Abstract
Visual search is an aspect of visual cognition that may be more impaired in Dementia with Lewy Bodies (DLB) than Alzheimer’s disease (AD). To assess this possibility, the present study compared patients with DLB (n=17), AD (n=30), or Parkinson’s disease with dementia (PDD; n=10) to non-demented patients with PD (n=18) and normal control (NC) participants (n=13) on single-feature and feature-conjunction visual search tasks. In the single-feature task participants had to determine if a target stimulus (i.e., a black dot) was present among 3, 6, or 12 distractor stimuli (i.e., white dots) that differed in one salient feature. In the feature-conjunction task participants had to determine if a target stimulus (i.e., a black circle) was present among 3, 6, or 12 distractor stimuli (i.e., white dots and black squares) that shared either of the target’s salient features. Results showed that target detection time in the single-feature task was not influenced by the number of distractors (i.e., “pop-out” effect) for any of the groups. In contrast, target detection time increased as the number of distractors increased in the feature-conjunction task for all groups, but more so for patients with AD or DLB than for any of the other groups. These results suggest that the single-feature search “pop-out” effect is preserved in DLB and AD patients, whereas ability to perform the feature-conjunction search is impaired. This pattern of preserved single-feature search with impaired feature-conjunction search is consistent with a deficit in feature binding that may be mediated by abnormalities in networks involving the dorsal occipito-parietal cortex.
Keywords: Visual Search, Visual Attention, Feature-Conjunction, Dementia with Lewy Bodies, Alzheimer’s Disease
1. INTRODUCTION
Negotiating a complex visual environment is a task that most people complete easily and with relatively little conscious effort. An important aspect of this activity is the ability to pick out a target among distractors while processing a visual scene. Visual search processes by which we recognize and detect objects in a complex scene have traditionally been divided into two components (Treisman & Gelade, 1980). One component, single-feature search, involves pre-attentive identification of a salient feature that distinguishes the target. This process is relatively automatic with multiple features of the scene processed in parallel (Treisman & Gelade, 1980). The amount of time needed to detect the target is generally constant no matter how many distracting stimuli are present. In essence, the target appears to “pop-out” from the background (Treisman & Gelade, 1980). The second component of visual search is feature conjunction. This aspect of visual search requires higher order visual processing as multiple features of the target (e.g., shape and color) must be conjoined before the target can be correctly discriminated from distractors that share one or the other of the salient features (Treisman & Gelade, 1980). Feature-conjunction search is an effortful process in which the environment is searched sequentially; as the number of distractors in the visual scene increases, so does the time needed to find the target (Treisman & Gelade, 1980).
Evidence suggests that neural correlates of single-feature and feature-conjunction search are distinct. Single-cell recordings in non-human primates indicate that single-feature search “pop-out” effects are modulated by cells in cortical area V4 at the occipital-temporal junction (Burrows & Moore, 2009). Consistent with this finding, patients with lesions in occipito-temporal cortex are impaired on single-feature search tasks but not on feature-conjunction search tasks (Humphreys, Freeman, & Muller, 1992). Feature-conjunction search, in contrast, is thought to be largely mediated by occipito-parietal cortex (Corbetta, Shulman, Miezin, & Petersen, 1995; Stemmler, Usher, & Niebur, 1995; Wachsmuth, Oram, & Perrett, 1994). Patients with occipito-parietal cortex lesions have impaired feature-conjunction search with preserved single-feature search (Atkinson & Braddick, 1989). Furthermore, when parietal cortex is inactivated by transcranial magnetic stimulation, feature-conjunction search is impaired but single-feature search is not (Ashbridge, Walsh, & Cowey, 1997; Walsh, Ellison, Battelli, & Cowey, 1998).
Patients with Alzheimer’s disease (AD) often have deficits in visual attention (Parasuraman, Greenwood, Haxby, & Grady, 1992; Perry & Hodges, 1999) and impaired performance on visual search tasks (Foster, Behrmann, & Stuss, 1999; Tales et al., 2002). The pathology of AD (e.g., neuritic plaques and neurofibrillary tangles in limbic and neocortical association areas) typically involves parietal (and parieto-occipital) cortex that may be important for feature-conjunction search. It does not, however, usually involve visual areas in the occipital cortex that are important for single-feature search. Accordingly, Foster et al. (1999) showed that patients with AD were impaired compared to controls in feature-conjunction search, but not in single-feature search (although their overall reaction time was generally slower). The same result was obtained by Tales et al. (2002) even after the attentional load of the two tasks was equated. Tales et al. (2002) proposed that AD patients have difficulty with feature-conjunction search because they cannot effectively conjoin multiple features of a stimulus due to disruption of the connections between the distinct cortical regions that process each feature (Morrison, Hof, & Bouras, 1991).
Dementia with Lewy Bodies (DLB) is an age-related neurodegenerative disorder often associated with AD. The disorder is characterized pathologically by Lewy bodies (i.e., α-synuclein positive neuronal inclusions) and neuron loss in subcortical brain areas typically affected by Parkinson’s disease (PD), and by Lewy bodies diffusely distributed in the neocortex. DLB commonly occurs with a variable admixture of AD pathology. Patients with DLB often display a pattern of neuropsychological deficits that is similar to that of patients with AD, but with disproportionately severe deficits in visuospatial ability (Ala, Hughes, Kyrouac, Ghobrial, & Elble, 2001; Collerton, Burn, McKeith, & O'Brien, 2003; Galasko, Katzman, Salmon, & Hansen, 1996; Mori et al., 2000; Salmon et al., 1996). When marked visuospatial impairment is absent early in the course of dementia, Lewy body pathology is less likely (Tiraboschi et al., 2006).
The prominent visuospatial deficit displayed by patients with DLB is consistent with neuroimaging evidence of structural and metabolic abnormalities in brain areas related to visual processing (Albin et al., 1996; Higuchi et al., 2000; Minoshima et al., 2001). Functional imaging studies using SPECT or PET show hypometabolism in posterior temporal, parietal and occipital cortical regions that is greater in DLB than in AD (Ishii et al., 2007; Kasama, Tachibana, Kawabata, & Yoshikawa, 2005; Mito et al., 2005). Structural imaging studies of patients with DLB have detected atrophy in these same cortical regions (Beyer, Larsen, & Aarsland, 2007). These loci of degenerative changes in the brains of patients with DLB are thought to contribute to their visuospatial deficits and may impact aspects of visual search.
Only one study to date has examined visual search mechanisms in DLB using both single-feature and feature-conjunction search tasks (Cormack, Gray, Ballard, & Tovee, 2004). In the single-feature (parallel search) condition, subjects were instructed to state whether a target red circle was present in a field of 2, 8, or 16 green circles that served as distractors. In the feature-conjunction (serial search) condition, subjects were instructed to state whether a target red circle was present among 2, 8, or 16 distractors that were green circles and red squares. The number of errors committed with each distractor set size was recorded. The speed of visual search was inferred by varying stimulus presentation times across 200 ms, 400 ms, and 800 ms. Results showed that in the feature-conjunction task the number of errors committed by patients with AD, patients with PD, and normal control (NC) participants increased with increasing numbers of distractors (or decreasing stimulus presentation times), a pattern indicative of serial search. Patients with DLB made more errors than the other groups, and made similar numbers of errors with each distractor set size or stimulus display duration. In the single-feature task NC participants made few errors and their performance was not influenced by distractor set size or duration of stimulus presentation (i.e., the “pop-out” effect). Patients with AD or PD made a slightly higher number of errors than NC participants, but only at the fastest stimulus display duration. Patients with DLB, in contrast, made significantly more errors than NC participants or patients with AD or PD regardless of stimulus duration or distractor set size. Cormack et al. (2004) concluded from these results that single-feature search is disproportionately impaired in patients with DLB, perhaps due to hypometabolism or other neuropathology in primary and secondary visual cortex in the occipital lobe.
Several factors make the interpretation of the findings of the Cormack et al. (2004) study uncertain. First, the visual scene was displayed very briefly (i.e., 200 to 800 ms) which may have precluded a full scan of the visual scene before its offset. Second, reaction time was not measured so the impact of the number and type of distractors on speed of search could only be inferred. If the maximally effective stimulus presentation time differed across groups, and this fell outside the range of the employed stimulus durations for some groups but not others, different patterns of performance across durations could emerge relative to the patterns that might be observed with reaction times. Finally, the impact of cognitive slowing on visual search was not addressed. Generalized cognitive slowing has been proposed as a mechanism underlying a variety of cognitive deficits in AD and DLB, including deficits in attention (Bailon, Roussel, Boucart, Krystkowiak, & Godefroy, 2010; Nebes & Brady, 1992). Qualitatively different deficits on search tasks in the two disorders could reflect greater cognitive slowing in DLB than in AD rather than a fundamental difference in visual attention.
Decreased attention in patients with AD is thought to contribute to performance deficits on a wide variety of cognitive (Nebes & Brady, 1989; Stuart-Hamilton, Rabbitt, & Huddy, 1988) and functional (Alberoni, Baddeley, Della Sala, Logie, & Spinnler, 1992; Camicioli, Howieson, Lehman, & Kaye, 1997) tasks. An even greater attentional deficit in patients with DLB could help explain why deficits in certain cognitive domains (e.g., executive functions, visual cognition) appear greater in DLB than AD (Salmon & Hamilton, 2005). Different patterns of performance on single-feature and feature-conjunction visual search tasks in DLB and AD could aid in differential diagnosis (Cormack et al., 2004). Understanding the nature of the visual search deficit in patients with DLB would also provide means to evaluate an important cognitive aspect of symptomatic treatment. In addition, delineation of the visual search deficits in patients with DLB should provide information about the extent of their posterior cortical abnormalities.
Therefore, in the present study we compared the performances of patients with DLB or AD on single-feature and feature-conjunction visual search tasks that varied the number of distractor stimuli across trials and presented each visual scene until a response was made or for a maximum of 3 seconds. Both response times and errors were measured. Simple reaction time to the onset of a single visual stimulus was also measured as a control for cognitive slowing. Patients with PD, with or without dementia, were included in the study to assess the impact of motoric and cognitive deficits associated with Lewy body pathology alone on visual search.
We expected patients with AD to show intact performance on single-feature search and impairment on feature-conjunction search, consistent with results from previously published studies (Foster et al., 1999; Tales et al., 2002). Patients with DLB, in contrast, were expected to be impaired on single-feature search, consistent with the results of Cormack et al. (2004), unless previous results were due to particularly slow scanning of very briefly presented visual scenes. The present methods allowed much longer scene presentations and used response times to assess processing speed. Because patients with DLB have a high rate of concomitant AD pathology (Armstrong, Cairns, & Lantos, 1998), it is also possible that DLB pathology exacerbates the impairment that AD patients display when visual search tasks require the conjunction of multiple features. Thus, patients with DLB may have deficits on both single-feature and feature-conjunction tasks that are greater than those of patients with AD.
2. METHODS
2.1 Participants
Ninety-five individuals participated in this study: 20 patients with DLB, 13 patients with PD and dementia (PDD), 18 non-demented patients with PD, 31 patients with AD, and 13 cognitively normal controls (NC). The DLB, PDD, AD and NC participants were recruited from the University of California, San Diego (UCSD) Shiley-Marcos Alzheimer’s Disease Research Center (ADRC). The PD patients were recruited from the UCSD Movement Disorders Clinic or from community neurologists. The patient groups consisted of clinically diagnosed individuals and those who died subsequent to testing and received a definite diagnosis of disease at autopsy. Neuropathologic confirmation at autopsy was obtained in 7 patients with DLB and 6 with AD. Neuropathologic diagnoses were made by a neuropathologist with expertise in AD, DLB and PD. Detailed neuropathologic methods and diagnostic procedures have been described previously (Hamilton et al., 2008). Consensus clinical diagnoses were based on published criteria and made by neurologist researchers with expertise in dementia and movement disorders. Probable DLB was diagnosed clinically using established criteria (McKeith et al., 1996; 2005) based on the presence of dementia and at least two of three additional core features of mild parkinsonism, well-formed visual hallucinations, and fluctuations in consciousness or attention. In all cases of DLB, cognitive decline was the presenting symptom and preceded or occurred in conjunction with mild parkinsonism. Idiopathic PD was clinically diagnosed by the presence of at least two of the cardinal motor signs of resting tremor, rigidity, and bradykinesia in accordance with established criteria (Hughes, Ben-Shlomo, Daniel, & Lees, 1992). Patients with atypical findings or secondary causes of PD were excluded. PD patients did not have sufficient cognitive or functional decline to warrant a diagnosis of dementia. The clinical diagnosis of PDD was based on the presence of at least two of the cardinal motor signs of PD, as well as objective cognitive deficits on neuropsychological tests and functional decline due to cognitive problems (Emre et al., 2007). Motor signs preceded cognitive decline by more than one year in all PDD cases. Probable AD was diagnosed according to criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA) (McKhann et al., 1984; 2012). Elderly NC participants were judged to be cognitively normal following extensive neurological, medical, psychiatric and neuropsychological assessment through the ADRC.
2.2 Procedure
Participants were tested individually in a quiet room with ambient lighting. Patients were tested with their usual and stable medication allowed. PD, PDD and DLB patients were evaluated during their “on” period. Motor functioning was assessed with Part III of the Unified Parkinson’s Disease Rating Scale (UPDRS) by a board-certified neurologist. A trained examiner administered the Mini-Mental State Examination (MMSE), Dementia Rating Scale (DRS; Mattis, 1988), and three visual attention tasks: a simple visual detection task, a single-feature visual search task, and a feature-conjunction visual search task. The three visual attention tasks were presented on a laptop computer with a 17” Dell Flat Panel LCD monitor. Participants viewed the screen from a distance of approximately 75 cm. Stimulus control, timing and response recording were controlled using E-Prime software. Responses consisted of a button press on a Psychology Software Tools 200a Serial Response box. In all cases, participants were instructed to use their dominant hand to press a 1 cm x 1 cm button on the response box. The response box was modified so that only one button (the far left) was accessible to reduce the likelihood of erroneous responses due to problems with fine motor control. Prior to beginning the test session, participants were familiarized with the response box.
Stimuli were a solid black circular dot (0.9° in diameter) for the simple visual detection task, solid black and solid white dots (each 0.9° in diameter) for the single-feature visual search task, and solid black and solid white dots (each 0.9° in diameter), as well as solid black squares (0.9° diagonal), for the feature-conjunction visual search task. All stimuli were presented against a gray background. Stimuli were created with Adobe Photoshop.
The simple visual detection task was presented first, followed by the single-feature and feature-conjunction visual search tasks. The order of the latter two tasks was counterbalanced across participants. A short rest period was provided between tasks.
2.2.1 Simple Visual Detection Task
Participants were instructed to respond with a button press as soon as they saw a single target stimulus (i.e., a black dot) appear on the screen. The position of the target was determined pseudo-randomly with the stipulation that the target could appear with equal likelihood in all quadrants of the display. The target remained on the screen until a response was made. Reaction time was measured in milliseconds (ms) on each of 20 trials. Five practice trials were initially presented to orient the participant to the task and ensure that they used the response box accurately.
2.2.2 Single-Feature Visual Search Task
Participants had to determine whether or not a target stimulus (i.e., a black dot) was present among 3, 6, or 12 distractor stimuli (i.e., white dots). The position of the target was determined pseudo-randomly, with the stipulation that it could appear with equal likelihood in all quadrants of the display. The distractor stimuli appeared at random locations throughout the display. There were a total of 120 trials: 60 target-present trials with 20 each having 3, 6 or 12 distractors, and 60 target-absent trials with 20 each having 4, 7, or 13 distractors. Thus, an equal number of stimuli appeared on target-present and target-absent trials. Trials were presented in four blocks of 30. Each block contained an equal number of the 6 trial types. The various trial types were presented randomly within blocks. The blocks were separated by a short rest period to reduce eye strain and visual fatigue.
At the start of the task, participants were familiarized with the target and distractor stimuli and instructed to press the response button as quickly as possible if the target dot was in the display. They were told not to respond if the target was absent. On each trial a fixation cross appeared for 500 ms in the center of the screen, followed immediately by the stimulus display. The stimulus display remained on-screen until the participant responded, or for a maximum of 3 seconds. Immediately after a response or after 3 seconds with no response, the fixation cross for the start of the next trial appeared. Accuracy was recorded for each trial. Reaction time was recorded for trials with a response. The primary measure of interest was reaction time on target-present trials. Therefore, if a target-present trial ended after three seconds without a response, the trial was re-administered (with the same display) at the end of the 30 trial block. Similarly, if an anticipatory response occurred less than 100 ms after stimulus presentation on a target-present trial, the trial was rejected and re-administered (with the same display) at the end of the 30 trial block. This was repeated until all 15 target-present trials in a block had a response. In this way, we could examine performance 1) based on only the first 30 trials in each block, assigning a maximum reaction time of 3000 ms to any of the 15 target-present trials without a response, 2) based only on the first 30 trials in each block with any of the 15 target-present trials without a response considered missing, or 3) based on the 15 target-present trials with a response that included those re-administered at the end of the 30-trial block.
2.2.3 Feature-Conjunction Visual Search Task
This task was designed exactly as the single-feature visual search task with the exception that there were two types of distractors on each trial, those that differed from the target (a black dot) only in luminance (i.e., white dots) and those that differed only in shape (i.e., black squares). Thus, participants had to determine whether or not a target stimulus (i.e., a black circle) was present among 3, 6, or 12 distractor stimuli (i.e., white dots and black squares). An equal number of white dot and black square distractors were presented on each trial, with the exception that two white dots and one black square appeared on target-present trials with three distractors (equal numbers of trials with 2 white dots and 1 black square, or 1 white dot and 2 black squares, as distractors occurred throughout the block). The position of the target was determined pseudo-randomly, with the stipulation that it appear with equal likelihood in all quadrants of the display. The distractor stimuli appeared at random locations throughout the display. There were a total of 120 trials: 60 target-present trials with 20 each with 3, 6 or 12 distractors, and 60 target-absent trials with 20 each with 4, 7, or 13 distractors. Trials were presented in four blocks of 30. Each block contained an equal number of the 6 trial types with the various trial types presented randomly within blocks. The blocks were separated by a short rest period to reduce eye strain and visual fatigue. All task procedures, trial methodology and parameters, and scoring were exactly as in the single-feature visual search task.
The research protocol was reviewed and approved by the human subjects review board at UCSD. Informed consent to participate in the study was obtained prior to testing from all participants or their caregivers consistent with California State law. Informed consent for autopsy was obtained at the time of death from the next of kin.
2.3 Data Analysis
Statistical analyses were completed using SPSSv20. Group differences in demographic characteristics and clinical test scores were tested using one-way analysis of variance (ANOVA). Partial eta-squared (pη2) was used to measure effect sizes. Post-hoc pair-wise group comparisons were made with Tukey’s Least Significant Difference (LSD) test (alpha for significance set at p<.05). Pair-wise group comparisons of gender distribution, prevalence of hallucinations, and prevalence of cholinesterase inhibitor or dopamine replacement medication use were made with χ2 tests.
2.3.1 Simple Visual Detection Task
Median reaction time was calculated for each subject after anticipatory responses that had occurred less than 100 ms after stimulus presentation were dropped. Group differences in median reaction times were tested using one-way analysis of variance (ANOVA). Partial eta-squared (pη2) was used to measure effect sizes. Post-hoc pair-wise group comparisons were made with Tukey’s Least Significant Difference (LSD) test (alpha for significance set at p<.05).
2.3.2 Single-Feature and Feature-Conjunction Visual Search Tasks
Data from the single-feature and feature-conjunction tasks were analyzed separately. Median reaction times on the 3 types of target-present trials (i.e., with 3, 6 or 12 distractors) were calculated for each subject after anticipatory responses that had occurred less than 100 ms after stimulus presentation were dropped. The primary analyses were based on the first 30 trials in each block, assigning a maximum reaction time of 3000 ms to any of the 15 target-present trials without a response (the other methods of handling target-present trials without a response produced similar patterns of results and are therefore not reported). The median reaction time data were subjected to repeated measures ANOVA with Group (between subjects) and Distractor Set Size (within subjects repeated measure) as factors. Partial eta-squared (pη2) was used to measure effect sizes. If the Group X Distractor Set Size interaction effect was significant, the interaction was explored by comparing groups on a difference score between reaction times with 3 versus 12 distractors (i.e., reaction time for 12 distractors minus reaction time for 3 distractors) using one-way ANOVA. Post-hoc pair-wise group comparisons were made with Tukey’s LSD test (alpha for significance set at p<.05).
To examine the impact of slowing on the single-feature and feature-conjunction visual search tasks, repeated measures analyses of covariance (ANCOVA) were performed on difference scores between reaction times for trials with 3 versus 12 distractors while reaction time on the Simple Visual Detection Task served as a covariate.
Group differences in the numbers of false negative errors (not responding on a target-present trial) made in the first 30 trials of each block of the Single-Feature and Feature-Conjunction Visual Search tasks were examined with repeated measures ANOVAs with Group (between subjects) and Distractor Set Size (within subjects repeated measure) as factors. Partial eta-squared (pη2) was used to measure effect sizes. The numbers of false-positive errors (responding on a target-absent trial) were too few to analyze.
3. RESULTS
Seven of the participants were unable to complete all components of the three visual attention tasks and were dropped from the analyses. Of these seven, one patient with AD and one patient with DLB had reaction times on the simple visual detection task that were more than 3 standard deviations slower than their group mean, and 2 DLB and 3 PDD patients made errors on 40% of the 30 initial trials in both blocks of either the single-feature or feature-conjunction visual search task (more than 2 standard deviations beyond their group mean). The final groups used in the analyses consisted of 17 patients with DLB, 10 patients with PDD, 18 non-demented patients with PD, 30 patients with AD, and 13 NC. The seven dropped participants had mean age (73.1 years), mean years of education (15.6 years), mean Mattis DRS score (114.3 points), percentage of men (71%), and percentage of DLB and PDD patients with hallucinations (60%) that were similar to those of the retained DLB, PDD and AD patients (see Table 1). The mean MMSE score of the dropped participants (19.9 points) was slightly lower than that of the retained DLB, PDD and AD participants.
Table 1.
Mean (Standard Deviation; SD) Age, Education, Mini-Mental State Exam (MMSE) and Uniform Parkinson’s Disease Rating Scale (UPDRS) scores for the final sample of Normal Control (NC) Participants and Patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer's Disease (AD). The percentage of males in each group, and the percentage of DLB, PDD and AD patients with past or current visual hallucinations, taking cholinesterase inhibitors, or taking dopamine replacement therapy are also shown.
| NC | PD | DLB | PDD | AD | |
|---|---|---|---|---|---|
| n = 13 | n = 18 | n = 17 | n = 10 | n = 30 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 72.0 (7.0) | 71.2 (7.0) | 73.4 (8.1) | 76.1 (8.4) | 75.5 (6.0) |
| Sex (% men) | 62% | 72% | 94% | 90% | 57% |
| Education | 17.2 (2.3) | 16.0 (3.1) | 15.5 (2.7) | 15.5 (1.7) | 15.3 (2.6) |
| MMSE | 29.2 (1.0) | 28.4 (1.8) | 23.2 (5.0) | 26.1 (1.8) | 22.1 (4.7) |
| Mattis DRS | 140.7 (1.8) | 139.7 (2.8) | 116.1 (14.2) | 126.7 (6.2) | 117.6 (14.1) |
| UPDRS | 2.2 (5.3) | 19.7 (6.9) | 22.3 (12.8) | 24.6 (10.9) | 3.2 (5.7) |
| Hallucinations | ----- | 33% | 75% | 30% | 7% |
| Cholinesterase Inhibitor | ----- | 11% | 77% | 50% | 80% |
| Dopamine Therapy* | ----- | 94% | 41% | 90% | 0% |
| LED* (mg) of those on Dopamine Therapy | ----- | 331.8 (191.6) | 422.6 (83.7) | 676.2 (360.7) | ----- |
LED = L-Dopa Equivalent Dosage (daily mg)
Demographic information, cognitive test scores and motor function scores for the five subject groups are presented in Table 1. The groups did not differ significantly in age (F(4,83)=1.52; p= .20) or education (F(4,83)=1.31; p= 0.27). The DLB, PDD and PD groups had a greater proportion of men than the AD and NC groups (χ2=9.84; p=0.04), but did not differ from each other in this regard. The AD and NC groups had similar proportions of men. As expected, PDD, DLB, and AD groups performed worse than PD and NC groups on measures of global cognitive functioning, the MMSE (F(4, 80) = 13.24; p<0.001) and the DRS (F(4,81) = 21.48; p<0.001). The three demented groups did not differ significantly from each other on these tests, nor did PD differ from NC subjects. The UPDRS scores were significantly different across groups (F(4,80)= 28.18; p<0.001). Patients with PD, PDD or DLB had more parkinsonian symptoms (i.e., higher UPDRS scores) than AD patients or NC participants, but did not differ from each other. The AD patients and NC participants did not differ on UPDRS scores. The proportion of individuals with visual hallucinations differed across groups (χ2=30.61; p<0.001). A greater proportion of patients had visual hallucinations in the DLB group than in any other group. There were no significant differences in the proportions of DLB, AD and PDD patients taking a cholinesterase inhibitor at the time of testing, and all three groups had a higher proportion than did the PD group (all p’s < 0.02). There was no significant difference in the proportion of PD and PDD patients on dopamine replacement medications at the time of testing, and both groups had higher proportions than did the DLB (all p’s < 0.02) group. The daily L-dopa equivalency dosage (LED; Tomlinson et al., 2010) of those patients taking dopamine replacement medications differed across the DLB, PDD and PD groups (F(2,33)=6.50; p=.004). PDD patients had higher LED than DLB or PD patients (all p’s < 0.05) whereas the latter two groups did not differ. A clinical neuroimaging study had been completed on almost all DLB (n=15) and AD (n=29) patients. These included 13 MRI scans and 2 CT scans in the DLB group and 20 MRI scans and 9 CT scans in the AD group. The clinical evaluation of each scan by a neuroradiologist noted “age-appropriate atrophy” in 14/29 (48%) AD (5 with white matter hyperintensities; WMH) and 9/15 (60%) DLB patients (4 with WMH); “mild global atrophy” in 11/29 (38%) AD (2 with WMH) and 5/15 (33%) DLB patients (2 with WMH); and “moderate/severe global atrophy” in 4/29 (14%) AD (3 with WMH) and 1/15 (7%) DLB patients (0 with WMH). Hippocampal atrophy was specifically noted in 1 DLB patient and 1 AD patient, and iron deposits in the basal ganglia were noted in 1 DLB patient. No mass effects or significant infarcts were noted except for a small acute infarct in the genu of the internal capsule in 1 DLB patient.
3.1 Simple Visual Detection Task
The average median reaction times produced by each group on the simple visual detection task are presented in Table 2. The groups differed significantly (F(4, 83) = 7.66; p<0.001; pη2=0.27) with DLB patients slower than all other groups, and AD patients slower than PD patients and NC participants. The PD, PDD and NC groups had similar reaction times. There were no significant correlations between reaction time and age, DRS score, or UPDRS score for any of the groups (all p’s > 0.05), with the exception of a significant correlation between reaction time and DRS score in the AD group (r = −.425; p=.02). Reaction times were not significantly different in DLB patients receiving (mean=440.0 ± 180.0; n=8) or not receiving dopamine replacement therapy (mean=586.7 ± 191.5; n=9) (t(15)=1.58; p=0.14). Furthermore, there was no significant correlation between LED and simple reaction time in those DLB receiving this therapy (r=.19; p = 0.66). UPDRS scores of DLB patients receiving (mean=29.0 ± 15.0) or not receiving dopamine therapy (mean=17.0 ± 8.3) were not significantly different (t(14)=2.04; p=0.06).
Table 2.
Mean (Standard Deviation; SD) of the median simple Reaction Time to the presentation of a single visual target stimulus for the Normal Control (NC) Participants and Patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer's Disease (AD). Reaction time is presented in milliseconds (msec).
| NC | PD | DLB | PDD | AD | |
|---|---|---|---|---|---|
| n = 13 | n = 18 | n = 17 | n = 10 | n = 30 | |
| Reaction Time (ms) | |||||
| Mean | 344.3 | 340.1 | 519.5 | 382.7 | 419.6 |
| SD | 54.6 | 51.2 | 194.7 | 63.7 | 92.0 |
3.2 Single-Feature Visual Search Task
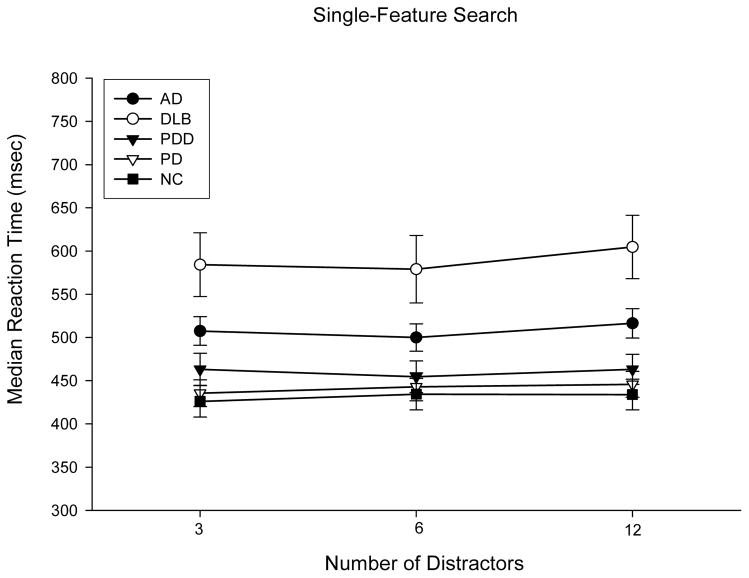
The average median reaction times produced by each group on the single-feature visual search task are presented as a function of distractor set size in Figure 1. A Group X Distractor Set Size (3, 6, or 12 distractors) repeated measures ANOVA revealed a significant main effect of group (F(4,83)=7.91; p<0.001; pη2=0.28), but no significant effect of distractor set size (F(2,82)=2.17,p=0.12; pη2=0.05) or Group X Distractor Set Size interaction effect (F(8,164)=0.58; p=0.80; pη2=0.03). Post-hoc analyses revealed that the DLB group performed the single-feature search task significantly slower than all other patient groups (p’s<0.001), while the other groups did not differ from each other. A repeated measures ANCOVA on difference scores between reaction times on trials with 3 versus 12 distractors, using median reaction time on the simple visual detection task as a covariate to control for simple search speed, revealed no significant Group effect (F(4,82)=0.21; p=0.93; pη2=0.01). Because patients with PDD were slightly less demented than patients with DLB or AD, a repeated measures ANCOVA on the difference scores was carried out for these groups using DRS score as a covariate to control for level of dementia. This analysis showed no significant difference in the difference scores for these three groups (F(2,51)=0.28; p=0.76; pη2=0.01).
Figure 1.
Mean (with standard error bars) of median reaction times in milliseconds (msec) on the single-feature visual search task as a function of distractor set size for Normal Control (NC) Participants and Patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer's Disease (AD).
The mean numbers of false negative (not responding on a target-present trial) and false-positive (responding on a target-absent trial) errors made in the first 30 trials of each block of the single-feature search task are shown as a function of Distractor Set size in Table 3. A Group X Distractor Set Size repeated measures ANOVA of false-negative errors revealed a significant main effect of Group (F(4,83)=2.93; p<0.05; pη2=0.12), but no effect of Distractor Set Size (F(1,83)=0.87; p=0.35; pη2=0.01) or interaction effect (F(4,83)=0.77; p=0.55; pη2=0.04). Post-hoc analyses showed that the DLB group made more false-negative errors than all other groups, but the mean number of false-negative errors in all participant groups (including DLB) was less than 1 error in each distinct distractor set size, so the overall error rate was minimal. The numbers of false-positive errors were too few to analyze.
Table 3.
Mean number of errors (and standard deviations) in the Single-Feature and Feature-Conjunction Visual Search Tasks made by Normal Control (NC) Participants and Patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer's Disease (AD). False Positive (FP; response when the target was not present) and False Negative (FN; no response when the target was present) errors are reported separately.
| NC | PD | DLB | PDD | AD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=13 | N=18 | N=17 | N=10 | N=30 | ||||||
|
|
||||||||||
| FP | FN | FP | FN | FP | FN | FP | FN | FP | FN | |
|
|
||||||||||
| Single Feature | ||||||||||
| 3 Distractors | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.0) | 0.0 (0.0) | 0.2 (0.6) | 0.1 (0.5) | 0.0 (0.0) | 0.1 (0.3) | 0.1 (0.3) | 0.2 (0.5) |
| 6 Distractors | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.2) | 0.3 (0.5) | 0.5 (1.5) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.3) | 0.1 (0.4) |
| 12 Distractors | 0.1 (0.3) | 0.1 (0.3) | 0.1 (0.2) | 0.0 (0.0) | 0.2 (0.8) | 0.4 (0.5) | 0.2 (0.4) | 0.0 (0.0) | 0.0 (0.2) | 0.2 (0.5) |
|
| ||||||||||
| Dual Feature | ||||||||||
| 3 Distractors | 0.2 (0.4) | 0.0 (0.0) | 0.3 (0.5) | 0.2 (0.7) | 0.4 (0.6) | 1.5 (1.5) | 0.7 (1.1) | 0.8 (1.7) | 0.5 (0.8) | 0.8 (1.3) |
| 6 Distractors | 0.2 (0.6) | 0.1 (0.8) | 0.3 (0.6) | 0.2 (0.5) | 0.4 (0.8) | 1.9 (2.0) | 0.9 (0.9) | 0.7 (1.3) | 0.4 (0.7) | 1.1 (1.6) |
| 12 Distractors | 0.2 (0.6) | 0.0 (0.0) | 0.1 (0.2) | 0.6 (1.0) | 0.4 (0.6) | 3.7 (3.8) | 0.5 (1.1) | 1.9 (2.4) | 0.5 (0.9) | 2.5 (3.6) |
3.3 Feature-Conjunction Visual Search Task
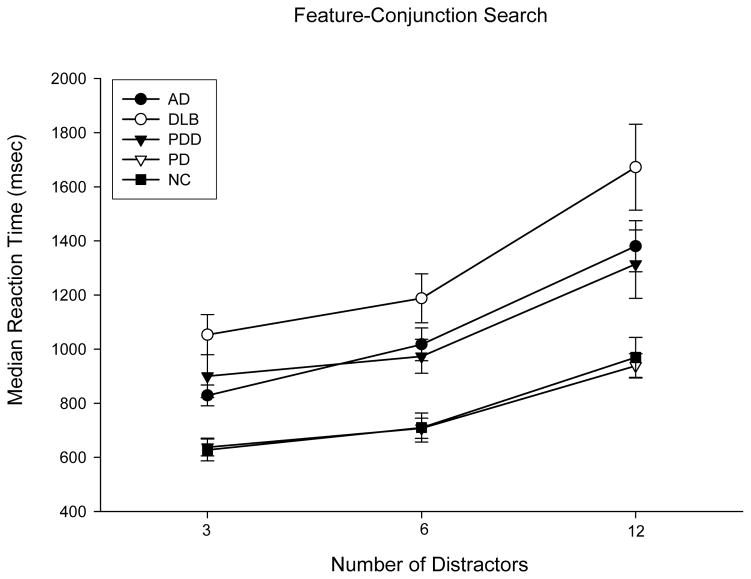
The average median reaction times produced by each group on the feature-conjunction visual search task are presented as a function of distractor set size in Figure 2. A Group X Distractor Set Size repeated measures ANOVA revealed significant main effects of Group (F(4,83)=9.45; p<0.001; pη2=0.81) and Distractor Set Size (F(2,82)=86.49,p<0.001; pη2=0.68), and a significant Group X Distractor Set Size interaction effect (F(8,164)=2.54; p=0.01; pη2=0.11).
Figure 2.
Mean (with standard error bars) of median reaction times in milliseconds (msec) on the feature-conjunction visual search task as a function of distractor set size for Normal Control (NC) Participants and Patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer’s Disease (AD).
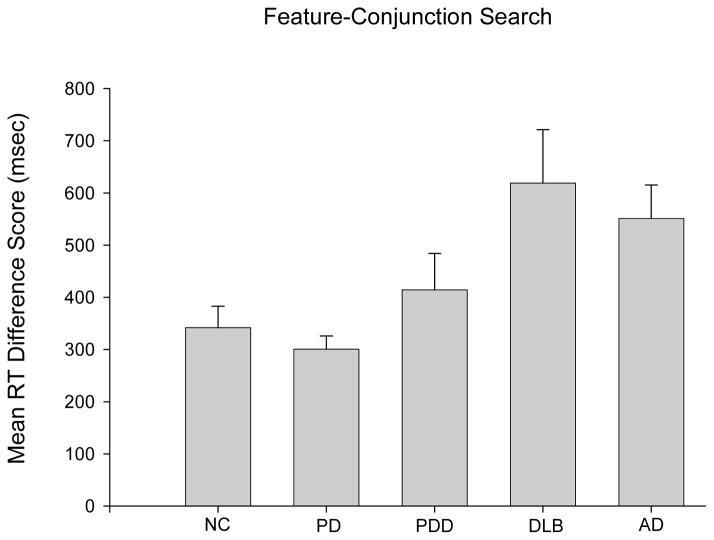
The interaction effect was explored by comparing difference scores between reaction times for trials with 3 versus 12 distractors (see Figure 3). All group difference scores were significantly greater than zero (all p’s<.001) indicating that all groups engaged in parallel search. A one-way ANOVA comparing difference scores across groups was significant (F(4,83)=3.81,p=0.007; pη2=0.15), and post-hoc pairwise comparisons showed that patients with AD and patients with DLB had similar difference scores that were greater than those of patients with PD or NC participants (p’s<0.05). PDD patients’ difference scores did not differ significantly from those of any other group. Difference scores of DLB patients receiving dopamine replacement therapy (mean=673.8 ± 523.99; n=6) or not receiving dopamine replacement therapy (mean=570.5 ± 423.7; n=9) were not significantly different (t(13) < 1).
Figure 3.
Mean (with standard error bars) of reaction time (RT) difference scores (difference between reaction time at a set size of 3 and a set size of 12) in milliseconds (msec) on the feature-conjunction visual search task for Normal Control (NC) participants, patients with Dementia with Lewy Bodies (DLB), Parkinson’s Disease with Dementia (PDD), Parkinson’s Disease (PD), or Alzheimer’s Disease.
A repeated measures ANCOVA on difference scores between reaction times on trials with 3 versus 12 distractors, using median reaction time on the simple visual detection task as a covariate to control for simple search speed, revealed no significant effect of Group (F(4,82)=1.48; p=0.22; pη2=0.07). Thus, the abnormal increase in reaction time that DLB and AD patients displayed as the number of distractors increased may be partially explained by generalized cognitive slowing (it should be noted, however, that the Group effect in the ANCOVA was significant when the other two methods of accounting for target-present trials without a response were used; i.e., counting these trials as missing or repeating these trials at the end of each block).
Because patients with PDD were slightly less demented than patients with DLB or AD, repeated measures ANCOVA on the difference scores were carried out for these groups using DRS score as a covariate to control for level of dementia. There was no significant difference in the difference scores for these three groups (F(2,51)=0.11; p=0.89; pη2=0.004).
The mean numbers of false negative (not responding on a target-present trial) and false-positive (responding on a target-absent trial) errors made in the first 30 trials of each block of the feature-conjunction search task are shown as a function of Distractor Set size in Table 3. A Group X Distractor Set Size repeated measures ANOVA of false-negative errors revealed a significant main effect of Group (F(4,83) = 5.42; p=0.001; pη2=0.21) and Distractor Set Size (F(1,83) = 15.95, p<0.001; pη2=0.16), but no interaction effect (F(4,83)=2.40; p=0.06; pη2=0.10). These analyses indicate that the number of errors increased with an increase in set size. Post-hoc analyses revealed that DLB and AD patients made similar numbers of errors, and these 2 groups made more false-negative errors than any other participant group (PD, NC, PDD). The numbers of false-positive errors were too few to analyze.
4. DISCUSSION
Although patients with DLB were generally slower to respond than NC participants, they showed no significant increase in time to detect a target as the number of distractor stimuli increased in the single-feature search task. This pattern of results is consistent with a “pop-out” effect indicative of parallel processing of multiple features of a visual scene. This “pop-out” effect was also observed in patients with AD and demented and non-demented patients with PD. The pattern of performance remained evident in all patient groups even after differences in simple reaction time were taken into account. These results suggest that visual cortex at the occipital-temporal junction remains functionally intact in patients with DLB since this aspect of the ventral visual processing stream is thought to mediate single-feature search “pop-out” effects (Lamme, 1995; Lee, Yang, Romero, & Mumford, 2002).
The present results differ from those of Cormack et al. (2004) who found that patients with DLB produced an increasing number of target-detection errors in a single-feature search task as the number of distractors increased in briefly presented visual scenes. This is most likely because the reaction time method used in the present study allowed the stimulus array to be displayed beyond the scanning threshold for all groups (as indicated by the very low error rates). In the study by Cormack and colleagues (2004), the visual scene was displayed very briefly (i.e., 200 to 800 ms) and individuals had to detect the target within that brief interval. Different levels of generalized cognitive slowing across patient groups could mean that slower patients were unable to perform a full scan of the visual scene before its offset and missed the target for that reason. Thus, greater cognitive slowing in patients with DLB compared to patients with AD could explain the different patterns of errors DLB and AD patients produced across stimulus durations. Cormack and colleagues did not have an independent measure of simple reaction time that would have allowed them to adjust their visual search results for group differences in speed of cognitive processing. The present study did have such a measure and showed that the “pop-out” effect was intact in patients with DLB even after the effects of generalized cognitive slowing (as revealed by abnormally slow simple reaction time) were taken into account.
Another factor that could contribute to the different single-feature search results obtained in the two studies is that the present study used luminance to distinguish between target and distractor stimuli (i.e., black versus white), whereas Cormack et al. (2004) used color (red versus green). Previous studies have shown that the development of DLB or PDD in individuals with rapid eye movement (REM) sleep behavior disorder is associated with abnormalities in color vision (Postuma et al., 2011). Thus, the task used by Cormack et al. (2004) to assess single-feature search may have been particularly difficult for patients with DLB. It is also possible that differences in characteristics of the participants in the present study and the study by Cormack et al. (2004) may have contributed to the different results. Patients with DLB in the present study were younger (mean age of 73.4 years) than those in the Cormack et al. study (79.7 years) and it is possible that age and DLB pathology interact to reduce search efficiency. Both studies also were based on clinically diagnosed DLB and AD, so they may have differed in the extent of DLB pathology in each cohort or in the proportion of DLB patients with significant concomitant AD pathology.
All groups showed an increase in response time as the number of distractor stimuli increased in the feature-conjunction visual search task. This pattern of results is consistent with a serial search process in which stimuli are examined one-by-one until the target is detected. Consistent with several previous studies (Foster et al., 1999; Tales et al., 2002), patients with AD showed a greater increase in response time than NC participants as the number of distractor stimuli increased. Foster et al. (1999) suggested that this impairment in feature-conjunction search might arise from deficits patients with AD have in shifting attention. However, when attentional demands were controlled across single-feature and feature-conjunction tasks, Tales et al. (2002) found that patients with AD remained impaired on only the feature-conjunction search task. The deficit in feature-conjunction search exhibited by patients with AD may reflect pathology in the occipito-parietal cortex thought to mediate this aspect of search (Corbetta et al., 1995; Stemmler et al., 1995; Wachsmuth et al., 1994).
Tales et al. (2002) suggested that the feature-conjunction visual search deficit exhibited by patients with AD might also be due to decreased interaction between cortically distinct visual processing streams (Ungerleider & Haxby, 1994). In order to effectively detect the target, information concerning object form (or shape), processed primarily in the ventral visual stream, must be integrated with information concerning luminance (black or white), processed primarily in the dorsal visual stream. Patients with AD may have a deficit in binding these features due to a breakdown in cortico-cortical connections between the visual processing streams. Consistent with this possibility, Festa and colleagues (Festa et al., 2005) showed that patients with AD performed as well as healthy older adults on a motion-luminance integration task that placed demands solely on one visual processing stream, but showed marked impairment compared to healthy adults when the task required integration of color and motion information that engaged distinct cortical processing streams.
Patients with DLB, like patients with AD, showed a greater increase in response times than patients with PD or NC participants as the number of distractors increased in the feature-conjunction visual search task. There was no difference in the amount of increase that occurred across distractor set size in the DLB and AD patient groups. There is some evidence that generalized cognitive slowing may partially explain the abnormal increase in reaction time that DLB and AD patients displayed in the feature-conjunction visual search task as the number of distractors increased. Generalized cognitive slowing suggests that if all cognitive operations are slowed to a similar extent, change in absolute reaction time to complete a task (e.g., detect a target) would increase as the number of cognitive operations needed to complete that task (e.g., examining each distractor stimulus in the feature-conjunction search) increases (e.g., from 3 to 12 distractors). The change would be greater in the slower group than in the faster group (Nebes & Brady, 1992). To account for this possibility, we compared groups on the difference scores between reaction times on trials with 3 versus 12 distractors while controlling for simple reaction time. This analysis showed no significant difference between the patient and control groups, which suggests that the effect may have been driven by cognitive slowing. It should be noted, however, that the abnormal increase in reaction time that DLB and AD patients displayed as the number of distractors increased remained significant after controlling for simple reaction time when the other two methods of accounting for target-present trials without a response were used in the analyses (i.e., counting these trials as missing or repeating these trials at the end of each block).
The similarity in the feature-conjunction visual search deficits exhibited by patients with DLB and AD may be related to neuropathology the two disorders share. Patients with DLB typically have some degree of concomitant AD pathology (Galasko et al., 1996) that may be driving their impairment in feature-conjunction search ability. The additional burden of Lewy body pathology in DLB may contribute to this deficit given that PDD patients did not differ significantly from DLB and AD patients, particularly after level of dementia was taken into account. However, Lewy body pathology may play a lesser role than AD pathology since non-demented patients with PD performed as well as NC participants. This interpretation of the results would be strengthened, however, by neuropathological studies which could help determine if the relative severity of AD and DLB pathology are associated with distinct patterns of performance on visual search tasks.
The particularly slow response times exhibited by patients with DLB on the single-feature and feature-conjunction visual search tasks appears to be a general feature of the condition and not specifically related to visual search. Although simple reaction times of patients with DLB were significantly slower than those of patients with AD, the two groups did not differ on either of the visual search tasks. Previous studies that have shown that dopamine replacement therapy improves finger tapping speed (Molloy et al., 2005) and subjective alertness (Molloy et al., 2006) in a minority of patients with DLB, but dopamine replacement therapy in patients with DLB did not significantly influence performance on reaction times in the simple visual detection task in the present study. Furthermore, there were no significant differences between DLB patients receiving or not receiving dopamine replacement therapy in the reaction time difference scores for trials with 3 versus 12 distractors in either the single-feature or feature-conjunction search tasks.
Overall error rates in both single-feature and feature-conjunction search tasks were extremely low. The mean number of errors across all distractor set size conditions was less than one per distractor set size in the single-feature search task, and less than four per distractor set size in the feature-conjunction search task. Thus, it is unlikely that false-positive or false-negative error rates significantly impacted the performance of any group on either the single-feature or feature-conjunction search task, or that group differences in response times were due to differences in speed-accuracy trade-off. The very low false positive error rate exhibited by the DLB patients is somewhat surprising given that 75% of these patients had reported visual hallucinations. However, in the usual case these reported hallucinations were sporadic, were not reported during the visual search test session, and were well-formed and not of a nature to interfere with the perception of the simple stimuli used in the visual search tasks.
Several limitations of the present study should be noted. First, participants failed to produce a response on some of the target-present trials in the feature-conjunction search task. Different analytic methods were used to mitigate this problem of missing reaction time data, but in future studies it may be better to extend the length of the trial duration. Second, the sample sizes for some of the groups (e.g., the patients with PDD) were relatively small and this limits the ability to make any strong claims about non-significant results (which could be due merely to a lack of power). Small sample size may also account for some unusual aspects of the patient characteristics such as the lower than expected percentage of PDD patients with reported visual hallucinations. Only 30% of the 10 PDD patients in the present study reported visual hallucinations compared to 40% to 85% of PDD patients in much larger studies (Fenelon & Alves, 2010; Fenelon, Machieux, Huon & Ziegler, 2000). Replication with larger numbers of patients with AD, DLB or PDD is warranted. Third, the diagnosis of DLB or AD was not autopsy verified so it is possible that there was some cross-contamination of groups. Even when consensus clinical criteria are carefully applied, some patients with a clinical diagnosis of probable AD have both AD and DLB pathology at autopsy (Hohl, Tiraboschi, Hansen, Thal, & Corey-Bloom, 2000). Unfortunately, available clinical neuroimaging results were not helpful in this regard since there was little difference in the proportion of AD and DLB patients who were classified as having age-appropriate atrophy, mild cerebral atrophy, or moderate/severe cerebral atrophy. High resolution MRI-based volumetric analyses that focus on the hippocampus and other medial temporal lobe structures have shown that hippocampal volume is reduced in AD compared to DLB (Barber, McKeith, Ballard, Gholkar & O’Brien, 2001; Firbank, Blamire, Teodorczuk, Teper, Burton, Mitra & O’Brien, 2010), but this level of imaging was not available in the present study. Therefore, replication of the present results in DLB and AD patients with pathological verification of diagnosis is needed. Neuropathological information would also allow examination of the relationship between the relative quantity or distribution of DLB and AD pathology and performance on single-feature and feature-conjunction visual search tasks.
Despite these limitations, the results of the present study demonstrate that patients with DLB perform similar to patients with AD on visual search tasks. The single-feature search “pop-out” effect was preserved in both DLB and AD patient groups. Both groups were impaired relative to NC participants (and non-demented patients with PD) in the ability to perform the feature-conjunction search task. The pattern of preserved single-feature search with impaired feature-conjunction search exhibited by patients with DLB or AD is also observed in patients with lesions in occipito-parietal cortex (Atkinson & Braddick, 1989). Taken together, these results suggest that hypometabolism and other pathology observed in the occipital cortex of patients with DLB (Albin et al., 1996; Ishii et al., 2007) may primarily involve dorsal occipito-parietal regions. Future studies with neuropathological information would be helpful in determining the varying contributions of AD and DLB pathology to the ability to perform visual search tasks.
Acknowledgments
The results of this study were presented, in part, at the April 2011 meeting of the American Academy of Neurology in Honolulu, HI. The study was supported by NIH grants NS049298, AG12963, AG039247 and AG05131 to the University of California, San Diego, and by a VA MERIT Review Grant to JVF. We thank the participants and staff of the UCSD Shiley-Marcos Alzheimer’s Disease Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70(4):483–488. doi: 10.1136/jnnp.70.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberoni M, Baddeley A, Della Sala S, Logie R, Spinnler H. Keeping Track of a Conversation - Impairments in Alzheimers-Disease. Int J Geriatr Psychiatry. 1992;7(9):639–646. doi: 10.1002/gps.930070905. [DOI] [Google Scholar]
- Albin RL, Minoshima S, D'Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47(2):462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. The spatial patterns of Lewy bodies, senile plaques, and neurofibrillary tangles in dementia with Lewy bodies. Exp Neurol. 1998;150(1):122–127. doi: 10.1006/exnr.1997.6761. [DOI] [PubMed] [Google Scholar]
- Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997;35(8):1121–1131. doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick OJ. 'Where' and 'what' in visual search. Perception. 1989;18(2):181–189. doi: 10.1068/p180181. [DOI] [PubMed] [Google Scholar]
- Bailon O, Roussel M, Boucart M, Krystkowiak P, Godefroy O. Psychomotor slowing in mild cognitive impairment, Alzheimer's disease and lewy body dementia: mechanisms and diagnostic value. Dement Geriatr Cogn Disord. 2010;29(5):388–396. doi: 10.1159/000305095. [DOI] [PubMed] [Google Scholar]
- Barber R, McKeith IG, Ballard C, Gholkar A, O’Brien JT. A comparison of medial amd lateral temporal lobe atrophy in Dementia with Lewy Bodies and Alzheimer’s disease: Magnetic resonance imaging volumetric study. Dement Geriatr Cogn Disord. 2001;12:198–205. doi: 10.1159/000051258. [DOI] [PubMed] [Google Scholar]
- Bertrand JA, Bedetti C, Postuma RB, Monchi O, Marchand DG, Jubault T, Gagnon JF. Color discrimination deficits in Parkinson’s disease are related to cognitive impairment and white matter alterations. Mov Disorders. 2012;27(14):1781–1788. doi: 10.1002/mds.25272. [DOI] [PubMed] [Google Scholar]
- Beyer MK, Larsen JP, Aarsland D. Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology. 2007;69(8):747–754. doi: 10.1212/01.wnl.0000269666.62598.1c. [DOI] [PubMed] [Google Scholar]
- Burrows BE, Moore T. Influence and limitations of popout in the selection of salient visual stimuli by area V4 neurons. J Neurosci. 2009;29(48):15169–15177. doi: 10.1523/JNEUROSCI.3710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: The effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48(4):955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- Collerton D, Burn D, McKeith I, O'Brien J. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord. 2003;16(4):229–237. doi: 10.1159/000072807. 72807. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270(5237):802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cormack F, Gray A, Ballard C, Tovee MJ. A failure of 'pop-out' in visual search tasks in dementia with Lewy Bodies as compared to Alzheimer's and Parkinson's disease. Int J Geriatr Psychiatry. 2004;19(8):763–772. doi: 10.1002/gps.1159. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, … Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Alves G. Epidemiology of psychosis in parkinson’s disease. J Neurol Sci. 2010;289:12–17. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Machieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- Festa EK, Insler RZ, Salmon DP, Paxton J, Hamilton JM, Heindel WC. Neocortical disconnectivity disrupts sensory integration in Alzheimer's disease. Neuropsychology. 2005;19(6):728–738. doi: 10.1037/0894-4105.19.6.728. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Blamire AM, Teodorczuk A, Teper E, Burton EJ, Mitra D, O’Brien JT. High resolution imaging of the medial temporal lobe in Alzheimer’s disease and Dementia with Lewy Bodies. J Alzheimers Dis. 2010;21:1129–1140. doi: 10.3233/jad-2010-100138. [DOI] [PubMed] [Google Scholar]
- Foster JK, Behrmann M, Stuss DT. Visual attention deficits in Alzheimer's disease: simple versus conjoined feature search. Neuropsychology. 1999;13(2):223–245. doi: 10.1037//0894-4105.13.2.223. [DOI] [PubMed] [Google Scholar]
- Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain Cogn. 1996;31(2):166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, … Thal LJ. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22(6):729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, … Sasaki H. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol. 2000;162(2):247–256. doi: 10.1006/exnr.2000.7342. [DOI] [PubMed] [Google Scholar]
- Hohl U, Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol. 2000;57(3):347–351. doi: 10.1001/archneur.57.3.347. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Freeman TA, Muller HJ. Lesioning a connectionist model of visual search: selective effects on distractor grouping. Can J Psychol. 1992;46(3):417–460. doi: 10.1037/h0084326. [DOI] [PubMed] [Google Scholar]
- Ishii K, Soma T, Kono AK, Sofue K, Miyamoto N, Yoshikawa T, … Murase K. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer's disease. J Nucl Med. 2007;48(5):704–711. doi: 10.2967/jnumed.106.035691. [DOI] [PubMed] [Google Scholar]
- Kasama S, Tachibana H, Kawabata K, Yoshikawa H. Cerebral blood flow in Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease according to three-dimensional stereotactic surface projection imaging. Dement Geriatr Cogn Disord. 2005;19(5–6):266–275. doi: 10.1159/000084551. [DOI] [PubMed] [Google Scholar]
- Lamme VA. The neurophysiology of figure-ground segregation in primary visual cortex. J Neurosci. 1995;15(2):1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Yang CF, Romero RD, Mumford D. Neural activity in early visual cortex reflects behavioral experience and higher-order perceptual saliency. Nat Neurosci. 2002;5(6):589–597. doi: 10.1038/nn860. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H … Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, … Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Sperling RA. Changing Diagnostic Concepts of Alzheimer's Disease. Alzheimer's Disease - Modernizing Concept, Biological Diagnosis and Therapy. 2012;28:115–121. [Google Scholar]
- Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50(3):358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- Mito Y, Yoshida K, Yabe I, Makino K, Hirotani M, Tashiro K, … Sasaki H. Brain 3D-SSP SPECT analysis in dementia with Lewy bodies, Parkinson's disease with and without dementia, and Alzheimer's disease. Clin Neurol Neurosurg. 2005;107(5):396–403. doi: 10.1016/j.clineuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76:1200–1203. doi: 10.1136/jnnp.2004.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SA, Rowan EN, O’Brien JT, McKeith IG, Wesnes K, Burn DJ. Effect of levodopa on cognitive function in Parkinson’s disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:1323–1328. doi: 10.1136/jnnp.2006.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, Hanihara T. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57(4):489–493. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Bouras C. An anatomic substrate for visual disconnection in Alzheimer's disease. Ann N Y Acad Sci. 1991;640:36–43. doi: 10.1111/j.1749-6632.1991.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Focused and divided attention in Alzheimer's disease. Cortex. 1989;25(2):305–315. doi: 10.1016/s0010-9452(89)80045-0. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Generalized cognitive slowing and severity of dementia in Alzheimer's disease: implications for the interpretation of response-time data. J Clin Exp Neuropsychol. 1992;14(2):317–326. doi: 10.1080/01688639208402831. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115(Pt 3):711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapdi eye movement sleep behavior disorder. Ann Neurol. 2011;69:811–818. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Galasko D, Hansen LA, Masliah E, Butters N, Thal LJ, Katzman R. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Hamilton JM. Neuropsychological changes in Dementia with Lewy Bodies. In: Burns A, Ames D, O’Brien JT, editors. Dementia. 3. London: Arnold Press; 2005. pp. 634–647. [Google Scholar]
- Stemmler M, Usher M, Niebur E. Lateral interactions in primary visual cortex: a model bridging physiology and psychophysics. Science. 1995;269(5232):1877–1880. doi: 10.1126/science.7569930. [DOI] [PubMed] [Google Scholar]
- Stuart-Hamilton IA, Rabbitt PM, Huddy A. The role of selective attention in the visuo-spatial memory of patients suffering from dementia of the Alzheimer type. Compr Gerontol B. 1988;2(3):129–134. [PubMed] [Google Scholar]
- Tales A, Butler SR, Fossey J, Gilchrist ID, Jones RW, Troscianko T. Visual search in Alzheimer's disease: a deficiency in processing conjunctions of features. Neuropsychologia. 2002;40(12):1849–1857. doi: 10.1016/s0028-3932(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129(Pt 3):729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disorders. 2010;25(15):2649–2685. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12(1):97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 'What' and 'where' in the human brain. Curr Opin Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E, Oram MW, Perrett DI. Recognition of objects and their component parts: responses of single units in the temporal cortex of the macaque. Cereb Cortex. 1994;4(5):509–522. doi: 10.1093/cercor/4.5.509. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc Biol Sci. 1998;265(1395):537–543. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]