ABSTRACT
Due to improved understanding of the role of bone morphogenetic protein 4 (BMP4) in an increasing number of diseases, the development of selective inhibitors of BMP4 is an attractive therapeutic option. The currently available BMP4 inhibitors are not suitable as therapeutics because of their low specificity and low effectiveness. Here, we compared newly generated anti-BMP4 llama-derived antibodies (VHHs) with 3 different types of commercially available BMP4 inhibitors, natural antagonists, small molecule BMPR inhibitors and conventional anti-BMP4 monoclonal antibodies. We found that the anti-BMP4 VHHs were as effective as the natural antagonist or small molecule inhibitors, but had higher specificity. We also showed that commercial anti-BMP4 antibodies were inferior in terms of both specificity and effectiveness. These findings might result from the fact that the VHHs C4C4 and C8C8 target a small region within the BMPR1 epitope of BMP4, whereas the commercial antibodies target other areas of the BMP4 molecule. Our results show that the newly developed anti-BMP4 VHHs are promising antibodies with better specificity and effectivity for inhibition of BMP4, making them an attractive tool for research and for therapeutic applications.
KEYWORDS: BMP inhibitors, BMP4, llama antibodies, Noggin, VHH
Introduction
Bone morphogenic proteins (BMPs) are multi-functional growth factors that belong to the transforming growth factor-β (TGF-β) superfamily. More than 20 members of the BMP subfamily have been described,1 and they can be classified in different subgroups, e.g., BMP2/4, BMP5/6/7/8a/8b, BMP9/10 and BMP12/13/14, depending on their amino acid sequence homology, structure and functions.2,3 BMP signals are mediated through 2 classes of transmembrane serine-threonine kinase receptors (BMPR), BMPR type I (BMPR1) and type II (BMPR2). Binding to BMPR2 receptors induces the activation of the receptor complex with the phosphorylation of the BMPR1, leading to intracellular activation of canonical Smad-dependent or non-canonical Smad-independent pathways, which include mitogen-activated proteins kinases (MAPK), p38 or ERK1/2, and Akt.1
BMP4 has recently been revealed as a crucial player in a wide variety of diseases. For instance, BMP4 was shown to have an essential role in the development of severe and progressive disease, such as pulmonary arterial hypertension, through regulation of Ca2+ signaling and activation of the Smad1/5/8, ERK1/2 and MAPK/p38 signaling pathways. 4 BMP4 has also been detected in atherosclerotic plaques, being an inducer of foam cell formation by attenuating cholesterol transporters expression.5 Furthermore, increased levels of BMP4, due to oxidative stress, are also present in intrauterine growth retardation, leading to inhibition of oligodendrocyte maturation and myelination.6 Interestingly, opposing roles have been found when evaluating the involvement of BMP4 in comparison with other BMPs in disease pathophysiology.7 For instance, BMP4 was shown to have an important pathologic role in hypoxic pulmonary hypertension, whereas BMP2 exerted a protective role in this disease.8 A similar effect can be seen in renal disease, where BMP4 involvement in the initiation and progression of diabetic changes in the kidney was shown,9 but BMP2 exerted a protective effect on renal damage.10 In other instances, BMP2 and BMP4 might act synergistically, such as in diabetes11 and ovarian cancer,12 and therefore concomitant inhibition of both BMP2 and BMP4 might be desired. Distinct roles of BMP4 with other BMPs have also been found in several neoplasms.13 For example, in gastric cancer BMP4 enhances migration and reduces cisplatin sensitivity,14 while BMP9 has tumor suppressor functions.15 Similarly, while BMP4 and BMP2 seem to facilitate metastasis and invasion in most cancers, BMP6 and BMP7 have a suppressive role in metastatic breast cancer and melanomas.16
If BMP4 inhibition is to be used as a therapy strategy, avoiding the side effects of inhibition of other BMPs is of major importance. Three major types of anti-BMP4 antagonists have been described, natural antagonists, small molecule inhibitors of BMP receptors and conventional anti-BMP4 antibodies. A plethora of natural antagonists regulate BMP function.17 The best studied group of extracellular BMP modulators is the cystine-knot group of BMP antagonists, which bind BMPs with high affinities and prevent their interaction with the receptors. Depending on the structure (size of the cystine knot), they are divided into 3 groups: the DAN family (Gremlin, Sclerostin), the twisted gastrulation (Tsg), and Chordin and Noggin. The interplay between BMPs and their antagonists is crucial in ultimately determining their effects. A degree of promiscuity exists between these antagonists and the BMPs they bind to and inhibit. Noggin is the BMP antagonist that has been most extensively studied and has been found to inhibit BMP2, BMP4, BMP5, BMP7, BMP13 and BMP14.18 For the other antagonists, only some BMPs have been tested.19
Advances in high-throughput screening in zebrafish have allowed the generation of small molecules that inhibit BMP receptors and therefore inhibit BMP4 signaling. Dorsomorphin (DM) was the first small molecule inhibitor developed. It inhibits the canonical SMAD pathway through binding to the BMPR1, Activin receptor-like kinase-2 (ALK2 or ActR-Ia), ALK3 (BMPR1a) and ALK6 (BMPR1b), blocking BMP-mediated Smad1/5/8 phosphorylation and target gene transcription.20 Besides its moderate efficiency this molecule is not specific, i.e., it also inhibits other signaling pathways, including AMP-activated protein kinase (AMPK), receptor tyrosine kinase for platelet-derived growth factor (PDGPRβ) and vascular endothelial growth factor type-II receptor (VEGFR-II).21-23 Structure-activity relationship (SAR) studies in DM permitted the generation of molecules with increased inhibitory activity by manipulating their chemical structure. The DM derivate LDN-193189 (LDN) is a more potent and specific inhibitor compared with DM, inhibiting more efficiently the activity of the BMP type I receptors ALK2 and ALK3 and having lower selectivity against ALK5 and VEGFR-II.22 Dorsomorphin homolog 1 (DMH1), a second generation molecule also derived from DM, targets the BMPR1 ALK2 and ALK3 more selectively, but not VEGF or AMPK signaling.23 Unlike DM and LDN, DMH1 has no inhibitory effect in the non-canonical pathways p38/MAPK.23 More recently, K02288 was generated.24 This small molecule, when compared with LDN in kinome-wide selectivity studies, showed a more potent and selective activity against ALK1 and ALK2, but not VEGF-II.24,25 However, in cell-based assays of BMP signaling, K02288 showed relatively weak potency and selectivity for the BMP receptors.26 Experiments have shown how these small molecules bind and inhibit signaling through the BMP receptors, yet a proper analysis of the different BMPs that they inhibit has not been properly completed.
Several conventional anti-BMP4 antibodies with high specificity to BMP4, and not BMP2, have been described. Whereas most are only used in research applications, such as Western blot or immunostaining, a few of them have been used for neutralization experiments with limited success due to their low efficacy.27-31 Llama-derived antibodies or VHH are small (∼15 kDa) fully functional antibodies that lack light chains. Due to their distinct structure, these stable antibodies can bind specifically and with great affinity to their antigens better than the conventional antibodies.32 We recently developed 2 selective anti-BMP4 VHH: C4C4 and C8C8.33 C4C4 showed remarkable BMP4 specificity, whereas C8C8 could bind and inhibit both BMP2 and BMP4 signals. Both antibodies were shown to bind to the BMPR1-binding area of BMP4. Whereas C4C4 bound to the BMP4-specific groove region, C8C8 bound to the BMP2/BMP4 pocket interface within the BMPR1 epitope. We also showed that both antibodies potently inhibit BMP4-mediated functions, such as modulating chemosensitivity in colorectal cancer cells.
In this study, we sought to compare these VHHs with the existing BMP4 antagonists and determine their level of specificity and effectiveness. We found that whereas natural antagonists and small molecules were able to inhibit signaling of many members of the BMP family, conventional anti-BMP4 antibodies were BMP4-specific, i.e., they could only inhibit BMP4-mediated signals. However, we found that the neutralization activities of these antibodies were inferior in comparison with the VHHs. Examination of their epitopes suggests that, in contrast to the VHHs, they do not target the BMPR1 binding region, offering an explanation for their low efficiency. Together, our results demonstrate that the newly generated anti-BMP4 VHHs represent a more specific and effective option for inhibition of BMP4, which could potentially be used for research or therapeutic purposes.
Results
Specificity of VHH compared to current inhibitors
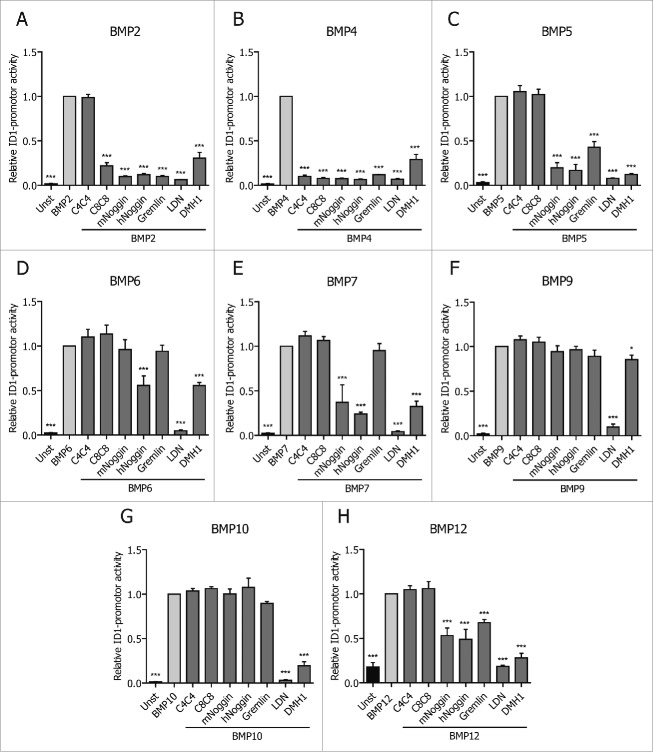
We recently generated 2 llama-derived BMP4-specific antibodies or VHHs, C4 and C8.33 VHHs are single domain antibodies only composed by the variable domain of the heavy chain of a llama-derived antibody, which is around 15 kDa in size. Due to their unique structure and small size, VHHs can specifically bind with high affinity to their antigens. We found that dimerization into biheads (C4C4 and C8C8) increased their effectiveness and affinity to BMP4, and therefore proceed to use those for our studies. Compared to Noggin, C4C4 and C8C8 exhibited increased specificity, as they only bind to BMP4 or BMP2 and 4, respectively. We wanted to determine whether this superiority would also extend to other natural antagonists as well as chemical inhibitors of BMP receptors. Therefore, we compared these 2 VHHs with 2 natural antagonists, Noggin and Gremlin, and 2 chemical BMPR inhibitors, LDN and DMH1, using a BMP response element (BRE)-luciferase reporter assay. In this assay, the activation of the BMP-SMAD dependent response gene ID1 was measured in luciferase reporter C2C12 myoblast cells.34 As expected, Fig. 1 confirms that specificity of C4C4 is restricted to BMP4 and of C8C8 to BMP2 and BMP4. In contrast, the other inhibitors are less selective. For instance, Noggin and Gremlin could greatly inhibit BMP2, BMP4 and BMP5 signals. Further, Noggin inhibited BMP6, BMP7 and BMP12-mediated SMAD pathways. The chemical inhibitors LDN and DMH1 were observed to be even less selective than Noggin, as they also inhibited BMP9 and BMP10-derived signals. At the concentrations tested, all the inhibitors were equally potent at inhibiting BMP4-derived signals. These results confirm that C4C4 and C8C8 are more specific than the other BMP inhibitors tested because they can neutralize only BMP4 or BMP2/BMP4-mediated SMAD signaling.
Figure 1.
VHH specificity compared to different inhibitors. C2C12 cells were activated with 50 ng/ml of hBMP2 (A), 10 ng/ml of hBMP4 (B), 200 ng/ml of hBMP5 (C), 50 ng/ml of hBMP6 (D), 200 ng/ml of hBMP7 (E), 25 ng/ml of hBMP9 (F), 25 ng/ml of hBMP10 (G) and 50 ng/ml of hBMP12 (H) for 16h. At the same time the inhibitors were added. VHH, mNoggin, hNoggin and Gremlin were added at a concentration of 500 ng/ml; LDN-193189 (LDN) at 0.5 μM and DMH1 at 0.2 μM.
Superior efficacy of VHH compared to current inhibitors
Several commercial anti-BMP4 antibodies have been reported. Whereas most have been used for research applications, some have been shown to have neutralization activities. For instance, clone 3H2, a mouse monoclonal antibody, was able to inhibit BMP4-mediated glial differentiation, 27 while anti-BMP4 clone 66119 was shown to inhibit BMP4-mediated smooth muscle cell proliferation.28 Surprisingly, the concentrations at which those antibodies were used in those and in other publications29-31 ranged between 1-10 μg/ml, in contrast to the nanomolar range of which the VHH were shown to be biologically active.33 To test whether these disparities are due to differences in experimental conditions or not, we compared the activity of C4C4 and C8C8 with current anti-BMP4 antibodies in the luciferase C2C12 system. These experiments confirmed that these antibodies are BMP4-specific, as only C8C8 inhibited BMP2-mediated signals (Fig. 2A). At antibody concentrations of 100 ng/ml, both VHHs completely inhibited BMP4-specific signals, whereas clone 66119 only inhibit 30% and clones 3H2 and M912262 only inhibited 1% of the BMP4-specific signal. Interestingly, 3 anti-BMP4s antibodies were unable to inhibit the BMP4 signals even at saturating concentrations of 10 μg/ml (Fig. 2B).
Figure 2.
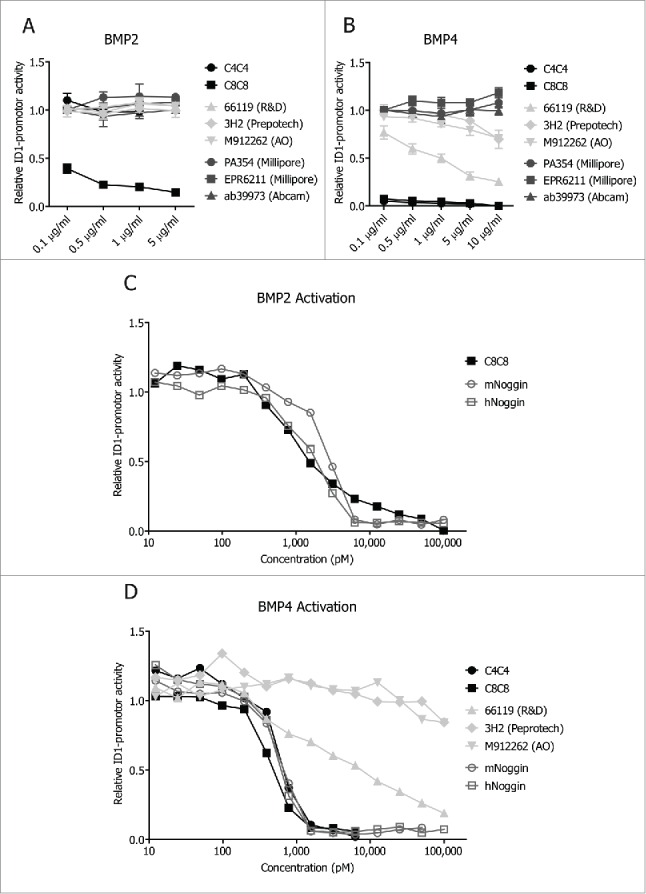
VHH efficacy compared to different available inhibitors. C2C12 cells were stimulated with 10 ng/ml of hBMP4 (A) or 50 ng/ml of BMP2 (B), and at the same time increasing concentrations of anti-BMP4 antibodies and VHHs were added (0.1 μg/ml to 5 μg/ml). IC50 curves of inhibition of BMP2-madiated signals by C8C8 and natural antagonists (C). IC50 curves of the inhibition of BMP4-mediated signals by VHHs, commercial anti-BMP4 antibodies and natural antagonists (D).
The superiority in functionality of the VHHs is also maintained at the molarity level, as shown by the IC50 curves of these antibodies (Fig. 2C – D). Remarkably the neutralization activities of C4C4 for BMP4 (IC50 ∼600 pM) and C8C8 for BMP4 (IC50 ∼470 pM) and BMP2 (IC50 = 1205 pM) are similar to those of mouse (BMP4 IC50 ∼615 pM; BMP2 IC50 ∼2550 pM) and human Noggin (BMP4 IC50 ∼540 pM and BMP2 IC50 ∼1690 pM) (Fig. 2C – D). The values of the IC50 of the commercial anti-BMP4 antibodies were not able to be determined because they were unable to completely inhibit BMP4 at the saturating concentrations tested (Fig. 2D). These results establish C4C4 and C8C8 as highly effective inhibitors with higher neutralization capabilities compared to current conventional anti-BMP4 antibodies.
Epitope mapping of commercial anti-BMP4 antibodies
Based on the BMP4 BRE-signal data, the anti-BMP4 antibodies were of 3 different types: 1) functionally inactive; 2) weak inhibitors; and 3) strong inhibitors. These results could be explained by differences in epitope binding. The active mature form of BMP4 is released after proteolytic cleavage of the secreted inactive form (pro-BMP4) (Fig. 3A). As only the active dimeric form of BMP4 is used in the C2C12 luciferase-based assays, it is likely that the lack of activity of some antibodies would be due to binding to pro-BMP4 rather than the active dimeric form. Indeed, when the mature dimers of BMP4 were immobilized in nitrocellulose membranes, they were not recognized by the functionally neutral antibodies (data not shown). In contrast, all the antibodies with functional activity detected a band of ∼34 kDa, which corresponds to the mature BMP4 dimer (Fig. 3A). In these blots, C8C8 also identified dimers of BMP2 (band at ∼26 kDa, Fig. 3B). Surprisingly, clones M9122623 and 3H2 also seemed to recognize BMP2 and BMP5. There was also a weak BMP5 band (31 kDa) observed when the clone 66119 was used. These results seem to indicate that whereas the inactive antibodies bind pro-BMP4, both weak and strong anti-BMP4 antibodies bind to the mature region of BMP4.
Figure 3.
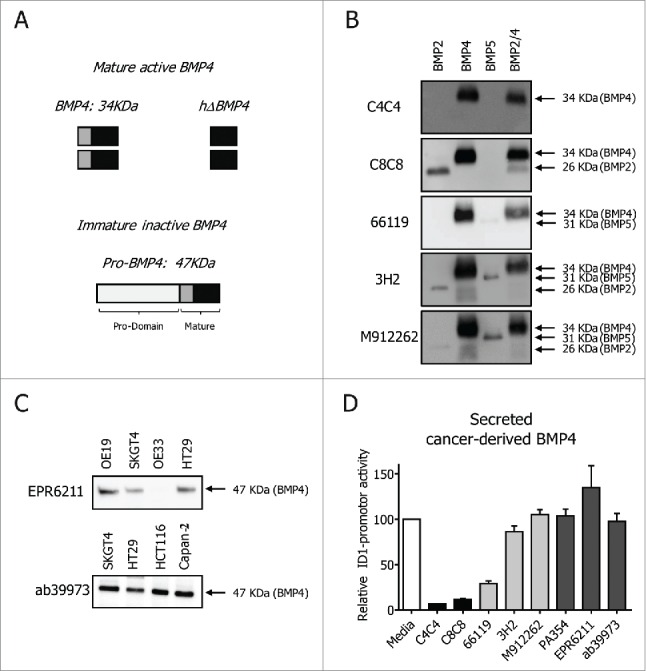
Epitope mapping of commercial anti-BMP4 antibodies. Schematic representation of BMP4: mature active BMP4 (BMP4 active dimer and BMP4 heparin domain) and immature inactive pro-BMP4 form (A). Western Blot detection of unreduced recombinant BMP proteins (B) and reduced cancer cell lines lysates (C). Conditioned media from HT29 was added to the C2C12 cells in the presence of 10 μg/ml of VHHs or anti-BMP4 antibodies (clone 66119, clone 3H2, clone PA354, clone and EPR6211 and antibody ab39973) (D).
To confirm that indeed the inactive antibodies bind to the long pro-BMP4, reduced lysates of different cancer cell lines were blotted with those antibodies (Fig. 3C). A single line of ∼47 kDa, corresponding to the reduced pro-BMP4, was observed. These results explain why these antibodies do not directly inhibit the signals of the active form of BMP4 in the C2C12 experimental setting, but they do not reflect the real biological significance of pro-BMP4 inhibition. It could be postulated that blocking the inactive form of BMP4 could indirectly result in a decreased BMP4 activity, because of blocking its cleavage into an active BMP4. We therefore incubated the supernatants of the colorectal cancer cell line HT29, known to produce and secrete BMP4,33 with the different antibodies (Fig. 3D). Addition of the antibodies that target pro-BMP4 had no effect on total BMP signal, indicating that these antibodies bind to a region within the pro-domain that does not interfere with proteolytic cleavage, and therefore have no biological effect on BMP4 function. As expected, the other anti-BMP4 antibodies inhibited endogenous BMP4 activity at different levels.
We previously showed that C4C4 and C8C8 target different areas within the BMP4 molecular interface that binds BMPR1a.33 We therefore wanted to test whether the superiority of the VHHs over the weak commercial anti-BMP4 antibodies was due to the specific targeting of the BMPR1 areas, as opposed to targeting other non-BMPR binding regions within the mature dimer. To that end, we used a mutated form of BMP4 (Figs. 3A and 4A), that lacks an N-terminal region containing several basic residues shown to be involved in binding to the extracellular matrix through interactions with heparin. 35 Using the C2C12 system, this mutated BMP4 (hΔBMP4) was shown to be less active in activating BMP4-mediated ID1 promoter activity than the full length mature dimer of BMP4 (Fig. 4A). As previously shown,33 the VHHs had no difficulty in inhibiting hΔBMP4-mediated activity, indicating that deletion of this region had no effect on binding of the VHHs to their epitopes on BMP4 (Fig. 4B). In contrast, deletion of the N-terminal part had an effect on the binding of the other antibodies to BMP4 (Fig. 4B). Clone 66119, for instance, was unable to inhibit hΔBMP4-mediated signals, demonstrating that the deleted region is indeed the epitope of this antibody. Interestingly, 3H2 and M912262 were able to inhibit hΔBMP4-mediated signals better than BMP4, indicating that this N-terminal domain interferes with their epitope (Fig. 4B). Together, these results suggest that the epitopes of the commercial antibodies are different than the VHHs. Thus, it could be postulated that targeting areas involved with BMPR1 binding, as opposed to targeting other areas within the mature dimeric form of BMP4, confers functional superiority to the anti-BMP4 VHHs.
Figure 4.
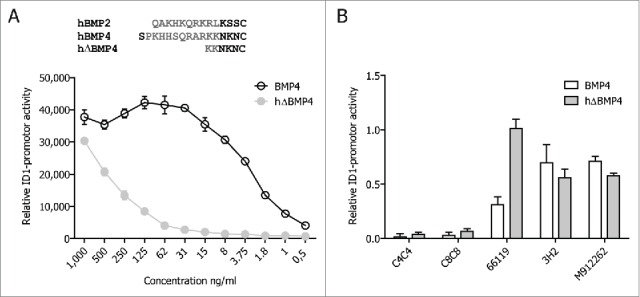
Epitope mapping with hΔBMP4. Schematic alignment of hBMP2, hBMP4 and mutated BMP4 (hΔBMP4) and comparison of relative functionality of BMP4 and hΔBMP4 (A). Comparison of BMP4 binding sites between antibodies. C2C12 cells were stimulated with 10 ng/ml of BMP4 or 1,000 ng/ml of hΔBMP4. At the same time the antibodies were added at a concentration of 10 μg/ml (B).
Discussion
Specific BMP4 inhibition is therapeutically attractive because BMP4 is involved in the pathogenesis of certain diseases such as renal disease,9 diabetic nephropathy,36 hypoxic pulmonary hypertension8 and gastric cancer,14 among others. However, BMP ligands and BMP receptors share remarkable functional and structural similarities, which has posed challenges for the development of specific BMP4 inhibitors with clinical potential. In this report, we compared the specificities and neutralization capabilities of current BMP inhibitors with recently designed anti-BMP4 llama-derived antibodies.33
Natural BMP antagonists are well-established inhibitors of BMP4, and some have been shown to be effective at inhibiting specific BMP4-mediated functions.37 However, several limitations curb their applications in the clinic for BMP4-specific diseases. Recombinant natural proteins may have very short half-life and poor bioavailability.38 For instance, BMP4-induced heterotopic ossification was only blocked if Noggin was delivered locally, but not systemically. However, when somatic cell gene transfer of a mutated form of Noggin that lacks its heparin-binding domain was systemically given, bone formation was effectively inhibited.38 Therefore, recombinant proteins need to be re-engineered for their use in the clinic, a process that can be cumbersome and expensive. Another complicating factor is that their activity is in turn tightly regulated by extracellular factors, including other BMP antagonists and other proteins, such as the metalloproteases Xolloid and Tolloid.39 Thus, if the expression of antagonists overlaps, activation of BMP signaling instead of inhibition might result. Also, additional mechanisms of action have been described for some antagonists, rendering these molecules highly unspecific at inhibiting BMP4 function. For instance, Chordin possesses BMP-independent functions due to its binding to cell surface proteins and alteration of cellular integrity.40 In some cases, BMP modulators possess such opposing modes of regulation that they can act both as anti- or pro-BMP molecules, as in the case of Tsg and Crossveinless-2 (CV2). Finally, some BMP antagonists are non-selective in nature and modulate signaling of not only several BMPs, but also of other members of the TGF-β family, as well as other signaling pathways such as Wnt.41
In our experiments, we found that natural Noggin and Gremlin are extremely potent at inhibiting BMP4-, BMP2- and BMP5-derived signals. They also inhibit the signals of other BMPs at varying degrees. For instance, we found that Noggin clearly inhibits BMP6 and BMP7. As these 2 BMPs have been shown to be protective in certain cancers,16 application of Noggin in the clinic might present detrimental effects. Interestingly, the inhibition of BMP7 was apparent with both human and mouse Noggin, whereas human, but not mouse, inhibited BMP6, and to lower levels than its inhibition of BMP7. This is in concordance with previous results.42
We also showed for the first time that both Noggin and Gremlin can inhibit BMP12-specific signals. Noggin and Gremlin bind to the ligands through several molecular interfaces that encompass both BMPR1a and also BMPR2 epitopes. These epitopes contain large areas of conserved amino acids between BMPs, which might explain their lack of specificity to certain BMPs.43,44
Since the characterization of Dorsomorphin (DM),20 much effort has been made to develop more specific small molecule inhibitors of type I BMP receptors that selectively target the BMP and not the TGF-β pathway. LDN-193189 and DMH1, are both DM-derivate with increased potencies and specificities over DM. LDN was recently shown to target both the BMP and TGF-β pathway, as well as several other kinases.45 DMH1, however, selectively inhibits Alk2 and Alk3, and has no effect on other BMP- or TGF-β-receptors.23 Most studies using these molecules have mainly compared their binding affinities and specificities toward their receptors, but their effects on the signaling pathways on the different BMPs was lacking. In this study, we analyzed their specificity toward a large panel of BMPs. These inhibitors were the least selective of all the compounds tested, as they were able to inhibit the signaling mediated by all 8 BMPs examined.
Our data also presents a comparison between Noggin and DMH1, revealing that natural antagonists are more selective than chemical inhibitors. This is in keeping with the fact that the small inhibitors target the receptors, rather than the ligands themselves. The so-called ligand-receptor promiscuity, by which BMP receptors can bind several ligands,46 renders the chemical inhibitors more promiscuous than the natural antagonists. Therefore BMP receptor inhibitors are also not fit to provide specific BMP4 inhibition and should not be used as therapy options in diseases in which BMP4 signaling is defective. Small molecule inhibitors can, however, potentially be used in the clinic for diseases in which total BMP signaling is affected. For instance, dysregulation of BMP signaling pathway is found in diseases such as fibrodysplasia ossificans progressiva (FOP) and diffuse intrinsic pontine glioma (DIPG), which are driven by mutations in the ACVR1 gene, vital for the ALK2 activation.22,26
Conventional anti-BMP4 antibodies have been previously reported and used both in research as well as in neutralization experiments.27,28 Their epitopes, however, have never been tested or disclosed, and their inhibitory capabilities were never compared. In this study, we aimed to decipher their binding epitopes and determine how these correlate with functionality. We selected antibodies commonly used in the literature. In particular, clone 66119 (MAB757 from R&D) has been mainly used to inhibit BMP4 function. Also, clone EPR6211 (Cat. No. ab124715; Abcam) is the one mostly used when detecting BMP4 by immunohistochemistry.47-59
Our study revealed 3 types of anti-BMP4 antibodies (Fig. 5). We showed that one group of antibodies that recognize pro-BMP4 are not suited at inhibiting BMP4 function. We demonstrated they cannot directly inhibit the functional activity of mature BMP4 because they do not bind to this active form. We also proved that their binding to pro-BMP4 does not affect its cleavage, and therefore its release into the active form, which leaves BMP4 activity in the system unchanged. Because these antibodies detect pro-BMP4, they are well suited to identify cells or tissues that secrete BMP4, and therefore can be used in research applications such as western blots or immunohistochemistry.
Figure 5.
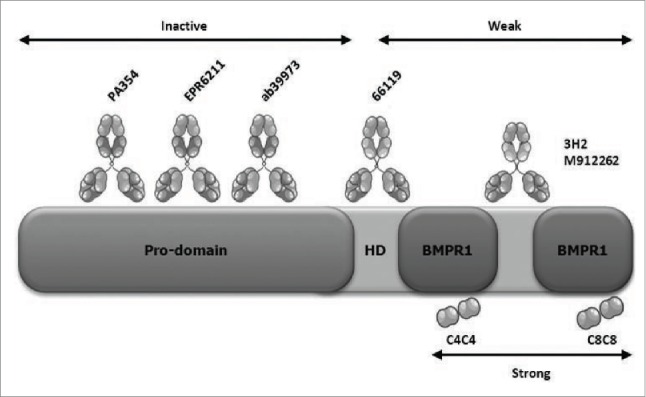
Schematic overview of binding determinants of BMP4 and the different anti-BMP4 tested. Existing anti-BMP4 antibodies engage their target via interactions through different domains within BMP4. Whereas C4C4 and C8C8 bind to the BMPR1a binding site, inactive antibodies bind to the pro-domain and weak antibodies bind to certain regions of the mature protein, such as the heparin domain (HD), that do not correspond to the BMPR1a binding site.
A second group of anti-BMP4 antibodies have weak inhibitory capabilities. Using protein gel blot, we found that all the antibodies in this group bind the mature region of BMP4. In particular, and as confirmed in previous results,33 clone 66119 binds to an N-terminal region of BMP4 that might correspond to the heparin domain of BMP4.35 Here, we prove that this antibody is remarkably less effective at inhibiting BMP4-mediated function than the VHHs. The other anti-BMP4 antibodies of this group were extremely poor at functionally inhibiting BMP4, and they bound other BMPs, such as BMP2 and BMP5. This suggests that their epitope lies within a region containing residues shared by these BMPs. Together, these results show that the antibodies in this group bind to a region within the mature BMP4 that is not functionally relevant, and that does not correspond to the BMPR1 binding region. Indeed, the finding that hΔBMP4 is still able to activate (albeit at much lesser concentrations) the ID1 promoter in C2C12 cells suggests that completely removal of this region is not sufficient to block the ability of BMP4 to activate its receptors. Further, we also exposed the low biological significance of these antibodies. When using a colorectal cancer cell line that exclusively secretes BMP4,33 we found that using a saturating amount of the commercially available antibodies produced minimal effects on BMP4 signaling. The significance of the epitope mapping of these commercial antibodies also extends beyond their applicability as neutralization antibodies. When using these antibodies for research purposes, caution in the interpretation of the results must be taken. For instance, if expression is being tested with antibodies that bind pro-BMP4, the results will depict BMP4 being secreted, but not necessarily active. If expression of active BMP4 is desired, then antibodies that target the mature region must be used.
The third group of antibodies tested included C4C4 and C8C8. These llama-derived antibodies raised against human BMP4 bind the BMPR1 epitope of BMP4 with high affinity (Fig. 5). The results herein show that these antibodies are superior both in specificity and effectiveness. To our knowledge, these are the first anti-BMP4 antibodies described to target the BMPR1-binding region of BMP4. It is therefore tempting to speculate that targeting this region provides these antibodies with superior ability to inhibit BMP4 function.
Concomitant inhibition of BMP2 and BMP4 might also be clinically attractive. Both BMPs are known to be involved in different biologic processes in various diseases. In a mouse model of diabetes, both BMP2 and BMP4 were shown to be elevated.11 In vitro experiments showed that BMP2 and BMP4 negatively regulate the proliferation of β cells and insulin secretion in mouse and human islets. In this setting, inhibition of BMP2 and BMP4 by C8C8 might represent a novel approach for protection and regeneration of adequate functional β cell mass in diabetes.11 Similarly, in ovarian carcinoma, BMP2 and BMP4 promote cancer growth with the formation of microcalcifications, which is commonly observed in ovarian tumors. When inhibiting BMP signaling in vitro and in vivo mesenchymal stem cell-promoted tumor growth is abrogated.12 These examples providence evidence for future therapeutic applications of C8C8.
Pharmacological inhibition of BMP4 function should, however, be undertaken with caution. BMP4 is a complex molecule that has been shown to have dual effects on, for instance, certain types of cancer.50 In breast cancer, BMP4 induces tumor progression activities such as migration and invasion, but suppresses cell growth.50 Similar results can be found for pancreatic cells.51 In colorectal cancer, mutations in the BMPR receptors or the canonical BMP transcription factor SMAD4 are associated with colorectal cancer progression, suggesting a tumor suppressive role for BMP signaling,52,53 although it was later shown that this functional bi-directionality depends on the mutational status of the colon cancer cells. For instance, loss of SMAD4 or mutations on p53 in colorectal cancer cells causes BMP signaling to switch from tumor suppressive to metastasis promoting.54 Our group has also shown that inhibition of BMP4 activity by C4C4 or C8C8 in SMAD4-negative colorectal cancer cells results in an increase in chemosensitivity. These results therefore suggest that inhibition of BMP4 is an attractive therapeutic strategy in colorectal cancers provided it is targeted to cancers presenting the right mutational profile. This might not only apply to colorectal malignancies but also other types of cancers. These findings also suggest that if pharmacological inhibition is given for diseases other than cancer, caution must be taken regarding its potential interplay with oncogenesis.
The clinical applicability of VHHs is currently being investigated in a wide array of diseases, such as inflammatory and infectious diseases, as well as oncology.55 The superiority of VHHs in comparison with conventional antibodies in terms of specificity and potency is well documented. For instance, VHHs raised against the respiratory syncytial virus (RSV)56 or tumor necrosis factor57 were found to have higher antagonist capabilities than conventional antibodies currently used in the clinic for RSV infections or rheumatoid arthritis, respectively. However, the decreased half-life of VHHs has remained a major obstacle for their introduction in the clinic.58 Several strategies are now being implemented to overcome these limitations. Such strategies include the generation of bivalent VHHs by the addition of albumin- or immunoglobulin-binding VHHs, resulting in half-lives of 2 or 9 days, respectively.55,57,59 Other approaches, such as addition of polyethylene glycol to the VHHs, have been shown to increase serum half-life and increase VHH functionality.60 These studies have therefore demonstrated that straightforward reengineering strategies can turn VHHs into effective therapeutic agents.
In conclusion, we showed that our recently generated llama-derived nanobodies specific for BMP4 are more effective and specific at inhibiting BMP4-mediated function than natural antagonists, small molecule BMP inhibitors and conventional anti-BMP4 antibodies. We have shown that other commercial anti-BMP4 antibodies are less potent and weakly bind to other BMPs. This is most likely due to binding to regions of the BMP4 molecule not involved in binding with the receptors. This might explain why the VHHs are superior. These VHHs are thus promising research and clinical tools to inhibit BMP4 function.
Methods
Cells and cell culture
Stably transfected C2C12 mouse myoblasts with BRE-luciferase vector containing mouse ID1 promoter (BMP Responsive Element) were kindly donated by Dr. L. Zilberberg and Dr. D. Rifkin.34 C2C12 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% of fetal bovine serum (FBS), 2 mM of glutamine, 100 units/ml of penicillin, 100 μg/ml of streptomycin and 700 μg/ml of G418 (InvivoGen SAS).
Esophageal adenocarcinoma cell lines OE19, OE33 and SKGT4 and colorectal adenocarcinoma cell line HT29 were cultured in Roswell Park Memorial Institute 1640 medium (RPMI) supplemented with 10% FBS, 2 mM of glutamine, 100 units/ml of penicillin and 100 μg/ml of streptomycin.
The colorectal adenocarcinoma cell line HCT116 and the pancreas adenocarcinoma cell line Capan-2 were cultured in DMEM supplemented with 10% FBS, 2 mM of glutamine, 100 units/ml of penicillin and 100 μg/ml of streptomycin. All carcinoma cell lines were cultured for at least 4 days, and medium changed every 2 to 3 days.
Western blotting
To determine the different forms of BMP4, recombinant BMP proteins and carcinoma cell lines lysate were used. Protein concentrations of cell lysates were determined using Pierce™ BCA Protein Assay Kit (Pierce Biotechnology), following the manufacturer's protocol. Equal amount of samples were loaded (between 8 and 30 μg/lane) on a 10% SDS-polyacrylamide gel and subsequently blotted onto PVDF transfer membranes. The membranes were blocked with blocking solution (5% non-fat milk + Tris-buffered saline with Tween® 20 (TBST) or 5% bovine serum albumin (BSA) + TBST) and subsequently incubated with the appropriate primary and secondary antibody. Afterwards, membranes were washed and incubate with chemiluminescent solution (Pierce™ ECL subtract, Pierce Biotechnology) and visualized using ImageQuant LAS 4000 (GE Healthcare Life Science). Densitometry analysis was performed using Image J 1.45s (Wayne Rasband, National Institutes of Health, USA).
Primary antibodies include: anti-BMP4 clone 66119 (1μg/ml; Cat. No. MAB757; R&D Systems), anti-BMP4 clone 3H2 (0.5μg/ml; Cat. No. 500-M121; Peprotech); anti-BMP4 clone M912262 (0.5μg/ml; Cat. No. ABIN934071; Antibodies-Online), anti-BMP4 Clone EPR6211 (0.1μg/ml; Cat. No. ab124715; Abcam), anti-BMP4 (0.8μg/ml; Cat No. ab39973; Abcam), C4C4 (0.1μg/ml) and C8C8 (1μg/ml). Secondary antibodies include: HRP-anti-rabbit (1:200; Cat. No. P0448; Dako), HRP-anti-mouse (1:2,000; Cat. No. P0447; Dako) and HRP-anti-llama (1:1,000; Cat. No. ab112786; Abcam).
Luciferase assay
C2C12 cells were cultured in 96-well plates at 5×103 cells/well. After overnight incubation, the cells were used for different experiments.
To analyze VHH specificity, C2C12 cells were stimulated with 10 ng/ml of hBMP4 (Cat. No. 314-BP-020; R&D Systems), 50 ng/ml of hBMP2 (Cat. No. 355-BM-010; R&D Systems), 200 ng/ml of hBMP5 (Cat. No. 615-BMC-020; R&D Systems), 50 ng/ml of hBMP6 (Cat. No. 506-BP-020; R&D Systems), 200 ng/ml of hBMP7 (Cat. No. 354-BP-010; R&D Systems), 25 ng/ml of hBMP9 (Cat. No. 3209-BP-010; R&D Systems), 25 ng/ml of hBMP10 (Cat. No. 2926-BP-025; R&D Systems), and 50 ng/ml of hBMP12 (Cat. No. 779-G7-010; R&D Systems). At the same time, different inhibitors were added: C4C4 and C8C8 at a concentration of 500 ng/ml; mNoggin (Cat. No. 1967-NG-025; R&D Systems), hNoggin (Cat. No. 6057-NG; R&D Systems) and mGremlin (cat#956-GR-050; R&D Systems) at 500 ng/ml; LDN-193189 at 0.5 μM (Cat. No. 11802; Cayman Chemical) and DMH1 at 0.2 μM (Cat. No. S7146; Selleckchem).
VHH efficacy was evaluated by stimulating cells with 10 ng/ml of hBMP4 and 50 ng/ml of hBMP2, with and without increasing concentrations (from 0.1 – 10 μg/ml) of VHHs or anti-BMP4 antibodies: clone 66119, clone 3H2, clone M912262, clone PA354-16.1.1 (Cat. No. MABD411; Millipore), Clone EPR6211, and anti-BMP4 (ab39973).
To determine the IC50 of each antibody, cells were stimulated with 10 ng/ml of hBMP4 or 50 ng/ml of hBMP2, with and without increasing molar concentrations (from 0.048 – 100,000 pM) of VHH, anti-BMP4 antibodies (clone 66119, clone 3H2 and clone M912262) and natural antagonists (mNoggin, and hNoggin).
Analysis of the different antibodies in the binding of endogenous BMP4 was performed by stimulated C2C12 cells with conditioned medium of HT-29 cell line with and without 10 μg/ml of VHHs and anti-BMP4 antibodies: clone 66119, clone 3H2, clone PA354-16.1.1, clone EPR6211 and Abcam antibodies ab39973.
To check the functional activity of different forms of BMP4, C2C12 cells were cultured with increasing concentrations (from 0.5 – 1,000 ng/ml) of BMP4 or the mutated form of BMP4 (hΔBMP4; Cat. No. AF-120-05ET; Peprotech). When indicated VHHs and anti-BMP4 antibodies were added at the same time at concentrations of 10 μg/ml.
All experiments were performed for a final volume of 100 μl of DMEM supplemented with 0.1% BSA, 2 mM of glutamine, 100 units/ml of penicillin and 100 μg/ml of streptomycin, in each well. Unstimulated cells were used as controls and wells with no cells used to normalize the background activity. Each condition was performed in triplicate. After 16 hours incubation, the luciferase activity was measured by adding 100 μl of luciferase substrate (Bright-Glo™ Luciferase assay System, Promega Benelux). After an incubation period of 5 minutes, luciferase activity was measured using Synergy HT Multi-Mode Microplate Reader (Biotek, Winooski, VT, United States). Data was normalized and represented as ratio to the activity of cells stimulated with only BMP.
Statistical analysis
All experimental data were carried out with at least 3 independent experiments. Data is expressed as mean ± standard error mean (SEM). Statistical analysis was analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. Probabilities of p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.001 (***) were considered to be statically significant. Statistical analysis was performed using GraphPad Prism 5.01 (GraphPad Software, Inc., CA USA).
Disclosure of potential conflicts of interest
A patent has been filed for the C4C4 and C8C8.
Funding
This work was supported by the European Research Council (ERC) starting and ERC POC grant: Targets4Barrett (S. Calpe, Ana C.P. Correia and K.K.Krishnadath).
References
- 1.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 2005; 16:251-63; PMID:15871923; http://dx.doi.org/ 10.1016/j.cytogfr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 2.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem 2010; 147:35-51; PMID:19762341; http://dx.doi.org/ 10.1093/jb/mvp148 [DOI] [PubMed] [Google Scholar]
- 3.Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Research 2015; 3:15005; PMID:26273537; http://dx.doi.org/ 10.1038/boneres.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang Y, Yang K, Tian L, Fu X, Wang Y, Sun Y, Jiang Q, Lu W, Wang J. BMP4 increases the expression of TRPC and basal [Ca2+]i via the p38MAPK and ERK1/2 pathways independent of BMPRII in PASMCs. PLoS One 2014; 9:e112695; PMID:25461595; http://dx.doi.org/ 10.1371/journal.pone.0112695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng J, Gao J, Li Y, Yang Y, Dang L, Ye Y, Deng J, Li A. BMP4 Enhances Foam Cell Formation by BMPR-2/Smad1/5/8 Signaling. International Journal of Molecular Sciences 2014; 15:5536; PMID:24690996; http://dx.doi.org/ 10.3390/ijms15045536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 2012; 71:640-53; PMID:22710965; http://dx.doi.org/ 10.1097/NEN.0b013e31825cfa81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandyopadhyay A, Yadav PS, Prashar P. BMP signaling in development and diseases: a pharmacological perspective. Biochem Pharmacol 2013; 85:857-64; PMID:23333766; http://dx.doi.org/ 10.1016/j.bcp.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Anderson L, Lowery JW, Frank DB, Novitskaya T, Jones M, Mortlock DP, Chandler RL, de Caestecker MP. Bmp2 and Bmp4 exert opposing effects in hypoxic pulmonary hypertension. 2010; PMID: 20042692; http://dx.doi.org/21471216 10.1152/ajpregu.00534.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tominaga T, Abe H, Ueda O, Goto C, Nakahara K, Murakami T, Matsubara T, Mima A, Nagai K, Araoka T, et al.. Activation of bone morphogenetic protein 4 signaling leads to glomerulosclerosis that mimics diabetic nephropathy. J Biol Chem 2011; 286:20109-16; PMID:21471216; http://dx.doi.org/ 10.1074/jbc.M110.179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y-L, Liu Y-S, Chuang L-Y, Guh J-Y, Lee T-C, Liao T-N, Hung M-Y, Chiang T-A. Bone Morphogenetic Protein-2 Antagonizes Renal Interstitial Fibrosis by Promoting Catabolism of Type I Transforming Growth Factor-β Receptors. Endocrinology 2009; 150:727-40; PMID:18832104; http://dx.doi.org/ 10.1210/en.2008-0090 [DOI] [PubMed] [Google Scholar]
- 11.Bruun C, Christensen GL, Jacobsen ML, Kanstrup MB, Jensen PR, Fjordvang H, Mandrup-Poulsen T, Billestrup N. Inhibition of beta cell growth and function by bone morphogenetic proteins. Diabetologia 2014; 57:2546-54; PMID:25260823; http://dx.doi.org/ 10.1007/s00125-014-3384-8 [DOI] [PubMed] [Google Scholar]
- 12.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, et al.. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 2011; 121:3206-19; PMID:21737876; http://dx.doi.org/ 10.1172/JCI45273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehata S, Yokoyama Y, Takahashi K, Miyazono K. Bi-directional roles of bone morphogenetic proteins in cancer: Another molecular Jekyll and Hyde? Pathology International 2013; 63:287-96; PMID:23782330; http://dx.doi.org/ 10.1111/pin.12067 [DOI] [PubMed] [Google Scholar]
- 14.Ivanova T, Zouridis H, Wu Y, Cheng LL, Tan IB, Gopalakrishnan V, Ooi CH, Lee J, Qin L, Wu J, et al.. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut 2013; 62:22-33; PMID:22535375; http://dx.doi.org/ 10.1136/gutjnl-2011-301113 [DOI] [PubMed] [Google Scholar]
- 15.Duan L, Ye L, Wu R, Wang H, Li X, Li H, Yuan S, Zha H, Sun H, Zhang Y, et al.. Inactivation of the Phosphatidylinositol 3-Kinase/Akt Pathway is Involved in BMP9-mediated Tumor-suppressive Effects in Gastric Cancer Cells. Journal of Cellular Biochemistry 2015; 116:1080-9; PMID:25640278; http://dx.doi.org/ 10.1002/jcb.25063 [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Morris RJ. The Yin and Yang of bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev 2010; 21:299-313; PMID:20688557; http://dx.doi.org/ 10.1016/j.cytogfr.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakin L, De Robertis EM. Extracellular regulation of BMP signaling. Curr Biol 2010; 20:R89-92; PMID:20144774; http://dx.doi.org/ 10.1016/j.cub.2009.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol 2011; 43:478-81; PMID:21256973; http://dx.doi.org/ 10.1016/j.biocel.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 19.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal 2011; 23:609-20; PMID:20959140; http://dx.doi.org/ 10.1016/j.cellsig.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 2008; 4:33-41; PMID:18026094; http://dx.doi.org/ 10.1038/nchembio.2007.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 2008; 18:4388-92; PMID:18621530; http://dx.doi.org/ 10.1016/j.bmcl.2008.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, et al.. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 2008; 14:1363-9; PMID:19029982; http://dx.doi.org/ 10.1038/nm.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In Vivo Structure−Activity Relationship Study of Dorsomorphin Analogues Identifies Selective VEGF and BMP Inhibitors. ACS Chemical Biology 2010; 5:245-53; PMID:20020776; http://dx.doi.org/ 10.1021/cb9002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanvitale CE, Kerr G, Chaikuad A, Ramel MC, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Yu PB, et al.. A new class of small molecule inhibitor of BMP signaling. PLoS One 2013; 8:e62721; PMID:23646137; http://dx.doi.org/ 10.1371/journal.pone.0062721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr G, Sheldon H, Chaikuad A, Alfano I, von Delft F, Bullock A, Harris A. A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis 2015; 18:209-17; PMID:25557927; http://dx.doi.org/ 10.1007/s10456-014-9457-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. Structure–Activity Relationship of 3, 5-Diaryl-2-aminopyridine ALK2 Inhibitors Reveals Unaltered Binding Affinity for Fibrodysplasia Ossificans Progressiva Causing Mutants. Journal of Medicinal Chemistry 2014; 57:7900-15; PMID:25101911; http://dx.doi.org/ 10.1021/jm501177w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak YD, Hendrix BJ, Sugaya K. Secreted type of amyloid precursor protein induces glial differentiation by stimulating the BMP/Smad signaling pathway. Biochem Biophys Res Commun 2014; 447:394-9; PMID:24727450; http://dx.doi.org/ 10.1016/j.bbrc.2014.03.139 [DOI] [PubMed] [Google Scholar]
- 28.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 2005; 97:496-504; PMID:16100039; http://dx.doi.org/ 10.1161/01.RES.0000181152.65534.07 [DOI] [PubMed] [Google Scholar]
- 29.Jeanpierre S, Nicolini FE, Kaniewski B, Dumontet C, Rimokh R, Puisieux A, Maguer-Satta V. BMP4 regulation of human megakaryocytic differentiation is involved in thrombopoietin signaling. Blood 2008; 112:3154-63; PMID:18664625; http://dx.doi.org/ 10.1182/blood-2008-03-145326 [DOI] [PubMed] [Google Scholar]
- 30.Bhattacherjee A, Rumi MA, Staecker H, Smith PG. Bone morphogenetic protein 4 mediates estrogen-regulated sensory axon plasticity in the adult female reproductive tract. J Neurosci 2013; 33:1050-61a; PMID:23325243; http://dx.doi.org/ 10.1523/JNEUROSCI.1704-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua H, Zhang YQ, Dabernat S, Kritzik M, Dietz D, Sterling L, Sarvetnick N. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem 2006; 281:13574-80; PMID:16547003; http://dx.doi.org/ 10.1074/jbc.M600526200 [DOI] [PubMed] [Google Scholar]
- 32.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 2013; 82:775-97; PMID:23495938; http://dx.doi.org/ 10.1146/annurev-biochem-063011-092449 [DOI] [PubMed] [Google Scholar]
- 33.Calpe S, Wagner K, El Khattabi M, Rutten L, Zimberlin C, Dolk E, Verrips CT, Medema JP, Spits H, Krishnadath KK. Effective inhibition of Bone Morphogenetic Protein function by highly specific llama-derived antibodies. Mol Cancer Ther 2015; 14(11):2527-40; PMID:26351325 [DOI] [PubMed] [Google Scholar]
- 34.Zilberberg L, ten Dijke P, Sakai LY, Rifkin DB. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol 2007; 8:41; PMID:17880711; http://dx.doi.org/ 10.1186/1471-2121-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol 2002; 12:205-9; PMID:11839272; http://dx.doi.org/ 10.1016/S0960-9822(01)00684-4 [DOI] [PubMed] [Google Scholar]
- 36.Matsubara T, Araki M, Abe H, Ueda O, Jishage K-I, Mima A, Goto C, Tominaga T, Kinosaki M, Kishi S, et al.. Bone Morphogenetic Protein 4 and Smad1 Mediate Extracellular Matrix Production in the Development of Diabetic Nephropathy. Diabetes 2015; 64:2978-90; PMID:25995358; http://dx.doi.org/ 10.2337/db14-0893 [DOI] [PubMed] [Google Scholar]
- 37.Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol 2015; 25:249-64; PMID:25592806; http://dx.doi.org/ 10.1016/j.tcb.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 38.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In Vivo somatic cell gene transfer of an engineered noggin mutein prevents BMP4-induced heterotopic ossification. 2003; PMID:146685029363949 [DOI] [PubMed] [Google Scholar]
- 39.Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 1997; 91:407-16; PMID:9363949; http://dx.doi.org/ 10.1016/S0092-8674(00)80424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B, Blair DG, Plisov S, Vasiliev G, Perantoni AO, Chen Q, Athanasiou M, Wu JY, Oppenheim JJ, Yang D. Cutting edge: bone morphogenetic protein antagonists Drm/Gremlin and Dan interact with Slits and act as negative regulators of monocyte chemotaxis. J Immunol 2004; 173:5914-7; PMID:15528323; http://dx.doi.org/ 10.4049/jimmunol.173.10.5914 [DOI] [PubMed] [Google Scholar]
- 41.Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev 2005; 16:309-17; PMID:15951218; http://dx.doi.org/ 10.1016/j.cytogfr.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 42.Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, Ten Dijke P, et al.. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem 2010; 285:12169-80; PMID:20048150; http://dx.doi.org/ 10.1074/jbc.M109.087197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirsch T, Nickel J, Sebald W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J 2000; 19:3314-24; PMID:10880444; http://dx.doi.org/ 10.1093/emboj/19.13.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Izpisua Belmonte JC, Choe S. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 2002; 420:636-42; PMID:12478285; http://dx.doi.org/ 10.1038/nature01245 [DOI] [PubMed] [Google Scholar]
- 45.Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal 2011; 23:1831-42; PMID:21740966; http://dx.doi.org/ 10.1016/j.cellsig.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 46.Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett 2012; 586:1846-59; PMID:22710174; http://dx.doi.org/ 10.1016/j.febslet.2012.02.043 [DOI] [PubMed] [Google Scholar]
- 47.Mari L, Milano F, Parikh K, Straub D, Everts V, Hoeben KK, Fockens P, Buttar NS, Krishnadath KK. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep 2014; 7:1197-210; PMID:24794431;http://dx.doi.org/ 10.1016/j.celrep.2014.03.074 [DOI] [PubMed] [Google Scholar]
- 48.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential Expression of Sonic Hedgehog Protein in Human Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Pathol Oncol Res 2015; 21:901-8; PMID:25740074; http://dx.doi.org/ 10.1007/s12253-015-9918-7 [DOI] [PubMed] [Google Scholar]
- 49.Hai Y, Sun M, Niu M, Yuan Q, Guo Y, Li Z, He Z. BMP4 promotes human Sertoli cell proliferation via Smad1/5 and ID2/3 pathway and its abnormality is associated with azoospermia. Discov Med 2015; 19:311-25; PMID:25977194 [PubMed] [Google Scholar]
- 50.Kallioniemi A. Bone morphogenetic protein 4-a fascinating regulator of cancer cell behavior. Cancer Genet 2012; 205:267-77; PMID:22749032; http://dx.doi.org/ 10.1016/j.cancergen.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 51.Virtanen S, Alarmo EL, Sandstrom S, Ampuja M, Kallioniemi A. Bone morphogenetic protein -4 and -5 in pancreatic cancer–novel bidirectional players. Exp Cell Res 2011; 317:2136-46; PMID:21704030; http://dx.doi.org/ 10.1016/j.yexcr.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 52.Kodach LL, Bleuming SA, Musler AR, Peppelenbosch MP, Hommes DW, van den Brink GR, van Noesel CJ, Offerhaus GJ, Hardwick JC. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer 2008; 112:300-6; PMID:18008360; http://dx.doi.org/ 10.1002/cncr.23160 [DOI] [PubMed] [Google Scholar]
- 53.Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, Dekker E, van den Brink GR, van Noesel CJ, Morreau H, et al.. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology 2008; 134:1332-41; PMID:18471510; http://dx.doi.org/ 10.1053/j.gastro.2008.02.059 [DOI] [PubMed] [Google Scholar]
- 54.Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, Hoogenboom JP, Biemond I, Verspaget HW, Hommes DW, de Rooij K, et al.. Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 2014; 147:196-208 e13; PMID:24704720; http://dx.doi.org/ 10.1053/j.gastro.2014.03.052 [DOI] [PubMed] [Google Scholar]
- 55.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 2007; 77:13-22; PMID:17704915; http://dx.doi.org/ 10.1007/s00253-007-1142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hultberg A, Temperton NJ, Rosseels V, Koenders M, Gonzalez-Pajuelo M, Schepens B, Ibanez LI, Vanlandschoot P, Schillemans J, Saunders M, et al.. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One 2011; 6:e17665; PMID:21483777; http://dx.doi.org/ 10.1371/journal.pone.0017665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coppieters K, Dreier T, Silence K, de Haard H, Lauwereys M, Casteels P, Beirnaert E, Jonckheere H, Van de Wiele C, Staelens L, et al.. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum 2006; 54:1856-66; PMID:16736523; http://dx.doi.org/ 10.1002/art.21827 [DOI] [PubMed] [Google Scholar]
- 58.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol 2011; 22:868-76; PMID:21862310; http://dx.doi.org/ 10.1016/j.copbio.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 59.Roovers RC, Laeremans T, Huang L, De Taeye S, Verkleij AJ, Revets H, de Haard HJ, van Bergen en Henegouwen PM. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies. Cancer Immunol Immunother 2007; 56:303-17; PMID:16738850; http://dx.doi.org/ 10.1007/s00262-006-0180-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harmsen MM, Van Solt CB, Fijten HP, Van Setten MC. Prolonged in vivo residence times of llama single-domain antibody fragments in pigs by binding to porcine immunoglobulins. Vaccine 2005; 23:4926-34; PMID:15992972; http://dx.doi.org/ 10.1016/j.vaccine.2005.05.017 [DOI] [PubMed] [Google Scholar]