Abstract
Objective
The gout working group at the Outcome Measures in Rheumatology (OMERACT) 12 meeting in 2014 aimed to determine which imaging modalities show the most promise for use as measurement instruments for outcomes in studies of people with chronic gout and to identify the key foci for future research about the performance of these imaging techniques with respect to the OMERACT filter 2.0.
Methods
During the gout session, a systematic literature review of the data addressing imaging modalities including plain radiography (XR), conventional computed tomography (CT), dual-energy computed tomography (DECT), magnetic resonance imaging (MRI), and ultrasound (US) and the fulfillment of the OMERACT filter 2.0 was presented.
Results
The working group identified 3 relevant domains for imaging in gout studies: urate deposition (tophus burden), joint inflammation, and structural joint damage.
Conclusion
The working group prioritized gaps in the data and identified a research agenda.
Key Indexing Terms: GOUT, OUTCOME MEASURES, MEDICAL IMAGING, OMERACT
Gout is the most common form of inflammatory arthritis and is caused by deposition of monosodium urate (MSU) crystals in and around joints, which provokes an intense inflammatory response1. Gout manifests as episodes of intense joint pain, swelling, and functional disability that may lead to persistent joint symptoms and structural joint damage2. At Outcome Measures in Rheumatology (OMERACT) meetings, joint damage imaging has been endorsed as a discretionary domain for outcome measurement in chronic gout studies3. However, no specific imaging instruments have been endorsed by OMERACT for use in gout studies. In the last decade there have been major advances in imaging in gout and so it was appropriate that the OMERACT gout working group focused on imaging modalities as outcome measures for use in chronic gout studies.
MATERIALS AND METHODS
At the meeting of the gout working group, a systematic literature review (SLR) was presented on the extent to which imaging modalities fulfill the OMERACT filter 2.0 for chronic gout studies. Briefly, a systematic search strategy was performed in electronic databases (PubMed, Medline, OVID) to evaluate plain radiography (XR), conventional computed tomography (CT), dual-energy computed tomography (DECT), magnetic resonance imaging (MRI), and ultrasound (US) for chronic gout. Bone scan and positron emission tomography were not considered because the working group decided that these techniques would be too infrequently used in patients with gout, or are difficult to access for outcomes research. The full results of the SLR will be published separately.
Discussion in the working group was focused to consider (1) features of gout that should be recorded using imaging, (2) best methods of measuring these features, (3) joints that should be imaged, and (4) the research agenda to ultimately achieve OMERACT endorsement for imaging as an outcome measure for chronic gout studies.
RESULTS
The SLR identified 74 articles that addressed how imaging modalities in chronic gout addressed aspects of the OMERACT filter 2.0 (XR n = 15, CT n = 10, DECT n = 18, MRI n = 16, and US n = 29). Some articles addressed more than 1 modality. The data are summarized in Table 1 but were presented in detail during the working group meeting. Imaging modalities were all classed within the “Pathophysiology” component of Filter 2.0.
Table 1.
Summary of data from systematic literature review addressing fulfillment of the OMERACT filter by imaging modalities in chronic gout.
| XR | CT | US | DECT | MRI | |
|---|---|---|---|---|---|
| Construct validity* | + | + | + | + | ± |
| Content validity† | + | + | + | + | ± |
| Criterion validity‡ | + | + | + | + | ± |
| Intrareader reliability§ | ± | + | ± | + | ± |
| Interreader reliability§ | + | + | + | + | ± |
| Discrimination in response to treatment | ± | − | ±¶ | − | − |
| Feasibility | + | ? | ? | ? | ? |
Considered present if imaging finding had been confirmed with an alternative imaging modality in at least 1 study.
Considered present if imaging findings measured part of the domain of interest [urate deposition (tophus burden), joint inflammation or structural joint damage].
Considered present if abnormality imaged had been directly confirmed with relevant histological assessment or by conformity with strict diagnostic criteria.
Considered fulfilled if intraclass correlation coefficient > 0.8. +: Data adequate in all domains the imaging modality can address; ±: some data available in all domains or data available only for some domains; −: insufficient or no data; ?: uncertainty because patient acceptability may be important but not yet addressed.
For tophus only, not the double contour sign. XR: radiography; CT: computed tomography; DECT: dual-energy computed tomography; MRI: magnetic resonance imaging; US: ultrasound.
During the gout working group, 3 key aspects of chronic gout for which an imaging modality could be relevant were identified, along with the previously identified domain to which they map (in parentheses): urate deposition (tophus burden), inflammation (joint inflammation), and structural damage (joint damage imaging).
Urate Deposition
MSU crystal deposition is the central pathogenic cause of gout. The usual primary outcome for interventional studies of patients with chronic gout is change in serum urate over time4, with urate deposition being as yet an unmeasured outcome. US, MRI, and CT can all identify nodular masses in subcutaneous tissue, joints, and tendons that have appearances recognizable as tophi. In US, for example, masses identified as tophi are usually hyperechoic with a heterogenous appearance, which may be calcified. These are often grouped together, have a poorly defined border, and may show postacoustic shadowing5. This appearance reflects the histological composition of tophi, which is of MSU crystals together with fibrovascular tissue and inflammatory cells6. DECT, in contrast, specifically identifies the MSU crystal component of the tophus. Plain film cannot directly identify tophi, but their presence is suggested by erosions or soft tissue opacities attributed to tophi. Thus US, MRI, CT, and DECT can potentially be used for measurement of urate deposition. US, MRI, and DECT all fulfill the truth components of the OMERACT filter, including the important direct confirmation of MSU crystals from structures identified as tophi on imaging7,8,9,10,11. These modalities also largely fulfill the discrimination components of the filter, although more data on sensitivity-to-change data are required for DECT and are lacking for MRI. High interreader reliability has been demonstrated for US across numerous anatomical locations.
Measurement of whole body urate deposition, by any modality, is not a feasible option in clinical studies. An alternative is quantification of tophus burden (as a surrogate for MSU crystal deposition), which could be measured in a single, representative area (joints and/or soft tissue) or a predetermined set of joints. US assessment of intraarticular and periarticular tophi has been demonstrated to have good intrareader and interreader reliability and sensitivity to change with successful urate-lowering over 12 months, discriminating between responders and nonresponders to urate-lowering therapies8. Therefore, US has the potential to quantify tophi in superficial areas and include both joints and relevant periarticular and subcutaneous locations; its setup costs are low, although operator cost and patient time are significant. MRI has the potential for repeat assessments, but access to facilities, high cost, and demands on patient time are significant barriers. In contrast, DECT provides a direct measure of MSU deposition. However, the issue of where to measure remains unclear because repeated imaging of multiple locations probably carries unacceptable radiation exposure for the clinical trial setting, and there may be limited access to DECT in many research centers. Overall, US and DECT appear to have most potential for measurement of urate burden. It is uncertain how the host tissue response and MSU crystal components of tophi interrelate and whether these components all respond similarly to ULT in most patients and at most locations, which may influence the use of US. Advantageously, DECT directly measures urate crystal deposition, but the ability to measure change over time in response to treatment has not yet been demonstrated.
US is also able to identify small amounts of MSU deposited on articular cartilage in the form of the double contour sign (DCS). This appearance can also be observed in individuals with asymptomatic hyperuricemia5. Some observers have reported that the DCS resolves with successful ULT12; however, this requires further systematic assessment. It is relevant to remember that the domain initially identified was tophus burden, which may be a different concept for this low level of MSU deposition.
Some key areas identified for future research on use of US and DECT in determining urate burden were responsiveness to ULT (in particular, detection of between-group differences in a randomized controlled trial setting), areas for assessment (a single joint vs a set and whether to include soft tissue tophi or a target tophus), and development of scoring systems to quantitate urate burden. The DCS may be most relevant in patient groups with less advanced gout, where large tophaceous deposits may not be present. Assessment of patient acceptability must also be addressed in future studies that aim to develop imaging modalities for use in clinical trials, especially where measurement of multiple sites is used.
Inflammation
Intermittent attacks of acute gout occur in intercritical and chronic gout; however, the periods between attacks appear to be characterized by ongoing inflammation as evidenced by hyperactive inflammatory cells, elevated systemic inflammatory proteins, and imaging evidence of inflammation1,2,3,13,14,15,16. This is identified in the discretionary domain of joint inflammation, for which no clinician-assessed outcome tools have been validated for chronic gout studies. Identification of inflammation using imaging is possible with US and MRI. MRI studies in patients with tophaceous gout have reported joint effusions, synovitis, tenosynovitis, and tophus with surrounding soft tissue or mild bone marrow edema (BME) and synovial enhancement after contrast4,5,16,17,18,19,20. MRI synovitis and BME have face validity as indicators of joint inflammation although construct validity data (MSU crystal identification and histological or biochemical evidence of local inflammation) are lacking, as are data to address discrimination. US can potentially identify evidence of inflammation in gout that includes joint effusion, synovial hypertrophy, power Doppler signal, and soft tissue edema. US-demonstrated synovitis has face validity for inflammation, yet it lacks construct validity data and discrimination data. When the 2 modalities are directly compared, MRI appears more sensitive but less specific than US for findings, suggesting inflammation6,16. This is especially important when considering the patients in chronic gout studies, who could range from patients with intercritical gout with no clinical inflammation between attacks, through to those with high tophi load and chronic indolent inflammation. Data systematically quantifying degree of imaging-identified inflammation in these differing stages of gout are not yet available, but such findings will be important in informing potential sites for assessment and sensitivity to change over time or with ULT, and these issues are import foci for future research.
Structural Damage
Chronic gout features typical radiographic changes of articular damage5,8,12,21,22, which are caused by tophi23. Most common findings on plain film are bone erosions, new bone formation, and joint space narrowing (JSN). These features are also identified by CT, DECT, and MRI, with US identifying only erosions and cartilage damage. A scoring system for XR based on the Sharp-van der Heijde method for rheumatoid arthritis shows excellent interobserver and intraobserver reliability and high correlation with expert opinion on the extent of joint damage. Extent of joint damage required only erosions and JSN to be scored. However, the rate of change in radiographic scores and the responsiveness of radiographic scores to therapy are largely unknown24. Erosion and cartilage damage assessment have intuitive face validity for XR, CT, DECT, MRI, and US; however, other aspects of the truth criterion are only partially fulfilled for erosions and cartilage damage for DECT, CT, and MRI while discrimination data, particularly for sensitivity to change, requires further data across all modalities. Because the concept of resolution or retardation of structural damage is analogous to “disease-modification,” it would seem that addressing these gaps in current knowledge, particularly sensitivity to change, is critical in developing imaging measurements of structural damage. Engaging with industry to ensure imaging modalities are included in the protocol of future interventional studies was considered a high priority, along with longitudinal observation data in gout cohorts.
Conclusion
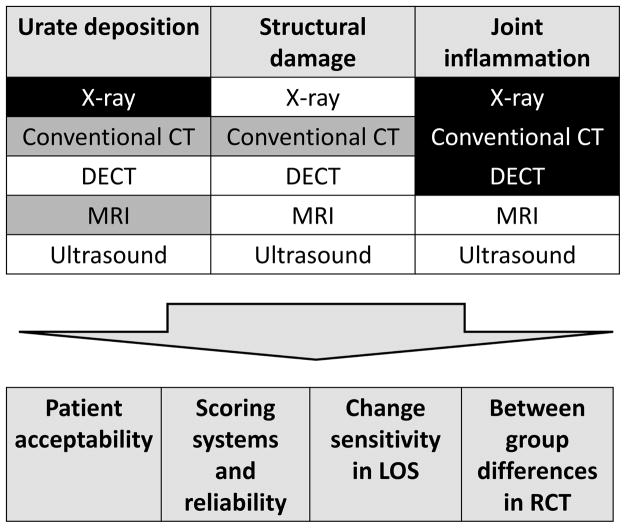
The working group attendees concluded that multiple imaging modalities need to be further developed for use as outcome measures in chronic gout because different modalities have relevance and potential for different domains. The prioritization of modalities with the most potential for each of the relevant domains and the key considerations for research agenda are summarized in Figure 1. More than 1 modality may need to be developed for each domain, to give flexibility in design of clinical trials, depending on the underlying research question. Inflammation in chronic gout has been underappreciated, and future therapeutic agents may have dual targets of urate lowering and control of inflammation, requiring more than 1 imaging modality to accurately assess changes in these domains. Further, the study population has a critical influence on the choice of imaging modality for an outcome measure. XR changes of chronic gout occur late, but US evidence of structural damage is identified earlier. Ideally, imaging outcome instruments will need to have similar performance characteristics at different stages of disease.
Figure 1.
Gout working group prioritization of imaging modalities with most potential for development in each of the 3 relevant domains (highest priority indicated as unshaded, possible modalities indicated in grey, and modalities not recommended for further development as black). Critical considerations for research agenda across all domains are indicated in lower panel. LOS: longitudinal observational study; RCT: randomized controlled trial; DECT: dual-energy computed tomography; CT: conventional computed tomography; MRI: magnetic resonance imaging.
It will not be feasible to image all potentially affected joints with any imaging modality. A data-driven strategy for choice of joints is required while assessment of a standard set of commonly affected joints versus symptomatic index joint(s) are options to be explored. The patient acceptability of different modalities also remains unclear, with duration and timing of imaging and radiation doses all likely to be important considerations. A description of the research agenda is shown in Table 2.
Table 2.
Gout special interest group proposal for a research agenda in the area of imaging for gout outcomes.
| General Question | Specific Project (examples) |
|---|---|
| Scoring systems and reliability | Investigation of the pathologic significance of US and DECT features that could contribute to an outcome score, in particular the double-contour sign, using histopathological correlation in patients with established gout Investigation of US and DECT features that could contribute to an outcome score, in patients with gout at various stages of disease Elaboration of a meaningful scoring system for US- and DECT-defined urate deposition, and testing of inter- and intraobserver reliability in early and established gout Investigation into the choice of joints required to construct a meaningful score for US and DECT, in patients with early and established gout, in the domains of urate burden and inflammation |
| Patient acceptability | Qualitative analysis of patient perceptions of discomfort, convenience, and outcome in gout patients undergoing routine US, DECT, XR, and MRI |
| Sensitivity to change in longitudinal outcome studies | Investigation into the longitudinal behavior of DECT-summated urate deposition in early untreated disease and in established disease after introduction of urate-lowering therapy Investigation of MRI features of inflammation over the short (acute attack) and long (intercritical periods) term in patients with active gout Investigation of US features of inflammation over the short term (acute attack) and urate deposition over the long term (inter-critical periods) in patients with active gout Determining whether XR damage scores change over time in patients with established gout and whether there are any patient or treatment factors associated with XR progression |
| Between-group differences in randomized controlled trials | Determining whether inflammation, urate deposition, or damage scores change in response to effective urate-lowering therapy in patients with established gout Investigation into whether observed changes in imaging scores in response to treatment are associated with changes in patient-reported outcomes, for example physical function |
XR: radiography; CT: computed tomography; DECT: dual-energy computed tomography; MRI: magnetic resonance imaging; US: ultrasound.
Acknowledgments
JAS is supported by grants from the Agency for Health Quality and Research Center for Education and Research on Therapeutics (AHRQ CERTs) U19 HS021110, US National Institute of Arthritis, Musculoskeletal and Skin Diseases P50 AR060772 and U34 AR062891, US National Institute on Aging U01 AG018947; US National Cancer Institute (NCI) U10 CA149950; the resources and use of facilities at the VA Medical Center at Birmingham, Alabama; and research contract CE-1304-6631 from the Patient Centered Outcomes Research Institute.
References
- 1.Lichtenstein L, Scott HW, Levin MH. Pathologic changes in gout; survey of eleven necropsied cases. Am J Pathol. 1956;32:871–95. [PMC free article] [PubMed] [Google Scholar]
- 2.Dalbeth N, So A. Hyperuricaemia and gout: state of the art and future perspectives. Ann Rheum Dis. 2010;69:1738–43. doi: 10.1136/ard.2010.136218. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. J Rheumatol. 2009;36:2342–5. doi: 10.3899/jrheum.090370. [DOI] [PubMed] [Google Scholar]
- 4.Stamp LK, Zhu X, Dalbeth N, Jordan S, Edwards NL, Taylor W. Serum urate as a soluble biomarker in chronic gout-evidence that serum urate fulfills the OMERACT validation criteria for soluble biomarkers. Semin Arthritis Rheum. 2011;40:483–500. doi: 10.1016/j.semarthrit.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Chowalloor PV, Keen HI. A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Ann Rheum Dis. 2013;72:638–45. doi: 10.1136/annrheumdis-2012-202301. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher HR. Pathology of the synovial membrane in gout. Light and electron microscopic studies. Interpretation of crystals in electron micrographs. Arthritis Rheum. 1975;18(Suppl):771–82. doi: 10.1002/art.1780180722. [DOI] [PubMed] [Google Scholar]
- 7.Naredo E, Uson J, Jimenez-Palop M, Martinez A, Vicente E, Brito E, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73:1522–8. doi: 10.1136/annrheumdis-2013-203487. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol. 2007;34:1888–93. [PubMed] [Google Scholar]
- 9.Ko K-H, Hsu Y-C, Lee H-S, Lee C-H, Huang G-S. Tophaceous gout of the knee. J Clin Rheumatol. 2010;16:209–14. doi: 10.1097/RHU.0b013e3181e92c38. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher HR, JR, Becker MA, Edwards NL, Palmer WE, MacDonald PA, Palo W, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60:408–14. doi: 10.1111/j.1368-5031.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 11.Gruber M, Bodner G, Rath E, Supp G, Weber M, Schueller-Weidekamm C. Dual-energy computed tomography compared with ultrasound in the diagnosis of gout. Rheumatol. 2013;53:173–9. doi: 10.1093/rheumatology/ket341. [DOI] [PubMed] [Google Scholar]
- 12.Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2009;30:495–503. doi: 10.1007/s00296-009-1002-8. [DOI] [PubMed] [Google Scholar]
- 13.Tugwell P, Boers M. OMERACT conference on outcome measures in rheumatoid arthritis clinical trials: conclusion. J Rheumatol. 1993;20:590. [PubMed] [Google Scholar]
- 14.Grainger R, McLaughlin RJ, Harrison AA, Harper JL. Hyperuricaemia elevates circulating CCL2 levels and primes monocyte trafficking in subjects with inter-critical gout. Rheumatol. 2012;52:1018–21. doi: 10.1093/rheumatology/kes326. [DOI] [PubMed] [Google Scholar]
- 15.Martin WJ, Grainger R, Harrison A, Harper JL. Differences in MSU-induced superoxide responses by neutrophils from gout subjects compared to healthy controls and a role for environmental inflammatory cytokines and hyperuricemia in neutrophil function and survival. J Rheumatol. 2010;37:1228–35. doi: 10.3899/jrheum.091080. [DOI] [PubMed] [Google Scholar]
- 16.Carter JD, Kedar RP, Anderson SR, Osorio AH, Albritton NL, Gnanashanmugam S, et al. An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatol. 2009;48:1442–6. doi: 10.1093/rheumatology/kep278. [DOI] [PubMed] [Google Scholar]
- 17.Zatarain E, Strand V. Monitoring disease activity of rheumatoid arthritis in clinical practice: contributions from clinical trials. Nat Clin Pract Rheumatol. 2006;2:611–8. doi: 10.1038/ncprheum0246. [DOI] [PubMed] [Google Scholar]
- 18.Yu JS, Chung C, Recht M, Dailiana T, Jurdi R. MR imaging of tophaceous gout. AJR Am J Roentgenol. 1997;168:523–7. doi: 10.2214/ajr.168.2.9016240. [DOI] [PubMed] [Google Scholar]
- 19.Horton SC, Walsh CAE, Emery P. Established rheumatoid arthritis: rationale for best practice: physicians’ perspective of how to realise tight control in clinical practice. Best Pract Res Clin Rheumatol. 2011;25:509–21. doi: 10.1016/j.berh.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Poh YJ, Dalbeth N, Doyle A, Mcqueen FM. Magnetic resonance imaging bone edema is not a major feature of gout unless there is concomitant osteomyelitis: 10-year findings from a high-prevalence population. J Rheumatol. 2011;38:2475–81. doi: 10.3899/jrheum.110477. [DOI] [PubMed] [Google Scholar]
- 21.Bloch C, Hermann G, Yü TF. A radiologic reevaluation of gout: a study of 2,000 patients. AJR Am J Roentgenol. 1980;134:781–7. doi: 10.2214/ajr.134.4.781. [DOI] [PubMed] [Google Scholar]
- 22.Watt I, Middlemiss H. The radiology of gout. Clin Radiol. 1975;26:27–36. doi: 10.1016/s0009-9260(75)80004-3. [DOI] [PubMed] [Google Scholar]
- 23.Dalbeth N, Clark B, Gregory K, Gamble G, Sheehan T, Doyle A, et al. Mechanisms of bone erosion in gout: a quantitative analysis using plain radiography and computed tomography. Ann Rheum Dis. 2009;68:1290–5. doi: 10.1136/ard.2008.094201. [DOI] [PubMed] [Google Scholar]
- 24.Dalbeth N, Clark B, McQueen F, Doyle A, Taylor W. Validation of a radiographic damage index in chronic gout. Arthritis Rheum. 2007;57:1067–73. doi: 10.1002/art.22891. [DOI] [PubMed] [Google Scholar]