Summary
The phagocytic clearance of dying cells in a tissue is a highly orchestrated series of intercellular events coordinated by a complex signaling network. Recent data from genetic, biochemical, and live imaging approaches have greatly enhanced our understanding of the dynamics of cell clearance and how the process is orchestrated at the cellular and tissue levels. We discuss how networks regulating apoptotic cell clearance are integrated to enable a rapid, efficient, and high-capacity clearance system within tissues.
Keywords: Phagocytosis, Apoptosis, Efferocytosis, Chemotaxis, Imaging, Signal transduction, Immunity
Introduction
The maintenance of tissue health and function requires the constant replacement of damaged or aged cells with new ones. In fact, on a daily basis the typical healthy adult is estimated to turn over via apoptosis approximately ~150 billion cells (out of the estimated 37.2 trillion cells in the human body), or roughly 0.4% of a body's cellular mass (Bianconi et al., 2013). Removal of these apoptotic cell corpses without damaging the healthy neighboring cells via phagocytosis thus plays a decisive role in cellular homeostasis. The phagocytic clearance of apoptotic cells (a process referred to as efferocytosis) plays key roles in embryonic development, organogenesis, tissue repair and immunity. Moreover, aberrant interstitial cell clearance is increasingly being viewed as both a cause and consequence of the pathobiology of many diseases (Elliott and Ravichandran, 2010; Green et al., 2016; S. Nagata et al., 2010; Poon et al., 2014b; Saas et al., 2013). Accordingly, there is growing interest in understanding the regulatory networks that control this dynamic and ubiquitous biophysical process.
Even in healthy tissues where cellular turnover rates are high (e.g. intestine, lung, bone marrow and thymus), uncleared apoptotic cells are relatively rare, suggesting that homeostatic efferocytosis operates at an impressive level of efficiency (Bailey et al., 2002; Braun, 1998; De Paepe, 2004; Elliott et al., 2009; 2010; Griswold, 1998; Lee et al., 2016; Liu and Keller, 2016; Nakanishi and Shiratsuchi, 2004; Park et al., 2011; Pittet and Weissleder, 2011; Shiratsuchi et al., 1997; Sunaga et al., 2013; Surh and Sprent, 1994). The mechanistic underpinnings of this efficiency are the focal point of a number of important, unanswered questions in the field: How is cell clearance orchestrated such that diverse signaling pathways facilitate efferocytosis? What is the capacity of the clearance system? What are the biophysical and spatiotemporal constraints for corpse recognition and removal? What are the constraints and unique features of cell clearance in specific tissue/disease contexts? What are the consequences of cell clearance on the metabolomics of cells and tissues? Over the past 20 years, we have gained an impressive amount of knowledge regarding the key molecular players and signaling pathways that regulate both cell death and efferocytosis. Moreover, we have recently begun to gain insight into how this complex and seamless biological process is carried out in real-time at the cellular and tissue levels in mammals. Increasingly sensitive, tractable intravital and in vitro microscopy techniques, together with new tissue-specific genetic probes have led to a number of recent advances in our understanding of apoptotic cell clearance in vivo. This has allowed us to begin to develop an integrated view of the physical and temporal constraints on cell clearance in a tissue context, which will have implications for cell clearance both under physiological and pathological conditions. This review highlights some of these recent advances. As there are a number of excellent reviews of the relevant technical advances (Elliott et al., 2010; Fourgeaud et al., 2016; Griswold, 1998; Liu and Keller, 2016; Lu et al., 2011; Mattocks and Tropepe, 2010; Nakanishi and Shiratsuchi, 2004; Park et al., 2011; Pittet and Weissleder, 2011; Shiratsuchi et al., 1997; Sierra et al., 2010), we will specifically focus here on findings that have helped reframe our conceptual understanding of this dynamic intercellular process and discuss how newer approaches can be applied to key unanswered questions in the field. While this review is focused primarily on cell clearance in the mammalian context, we acknowledge the invaluable contribution of many elegant genetic and intravital imaging studies from the nematode C. elegans, fruit fly Drosophila melanogaster, and the zebra fish Danio rerio models in developing our understanding of cell clearance in vivo.
Meeting Up: How Phagocytes Find Dying Cells
The first and most obvious requirement for efficient apoptotic cell clearance is to bring the apoptotic cell and phagocyte near enough to facilitate physical interaction between the cells. This proximity is facilitated in three different ways: adjacency, phagocyte migration, and the more recently recognized concept of apoptotic cell motility. Although useful for categorization, these mechanisms are not mutually exclusive, but rather likely act in concert to influence efficient cell clearance in the interstitium (Desch et al., 2011; Fourgeaud et al., 2016; Fujimori et al., 2015; Jenkins et al., 2011; Juncadella et al., 2012; Larson et al., 2016; Lee et al., 2016; Lu et al., 2011; Mattocks and Tropepe, 2010; Okabe and Medzhitov, 2014; Rosas et al., 2014; Sierra et al., 2010; Yang et al., 2015). Interstitial cell clearance is frequently carried out by neighboring or adjacent phagocytes that are of non-hematopoietic origin, such as epithelial cells in the lung and gut, and mesenchymal cells in the developing embryo (Juncadella et al., 2012; Lee et al., 2016; Wood et al., 2000). The efficiency and capacity of these so-called “non-professional” phagocytes to clear dying cells is typically much less than that of “professional” phagocytes of hematopoietic origin such as macrophages and dendritic cells. The roles of professional versus non-professional phagocytes in the clearance of dying cells has been discussed at length in several recent reviews (Arandjelovic and Ravichandran, 2015; Desch et al., 2011; Green et al., 2016). Here, we focus on spatiotemporal features related to motile, professional phagocytes that are important to establish the phagocyte-apoptotic cell interactions required for the highly efficient removal of dead cells.
Possible relevance of phagocyte positioning within the interstitium for apoptotic cell clearance
Most tissues are interspersed with networks of hematopoietic phagocytes, including macrophages, monocytes, and dendritic cells (Davies et al., 2013; Dzhagalov et al., 2013; H.-J. Kim et al., 2010; Okabe and Medzhitov, 2015; Perdiguero and Geissmann, 2015; Westphalen et al., 2014). These cells act as immune sentinels for infection and tissue damage and are also key mediators of dead cell clearance. However, in most tissues, professional phagocytes are greatly outnumbered by the non-phagocytic cells in the organ. Therefore, the positioning of these phagocytes within a tissue is likely important for maximizing their opportunity for interaction with dying cells. For example, in sinusoidal tissues like bone marrow, spleen, and liver, the tissue-resident macrophages are positioned either within or just exterior to the arterial sinus. While these macrophages can engulf apoptotic cells (e.g. aged neutrophils in the bone marrow and hepatocyte corpses in the liver (Arandjelovic and Ravichandran, 2015; Casanova-Acebes et al., 2013; Furze and Rankin, 2008; Juncadella et al., 2012; Suratt et al., 2004)), their primary function is thought to be the clearance of damaged or effete red blood cells (RBC). By contrast, interstitial positioning of macrophages and dendritic cells (DC) for engulfment of nucleated cells appears to be highly dependent on the nature of the cellular environment and function of the tissue. This is particularly true for lymphoid organs, where lymphocyte development, activation and subsequent contraction of immune effector cells lead to large numbers of apoptotic leukocytes (Garrod et al., 2012; Gautier et al., 2012; Klein et al., 2014; LeBien and Tedder, 2008; Okabe and Medzhitov, 2015; Perdiguero and Geissmann, 2015). In these tissues, macrophages and dendritic cells appear to be pre-positioned at locations where apoptotic cells accumulate or are likely to occur based on the nature of death stimuli in the tissue. For example, during an adaptive immune response, tingible body macrophages are located at the light/dark border of the germinal centers in the spleen and lymph nodes where they capture proliferating B cells undergoing apoptosis due to low affinity or self-reactivity (Gray and Cyster, 2012; Hanayama et al., 2004; Headland and Norling, 2015; N. D. Kim and Luster, 2015; Muñoz et al., 2015; Newson et al., 2014; Serhan, 2014; Vinuesa et al., 2009). T lymphocyte development in the thymus results in large numbers of apoptotic T cells, where thymic macrophages, and to a lesser extent dendritic cells, are sparse in numbers (~1% of total thymic cells) but are positioned in small clusters throughout the organ, providing widespread efferocytic coverage through the tissue (Dzhagalov et al., 2013; H.-J. Kim et al., 2010; Tacke et al., 2015). The CD169+ macrophages, the predominant efferocytes in the bone marrow, are located within dense cellular regions adjacent to the sinuses (Bianconi et al., 2013; Morrison and Scadden, 2014). These macrophages appear optimally located to multi-task in the engulfment of apoptotic B cells, aged neutrophils and erythrocytes.
In some non-lymphoid tissues where moderate to high rates of apoptosis are normal, “specialized” phagocytes appear positioned to maximize the opportunity for encountering apoptotic cells. In this context, “specialized” refers to a population of tissue-resident phagocytes (of either hematopoietic or non-hematopoietic origin) that have evolved highly unique gene expression and functional characteristics that support the function of one type of tissue. For example, in the seminiferous tubules of the testes, approximately 75% of developing germ cells will undergo apoptosis before ever becoming mature sperm (Bailey et al., 2002; Braun, 1998; De Paepe, 2004; Elliott et al., 2009; Lee et al., 2016; Sunaga et al., 2013; Surh and Sprent, 1994). Sertoli cells are specialized phagocytes attached to the basal lamina of the seminiferous tubules that extend their processes toward the lumen, forming a network of membrane structures that intertwine with spermatagonia and can recognize and rapidly engulf specifically those germ cells undergoing apoptosis (Elliott et al., 2010; Griswold, 1998; Liu and Keller, 2016; Nakanishi and Shiratsuchi, 2004; Park et al., 2011; Pittet and Weissleder, 2011; Shiratsuchi et al., 1997). Similarly, in the brain, ongoing neurogenesis in the hippocampus features moderate rates of apoptosis, involving hundreds of developing neurons. Microglia, a specialized brain phagocyte, line the dentate gyrus and mediate engulfment of developing neurons that undergo apoptosis (Desch et al., 2011; Fourgeaud et al., 2016; Fujimori et al., 2015; Juncadella et al., 2012; Larson et al., 2016; Lee et al., 2016; Lu et al., 2011; Mattocks and Tropepe, 2010; Sierra et al., 2010; Yang et al., 2015). In contrast, in the pleural cavities, sentinel, tissue-resident macrophages can move freely or are loosely adhered to the membrane surfaces, enabling rapid response to pathogens outside the lung and visceral organs (Arandjelovic and Ravichandran, 2015; Green et al., 2016; Jenkins et al., 2011; Okabe and Medzhitov, 2014; Rosas et al., 2014). Within the lung and airways, specialized tissue-resident macrophages and dendritic cells are integrated into the epithelial layer that lines the airway. A subset of migratory lung DC (CD103+/CD11c+/MHCIIhi) appear to be the dominant DC subtype that captures apoptotic cells in the lung and can traffic to the draining lymph node for antigen presentation to naïve T cells (Davies et al., 2013; Desch et al., 2011; Dzhagalov et al., 2013; H.-J. Kim et al., 2010; Okabe and Medzhitov, 2015; Perdiguero and Geissmann, 2015). Recently, Bhattacharya and colleagues used intravital microscopy to demonstrate that a subset of alveolar macrophages forms gap junction channels with epithelial cells, enabling calcium-dependent signaling between macrophages and across many cell widths (Arandjelovic and Ravichandran, 2015; Casanova-Acebes et al., 2013; Furze and Rankin, 2008; Suratt et al., 2004; Westphalen et al., 2014). This is important, as unlike many tissue-resident macrophages, alveolar macrophages are mostly sessile. Considering the critical role of airway epithelial cells in the clearance of apoptotic cells during inflammation and allergic responses, it is possible that both professional and non-professional phagocytes operate in concert to mediate efficient cell clearance and immune responses (Garrod et al., 2012; Juncadella et al., 2012; Klein et al., 2014; LeBien and Tedder, 2008). Although emerging evidence strongly suggests that tissue-resident phagocytes receive interstitial localization and positioning cues, the source and identity of these cues remains poorly understood. In light of the recent flood of information regarding the ontogeny and tissue-specific development of macrophages and dendritic cells (Gautier et al., 2012; Gray and Cyster, 2012; Hanayama et al., 2004; Okabe and Medzhitov, 2015; Perdiguero and Geissmann, 2015; Vinuesa et al., 2009), it seems likely that we are poised to apply new, sophisticated genetic models and gene expression analyses to understand how the positioning of tissue-resident interstitial phagocytes occurs and how the environmental, phenotypic, and functional heterogeneity are interconnected (Guilliams and van de Laar, 2015; van de Laar et al., 2016).
In contrast to homeostatic cell clearance, disease-associated tissue damage can lead to multiple waves of cell death, and the importance of cell clearance in these tissues is increasingly being understood as a key event in resolution, tissue repair, and the restoration of homeostatic tissue function (Dzhagalov et al., 2013; Headland and Norling, 2015; H.-J. Kim et al., 2010; N. D. Kim and Luster, 2015; Muñoz et al., 2015; Newson et al., 2014; Serhan, 2014; Tacke et al., 2015). In addition, tissue damage/infection also attracts circulating myeloid-derived phagocytes into the tissue to clear dying cells. Once in the tissue, monocytes can differentiate into a wide array of phagocytic phenotypes and may subsequently contribute to clearance of apoptotic cells (such as M1- and M2-like) (Basil and Levy, 2015; Headland and Norling, 2015; Larson et al., 2016; Newson et al., 2014; Serhan, 2014; Stanford et al., 2014). In these cases, localization of recruited phagocytes to apoptotic corpses is due to many different types of factors, including find-me signals, danger signals, and chemokines (Junger, 2011; Kolaczkowska and Kubes, 2013; Serhan, 2014). While it appears that both tissue-resident and recruited phagocytes contribute to the clearance of apoptotic cells in a damaged/infected tissues (Gautier et al., 2013; Uderhardt et al., 2012), the relative importance of each phagocyte type on the overall removal of dead cells in the tissue is still being worked out.
Communication Signals
The mobility of immune cells is a fundamental property of metazoan immunity that enables rapid and site-specific defense and wound-healing responses throughout the body. The movement of immune cells to and within tissues is controlled by attraction and repulsion factors produced by cells within local tissue environments, including chemokines, cytokines and soluble metabolites (e.g. lipids, nucleic acids). Like other immune cells, phagocytes rely on a range of different soluble cues emanating from apoptotic cells to sense and properly locate apoptotic cells. Activation of executioner caspases during apoptosis leads to the release of these soluble chemoattractants, called find-me signals (Lauber et al., 2004; Ravichandran and Lorenz, 2007). Find-me signals have been shown to enhance the localization of phagocytes to dying cells, and to date, at least four distinct find-me signal-receptor pathways have been identified primarily through in vitro studies (Figure 1) (Elliott et al., 2009; Gude et al., 2008; Lauber et al., 2003; Luo et al., 2016; Truman et al., 2008). Importantly, three of these have been reported to contribute to efferocytosis in vivo: extracellular nucleotides ATP and UTP, CX3CL1, and sphingosine-1-phosphate (S1P) (Elliott et al., 2009; Luo et al., 2016; Truman et al., 2008). However, due to the distinct experimental systems used to identify each of these factors as well as differences in the timing of release during cell death (Figure 1), it remains a challenge to understand the specific tissue settings where one or more of these factors would be operational, and how multiple find-me signals, when present concurrently, might be integrated to affect cell clearance.
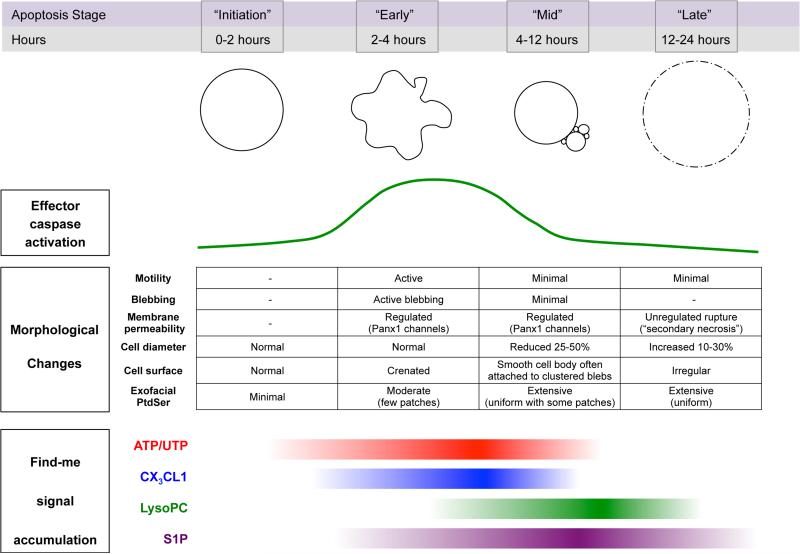
Figure 1. Spatiotemporal characteristics of apoptotic cells.
Generalized depiction of the temporal relationships among key features of apoptotic cells at different stages after induction of apoptosis. Effector caspase activation (i.e. caspases 3 and 7) and prominent morphological changes in apoptotic cells are shown in relationship to the accumulation of the four main find-me signals identified to date: ATP/UTP, CX3CL1, lysophosphatidycholine (LysoPC), and sphingosine-1-phosphate.
As depicted in Figure 1, established find-me signals can be categorized based on when they accumulate at physiologically relevant levels following the induction of apoptosis (i.e. early, mid, and late apoptosis). Initiation of apoptosis occurs upon receipt of a death stimulus such as death receptor ligation (e.g. CD95-CD95L), nuclear DNA damage or mitochondrial stress. Cleavage and activation of caspases 3 and 7 triggers an irreversible cascade of enzymatic changes that result in the morphological and biochemical features of apoptosis. Of the find-me signals identified to date, the release of nucleotides (i.e. ATP and UTP) from the cytoplasm of apoptotic cells coincides most closely with the activation of caspases, occurring in nearly parallel kinetics with the accumulation of activated executioner caspases (Chekeni et al., 2010; Elliott et al., 2009; Poon et al., 2014a; Qu et al., 2011). Subsequent mechanistic studies confirmed that caspase-3/7 act on pannexin-1 channels: cleavage of the C-terminal tail of pannexin-1 monomers led to the opening of the hexameric channels, allowing for the extracellular release of cytosolic nucleotides (Chekeni et al., 2010; Poon et al., 2014a; Qu et al., 2011). Apoptotic cell-derived extracellular nucleotides are detected by macrophages and other immune phagocytes via the P2Y2 G-protein coupled receptor (GPCR) (Elliott et al., 2009). P2Y2 is expressed at relatively high levels on numerous myeloid cell populations in mice and humans, including tissue-resident macrophages in the lung and peritoneum of mice (Chen et al., 2006; Gautier et al., 2012; Kaufmann et al., 2005; Kronlage et al., 2010). With high sensitivity to both ATP and UTP (maximal responses in ~0.1μM range) (Junger, 2011), P2Y2 has been shown to promote vascular adhesion, motility, and apoptotic cell clearance in a number of different studies of acute and long term inflammation and adaptive immune responses (Elliott et al., 2009; Idzko et al., 2007; Jin et al., 2014; Klämbt et al., 2015; Ma et al., 2013b; McDonald et al., 2010; Shah et al., 2014; Wang and Kubes, 2016). In the context of cell clearance, depletion of extracellular nucleotides or deletion of P2Y2 in mice was shown to cause a delay in the clearance of apoptotic cells by macrophages in the thymus (Elliott et al., 2009). Similarly, UDP released by dying hippocampal neurons stimulates phagocytic clearance by microglia in vivo (Koizumi et al., 2007). Another find-me cue released in the early stages of apoptosis is CX3CL1/fractalkine. Identified by Gregory and colleagues, CX3CL1 was released by apoptotic Burkitt lymphoma cells in a manner that coincides temporally with early cellular hallmarks of apoptosis (Figure 1)(Truman et al., 2008). Recognition of CX3CL1 from apoptotic cells by the GPCR CX3CR1 was critical for recruitment of macrophages to the splenic germinal centers of mice in vivo (Truman et al., 2008). Thus, nucleotides and CX3CL1 are released early by apoptotic lymphocytes and can play a role in the rapid and efficient clearance of apoptotic cell in tissues.
Two other prominent find-me signals, lysophosphatidylcholine (LysoPC) and S1P, accumulate to significant levels later in the apoptotic process (4-12 hours after induction) (Figure 1). LysoPC is a potent chemoattractant for monocytes and macrophages in vitro and in vivo (Hoffman et al., 1982; Ousman and David, 2000), and Lauber et al. reported that a number of transformed cell lines release LysoPC during apoptosis via caspase-mediated activation of calcium-independent phospholipase A2 (iPLA2) (Lauber et al., 2003). More recently, another chemotactic lipid, S1P, was shown to accumulate extracellularly following the induction of apoptosis, and S1P is also a potent macrophage chemoattractant in vitro (Gude et al., 2008; Luo et al., 2016; Weigert et al., 2010; 2006). Although the mechanisms of S1P release during cell death are not fully understood, it is interesting to note that Weigert et al. found that S1P release by apoptotic cells was associated with cleavage and activation of the inflammatory caspase, caspase-1, and that this release could be partially inhibited using the putative caspase-1 inhibitor YVAD (Weigert et al., 2010). Considering the timing of S1P accumulation following apoptosis induction (> 6 hours) and the well-described role of casp-1 in the execution of regulated necrosis (i.e. pyroptosis) it is possible that S1P may function as a find-me signal for the clearance of secondarily necrotic cells. Finally, additional find-me signals were recently reported by Cullen et al, who found that HeLa cells induced to apoptose by CD95 stimulation released chemokines MCP-1 and IL-8, both known myeloid/phagocyte chemoattractants (Cullen et al., 2013). However, these find-me cues appear more relevant for necrotic cell clearance, as the authors showed that chemokine release was due to caspase-independent necrosis. Along these lines, another necrotic cell find-me signal, cleaved annexin A1, was reported by Blume et al. to accumulate in the supernatants of late apoptotic or secondarily necrotic cells and could stimulate migration of myeloid cells in vitro (Blume et al., 2011).
The best in vivo evidence to date for the importance of find-me signaling in phagocyte recruitment and efficient cell clearance comes from studies where find-me signal receptors, all of which are GPCRs, have been ablated genetically or pharmacologically in mice. Inhibition or ablation of the ATP/UTP receptor P2Y2 in mice results in a significant inhibition in the recruitment of myeloid phagocytes in vivo as well as a delay in the clearance of apoptotic thymocytes (Elliott et al., 2009). In a separate study, deletion of P2Y2 significantly reduced the accumulation of macrophages infiltrating fibrosarcoma tumor tissues following apoptosis-inducing anthracycline chemotherapy (Ma et al., 2013a). Similarly, deletion of the fractalkine receptor CX3CR1 reduces the accumulation of tingible body macrophages in the splenic follicles, although an increase in uncleared apoptotic cells was not observed at the time points used in this study (Truman et al., 2008). Receptors for S1P (S1PR1-5) and lysoPC (G2A) have been implicated in autoimmunity, but their role in the clearance of apoptotic cells in vivo remains to be determined (reviewed in (Green et al., 2016; Medina and Ravichandran, 2016)).
Apoptotic cell motility
While the movement of phagocytes toward apoptotic cells has been studied extensively, there is now evidence to support the idea that the motile behavior of apoptotic cells themselves might contribute to cell clearance. Apoptosis is an active rather than a passive dismantling of the cell, and cells undergoing apoptosis often display rapid and dynamic alterations of the cytoskeleton that lead to the membrane blebbing and motility patterns characteristic of apoptosis (the so-called ‘dance of death’) (Figure 1). Many of these morphological and motile behaviors are driven by the caspase-mediated activation of rho-associated coiled-coil-containing protein kinase 1 (ROCK1). Caspase-3 cleavage of the autoinhibitory domain of ROCK1 leads to constitutive kinase activity, myosin light chain (MLC) phosphorylation, actomyosin contraction, and blebbing (Atkin-Smith et al., 2015; Coleman et al., 2001; Sebbagh et al., 2001). More recently, intravital imaging studies in vertebrates have confirmed this ‘dance of death’ behavior in vivo. Using a transgenic mouse line expressing a FRET-based caspase-3 activation reporter, Yamaguchi et al. observed that intact embryonic cells undergoing apoptosis, as detected by FRET, carried out a rapid and somewhat erratic motility pattern along the neural crest for up to three hours (Yamaguchi et al., 2011). A subsequent report similarly showed that apoptotic neural cells in the developing zebra fish brain were very motile for up to three hours and frequently moved several cell diameters interstitially after becoming positive for surface phosphatidylserine (PtdSer) (van Ham et al., 2012). Similarly, intravital imaging of mouse thymic explants during negative selection revealed that the thymic macrophages were relatively sessile and that the apoptotic thymocytes themselves moved rapidly (apparently non-directionally) until encountering a macrophage and being engulfed (Dzhagalov et al., 2013). Also, during skeletal myoblast fusion, the emerging apoptotic myoblasts appear to move around in close apposition to the growing myotube without being engulfed (Hochreiter-Hufford et al., 2013). Conceptually, it seems possible apoptotic cell movement could enhance the ability of phagocytes to locate them, or alternatively might allow for aggregation of multiple apoptotic cells into a common area in the tissue, thereby facilitating clearance in tissues where professional phagocytes are sparse. In support of this, previous studies have shown that unengulfed apoptotic cells are often seen in clusters at specific sites within the tissue context (Elliott et al., 2009; Surh and Sprent, 1994; Wood et al., 2000). The molecular basis of this clustering or its relevance to cell clearance is currently unknown. Together these studies provide new ways to consider how apoptotic cells might be cleared in a tissue.
Possible integration of find-me signals during interstitial cell clearance
Considering the diversity of find-me signals identified to date, it will be important to understand distinct roles for each of these factors in efferocytosis as well as determine how these signals are integrated into the overall process of cell clearance in the tissue. Find-me signals released early, such as nucleotides and CX3CL1, which do not appear to require active metabolic synthesis or gene expression, may provide key ‘alert’ information to neighboring phagocytes indicating apoptotic cell stress nearby. Factors that appear later and persist longer, such as LysoPC and S1P, might provide more stable cues that can stimulate recruitment of more distant phagocytes in settings where there are moderate to high levels of apoptosis. Another purpose for multiple and varied find-me signals may be that the condition the phagocytes or local tissue environments may need to be primed or altered in ways that enhance phagocyte mediated clearance. One recent example of this was the finding that S1P release by apoptotic cells triggers phagocytes to produce and release erythropoietin (EPO), which in an autocrine/paracrine manner activated PPARγ-dependent transcription of engulfment genes such as Mfge8 and Mertk (Luo et al., 2016).
Dynamics of Selective Uptake
In a steady state tissue context, the phagocyte must specifically recognize and ingest the few dying cells among the sea of living cells. Accordingly, the physical interaction of phagocytes and target cells is regulated by a series of dynamic and temporally restricted checkpoints that function to ensure rapid clearance of appropriate targets. Dying cells undergo a continuum of changes on their surface and intracellularly that convey their state of viability. A canonical feature of apoptosis is the rapid, caspase-dependent exposure of PtdSer to the outer leaflet of the apoptotic cell plasma membrane. Exposed PtdSer is subsequently recognized by one or more of a cadre of heterogeneous surface receptors expressed on phagocytes (reviewed in (Green et al., 2016; Gregory and Pound, 2010; Hochreiter-Hufford and Ravichandran, 2013; Penberthy and Ravichandran, 2015). However, PtdSer recognition alone is likely not always sufficient to stimulate engulfment, as the duration of PtdSer exposure as well as additional eat-me ligands exposed can work in concert to help the phagocyte properly recognize and engage an apoptotic cell. It has become clear that both the dying cell and the phagocyte provide key spatiotemporal information that ultimately conveys the tissue and cell-specific nature of the dying cell.
The clearance code
Upon initiation of apoptosis, activated effector caspases cleave numerous substrates that result in the biochemical and morphological changes characteristic of apoptotic cell death (Figure 1). Among the most ubiquitous and best studied of these is the exposure of PtdSer on the exofacial leaflet of the plasma membrane (Fadeel and Xue, 2009; Fadok et al., 1992; Segawa and S. Nagata, 2015). In viable cells, PtdSer (and the other major aminophospholipids, such as phosphatidylethanolamine) are maintained in the cytoplasmic side of the membrane due to the enzymatic activity of flippases that shuttle PtdSer from the outer to the inner leaflet (Arandjelovic and Ravichandran, 2015; G. C. Brown and Neher, 2012; Segawa and S. Nagata, 2015). A member of the P4-type ATPse, ATP11C, was recently identified in a haploid screen for regulators of PtdSer exposure (Segawa et al., 2014). Cleavage of ATP11C by caspase-3/7 leads to irreversible loss of flippase activity, which is required for exposure of PtdSer during apoptosis. By contrast, scramblases, including the recently identified Xkr8, are a class of enzymes that drive PtdSer from the inner to outer leaflet (Suzuki et al., 2013; 2014). In the case of Xkr8, caspase-3/7 cleaves the C-terminal intracellular tail, leading to constitutive scramblase activity and PtdSer exposure on the cell surface.
It is important to note that the kinetics of caspase-3/7 activation in cells following a death stimulus are highly variable and depend greatly on the cell type and death stimulus. However, the measurement of caspase-3/7 activation in cells in real-time has been studied using sensitive fluorescent reporters (e.g. Casp3-FRET, FLICA). Results from these experiments indicate that although the time from delivery of a death stimulus to the first signs of effector caspase activation can vary widely, once caspase-3/7 are activated, maximal caspase activation is achieved rapidly (~1-4 hours) and that many of the characteristic features of apoptosis that are relevant to cell clearance are visible, including membrane blebbing, PtdSer exposure, and Pannexin-1-dependent nucleotide release (Chekeni et al., 2010; Croker et al., 2011; Poon et al., 2014a). Thus, irreversible apoptosis is characterized by a rapid, intense and temporally linked series of biochemical and cellular events that provide cues to local phagocytes of the certain demise of the cell and thus the need for rapid clearance.
Even within the short time between minimal and maximal effector caspase activation, the exposure of eat-me signals on the dying cell occurs on a continuum of dynamic, distinct changes. In the case of PtdSer exposure, studies in lower organisms and mammalian cells have shown PtdSer on the outer leaflet of apoptotic cells can occur in aggregates or patches on the surface (Croker et al., 2011; Fairn et al., 2011; Gardai et al., 2005; van Ham et al., 2012). Consequently, localization of soluble PtdSer-binding proteins, like annexin-V, calreticulin, Gas6, and MFGE8, can also appear as patches on the surface of apoptotic cells. Interestingly, the earliest PtdSer molecules observed on apoptotic cells are present in one or two patches on the cell, with PtdSer becoming more uniform across the cell membrane as apoptosis progresses. Although the mechanisms responsible for the appearance or localization of PtdSer patches on apoptotic cells have not been precisely defined, it seems likely that the punctate localization of flippases and scramblases within the membrane are involved (Segawa et al., 2014; Suzuki et al., 2013). Functionally, the appearance of PtdSer-dense aggregates on early stage apoptotic cells, in combination with the blebbing activity, may contribute to the efficiency of clearance by enhancing the recognition and binding of PtdSer receptors on the phagocyte (discussed in more detail below). In addition to PtdSer, other changes on the surface of apoptotic cells contribute to recognition and likely convey important spatiotemporal information about the status of the target cell to phagocytes. These include ICAM-3, C1q, oxidized lipids and alterations in the glycocalyx/glycosylation patterns (Bilyy et al., 2011; Devitt et al., 1998; 2003; Fadok et al., 1998; Galvan et al., 2012; Torr et al., 2011; Ucker et al., 2012).
In contrast to the eat-me signals, some surface proteins can act as indicators of viability and act to suppress phagocytosis. These so-called “don't eat-me” signals include adhesion-related proteins CD47 and CD31. Originally identified as a viability marker on erythrocytes, CD47 has since been shown to be expressed on many different types of viable nucleated cells where it can interact with the inhibitory receptor SIRP1α on phagocytes to restrain the engagement of the phagocytic machinery (Willingham et al., 2012). While CD47 levels decrease as RBCs age, its downregulation during apoptosis has been observed, but is not a universal feature of apoptosis, and in many cases is not sufficient to override PtdSer-driven engulfment of apoptotic cells. In recent years, CD47 has become a potential therapeutic candidate for tumor cell eradication, as many different types of transformed cells express high levels of CD47, and antibodies targeting CD47 have shown promise in promoting clearance of tumor cells (Chao et al., 2010; 2011). Similarly, CD31 on viable cells can interact with CD31 on phagocytes to provide a spatially confined repulsion signal to prevent engulfment (S. Brown et al., 2002). Although CD31 expression varies widely on different cell types, certain leukocytes require the downregulation of CD31 for efficient efferocytosis to proceed (S. Brown et al., 2002).
Apoptosis leads to a wide array of changes to the cell surface. Understanding the specific molecular characteristics of the apoptotic cell surface at different times throughout apoptosis will likely provide important contextual information to aid in our understanding of distinct mechanisms and consequences of apoptotic cells clearance.
Quality control checkpoints during engulfment
Despite being recognized as the canonical eat-me signal in efferocytosis, surface PtdSer is also seen on many different ‘viable’ cell types in a number of biological settings involving neither apoptosis nor cell clearance. Perhaps the most widely accepted model of engulfment, called ‘tether-and-tickle’, proposes that successful engulfment of apoptotic cells occurs in two molecularly distinct steps. Tethering receptors such as Tim4 can bind PtdSer with a relatively high affinity (Kd ~2nM) (Miyanishi et al., 2007); however, Tim4 is unable to signal on its own to the engulfment machinery (Park et al., 2009), and additional engulfment receptors are required to ‘tickle’ the engulfment signaling pathways to mediate corpse internalization. In fact, a recent study in zebra fish showed that homologues of Tim4 and Bai1 worked concurrently to facilitate engulfment (Mazaheri et al., 2014). Interestingly, the affinity of other PtdSer-bridging proteins such as Gas6 (which link to the Mer tyrosine kinase receptor (Mer-TK)) are considerably lower that than of Tim4 (Kd 20-50nM) (Baroni et al., 2010; K. Nagata et al., 1996). Consistent with this notion, it has been shown in peritoneal macrophages that both Tim4 and Mer-TK worked concurrently to facilitate corpse uptake ex vivo (Nishi et al., 2014; Toda et al., 2012). However, a recent study by Dransfield et al. demonstrated that bone marrow-derived macrophages, which normally express Mer-TK but not Tim4, use Mer-TK for both tethering and tickling (Dransfield et al., 2015). These findings support the broader notion that the mechanisms that underlie the dynamics of engulfment are highly dependent on the subset of phagocytes being investigated. Further, in cells with high phospholipid scramblase activity (via calcium-induced activation of the lipid scramblase TMEM16F), this is not sufficient to induce engulfment of these PtdSer exposed cells; this is now thought to be due to PtdSer exposure being too transient with the exposed PtdSer being quickly flipped back to the inner leaflet by the aminophospholipid translocases (Segawa and S. Nagata, 2015; Segawa et al., 2011). This raises the dynamics of PtdSer exposure on apoptotic cells as another variable, with the possibility that tethering receptors like Tim4 could play a vital role in determining the viability of the target cell, i.e. PtdSer recognition by phagocytes only leads to internalization under conditions where PtdSer aggregation induces stronger and more persistent interactions between the target and the phagocyte.
Shifting Gears: How Motile Phagocytes Toggle between Motility and Engulfment
Efficient engulfment of apoptotic corpses by motile phagocytes requires the execution of two distinct but related cellular functions – migration toward the corpse and subsequent phagocytosis (Figure 2). Both of these energy-intensive processes involve large-scale reorganization of the phagocyte cytoskeleton and overlapping signaling pathways that regulate these changes. However, it is unclear how these events are integrated and act in synergistic and/or antagonistic manners. In addition, the complexity of soluble and cell-associated signals in a tissue context must be appropriately interpreted by the phagocyte to ensure efficient clearance of non-viable targets. A number of recent studies have provided important insights that are beginning to define these relationships.
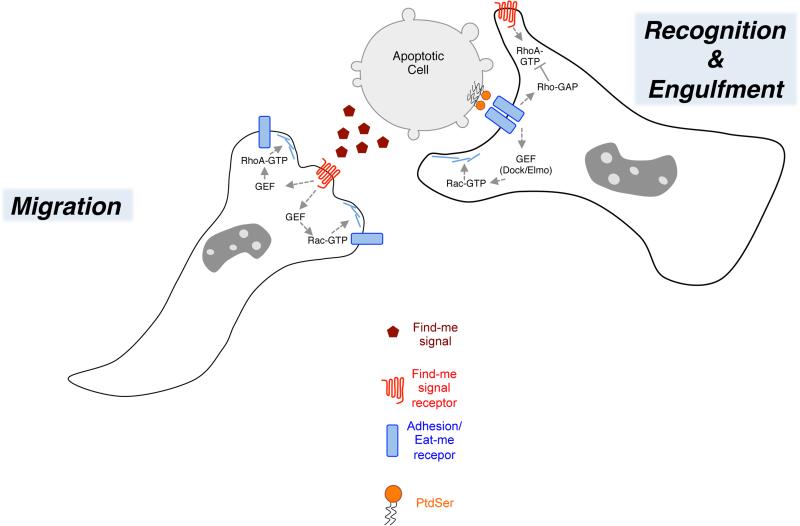
Figure 2. Model for hand-off of Rho signaling during transition from find-me to eat-me stages of efferocytosis.
Detection of find-me signals by GPCR receptors on phagocytes stimulates migration and/or motility behavior in a manner dependent on RhoA and possibly Rac1 activation. During the find-me stage, some eat-me receptors, for example αvβ3/5 integrins, might function in adhesion and cell motility. Upon encountering the apoptotic target, adhesion receptors can function to recognize PtdSer on the surface of the apoptotic cells, either directly or indirectly via bridging proteins like MFG-E8 or Gas6, and reorient in a putative phagocytic synapse at the site of intercellular contact between the apoptotic cell and phagocyte. RhoA-GTP is likely inhibited or spatially confined during this stage to prevent antagonism with Rac that would impair Rac-GTP-dependent actin polymerization and phagocytic cup formation.
Surface proteins that function in motility and phagocytosis
Once the find-me signals have provided the cues necessary for phagocytes to move near a dying target, the phagocyte must arrest its migration to physically engage the target cells. Several lines of recent evidence suggest that a migration/arrest cycle is very likely operative in phagocytes to mediate efferocytosis. Indeed, a number of molecular pathways that control adhesion and migration also function in many instances as key mediators of efferocytosis. In fact, the first apoptotic cell recognition receptor identified on phagocytes was the vitronectin-binding integrin αvβ3/5, which is well linked to cell migration and also doubles as an apoptotic cell receptor via binding to MFGE8, a PtdSer binding protein (Hanayama et al., 2004; 2002; Hynes, 2002; Overstreet et al., 2013; Savill et al., 1990). In addition, another PtdSer receptor, BAI1, was originally identified as an adhesion-type GPCR expressed in brain tissues (Nishimori et al., 1997; Park et al., 2007). Additional surface proteins that function in both motility and efferocytosis in mammals include, ICAM-3, LRP1, CD14 and CD36 (Devitt et al., 2003; Gardai et al., 2005; Ogden et al., 2001; Savill et al., 1992; Torr et al., 2011). It is important to note that this dual functionality is an evolutionarily conserved feature of efferocytosis, as numerous studies have identified similar functional overlap of surface proteins in C. elegans (integrin homologues Ina1 and Ina2) and in Drosophila (MEGF-10 homologue Draper, and CD36 homologue Croquemort) (Franc et al., 1996; Hsu and Wu, 2010; MacDonald et al., 2006).
Intracellular signaling networks that function in motility and phagocytosis
The canonical Rho GTPases Rac1, Cdc42 and RhoA are well known to coordinate the actin dynamics that drive cell motility and phagocytosis (Heasman and Ridley, 2008; Ravichandran and Lorenz, 2007; Rossman et al., 2005) (Figure 2). Activation of Rac1 (i.e. Rac1-GTP) is an essential, evolutionarily conserved mediator of the actin polymerization required to form the phagocytic cup and F-actin-rich membrane protrusions that extend around and envelop the target cell (Kinchen and Ravichandran, 2010; 2007; Nakaya et al., 2008; 2006). Activation of Rac downstream of apoptotic cell recognition is mediated by the recruitment and activation of guanine nucleotide exchange factors (Rac-GEFs). One of the most important and evolutionarily conserved Rac-GEFs in efferocytosis is the bipartite Dock1-Elmo complex (Gumienny et al., 2001). In fact Elmo (which stands for ‘engulfment and cell motility’) was identified to regulate gonadal distal tip cell migration and engulfment of somatic and germ cell corpses in C. elegans (Gumienny et al., 2001). Similarly, mammalian Elmo1 and Dock are crucial for mammalian apoptotic cell engulfment and cell migration in vivo and in vitro (Brugnera et al., 2002; Côté and Vuori, 2007; Elliott et al., 2010; Grimsley et al., 2004; Park et al., 2007; Stevenson et al., 2014). Importantly, signaling through multiple chemotaxis and efferocytosis receptors converge at the Dock-Elmo-Rac module, indicating the essential role of this dual-function signaling complex in cell migration and phagocytosis.
Although the control of migration and phagocytosis by common signaling networks is known, it is not clear how these pathways are modulated to allow for the migration/arrest/engulfment cycle of motile efferocytes. However, the likely key to toggling between migration and phagocytosis occurs at the level of Rac and RhoA antagonism and crosstalk (Figure 2). Many studies have shown that RhoA-GTP inhibits phagocytosis of apoptotic cells (Nakaya et al., 2006; Tosello-Trampont, 2003). In the context of cell migration, RhoA-GTP activates FilGAP, a filamin A-binding Rac1-GAP, leading to actomyosin contraction and inhibition of Rac-driven actin polymerization (Ohta et al., 2006). Although the role of FilGAP in apoptotic cell engulfment is presently unknown, its function as a toggling mechanism for the transition from Rac-dependent mesenchymal to RhoA-dependent amoeboid movement raises the possibility that a similar mechanism could underlie the transition from the polarized and directed migration of phagocytes induced by find-me signals to a partial arrest and probing behavior necessary for phagocytes to engage with apoptotic cells. Studies using FRET-based Rho GTPase activation reporter probes have produced a wealth of new information on the spatiotemporal dynamics of Rho and Rac activation during cell motility and efferocytosis that have altered the paradigm for understanding the nature of interplay and crosstalk between these signaling molecules (Machacek et al., 2009; Nakaya et al., 2008; Pertz et al., 2006). In the case of efferocytosis, a study by Nagata and colleagues using Rho and Rac FRET reporters revealed that active Rac rapidly accumulates at the site of phagocytic cup formation (Nakaya et al., 2008). Interestingly, it was observed (using wild-type and constitutively active Rac) that regions of the phagocyte membrane enriched for Rac-GTP acted as ‘portals’ for the engulfment of successive targets. However, since closure of the phagocytic cup and internalization of the target are hampered by constitutively active Rac (as has been elegantly shown for larger versus smaller particle uptake), the mechanical act of corpse internalization requires the spatially-defined termination of Rac signaling (Schlam et al., 2015). In contrast to Rac, the role of RhoA in efferocytosis is more nuanced. It is clear that globally activating RhoA (i.e. RhoA-GTP) in cells inhibits the phagocytosis of apoptotic cells; as a corollary, global inhibition of RhoA has an overall net positive effect on efferocytosis (Nakaya et al., 2006; Tosello-Trampont, 2003). However, the spatially restricted activation of RhoA appears to be important for controlling the closure of the phagocytic cup and internalization of the corpse within the phagolysosome. Thus, crosstalk between Rac and RhoA is a dynamic and essential element of efficient engulfment. The molecular regulation of this crosstalk during efferocytosis remains an important, but poorly understood area of apoptotic cell clearance.
Interstitial Cell Clearance
Given our current understanding of find-me and eat-me signals in the courting that occurs between dying cells and phagocytes, how might phagocytes ‘sense’ the need to arrest migration and engage the engulfment machinery in a tissue context? Even though it is common to consider find-me and eat-me signals as distinct, it is very likely that in vivo these events likely occur within a continuum. Efferocytosis in real-time likely involves a seamless integration of receptors that mediate the sensing of find-me to eat-me signals. Based on analogous biological systems, the physical localization of these surface proteins might be akin to a ‘phagocytic synapse.’ Such a synapse may be important for coordinating the signaling molecules on the phagocyte and to ensure maximum efficiency of target identification and uptake. Indeed, intercellular signaling synapses are observed in numerous biological contexts, including the well-detailed examples in the nervous and immune systems. These synapses are known to be effective means for cells to focus signaling information between cells to specific sites within a cell, and subsequently translate the various go/no-go signals from different receptors to a specific cellular response. Considering the complexity of signals that control phagocyte morphology during efferocytosis, it is an exciting possibility that such a phagocytic synapse containing find-me receptors and eat-me receptors would function in the dynamic movement of the phagocytes, selective sensing of apoptotic cells (and avoiding viable cells) within tissues, and the subsequent efficient corpse removal (Figure 2). Signaling through the find-me receptors P2Y2, S1PR and G2A has been shown to stimulate RhoA activation (Donati and Bruni, 2006; Kabarowski et al., 2000; Liao et al., 2007). Thus, RhoA-dependent migration in response to a find-me cue will need to be overcome/turned off so that the essential Rac-dependent engulfment processes can occur. Whether these surface receptors use the engulfment synapse and the Rho/Rac antagonism to transition between migration, arrest, and subsequent target uptake, i.e. a hand-off in integrating find-me and eat-me cues, remains to be determined, and promises to be a fascinating area of future investigation (Figure 2).
Future challenges in defining apoptotic cell clearance in tissues
Our first insights into the complex intercellular interactions that underlie cell death and clearance in the tissue came from the pioneering work of Elie Metchnikoff 100 years ago. By light microscopy, Metchnikoff observed the role of phagocytes (motile and sessile) in the removal of damaged and unnecessary cells within inflamed tissues and during development in a number of different metazoan species (Metschnikoff, 1891; Tauber, 2003). The notion of regulated cell death as a component of cellular turnover was cemented in 1972 by the landmark study of Kerr et al. in which the term “apoptosis” was coined (Kerr et al., 1972). Using electron microscopy of human and rodent tissues, this group solidified the notion of interstitial cell death as an integral part of cellular turnover (Kerr et al., 1972). Further, the authors observed the engulfment of these apoptotic cells by tissue macrophages (‘histiocytes’). While we have made tremendous progress since these early studies in deciphering the key steps in apoptotic cell clearance, the scientific problem remains a beautiful one, with broad implications for basic physiology and many forms of diseases. A key challenge moving forward is the wide variety of tissue environments and the many different molecular mechanisms for recognition of dying cells that make it difficult to develop and apply broadly useful strategies for tracking and targeting efferocytosis in vivo (Arandjelovic and Ravichandran, 2015; Green et al., 2016; Hochreiter-Hufford and Ravichandran, 2013). The heterogeneity of cell clearance mechanisms in mammalian tissues was appreciated early on, and over the past 15 years investigators have generally focused on identifying the clearance mechanisms relevant for specific tissues and biological or disease states (reviewed in (Elliott and Ravichandran, 2010; Green et al., 2016; Poon et al., 2014b). For example, using intravital imaging a recent report by Lemke and colleagues suggested that microglial process extension in response to vascular injury was impaired in the absence of the efferocytosis receptors Axl and MerTK (Fourgeaud et al., 2016). Similarly, intravital imaging of peritoneal macrophages revealed that they remain relatively sessile but execute dead cell clearance via extension of pseudopodia that gradually disassemble and engulf dead cell material (Wang and Kubes, 2016). Similarly, Casanova-Acebes used multiphoton imaging of calvarial bone marrow to track aged neutrophils as they return from circulation to the bone marrow, and were found to be engulfed by CD169+ bone marrow macrophages rapidly (Casanova-Acebes et al., 2013). Thus, although few in number, these studies highlight the possibilities for illuminating the true nature of phagocyte-apoptotic cell interactions that are important for interstitial cell clearance (Figure 3). Nevertheless, the reality is that there is tremendous heterogeneity at the dying cell and phagocyte levels. At the target level, there are different forms of apoptosis, different cell types undergo death at different rates, and different targets likely use different sets of find-me and eat-me cues. Further, the cells undergoing developmental apoptosis versus damage-induced apoptosis are certain to have additional differences in the markers they expose. From the phagocyte end, there are professional, non-professional, and specialized phagocytes, and they likely utilize distinct as well as overlapping sets of phagocytic receptors. Moreover, in many tissues, professional and non-professional phagocytes often reside nearby and how they may communicate with each other to divvy up their engulfment roles is a fascinating problem that remains to be addressed. Nevertheless, the recent progress in understanding the dynamics of cell clearance is already providing insights that were not apparent a few years ago, and the pace of learning about cell clearance in vivo is only going to increase, as the techniques and the power of our investigations continue to expand rapidly.
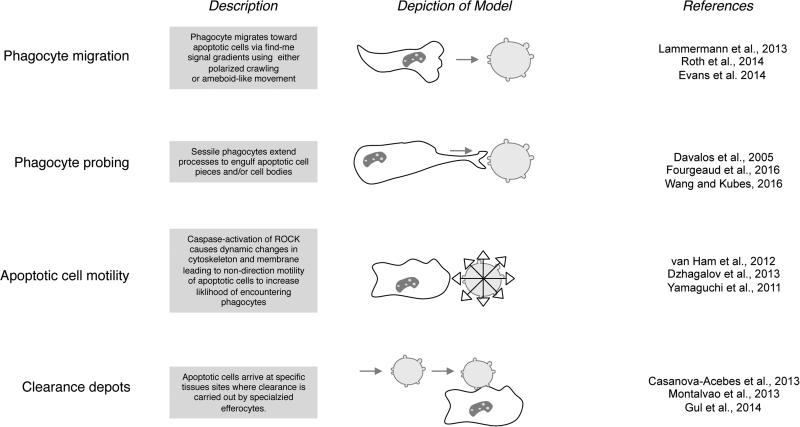
Figure 3. Different modes of phagocyte and apoptotic cell movement that can promote intercellular interaction and efferocytosis in a tissue.
The four modes of efferocytosis related cell movements described are based on data from the indicated studies using intravital microscopy.
Acknowledgements
K.S.R was supported by U.S. National Institutes of Health via GM064709, GM107848, HD74981, and HL120840) and M.R.E. was supported by US Nationals Institutes of Health via AI114554. While we have cited the works of many of our colleagues in the field, we apologize to those whose work could not be cited due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nature Immunology. 2015;16:907–917. doi: 10.1038/ni.3253. doi:10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IKH. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439. doi: 10.1038/ncomms8439. doi:10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- Baroni M, Pavani G, Marescotti D, Kaabache T, Borgel D, Gandrille S, Marchetti G, Legnani C, D'Angelo A, Pinotti M, Bernardi F. Membrane binding and anticoagulant properties of protein S natural variants. Thrombosis Research. 2010;125:e33–9. doi: 10.1016/j.thromres.2009.09.015. doi:10.1016/j.thromres.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2015 doi: 10.1038/nri.2015.4. doi:10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S. An estimation of the number of cells in the human body. Annals of Human Biology. 2013;40:463–471. doi: 10.3109/03014460.2013.807878. doi:10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- Bilyy RO, Shkandina T, Tomin A, Muñoz LE, Franz S, Antonyuk V, Kit YY, Zirngibl M, Fuernrohr BG, Janko C, Lauber K, Schiller M, Schett G, Stoika RS, Herrmann M. Macrophages discriminate glycosylation patterns of apoptotic cell-derived microparticles. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M111.273144. doi:10.1074/jbc.M111.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume KE, Soeroes S, Keppeler H, Stevanovic S, Kretschmer D, Rautenberg M, Wesselborg S, Lauber K. Cleavage of Annexin A1 by ADAM10 during Secondary Necrosis Generates a Monocytic “Find-Me” Signal. The Journal of Immunology. 2011 doi: 10.4049/jimmunol.1004073. doi:10.4049/jimmunol.1004073. [DOI] [PubMed] [Google Scholar]
- Braun RE. Every sperm is sacred--or is it? Nat Genet. 1998;18:202–204. doi: 10.1038/ng0398-202. doi:10.1038/ng0398-202. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: 'phagoptosis'. Trends Biochem. Sci. 2012;37:325–332. doi: 10.1016/j.tibs.2012.05.002. doi:10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. doi:10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. doi:10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-Gonzalez N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. doi:10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. doi:10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2011;12:58–67. doi: 10.1038/nrc3171. doi:10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. doi:10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. doi:10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. doi:10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Côté J-F, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. doi:10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang J-G, White M, Lackovic K, Cengia LH, O'Reilly LA, Bouillet P, Cory S, Strasser A, Roberts AW. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. doi:10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, Lavelle EC, Leverkus M, Martin SJ. Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Molecular Cell. 2013;49:1034–1048. doi: 10.1016/j.molcel.2013.01.025. doi:10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. doi:10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nature Immunology. 2013;14:986–995. doi: 10.1038/ni.2705. doi:10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe ME. Fas/FasL-mediated apoptosis in perinatal murine lungs. AJP: Lung Cellular and Molecular Physiology. 2004;287:L730–L742. doi: 10.1152/ajplung.00120.2004. doi:10.1152/ajplung.00120.2004. [DOI] [PubMed] [Google Scholar]
- Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. Journal of Experimental Medicine. 2011 doi: 10.1084/jem.20110538. doi:10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. doi:10.1038/33169. [DOI] [PubMed] [Google Scholar]
- Devitt A, Pierce S, Oldreive C, Shingler WH, Gregory CD. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death Differ. 2003;10:371–382. doi: 10.1038/sj.cdd.4401168. doi:10.1038/sj.cdd.4401168. [DOI] [PubMed] [Google Scholar]
- Donati C, Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: implications in its biological response. Biochim. Biophys. Acta. 2006;1758:2037–2048. doi: 10.1016/j.bbamem.2006.06.015. doi:10.1016/j.bbamem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Zagórska A, Lew ED, Michail K, Lemke G. Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell Death and Disease. 2015;6:e1646. doi: 10.1038/cddis.2015.18. doi:10.1038/cddis.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhagalov IL, Chen KG, Herzmark P, Robey EA. Elimination of self-reactive T cells in the thymus: a timeline for negative selection. Plos Biol. 2013;11:e1001566. doi: 10.1371/journal.pbio.1001566. doi:10.1371/journal.pbio.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. doi:10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. doi:10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. doi:10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. doi:10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009;44:264–277. doi: 10.1080/10409230903193307. doi:10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J. Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 2011;194:257–275. doi: 10.1083/jcb.201012028. doi:10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Través PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagórska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016 doi: 10.1038/nature17630. doi:10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Grabiec AM, Kaur M, Bell TJ, Fujino N, Cook PC, Svedberg FR, MacDonald AS, Maciewicz RA, Singh D, Hussell T. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. 2015;8:1021–1030. doi: 10.1038/mi.2014.129. doi:10.1038/mi.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. The FASEB Journal. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. doi:10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan MD, Greenlee-Wacker MC, Bohlson SS. C1q and phagocytosis: the perfect complement to a good meal. Journal of Leukocyte Biology. 2012 doi: 10.1189/jlb.0212099. doi:10.1189/jlb.0212099. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. doi:10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Garrod KR, Moreau HD, Garcia Z, Lemaître F, Bouvier I, Albert ML, Bousso P. Dissecting T Cell Contraction In Vivo Using a Genetically Encoded Reporter of Apoptosis. Cell Rep. 2012 doi: 10.1016/j.celrep.2012.10.015. doi:10.1016/j.celrep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013 doi: 10.1182/blood-2013-01-478206. doi:10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua W-J, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology. 2012:1–13. doi: 10.1038/ni.2419. doi:10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4:424–436. doi: 10.1159/000337007. doi:10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016 doi: 10.1038/cdd.2015.172. doi:10.1038/cdd.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J. Pathol. 2010;223:178–195. doi: 10.1002/path.2792. doi:10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J. Biol. Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. doi:10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. doi:10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. The FASEB Journal. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. doi:10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, van de Laar L. A Hitchhiker's Guide to Myeloid Cell Subsets: Practical Implementation of a Novel Mononuclear Phagocyte Classification System. Front Immunol. 2015;6:406. doi: 10.3389/fimmu.2015.00406. doi:10.3389/fimmu.2015.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, Schedl T, Qin Y, Van Aelst L, Hengartner MO, Ravichandran KS. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Gül N, Babes L, Siegmund K, Korthouwer R, Bögels M, Braster R, Vidarsson G, Hagen ten, T.L.M., Kubes P, van Egmond M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. Journal of Clinical Investigation. 2014;124:812–823. doi: 10.1172/JCI66776. doi:10.1172/JCI66776DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. doi:10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. doi:10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015;27:149–160. doi: 10.1016/j.smim.2015.03.014. doi:10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. doi:10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor Perspectives in Biology 5. 2013 doi: 10.1101/cshperspect.a008748. doi:10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. doi:10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RD, Kligerman M, Sundt TM, Anderson ND, Shin HS. Stereospecific chemoattraction of lymphoblastic cells by gradients of lysophosphatidylcholine. Proc. Natl. Acad. Sci. U.S.A. 1982;79:3285–3289. doi: 10.1073/pnas.79.10.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T-Y, Wu Y-C. Engulfment of Apoptotic Cells in C. elegans Is Mediated by Integrin α/SRC Signaling. Current Biology. 2010;20:477–486. doi: 10.1016/j.cub.2010.01.062. doi:10.1016/j.cub.2010.01.062. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MAM, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. doi:10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local Macrophage Proliferation, Rather than Recruitment from the Blood, Is a Signature of TH2 Inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. doi:10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim H-J. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014;16:R77. doi: 10.1186/bcr3694. doi:10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2012 doi: 10.1038/nature11714. doi:10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011;11:201–212. doi: 10.1038/nri2938. doi:10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarowski JH, Feramisco JD, Le LQ, Gu JL, Luoh SW, Simon MI, Witte ON. Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12109–12114. doi: 10.1073/pnas.97.22.12109. doi:10.1073/pnas.97.22.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Musset B, Limberg SH, Renigunta V, Sus R, Dalpke AH, Heeg KM, Robaye B, Hanley PJ. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J. Biol. Chem. 2005;280:32459–32467. doi: 10.1074/jbc.M505301200. doi:10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-J, Alonzo ES, Dorothee G, Pollard JW, Sant'Angelo DB. Selective Depletion of Eosinophils or Neutrophils in Mice Impacts the Efficiency of Apoptotic Cell Clearance in the Thymus. PLoS ONE. 2010;5:e11439. doi: 10.1371/journal.pone.0011439. doi:10.1371/journal.pone.0011439.g010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends in Immunology. 2015;36:547–555. doi: 10.1016/j.it.2015.07.007. doi:10.1016/j.it.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464:778–782. doi: 10.1038/nature08853. doi:10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Journey to the grave: signaling events regulating removal of apoptotic cells. Journal of Cell Science. 2007;120:2143–2149. doi: 10.1242/jcs.03463. doi:10.1242/jcs.03463. [DOI] [PubMed] [Google Scholar]
- Klämbt V, Wohlfeil SA, Schwab L, Hülsdünker J, Ayata K, Apostolova P, Schmitt-Graeff A, Dierbach H, Prinz G, Follo M, Prinz M, Idzko M, Zeiser R. A Novel Function for P2Y2 in Myeloid Recipient-Derived Cells during Graft-versus-Host Disease. The Journal of Immunology. 2015;195:5795–5804. doi: 10.4049/jimmunol.1501357. doi:10.4049/jimmunol.1501357. [DOI] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat. Rev. Immunol. 2014;14:377–391. doi: 10.1038/nri3667. doi:10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. doi:10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. doi:10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schon P, Schwab A, Hanley PJ. Autocrine Purinergic Receptor Signaling Is Essential for Macrophage Chemotaxis. Science Signaling. 2010;3:ra55–ra55. doi: 10.1126/scisignal.2000588. doi:10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, Leach SM, Henson PM, Jakubzick CV. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016 doi: 10.1038/cdd.2016.24. doi:10.1038/cdd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Molecular Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013 doi: 10.1038/nature12175. doi:10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. doi:10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Penberthy KK, Wheeler KM, Juncadella IJ, Vandenabeele P, Lysiak JJ, Ravichandran KS. Boosting Apoptotic Cell Clearance by Colonic Epithelial Cells Attenuates Inflammation In Vivo. Immunity. 2016 doi: 10.1016/j.immuni.2016.02.005. doi:10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J. Cell. Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. doi:10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Keller PJ. Emerging Imaging and Genomic Tools for Developmental Systems Biology. Developmental Cell. 2016;36:597–610. doi: 10.1016/j.devcel.2016.02.016. doi:10.1016/j.devcel.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature Cell Biology. 2011;13:1–9. doi: 10.1038/ncb2299. doi:10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, Liu Y, Liu Y, Jiang M, Zhang Z, Wu Y. Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity. 2016;44:287–302. doi: 10.1016/j.immuni.2016.01.002. doi:10.1016/j.immuni.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013a;38:729–741. doi: 10.1016/j.immuni.2013.03.003. doi:10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ma Y, Adjemian S, Yang H, Catani JPP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, Galluzzi L, Zitvogel L, Kroemer G. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. oncoimmunology. 2013b;2:e24568. doi: 10.4161/onci.24568. doi:10.4161/onci.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. doi:10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009:1–5. doi: 10.1038/nature08242. doi:10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattocks M, Tropepe V. Waste management and adult neurogenesis. Cell Stem Cell. 2010;7:421–422. doi: 10.1016/j.stem.2010.09.008. doi:10.1016/j.stem.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Mazaheri F, Breus O, Durdu S, Haas P, Wittbrodt J, Gilmour D, Peri F. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat Commun. 2014;5:4046. doi: 10.1038/ncomms5046. doi:10.1038/ncomms5046. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. doi:10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Medina CB, Ravichandran KS. Do not let death do us part: “find-me” signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016 doi: 10.1038/cdd.2016.13. doi:10.1038/cdd.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metschnikoff E. Lecture on Phagocytosis and Immunity. Br Med J. 1891;1:213–217. doi: 10.1136/bmj.1.1570.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. doi:10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, van Rooijen N, Bousso P. The mechanism of anti-CD20–mediated B cell depletion revealed by intravital imaging. Journal of Clinical Investigation. 2013;123:5098–5103. doi: 10.1172/JCI70972. doi:10.1172/JCI70972DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. doi:10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz LE, Berens C, Lauber K, Gaipl US, Herrmann M. Apoptotic cell clearance and its role in the origin and resolution of chronic inflammation. Front Immunol. 2015;6:139. doi: 10.3389/fimmu.2015.00139. doi:10.3389/fimmu.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, MIZUNO K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the Clearance of Dead Cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. doi:10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol. Pharm. Bull. 2004;27:13–16. doi: 10.1248/bpb.27.13. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Kitano M, Matsuda M, Nagata S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proceedings of the National Academy of Sciences. 2008;105:9198–9203. doi: 10.1073/pnas.0803677105. doi:10.1073/pnas.0803677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. doi:10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- Newson J, Stables M, Karra E, Arce-Vargas F, Quezada S, Motwani M, Mack M, Yona S, Audzevich T, Gilroy DW. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood. 2014;124:1748–1764. doi: 10.1182/blood-2014-03-562710. doi:10.1182/blood-2014-03-562710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi C, Toda S, Segawa K, Nagata S. Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Molecular and Cellular Biology. 2014;34:1512–1520. doi: 10.1128/MCB.01394-13. doi:10.1128/MCB.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori H, Shiratsuchi T, Urano T, Kimura Y, Kiyono K, Tatsumi K, Yoshida S, Ono M, Kuwano M, Nakamura Y, Tokino T. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene. 1997;15:2145–2150. doi: 10.1038/sj.onc.1201542. [DOI] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. doi:10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nature Immunology. 2015;17:9–17. doi: 10.1038/ni.3320. doi:10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. doi:10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia. 2000;30:92–104. [PubMed] [Google Scholar]
- Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun Y-M, Lambert K, Acharya M, Billroth-Maclurg AC, Rosenberg AF, Topham DJ, Yagita H, Kim M, Lacy-Hulbert A, Meier-Schellersheim M, Fowell DJ. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin αV. Nature Immunology. 2013;14:949–958. doi: 10.1038/ni.2682. doi:10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, Collins S, Lysiak JJ, Hoehn KL, Ravichandran KS. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011:1–7. doi: 10.1038/nature10340. doi:10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 2009;19:346–351. doi: 10.1016/j.cub.2009.01.042. doi:10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont A-C, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. doi:10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2015;269:44–59. doi: 10.1111/imr.12376. doi:10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nature Immunology. 2015;17:2–8. doi: 10.1038/ni.3341. doi:10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature Cell Biology. 2006;440:1069–1072. doi: 10.1038/nature04665. doi:10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–991. doi: 10.1016/j.cell.2011.11.004. doi:10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IKH, Chiu Y-H, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014a;507:329–334. doi: 10.1038/nature13147. doi:10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014b doi: 10.1038/nri3607. doi:10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 Is Required for ATP Release during Apoptosis but Not for Inflammasome Activation. The Journal of Immunology. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. doi:10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 2007;7:964–974. doi: 10.1038/nri2214. doi:10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Rosas M, Davies LC, Giles PJ, Liao C-T, Kharfan B, Stone TC, O'Donnell VB, Fraser DJ, Jones SA, Taylor PR. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. doi:10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. doi:10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. doi:10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saas P, Kaminski S, Perruche S. Prospects of apoptotic cell-based therapies for transplantation and inflammatory diseases. Immunotherapy. 2013;5:1055–1073. doi: 10.2217/imt.13.103. doi:10.2217/imt.13.103. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. doi:10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. Journal of Clinical Investigation. 1992;90:1513–1522. doi: 10.1172/JCI116019. doi:10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam D, Bagshaw RD, Freeman SA, Collins RF, Pawson T, Fairn GD, Grinstein S. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun. 2015;6:8623. doi: 10.1038/ncomms9623. doi:10.1038/ncomms9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. doi:10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. doi:10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- Segawa K, Nagata S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2015.08.003. doi:10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1114799108. doi:10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. doi:10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Romero F, Stafstrom W, Duong M, Summer R. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. AJP: Lung Cellular and Molecular Physiology. 2014;306:L152–61. doi: 10.1152/ajplung.00229.2013. doi:10.1152/ajplung.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J. Biol. Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. doi:10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JC, Young C, Hicks D, Owens P, Williams A, Vaught DB, Morrison MM, Lim J, Williams M, Brantley-Sieders DM, Balko JM, Tonetti D, Earp HS, Cook RS. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. Journal of Clinical Investigation. 2014;124:4737–4752. doi: 10.1172/JCI76375. doi:10.1172/JCI76375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson C, la Rosa, de G, Anderson CS, Murphy PS, Capece T, Kim M, Elliott MR. Essential role of elmo1 in dock2-dependent lymphocyte migration. The Journal of Immunology. 2014;192:6062–6070. doi: 10.4049/jimmunol.1303348. doi:10.4049/jimmunol.1303348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaga H, Matsui H, Ueno M, Maeno T, Iso T, Syamsunarno MRAA, Anjo S, Matsuzaka T, Shimano H, Yokoyama T, Kurabayashi M. Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nat Commun. 2013;4:2563. doi: 10.1038/ncomms3563. doi:10.1038/ncomms3563. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo J-A, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. doi:10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. doi:10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–406. doi: 10.1126/science.1236758. doi:10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Imanishi E, Nagata S. Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.583419. doi:10.1074/jbc.M114.583419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke R, Hilgendorf I, Garner H, Waterborg C, Park K, Nowyhed H, Hanna RN, Wu R, Swirski FK, Geissmann F, Hedrick CC. The transcription factor NR4A1 is essential for the development of a novel macrophage subset in the thymus. Sci Rep. 2015;5:10055. doi: 10.1038/srep10055. doi:10.1038/srep10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber AI. Metchnikoff and the phagocytosis theory. Nature reviews. Molecular cell biology. 2003 doi: 10.1038/nrm1244. doi:10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Molecular and Cellular Biology. 2012;32:118–125. doi: 10.1128/MCB.05993-11. doi:10.1128/MCB.05993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torr EE, Gardner DH, Thomas L, Goodall DM, Bielemeier A, Willetts R, Griffiths HR, Marshall LJ, Devitt A. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death and Differentiation. 2011:1–9. doi: 10.1038/cdd.2011.167. doi:10.1038/cdd.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosello-Trampont AC. Engulfment of Apoptotic Cells Is Negatively Regulated by Rho-mediated Signaling. Journal of Biological Chemistry. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. doi:10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. doi:10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- Ucker DS, Jain MR, Pattabiraman G, Palasiewicz K, Birge RB, Li H. Externalized glycolytic enzymes are novel, conserved, and early biomarkers of apoptosis. Journal of Biological Chemistry. 2012;287:10325–10343. doi: 10.1074/jbc.M111.314971. doi:10.1074/jbc.M111.314971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, Sarter K, Frey B, Rothe T, Voll R, Nimmerjahn F, Bochkov VN, Schett G, Krönke G. 12/15-Lipoxygenase Orchestrates the Clearance of Apoptotic Cells and Maintains Immunologic Tolerance. Immunity. 2012 doi: 10.1016/j.immuni.2012.03.010. doi:10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, Guilliams M. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity. 2016;44:755–768. doi: 10.1016/j.immuni.2016.02.017. doi:10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Kokel D, Peterson RT. Apoptotic Cells Are Cleared by Directional Migration and elmo1- Dependent Macrophage Engulfment. Curr. Biol. 2012 doi: 10.1016/j.cub.2012.03.027. doi:10.1016/j.cub.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 2009;9:845–857. doi: 10.1038/nri2637. doi:10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016 doi: 10.1016/j.cell.2016.03.009. doi:10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Weigert A, Cremer S, Schmidt MV, Knethen, von A, Angioni C, Geisslinger G, Brune B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–3540. doi: 10.1182/blood-2009-10-243444. doi:10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- Weigert A, Johann AM, Knethen, von A, Schmidt H, Geisslinger G, Brüne B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. doi:10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. doi:10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Volkmer J-P, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras- Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua M-S, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PØ, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NNH, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U.S.A. 2012 doi: 10.1073/pnas.1121623109. doi:10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, Miura M. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J. Cell Biol. 2011;195:1047–1060. doi: 10.1083/jcb.201104057. doi:10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao L-L, Ichimura T, Kuchroo V, Bonventre JV. KIM-1–mediated phagocytosis reduces acute injury to the kidney. Journal of Clinical Investigation. 2015;125:1620–1636. doi: 10.1172/JCI75417. doi:10.1172/JCI75417DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]