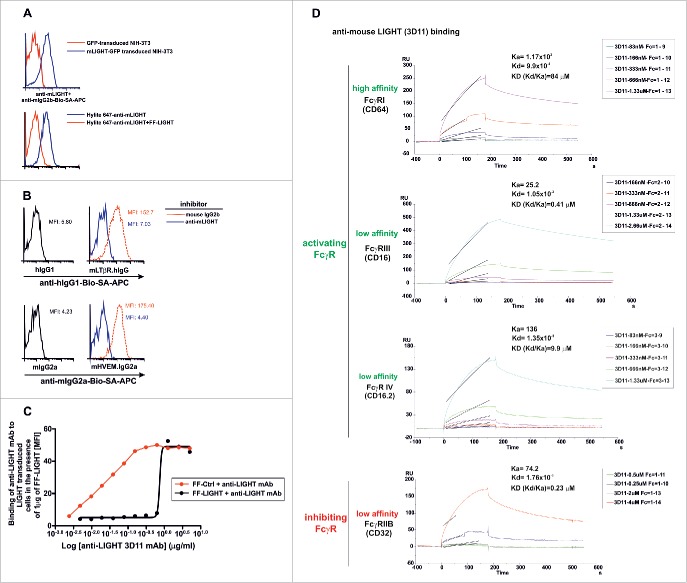
Effective blockade of LIGHT/LTβR and LIGHT/HVEM interactions using a mouse anti-mouse LIGHT monoclonal antibody. 2.5 × 105 NIH-3T3 cells transduced with GFP-tagged murine LIGHT (blue solid lines) or GFP-transduced NIH-3T3 cells (red solid lines) were incubated with a mouse anti-mouse LIGHT mAb 3D11. After an incubation step, antibody binding was revealed with biotinylated rat anti-mouse IgG2b followed by allophycocyanin-coupled streptavidin (A, upper panel). To further demonstrate the specificity of the anti-LIGHT mAb, 1 μg/well of Hilyte 647-labeled anti-LIGHT mAb alone (blue solid line) or 1 μg/well Hilyte 647-labeled anti-LIGHT mAb preincubated with 2 μg/well FF-LIGHT fusion protein (red solid line) were added to LIGHT-GFP transduced NIH 3T3 cells (A, lower panel). (B) 2.5 × 105 LIGHT-transduced NIH-3T3 cells were pre-incubated for 30 min at room temperature with anti-LIGHT mAb (clone 3D11, blue solid lines) or mouse IgG2b isotype control (red dotted lines). Then, cells were stained with LTβR-Ig (upper panel) or HVEM-Ig (lower panel). Binding of LTβR-Ig was revealed with biotinylated anti-hIgG and binding of HVEM-Ig was revealed with biotinylated anti-mouse IgG2a, both followed by allophycocyanin-coupled streptavidin. The baseline background staining is represented by hIgG1 Fc fragment (upper panel, black solid lines) to LIGHT-transduced NIH-3T3 cells or the binding of the Fc fragment mIgG2a (lower panel, black solid lines). The mean fluorescence intensity (MFI) is indicated in each plot. (C) Serial dilutions of anti-LIGHT mAb (clone 3D11) were preincubated with a fixed amount of 1 μg/well of soluble recombinant FF control (red solid line) or FF-LIGHT protein (black solid line). Then, 1 × 105 LIGHT-transduced NIH-3T3 were added to the reaction. After a washing step, a biotinylated rat anti-mouse IgG2b conjugate was further incubated and developed by SA-APC. The mean fluorescence intensity (MFI) binding of anti-LIGHT mAb to LIGHT transduced cells in the presence of FF control or FF-LIGHT is plotted. (D) The association and dissociation constant rates of 3D11 mAb binding to distinct immobilized mouse FcγR were calculated by surface plasmon resonance and from those values the equilibrium dissociation constant KD for each of them was determined.