Abstract
Objectives
This study aimed to estimate rates of cancer or opportunistic infection in patients with psoriatic arthritis (PsA) compared with patients without PsA.
Methods
Using the Clinical Practice Research Datalink, we conducted a cohort study of patients with a PsA diagnosis and patients without such diagnosis, matched on age, sex, general practice, and calendar time, to assess the incidence of cancers (solid, hematologic, and nonmelanoma skin cancer) and opportunistic infections. We estimated incidence rates (IRs) and IR ratios (IRRs) with 95% confidence intervals (CIs) for each outcome and stratified results in the PsA cohort by receipt of systemic PsA drugs.
Results
The rate of hematologic cancer was slightly higher in the PsA cohort compared with the non-PsA cohort (IRR, 1.52; 95% CI, 1.10–2.10), whereas the rates of solid cancer and of nonmelanoma skin cancer were similar between the PsA and non-PsA cohorts (IRR, 1.01; 95% CI, 0.90–1.13; and IRR, 0.97; 95% CI, 0.82–1.14, respectively). Incidence rates were higher for PsA patients who received prescriptions for PsA drugs compared with those who did not. The IRs for infection were higher in the PsA compared with the non-PsA cohort (IRR, 1.39; 95% CI, 1.31–1.47) and were significantly higher in patients who received prescriptions (IRR, 1.71; 95% CI, 1.52–1.91).
Conclusions
The rates of solid and nonmelanoma skin cancers were similar in patients with PsA compared with patients without PsA, but the rates of hematologic cancer and opportunistic infections were higher in patients with PsA. In patients with PsA, rates of all outcomes were higher among those who received prescriptions for systemic PsA therapy.
Key Words: cancer, malignancy, opportunistic infection, psoriatic arthritis
Increased risks for malignancy and infections in patients with rheumatoid arthritis and psoriasis have been reported extensively in the literature. However, less is known regarding the frequency of malignancies or infections in patients with psoriatic arthritis (PsA).
There are a limited number of published studies that report rates of malignancy in patients with PsA to date. These studies report similar rates of cancer in patients with PsA compared with the general population1 or compared with patients with rheumatoid arthritis.2,3 One study reported that the risk of lymphoma in patients with PsA who used anti–tumor necrosis factor (anti-TNF) drugs was not elevated in comparison with patients with rheumatoid arthritis, but not much is known about the risk of cancer with the use of other PsA treatments.2
The rate of opportunistic infections in the PsA population is unknown, but several published studies have estimated the rate of infections in patients with psoriasis or rheumatoid arthritis. A recent analysis of a US health care database reported serious hospital infection rates in patients with psoriasis ranging from 165/10,000 person-years (PYs) for patients receiving phototherapy to 262/10,000 PYs for patients receiving anti-TNF.4 A biological therapy registry in France reported 45 events of opportunistic infection among 43 patients receiving anti-TNF for several indications, including PsA,5 of which, 40% of the infections were viral, 33% were bacterial, 22% were fungal, and 4% were parasitic. Ten patients (23%) were admitted to intensive care units, and 4 patients (9%) died. A retrospective analysis of treated patients with rheumatoid arthritis and spondyloarthopathy (which included PsA) at one medical center in Rome found a high proportion of infection (52%) with incidence rates (IRs) of 36.3/100 PYs, which varied depending on the treatment received, from 12.4/100 person-years for patients receiving disease-modifying antirheumatic drugs (DMARDs) to 62.7/100 PYs for patients receiving anti-TNF, with a mean incidence of 36.3/100 PYs.6
The objective of this study was to estimate the rate of solid, hematologic, and nonmelanoma skin cancers and of opportunistic infections among patients with PsA compared with matched patients without PsA or psoriasis using the UK Clinical Practice Research Datalink (CPRD). For patients with PsA, we also estimated rates of these diseases by type of systemic PsA drugs received.
METHODS
Data Source
The UK CPRD is a large, longitudinal, population-based electronic medical record database that contains data on approximately 10 million people. The UK National Health Service provides universal coverage; therefore, the database contains a representative sample of the UK general population.7 Participating general practitioners (GPs) contribute data in an anonymous format including medical diagnoses, physical findings, symptoms, details of hospital stays and specialist visits, and deaths. Several validation studies have been published on the accuracy of information recorded in the CPRD,8–10 which indicate that the data are of high accuracy with regard to recorded clinical diagnoses with more than 90% of information from the manual medical records present in the GP's office recorded on the computer.
Study Design and Cohort Identification
This cohort study was conducted using the CPRD data from January 1, 1988, through December 31, 2012. The PsA cohort included all patients in the CPRD with a diagnosis of PsA who had at least 1 year of medical information in their record before the first PsA diagnosis code. One year of history was required to ensure that we identified patients with new diagnoses of PsA that were not diagnosed before the start of the patient record. The cohort entry date was the date of the first recorded PsA diagnosis. For each PsA patient, we identified up to 10 patients with no recorded diagnoses of psoriasis or PsA (henceforth referred to as the non-PsA cohort) matched on age, sex, and general practice attended. All patients without PsA were required to be present in the database on the cohort entry date of their matched patient with PsA, and the cohort entry date of each patient with PsA was assigned to the matched patient without PsA.
Separate subcohorts were identified for each outcome of interest (ie, solid and hematologic cancer, nonmelanoma skin cancer, and opportunistic infections). For solid and hematologic cancers, any patient who had a diagnosis of a solid or hematologic cancer before the cohort entry date was excluded from that subcohort. We did not exclude any patients from the nonmelanoma skin cancer subcohort or patients from the opportunistic infection subcohort because previous diagnoses were not thought to influence development of these outcomes at a later date. In each subcohort, patient follow-up began at the cohort entry date and continued until the end of the study period, end of registration with the practice, death, or until they developed the outcome of interest. The accumulated time was expressed as PYs either exposed to PsA or not exposed.
Case Identification
Diagnoses of interest were identified using automated searches of the electronic records of all patients in each subcohort. For each outcome included in the study, we reviewed samples of electronic medical records to review the accuracy of our case selection process. A patient was identified as a case if he or she had a diagnosis of the outcome of interest (solid, hematologic, nonmelanoma skin cancer or opportunistic infection) recorded after the cohort entry date. If a patient had more than one diagnosis of the outcome of interest under study, we included the first to occur. Opportunistic infections under evaluation were pneumocystis pneumonia, cryptospodiosis, tuberculosis, diseases caused by mycobacteria, bartonellosis, leukoencephalopathy, candidiasis, cryptococcis, other mycoses, cytomegaloviral disease, herpes simplex, herpes zoster, human papilloma virus, viral hepatitis, Epstein-Barr virus, histoplasmosis, or toxoplasmosis.
Stratification by Receipt of PsA Systemic Therapy
Among those with PsA, we further evaluated the effects of having treated versus untreated PsA. For each patient in the PsA cohort, we accumulated person time on any PsA systemic therapy and for nonexposed time. Systemic therapies included DMARDs/biologics (eg, methotrexate, sulfasalazine, and adalimumab), immunosuppressants (eg, azathioprine and leflunomide), and corticosteroids (codes are available upon request). For the analysis of the cancer outcome, person time of exposure to PsA therapy was accrued from the date of the first prescription through the end of follow-up or until the date of the cancer diagnosis minus 1 year (the induction period) for cancer cases. People who received only 1 prescription were considered unexposed. We conducted a sensitivity analysis to assess the appropriateness of this induction period definition by expanding the induction period to 2 years before the cancer diagnosis date. Analyses were further stratified by type of PsA therapy prescribed. A patient could be exposed to more than 1 type of therapy.
For the analysis of the opportunistic infection outcome, person time of exposure to these drugs was accrued starting at the date of the prescription for a PsA therapy through the end of the supply (based on the amount prescribed divided by the number of times per day) plus 30 days. Time during follow-up was treated as not exposed to systemic therapy when no drug prescriptions were issued. To assess the appropriateness of this exposure time window definition, we conducted sensitivity analyses where the period after the end of supply was extended to 90 days. Analyses were further stratified by type of PsA therapy prescribed. A patient contributed person time to 1 type of therapy until the end of supply plus 30 days for each therapy separately, and a patient could be exposed to more than 1 type of therapy at a time.
Statistical Analysis
Within each subcohort, we estimated IRs per 1000 PYs and IR ratios (IRRs) with 95% confidence intervals (CIs) for PsA compared with non-PsA cohorts and stratified by sex and age (<30, 30–49, 50–69, ≥70 years). We estimated the risk (cumulative hazard function) of each study outcome using the Kaplan-Meier method and tested the differences using log rank test. We stratified the rates of nonmelanoma skin cancer by whether patients had a previous nonmelanoma skin cancer diagnosis and, separately, if they had a diagnosis of solid or hematologic tumor before cohort entry. In the PsA cohort, the results were stratified by receipt or nonreceipt of prescriptions for systemic PsA therapy and by the type of drug prescribed. Statistical analyses were performed using SAS release 9.2 (SAS Institute Inc, Cary NC).
The protocol for this study was reviewed and approved by the Independent Scientific Advisory Committee of the CPRD.
RESULTS
Solid and Hematologic Cancers
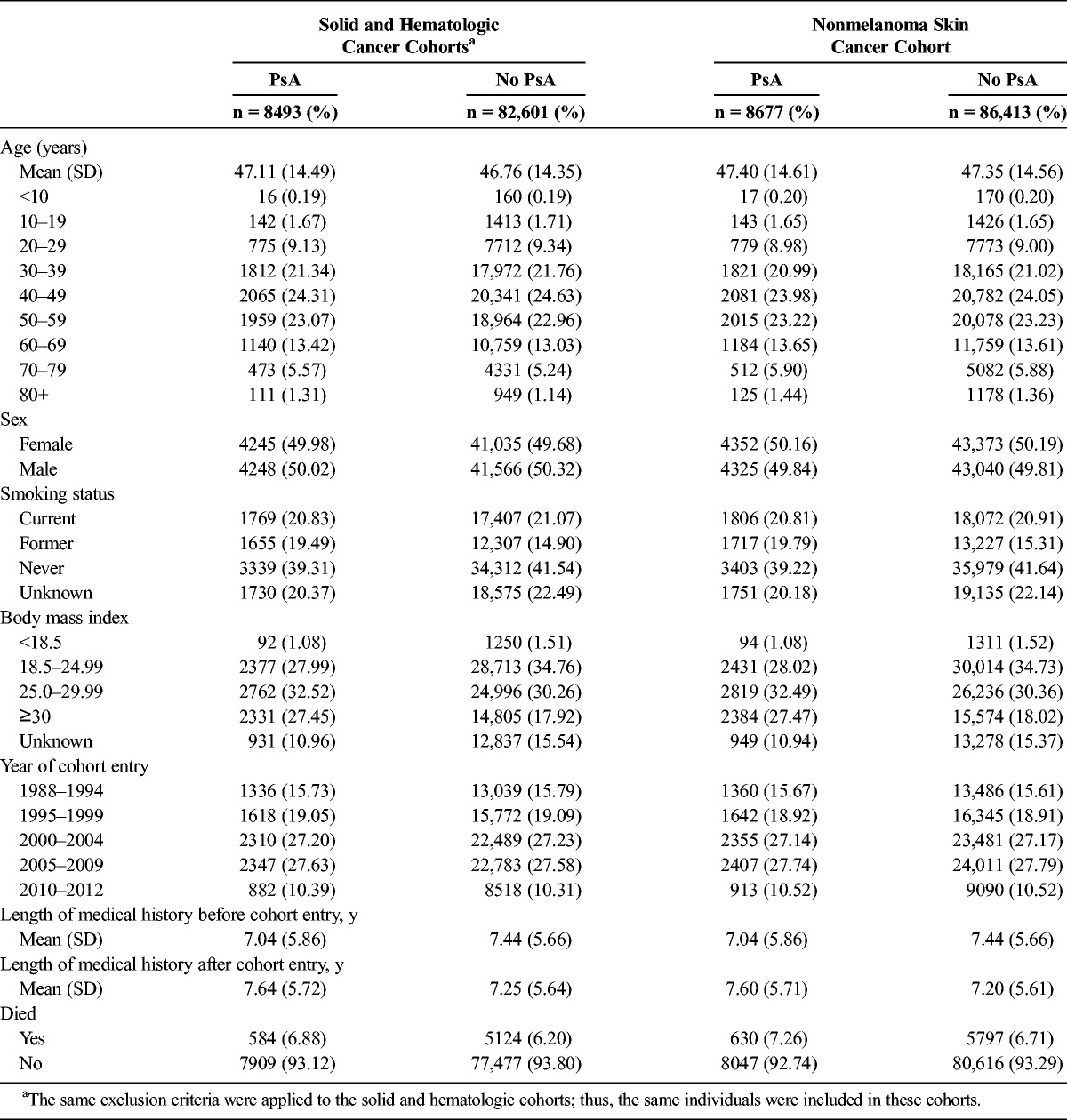
We identified 8493 patients with PsA and 82,601 patients without PsA in the solid and hematologic cancer subcohorts after exclusions. These two cohorts are composed of the same individuals because in both we excluded any person with a diagnosis of solid or hematologic cancer before cohort entry. Their characteristics are presented in Table 1.
TABLE 1.
Distribution of Characteristics of Patients With and Without PsA: Cancer Subcohorts
The rate of incident solid cancer was similar between the PsA cohort and the non-PsA cohort (5.4/1000 PYs [95% CI, 4.8–6.0] vs 5.3/1000 PYs [95% CI, 5.2–5.5]: IRR, 1.01 [95% CI, 0.90–1.13]) (Table 2). Rates were lowest for the youngest age group (<30) and increased with increasing age in both PsA and non-PsA cohorts. The analysis of cumulative hazards demonstrated that there was no statistically significant difference between the PsA and non-PsA cohorts in the solid cancer analysis (P > 0.9) (Fig. 1).
TABLE 2.
Incident Rates of Cancers in Patients With and Without PsA
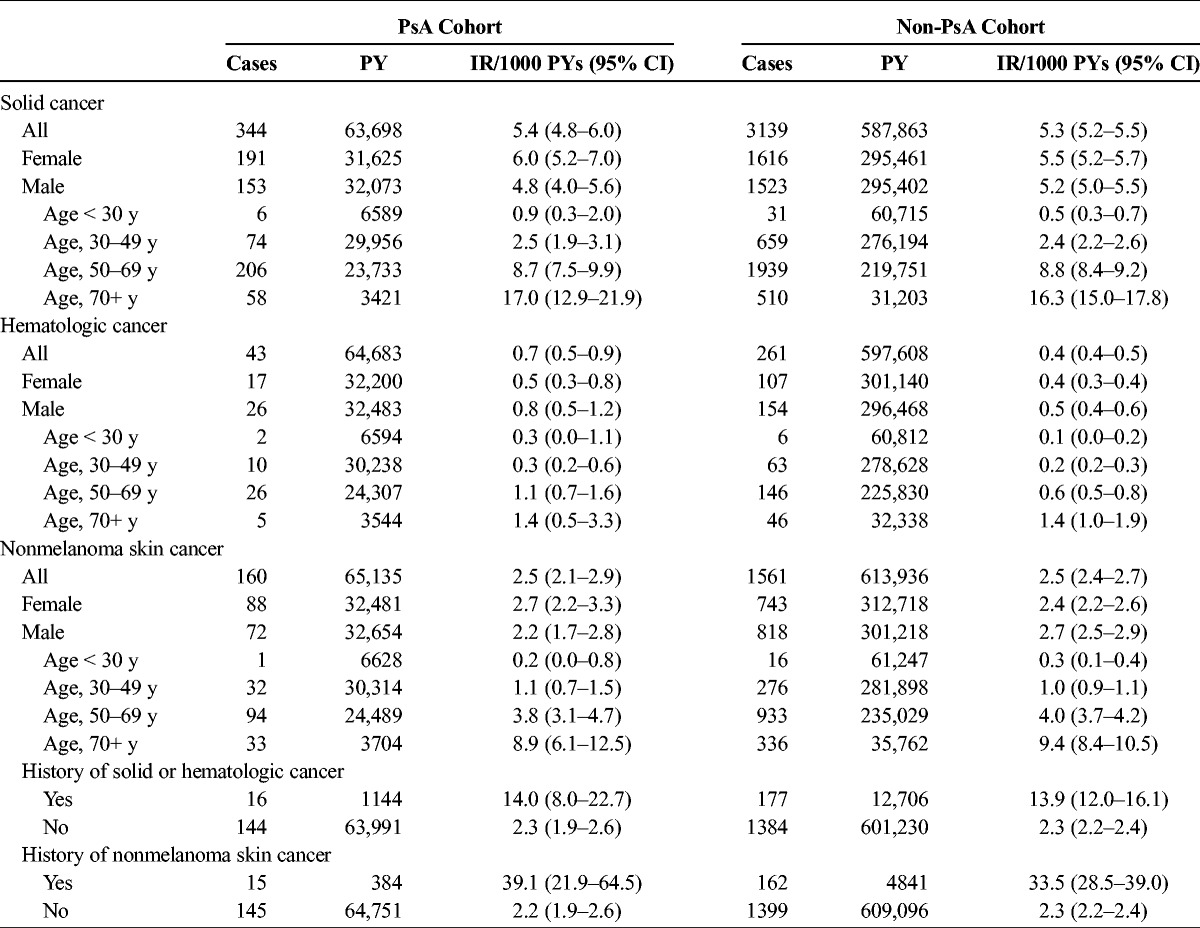
FIGURE 1.
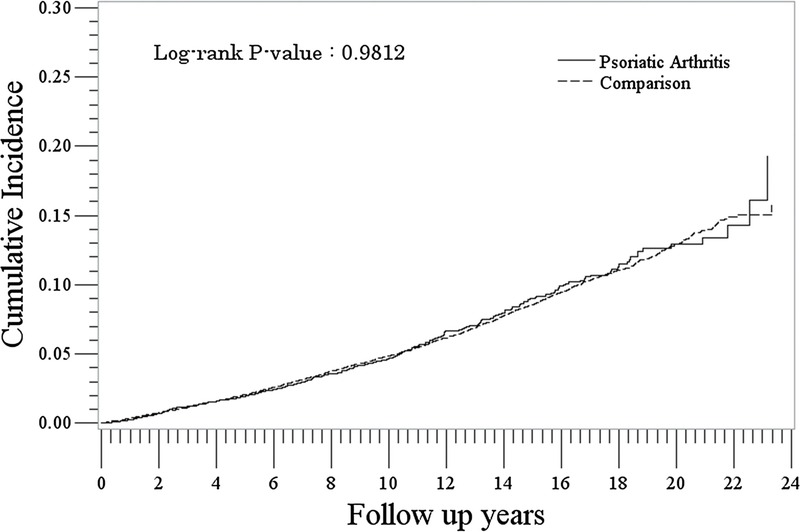
Cumulative incidence of solid cancer in the PsA and comparison cohorts.
The rate of incident hematologic cancer was slightly higher in the PsA cohort compared with the non-PsA cohort (0.7/1000 PYs [95% CI, 0.5–0.9] vs 0.4/1000 PYs [95% CI, 0.4–0.5]: IRR, 1.52 [95% CI, 1.10–2.10]) (Table 2). Rates were lowest for the youngest age group (<30) and increased with increasing age in both PsA and non-PsA cohorts. The difference in risk/hazard was very small but significant in the hematologic cancer analysis of cumulative hazards (P = 0.011) (Fig. 2).
FIGURE 2.
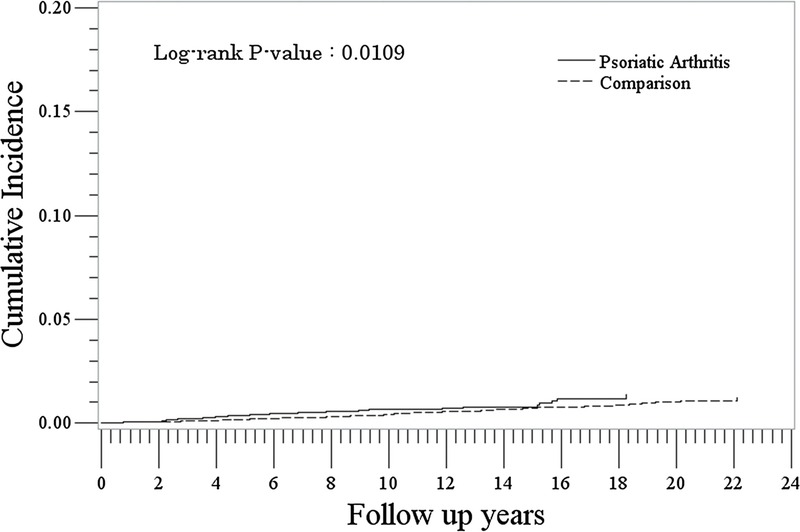
Cumulative incidence of hematologic cancer in the PsA and comparison cohorts.
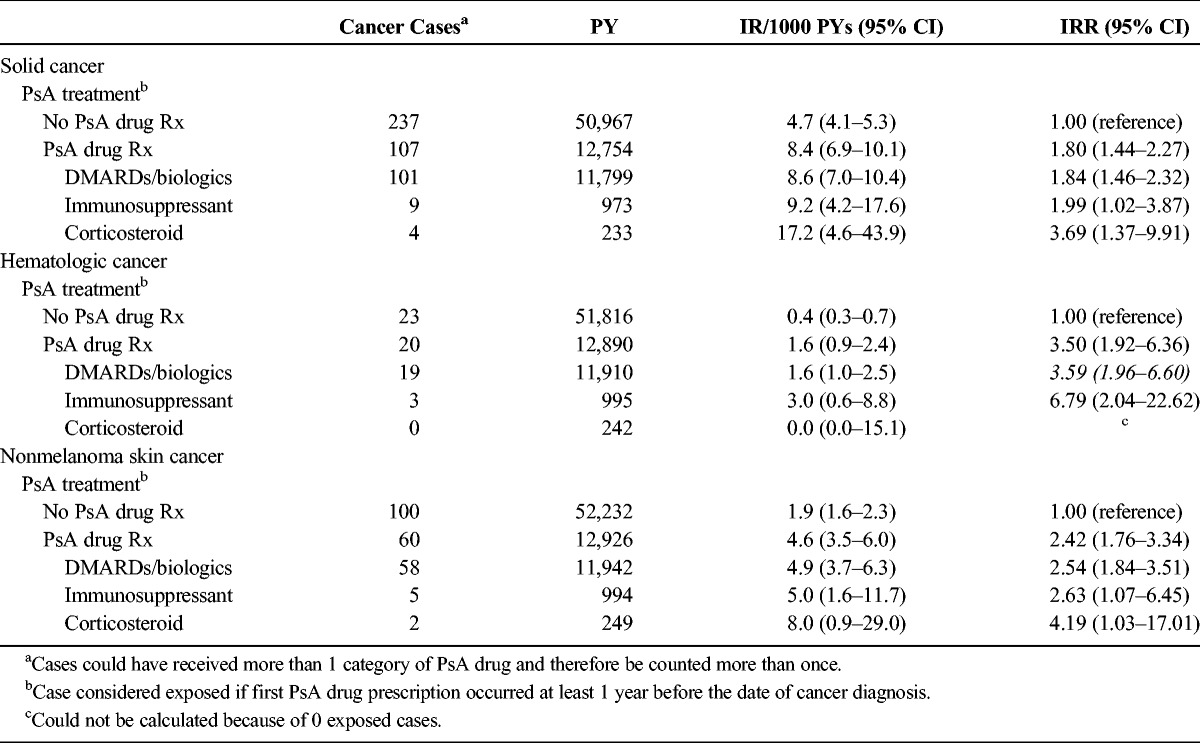
In the PsA cohort, rates of incident solid cancer were higher in patients who received prescriptions for PsA drugs compared with those who did not (IRs, 8.4/1000 PYs [95% CI, 6.9–10.1] and 4.7/1000 PYs [95% CI, 4.1–5.3], respectively: IRR, 1.80 [95% CI, 1.44–2.27]) (Table 3). Similarly, rates of incident hematologic cancer were higher in patients with PsA who received prescriptions for PsA drugs compared with those who did not (IRs, 1.6 [95% CI, 0.9–2.4] and 0.4 [95% CI, 0.3–0.7], respectively; IRR, 3.50 [95% CI, 1.92–6.36]) (Table 3). For both solid and hematologic cancer, patients who were prescribed DMARDs/biologics had the lowest rates of these cancers. The results did not differ when we expanded the induction period to 2 years before the cancer diagnosis in the sensitivity analyses.
TABLE 3.
Rates of Solid, Hematologic, and Nonmelanoma Skin Cancer in Patients With PsA, Stratified by Receipt of PsA Drug Prescriptions
Nonmelanoma Skin Cancer
We identified 8677 patients with PsA and 86,413 patients without PsA in the nonmelanoma skin cancer subcohort. Their characteristics are presented in Table 1. The rate of nonmelanoma skin cancer was similar between the PsA cohort and non-PsA cohort ([2.5/1000 PYs [95% CI, 2.1–2.9] vs 2.5/1000 PYs [95% CI, 2.4–2.7]: IRR, 0.97 [95% CI, 0.82–1.14]) (Table 2). Rates were lowest for the youngest age group (<30) and increased with increasing age in both PsA and non-PsA cohorts. Rates of nonmelanoma skin cancer were highest among patients with a history of solid or hematologic cancer or a history of previous nonmelanoma skin cancer; however, these rates were not significantly different between the PsA and non-PsA cohorts. The analysis of cumulative hazards demonstrated that there was no statistically significant difference between the PsA and non-PsA cohorts in the nonmelanoma skin cancer analysis (P = 0.61) (Fig. 3).
FIGURE 3.
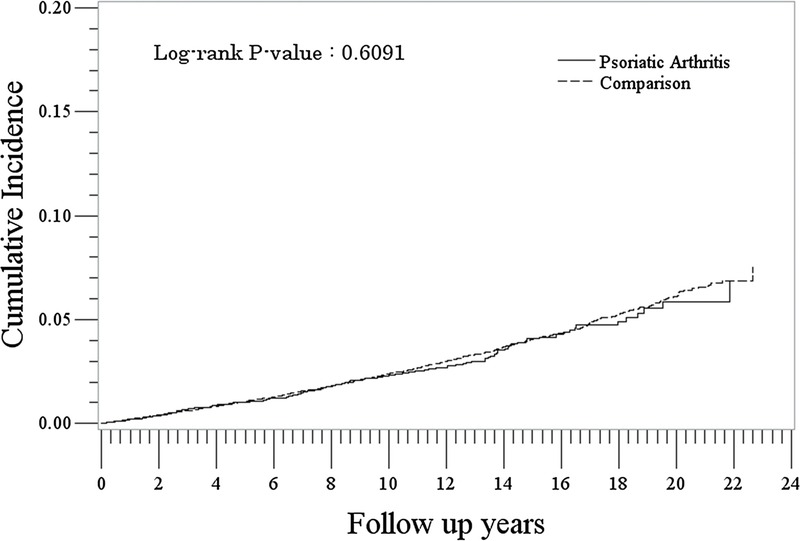
Cumulative incidence of nonmelanoma skin cancer in the PsA and comparison cohorts.
In the PsA cohort, rates of nonmelanoma skin cancer were higher in patients who received prescriptions for PsA drugs compared with those who did not (IRs, 4.6/1000 PYs [95% CI, 3.5–6.0] and 1.9/1000 PYs [95% CI, 1.6–2.3], respectively: IRR, 2.42 [95% CI, 1.76–3.34]) (Table 3). Rates were highest among those treated with corticosteroids (8.0/1000 PYs [95% CI, 0.9–29.0]) and similar among patients who were prescribed DMARDs/biologics or immunosuppressants (4.9/1000 PYs [95% CI, 3.7–6.3] and 5.0/1000 PYs [95% CI, 1.6–11.7]). The results did not differ when we expanded the induction period to 2 years before the cancer diagnosis in the sensitivity analyses.
Opportunistic Infections
We identified 8677 patients with PsA and 86,413 patients without PsA in the opportunistic infection subcohort. Their characteristics are presented in Table 4. The rate of infection was higher in the PsA cohort compared with the non-PsA cohort (24.6/1000 PYs [95% CI, 23.3–25.9] vs 17.7/1000 PYs [95% CI, 17.1–18.1]: IRR, 1.39 [95% CI, 1.31–1.47]) (Table 5). In both PsA and non-PsA cohorts, rates were more than double for females compared with males and highest in the youngest and oldest age categories. Rates of infection were consistently higher in the PsA cohort compared with the non-PsA cohort at each time point after cohort entry (P < 0.0001) (Fig. 4). Most infections were cryptospodiosis, cryptococcosis, or other mycoses.
TABLE 4.
Distribution of Characteristics of Patients With and Without PsA—Opportunistic Infection Subcohort
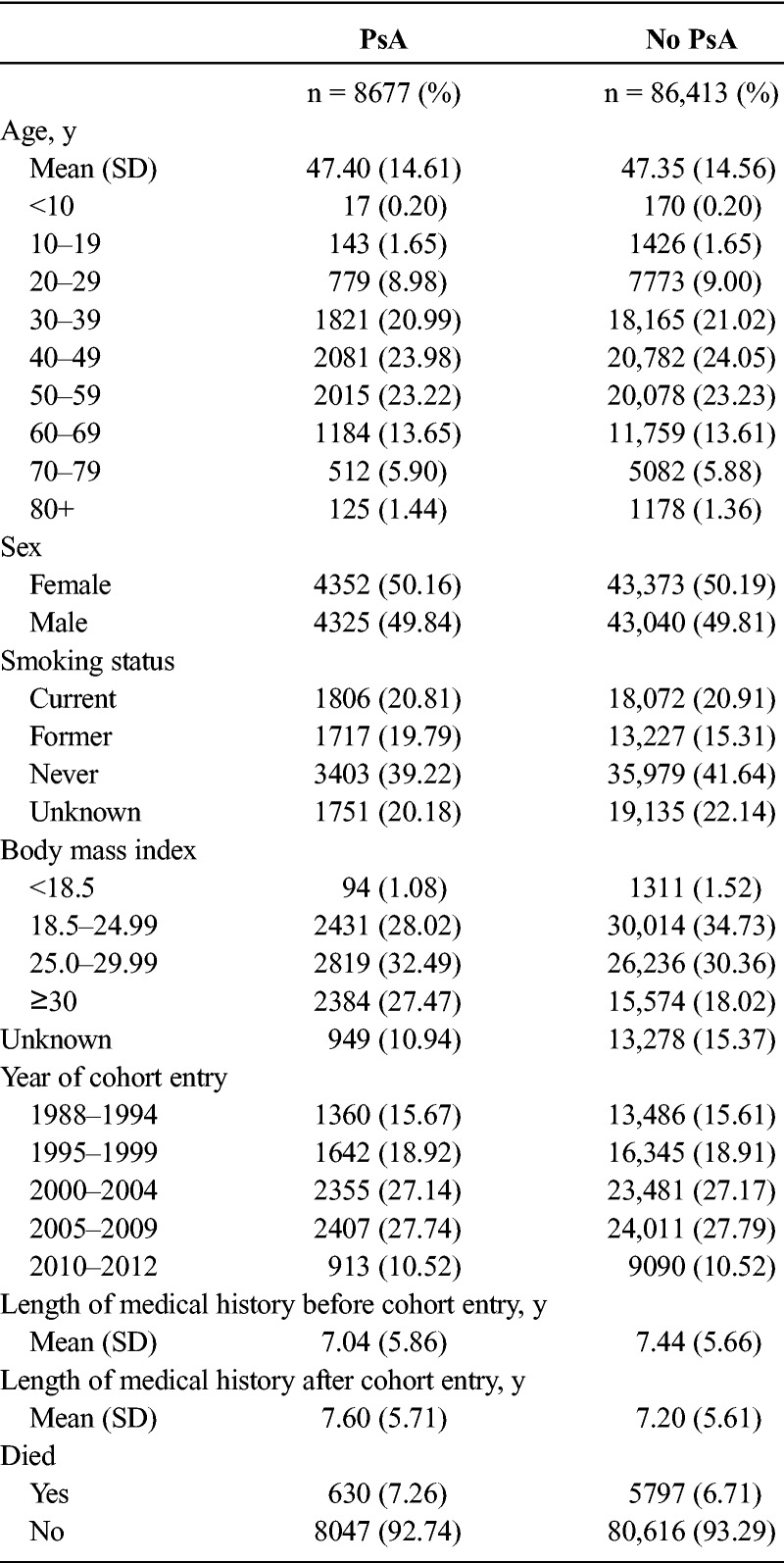
TABLE 5.
Rates of Incident Opportunistic Infection in Patients With and Without PsA
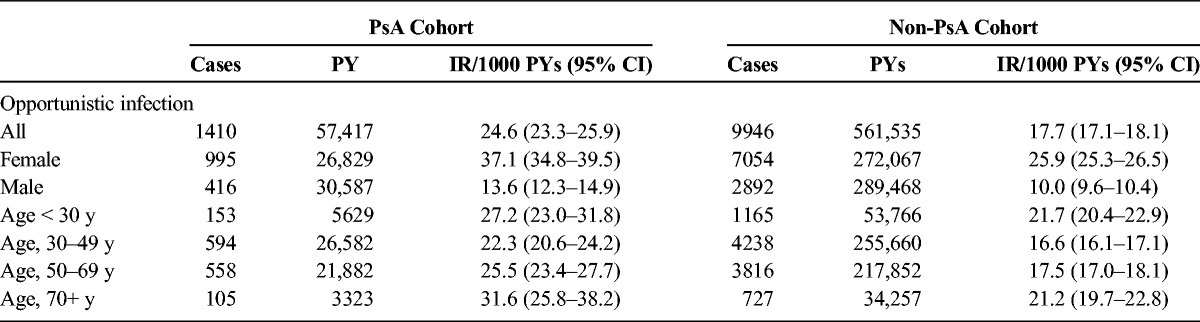
FIGURE 4.
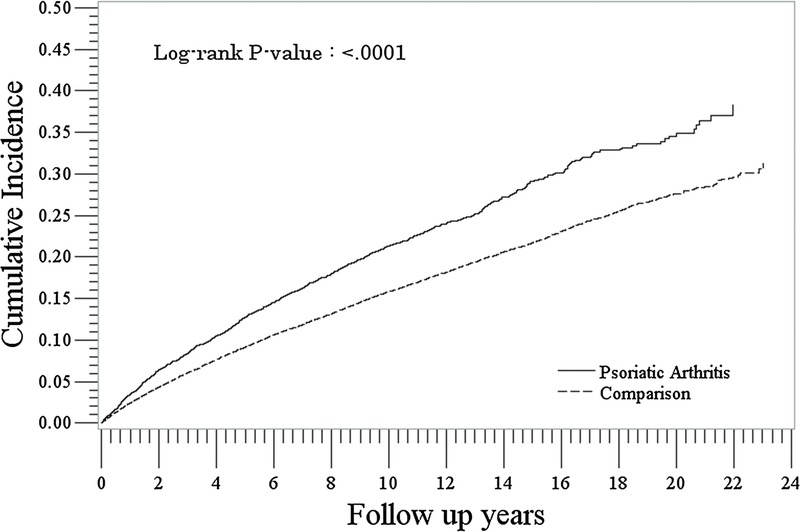
Cumulative incidence of opportunistic infections in the PsA and comparison cohorts.
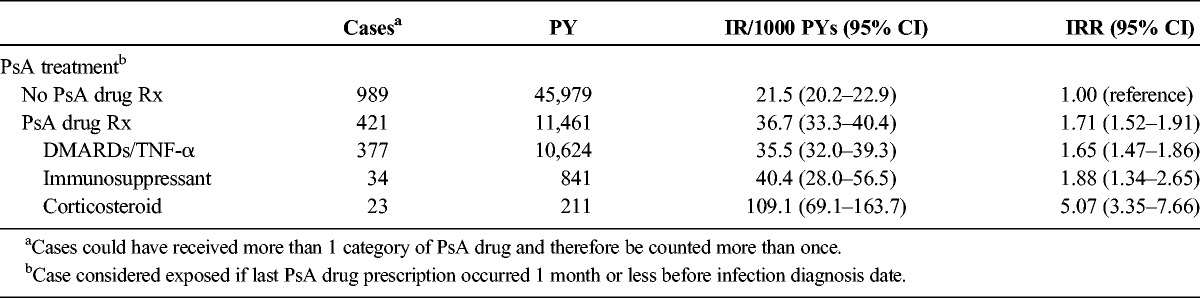
In the PsA cohort, rates of opportunistic infection were significantly higher in patients who received prescriptions for PsA drugs compared with those who did not (IRs, 36.1/1000 PYs [95% CI, 33.3–40.4] and 21.5/1000 PYs [95% CI, 20.2–22.9], respectively: IRR, 1.71 [95% CI, 1.52–1.91]) (Table 6). Rates for users of DMARDs/biologics were similar to the rates in users of immunosuppressants, but the rate was higher in current users of corticosteroids. The results did not differ when we redefined the exposure window to 90 days in the sensitivity analyses.
TABLE 6.
Rates of Opportunistic Infection in Patients With PsA, Stratified by Receipt of PsA Drug Prescriptions
DISCUSSION
Rates of hematologic cancer were higher in patients with PsA compared with patients without PsA, whereas rates of solid cancers and nonmelanoma skin cancers were similar for the 2 groups. Rates of all cancers were higher in patients with PsA who received prescriptions for systemic treatments (DMARDs/biologics, immunosuppressants, and corticosteroids), although the rates did not differ by type of treatment prescribed. These results are similar to other studies. Rohekar et al1 also reported similar rates of all cancer compared with the general population; however, their results suggested more breast cancers (standardized incidence ratio, 1.55; 95% CI, 0.92–2.62) and fewer hematologic cancers (standardized incidence ratio, 0.69; 95% CI, 0.26–1.83) among PsA patients. Similar to our study, a study in Sweden reported a slightly higher hazard ratio (1.2; 95% CI, 0.9–1.7) of lymphoma in PsA patients compared with the general population.2 A recent analysis of a US population reported similar incidence of malignancies in both patients with PsA and patients with rheumatoid arthritis (5.6/1000 PYs).3 Reported IRs of solid, hematologic, and nonmelanoma skin cancers in patients with PsA in this US study (2.8/1000 PYs, 0.7/1000 PYs, and 3.5/1000 PYs, respectively) were similar to those we found in our analysis.
In our study, rates of opportunistic infection were higher in patients with PsA compared with patients without PsA and highest among PsA patients who received prescriptions for systemic therapy. Rates were similar in current users of DMARDs/biologics and immunosuppressants but were higher among current users of corticosteroids. These results suggest that exposure to systemic treatment increases the rate of opportunistic infection, yet untreated patients with PsA also had a higher rate of infection compared with that of patients without PsA (21.5/1000 PYs non-treated PsA, 17.7/1000 PYs non-PsA). Our results are similar to published results of a cohort of patients with psoriasis in an administrative database where hospital infection events ranged from 16.5/1000 PYs in phototherapy exposed to 26.2/1000 PYs for anti–TNF-αs exposed.4 The finding of the highest IRR in the corticosteroid group is similar to the results from Germano et al,6 where the highest IRR for opportunistic infection (3.6; 95% CI, 1.58–8.20) was found among patients treated with DMARDs and corticosteroids compared with DMARDs alone. Combined with our results, this suggests that patients with PsA treated with corticosteroids have the highest risk for opportunistic infection.
Our population-based study had a number of strengths. We used a very large and well-established, validated, longitudinal primary care database, the CPRD, which is known for its high accuracy and completeness of diagnoses. The mean length of follow-up was more than 7 years in both the PsA and non-PsA cohorts for each outcome of interest. By excluding patients who had less than 1 year in their history before the index date, we reduced the risk of including patients with prevalent, rather than incident, PsA.
There were a few potential limitations to consider. First, it is possible that we included some people who did not actually have PsA. Results of validation studies have indicated that the diagnosis of psoriasis and PsA in the CPRD has high positive predictive value.11,12 In addition, we reviewed the electronic medical records of the patients with PsA and assessed the presence of and number of PsA diagnoses, codes for PsA symptoms, and treatments for PsA to provide confidence in the PsA diagnosis. We found that approximately 98% of potential patients with PsA identified had at least one of these supporting codes, so we are confident that the vast majority of people in the PsA cohort did have the disease. To be eligible for inclusion in the PsA cohort, a patient had to have a recorded diagnosis of PsA; thus, if a GP did not record the diagnosis, the patient was not included in the study. Therefore, the results of this study are generalized to patients with diagnosed PsA that comes to the attention of the GP. Second, the CPRD does not contain information on PsA severity or activity. Patients with PsA who receive treatments are likely to have more severe or active disease than patients with PsA who do not; thus, the elevated risks of observed among treated patients with PsA compared with patients with PsA who did not receive treatment may be explained by disease severity or activity. Third, it is possible that some patients with PsA stopped taking systemic PsA therapies sooner than was assumed in our estimation of person time. To assess this, we conducted sensitivity analyses where we extended and shortened the exposure time window, and there were no material differences in the rates of opportunistic infection in these analyses. Finally, in the United Kingdom, biologics are often prescribed by consultants (specialists) and thus may not be captured in the GP record; thus, it is possible that we misclassified some exposed person time as nonexposed. The incomplete capture of exposure to biologics also limits our ability to separate independent effects of biologics from DMARDs.
In summary, we found that the rates of solid and nonmelanoma skin cancer were similar in patients with PsA compared with patients without PsA, but rates of hematologic cancer and opportunistic infections were higher in patients with PsA. In patients with PsA, rates of all outcomes were higher among those who received prescriptions for systemic PsA therapy.
Footnotes
This study was sponsored by Celgene Corporation. Hagberg, Li, and Jick received funding from Celgene Corporation to conduct the study. Peng, Shah, and Paris are all employees of and own stock/have stock options in Celgene Corporation.
REFERENCES
- 1.Rohekar S, Tom BD, Hassa A, et al. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum. 2008;58:82–87. [DOI] [PubMed] [Google Scholar]
- 2.Hellgren K, Smedby KE, Backlin C, et al. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol. 2014;66:1282–1290. [DOI] [PubMed] [Google Scholar]
- 3.Gross RL, Schwartzman-Morris JS, Krathen M, et al. A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheumatol. 2014;66:1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimball AB, Schenfeld J, Accortt NA, et al. Incidence rates of malignancies and hospitalized infectious events in patients with psoriasis with or without treatment and a general population in the U.S.A.: 2005–09. Br J Dermatol. 2014;170:366–373. [DOI] [PubMed] [Google Scholar]
- 5.Salmon-Ceron D, Tubach F, Lortholary O, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–623. [DOI] [PubMed] [Google Scholar]
- 6.Germano V, Cattaruzza MS, Osborn J, et al. Infection risk in rheumatoid arthritis and spondyloarthropathy patients under treatment with DMARDs, corticosteroids and TNF-α antagonists. J Transl Med. 2014;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson DH, Sherman V, Hollowell J. The General Practice Research Database. Scientific and Ethical Advisory Group. Q J Med. 1998;91:445–452. [DOI] [PubMed] [Google Scholar]
- 8.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerized data resource in the United Kingdom. BMJ. 1991;302:766–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jick H, Terris BZ, Derby LE, et al. Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiol Drug Saf. 1992;1:347–349. [Google Scholar]
- 10.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23:686–689. [DOI] [PubMed] [Google Scholar]
- 11.Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–1565. [DOI] [PubMed] [Google Scholar]
- 12.Gelfand JM, Wang X, Qing L, et al. Epidemiology and treatment patterns of psoriasis in the General Practice Research Database (GPRD). Pharmacoepidemiol Drug Saf. 2005;14(Suppl):S23. [Google Scholar]


