Abstract
In trials evaluating the immune responses to Bacille of Calmette-Guérin (BCG), the genetic background and the nutritional status are host-related factors that could affect the heterogeneity in these parameters. The IFNG+874 A/T (rs 62559044) polymorphism has been reported to influence the IFN-γ production by BCG-vaccinated individuals challenged in vitro with mycobacterial antigens. The body mass index (BMI) is a proxy for the nutritional status and has been associated both with the susceptibility to tuberculosis and with the IFN-γ response. We show that although the IFNG+874 A/T polymorphism was not associated with the heterogeneity of IFN-γ production in a randomized controlled trial that evaluated long-term immune responses to BCG revaccination previously conducted in Salvador, Bahia, Brazil, the effect of this polymorphism on the observed increase in IFN-γ production among revaccinated subjects was adjusted in individuals with a low BMI.
Introduction
The Bacille of Calmette-Guérin (BCG) vaccine has been the subject of numerous efficacy trials and epidemiological studies conducted over several decades. These trials indicate that the neonatal vaccination with BCG has 40–80% protective efficacy against tuberculosis (TB), being particularly effective against the meningeal and miliary forms of the disease, and its efficacy against the pulmonary form varies geographically [1]. New vaccines against TB are under trial [2], and the establishment of biomarkers of vaccine-induced protection could accelerate their evaluation. The study of the immune response to BCG in vaccine trials can yield important insights relative to the appropriateness of putative biomarkers of protection against TB, given the fact that BCG is the only vaccine to date proven to be protective against the disease. A putative marker related with the anti-mycobacterial response is IFN-γ. There is evidence relating the IFN-γ production with reduced disease burden in experimental disease [3] and in humans [4–6], but the in vitro IFN-γ production against mycobacterial antigen(s) and the expansion of IFN-γ-producing antigen-specific cells do not always correlate with vaccine-induced protection against TB [7,8].
The genetic background of BCG-vaccinated individuals may influence the amplitude of the post-vaccination IFN-γ response. The T>A single nucleotide polymorphism (SNP) of IFNG in the +874 position is one of the best studied polymorphisms that affect the IFN-γ production [9], and it has been associated both with the occurrence of active TB [10] and with lower IFN-γ production in TB patients [9] and in BCG-vaccinated children [11]. Likewise, malnutrition has been related with an increased risk of tuberculosis development [12]. A recent study conducted in Taiwan showed that body mass index (BMI) values at the beginning of the anti-tuberculosis treatment related with mortality caused by tuberculosis among male patients [13]. Also the production of pro-inflammatory cytokines including IFN-γ is impaired in Mycobacterium tuberculosis-infected underweight individuals [14,15].
A randomized controlled trial to evaluate the immune response to BCG revaccination was conducted in Salvador, Bahia, Brazil [16]. The volunteers enrolled in the trial were undergraduate students from two universities with a short age range, without TB infection, with high socioeconomic level, no HIV infection, no contact with tuberculosis patients and with normal complete blood counts and hematocrit. The IFN-γ response to BCG revaccination in the interval of two months after the intervention varied widely (from 0.1 to 100 fold in the revaccinated group, compared with a range of 0.1–7.1 in the control group), and was associated with the capacity of producing high levels of IFN-γ at 12-months follow-up. The study was not designed to make it possible to correlate the IFN-γ response with protection conferred by revaccination. In a previous trial conducted in the same city BCG revaccination had a modest protective effect over the neonatal BCG vaccine [17]. We discuss the possible role of IFNG +874 polymorphism and BMI on the observed heterogeneous IFN-γ production to in vitro stimulation with mycobacterial antigens in this trial [16].
Materials and Methods
Recruitment and study design
To investigate the association of the IFNG+874 T>A polymorphism with the IFN-γ response to Mycobacterium tuberculosis we compared four groups:
Twenty-nine volunteers with distinguishable BCG vaccination scars randomly assigned to the control group in a randomized controlled trial to assess the IFN-γ response after BCG revaccination performed in Salvador, Bahia, Brazil [16].
Forty-six volunteers with distinguishable BCG vaccination scars randomly assigned to be revaccinated in the same trial [16].
LTBI, comprised of 66 latently infected individuals identified by routine tuberculin skin test of undergraduate health care students involved in patients’ follow-up at the Hospital Especializado Octavio Mangabeira, Salvador, Bahia, Brazil (the state reference hospital for tuberculosis diagnosis and treatment). Subjects were considered positive if they had induration above 10 mm.
TB, comprised of 93 individuals with active pulmonary tuberculous disease, newly diagnosed by positive smear and/or culture for Mycobacterium tuberculosis at the Hospital Especializado Octavio Mangabeira.
This study was approved by the Ethical Committee of the Centro de Pesquisas Goncalo Moniz (CEP-CPQGM/FIOCRUZ, CAAE: 0015.0.225.000–10 and 0005.0.225.000–11), and complied with the ethical principles contained in the Brazilian National Health Council Resolution 196/96 Guidelines.
Cultures and IFN-y production
Whole blood cultures were performed from vacuum-collected heparin treated blood, with or without mycobacterial antigen (10 μg/ml of Mycobacterium tuberculosis H37Rv culture lysate, Mtb, kindly provided by the Colorado State University, USA as part of NIH, NIAID Contract No. HHSN266200400091C, entitled "Tuberculosis Vaccine Testing and Research Materials", which was awarded to Colorado State University), and IFN-γ was measured in the culture supernatants as described in [16].
Genotyping
Genomic DNA was successfully obtained for 25 revaccinated (8 male, 32%), 15 control (5 male, 33%), 66 LTBI (18 male, 27%) and 93 TB (64 male, 69%) volunteers. In this sample, height and weight values were not registered for one control (female) and five TB (3 male) participants. Height was not registered for one revaccinated (female), one LTBI (female), and 13 TB (7 male) participants. Weight was not registered for one TB (male) participant. Baseline IFN-γ production was evaluated for all revaccinated and control volunteers, as well as for 57 LTBI (among which 11 participants with values of IFN-γ in stimulated cultures that exceeded the maximum optical density of the standard curve), and 45 TB participants. IFN-γ production 2 months after intervention was available for 23 revaccinated and 8 control participants. Genomic DNA was obtained using the phenol-chloroform method. The IFNG+874 A/T polymorphism was investigated by Amplification Refractory Mutation System (ARMS-PCR) as previously described [18]. Briefly, to assess the presence of the A Allele, the primer IFN-γ G: 5’-TCA ACA AAG CTG ATA CTC CA-3’; and the primer IFN-γ A: 5’-TTC TTA CAA CAC AAA ATC AAA TCA-3’ were used. For the T Allele, the primer IFN-γ G was used with the primer IFN-γ T: 5’-TTC TTA CAA CAC AAA ATC AAA TCT-3’. Both reactions produce a 295-bp fragment. Amplification was performed under the following conditions: 12 μL reaction containing approximately 100 ng genomic DNA, 1μM of each primer pair, 10X buffer, MgCl2 50 mM, dNTP 25 mM and 5U/μL of Taq polymerase. The interpretation of the results was based on the presence or absence of the amplified product, which was confirmed on 2% agarose gel stained with ethidium bromide (1μg/mL).
Statistical Analyses
The cytokine levels and ratios were compared between 2 groups using the Mann-Whitney U test for unpaired samples. All genotypes were tested for the Hardy–Weinberg equilibrium using a chi-square-test between observed and expected numbers. The Fisher’s exact test was applied to compare IFNG +874T/A SNP mutation frequency between the groups. The tentative Poisson model was used to evaluate the interaction among the IFN-y production and the variables genotype, sex and BMI. To assess the association among the genotypes AA and TT/TA and the ratio in the IFN-γ production (T0/T2 ≥3,3) we performed the Poisson regression. The Crude Coefficient from Poisson regression model was calculated to analyze the influence of the genotypes on the IFN-y production [19,20]. We evaluated the quality of model adjustment through Akaike information criterion (AIC), residual analysis and Variance inflation factor (Vif) [21]. The analyses were performed only in the revaccinated group, restricted to the most important variables, given the sample size. Age, body mass index (BMI [22]), and cytokine levels of the volunteers are presented as mean (95% confidence interval, CI) values. Databanks were mounted in EpiData Entry (version, EpiData Association, Denmark), and data were processed and analyzed using R (version 3.1) [23], EpiData Analysis (version) and Prism (version, GraphPad Inc., San Diego, CA).
Results and Discussion
Association between the IFN-γ production and the IFNG+874 polymorphism
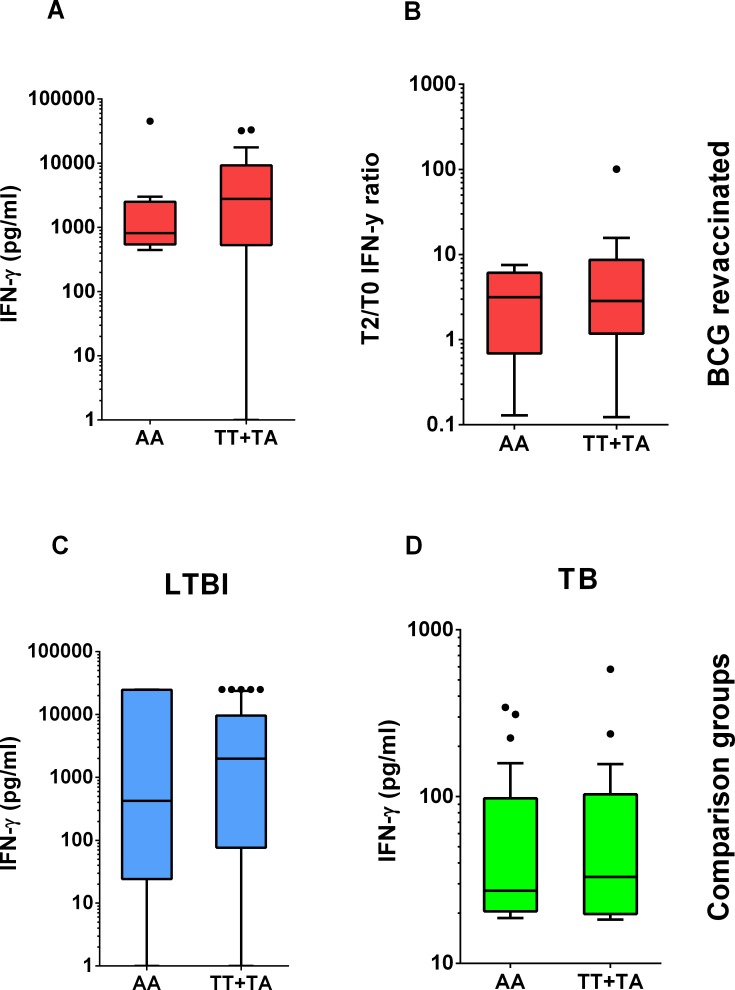
The allelic and genotypic frequencies for the IFNG+874 polymorphism agreed with those found for the Brazilian population [18,24,25] and elsewhere [26,27]. The A allele was the most frequent and there were no differences in its frequency between the groups evaluated (Table 1). The TA genotype was the most frequent, followed by the AA genotype. The genotypic frequencies also did not differ between the groups. The individuals in all groups were stratified according to the IFNG+874 genotype: AA and TT/TA. The IFN-γ production at baseline (Fig 1A) and the ratio of the IFN-γ concentration measured in the 2-month follow-up culture divided by the IFN-γ concentration measured in the baseline culture (T2/T0 IFN-γ ratio) (Fig 1B) did not differ between these two strata for the revaccinated subjects. Individuals with T2/T0 IFN-γ ratio above the cut-off of 3.262 ("high-ratio") were 4.7 times more likely to produce IFN-γ above the median for the revaccinated group one year after intervention than individuals with T2/T0 IFN-γ ratio below this cut-off (“low ratio”) [16]. Furthermore, high-ratio revaccinated individuals were 7.1 times more likely to have IFN-γ production above that found for controls plus twice the standard deviation one year after the intervention [16].
Table 1. Distribution of genotypic and allelic frequencies of the IFNG+874T/A in the individuals involved in the BCG revaccination trial and in the comparison groups (LTBI and TB).
| Genotype frequencya | Allele frequencyb | ||||
|---|---|---|---|---|---|
| TT (%) | TA (%) | AA (%) | T (%) | A (%) | |
| Revaccinated (N = 25) | 2 (8) | 15 (60) | 8 (32) | 19 (38) | 31 (62) |
| Controls (N = 15) | 1 (7) | 10 (67) | 4 (27) | 12 (40) | 18 (60) |
| LTBI (N = 66) | 7 (11) | 39 (59) | 20 (30) | 53 (40) | 79 (60) |
| TB (N = 93) | 11 (12) | 47 (51) | 35 (38) | 69 (37) | 117 (63) |
| Total (N = 199) | 21 (11) | 111 (56) | 67 (34) | 153 (38) | 245 (62) |
aComparison between revaccinated and controls: χ2 = 0.1778, P = 1.0000; Comparison between all groups: χ2 = 2.395, P = 0.8801, AA vs TA+TT: χ2 = 1.350, P = 0.9981.
bComparison between revaccinated and controls: χ2 = 0.03160, P = 1.0000; Comparison between all groups: χ2 = 0.3402, P = 0.9523.
Fig 1.
Genotype distribution of IFN-γ production (A, C, D) or T2/T0 IFN-γ ratio (B) in whole blood cultures stimulated with mycobacterial antigen. (A, B) BCG-revaccinated subjects; A depicts IFN-γ production at baseline. (C) LTBI volunteers. (D) TB volunteers.
LTBI (Fig 1C) and TB (Fig 1D) volunteers also did not present differences in IFN-γ production according to the IFNG+874 genotype. We speculate that polymorphisms in other positions of the IFNG gene probably also did not account for the heterogeneous IFN-γ response in our population because their frequency is low [28–30]. A recent study using genome-wide linkage analysis showed that the IFN-γ response of contacts of tuberculosis patients to both BCG and PPD stimulation in vitro related with the 8q11.2-8q22 region, where 108 genes are encoded including IL7 and LY96 [31].
Multivariate analysis of variables influencing the heterogeneity of the IFN-γ response
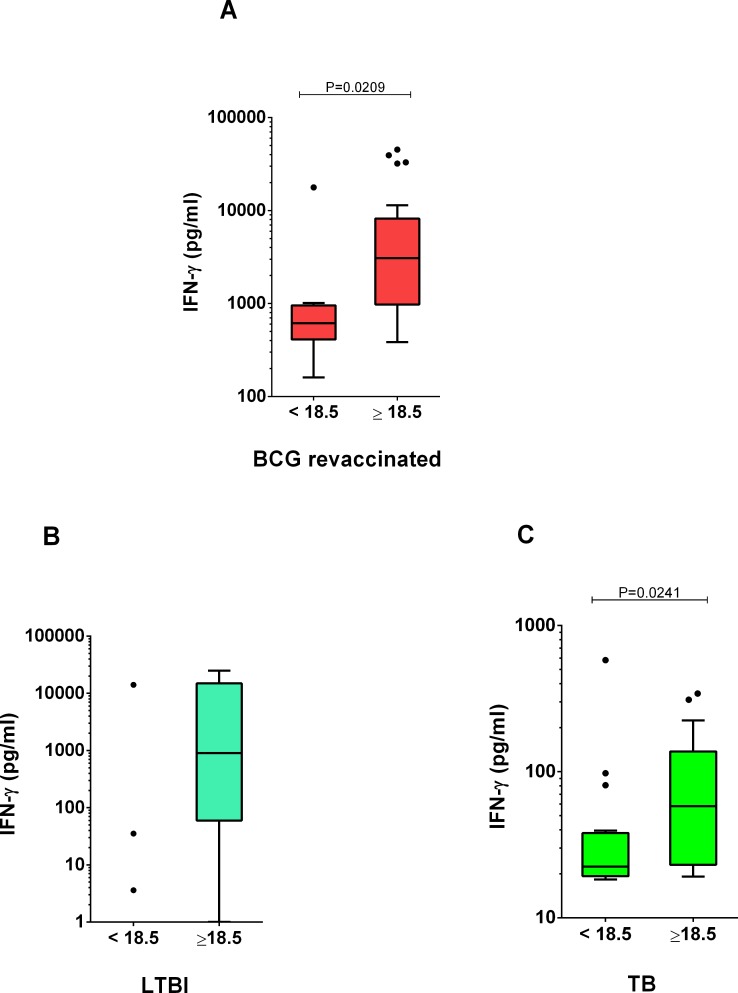
BMI did not correlate with T2/T0 IFN-γ ratio among revaccinated subjects (Spearman r = -0.0707, P = 0.7368). However, individuals in the revaccinated group with normal BMI had significantly higher IFN-γ production at baseline when compared with low BMI subjects (Fig 2A). Only three individuals with LTBI had low BMI (Fig 2B). The association between BMI and IFN-γ production was also true for the comparison group TB (Fig 2C). Therefore, we assessed the association between the T2/T0 IFN-γ ratio and the variables BMI and genotype using a multivariate model.
Fig 2. IFN-γ production in whole blood cultures stimulated with mycobacterial antigen stratified by subjects’ BMI.
(A) BCG-revaccinated subjects’ baseline cultures. (B) LTBI volunteers. (C) TB volunteers. *P<0.05.
The results of the non-adjusted model indicated that the genotype AA increased the relative risk of belonging to the low-ratio group by 2% (Table 2). When the relative risk was adjusted by the BMI we observed that having a low BMI combined with the genotype AA decreased the relative risk of belonging to the low-ratio group by 11%. Significant interaction between a polymorphism in the beta chain of the IFN-γ receptor and BMI values influencing the risk of non-Hodgkin lymphoma among women has been reported [32]. We have not found interaction between the polymorphism in IFNG+874 locus and BMI (β = 0.154; RR = 1.17, P = 0.92), however we found that BMI potentially confounded the association between the IFNG+874 polymorphism and the T2/T0 IFN-γ ratio (Table 2) diminishing the relative risk (RR) from 1.02 to 0.89 (a moderate reduction of 12.8%). We believe it would be cautious to consider BMI when describing the association between polymorphisms in the IFN-γ axis and the IFN-γ response. We suggest that further investigation is needed to address the influence of nutrition on the effect of these polymorphisms when evaluating clinically relevant endpoints in the response to M. tuberculosis, also further dissecting pathways related with body composition [33].
Table 2. Multivariate analysis of the association between presenting a T2/T0 IFN-γ ratio below 3.262 (low-ratio) and the variables BMI and IFNG+874 genotype.
| Variable | Crude Coefficient | Crude RR | P | Adjusted Coefficient | Adjusted RR | P |
|---|---|---|---|---|---|---|
| (Intercepto) | -0,5754 | - | 0,0840 | -0,549 | - | 0,1300 |
| genotype | 0,0157 | 0,0979 | -0,109 | 0,8700 | ||
| TT+TA | 1 | 1 | ||||
| AA | 1,02 | 0,89 | ||||
| BMI | - | -0,109 | 0,8700 | |||
| ≥ 18.5 | - | 1 | ||||
| < 18.5 | - | 0,89 |
Note: Akaike information criterion (AIC): crude = 44,83; adjusted = 44,49. Residues analysis: mean (variance) for the crude model: -0,007 (1,05); for the adjusted model: -0,004 (1,07). Vif: absence of co-linearity between BMI and genotype.
Conclusions
BMI and genotype may influence the IFN-γ response of individuals in tuberculosis vaccine trials, but these variables were not able to explain most of the variation found in the response to the vaccine among these subjects. It is cautious to consider BMI values in studies that address the importance of the genetic background in the immune response against mycobacteria.
Supporting Information
Dots represent missing data.
(XLSX)
Acknowledgments
To Beatriz Muller, Priscila Miranda, Gislaine Aparecida Lacerda and Paula Fernanda in contributing for technical support. To Bruno de Bezerril Andrade for critically reviewing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fundacao de Amparo a Pesquisa do Estado da Bahia—FAPESB (002/03-PPP). ELC received a scholarship from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior—CAPES. ESO and MSR received scholarships from FAPESB. TB was a senior investigator from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico—CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58: 470–480. 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Kaufmann SHE. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014;4 10.1101/cshperspect.a018523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, et al. Multifunctional, High-Level Cytokine-Producing Th1 Cells in the Lung, but Not Spleen, Correlate with Protection against Mycobacterium tuberculosis Aerosol Challenge in Mice. J Immunol. 2008;181: 4955–4964. 10.4049/jimmunol.181.7.4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359: 1393–1401. 10.1016/S0140-6736(02)08353-8 [DOI] [PubMed] [Google Scholar]
- 5.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, et al. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204: 1075–1085. 10.1093/infdis/jir515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur Y-G, Gorak-Stolinska P, Lalor MK, Mvula H, Floyd S, Raynes J, et al. Factors affecting immunogenicity of BCG in infants, a study in Malawi, The Gambia and the UK. BMC Infect Dis. 2014;14: 184 10.1186/1471-2334-14-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz A, Torrado E, Carmona J, Fraga AG, Costa P, Rodrigues F, et al. BCG vaccination-induced long-lasting control of Mycobacterium tuberculosis correlates with the accumulation of a novel population of CD4+IL-17+TNF+IL-2+ T cells. Vaccine. 2015;33: 85–91. 10.1016/j.vaccine.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol CVI. 2015;22: 258–266. 10.1128/CVI.00721-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallakci N, Coskun M, Berber Z, Gürkan F, Kocamaz H, Uysal G, et al. Interferon-gamma gene+874T-A polymorphism is associated with tuberculosis and gamma interferon response. Tuberc Edinb Scotl. 2007;87: 225–230. 10.1016/j.tube.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque AC de, Rocha LQ, Batista AH de M, Teixeira AB, Santos DB dos, Nogueira N a. P. Association of polymorphism +874 A/T of interferon-γ and susceptibility to the development of tuberculosis: meta-analysis. Eur J Clin Microbiol Infect Dis. 2012;31: 2887–2895. 10.1007/s10096-012-1660-4 [DOI] [PubMed] [Google Scholar]
- 11.Anuradha B, Rakh SS, Ishaq M, Murthy KJR, Valluri VL. Interferon-gamma Low producer genotype +874 overrepresented in Bacillus Calmette-Guerin nonresponding children. Pediatr Infect Dis J. 2008;27: 325–329. 10.1097/INF.0b013e31816099e6 [DOI] [PubMed] [Google Scholar]
- 12.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2004;8: 286–298. [PubMed] [Google Scholar]
- 13.Yen Y-F, Chuang P-H, Yen M-Y, Lin S-Y, Chuang P, Yuan M-J, et al. Association of Body Mass Index With Tuberculosis Mortality: A Population-Based Follow-Up Study. Medicine (Baltimore). 2016;95: e2300 10.1097/MD.0000000000002300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anuradha R, Munisankar S, Bhootra Y, Kumar NP, Dolla C, Kumaran P, et al. Coexistent Malnutrition Is Associated with Perturbations in Systemic and Antigen-Specific Cytokine Responses in Latent Tuberculosis Infection. Clin Vaccine Immunol CVI. 2016;23: 339–345. 10.1128/CVI.00009-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaviya N, Budambula V, Webale MK, Were T. Circulating Interferon-Gamma Levels Are Associated with Low Body Weight in Newly Diagnosed Kenyan Non-Substance Using Tuberculosis Individuals. Interdiscip Perspect Infect Dis. 2016;2016: 9415364 10.1155/2016/9415364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira ES, Marinho JM, Barbosa T, study group. Interferon-gamma production by mononuclear cells in Bacille Calmette-Guérin-revaccinated healthy volunteers predicted long-term antimycobacterial responses in a randomized controlled trial. Vaccine. 2013;31: 3778–3782. 10.1016/j.vaccine.2013.04.079 [DOI] [PubMed] [Google Scholar]
- 17.Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant’Anna C, et al. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine. 2011;29: 4875–4877. 10.1016/j.vaccine.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 18.Amim LHLV, Pacheco AG, Fonseca-Costa J, Loredo CS, Rabahi MF, Melo MH, et al. Role of IFN-gamma +874 T/A single nucleotide polymorphism in the tuberculosis outcome among Brazilians subjects. Mol Biol Rep. 2008;35: 563–566. 10.1007/s11033-007-9123-1 [DOI] [PubMed] [Google Scholar]
- 19.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3: 21 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159: 702–706. [DOI] [PubMed] [Google Scholar]
- 21.Bozdogan H. Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52: 345–370. 10.1007/BF02294361 [DOI] [Google Scholar]
- 22.World Health Organization. WHO | Physical status: the use and interpretation of anthropometry [Internet]. Geneva, Switzerland: World Health Organization; 1995. Report No.: ISSN 0512-3054. Available: http://www.who.int/childgrowth/publications/physical_status/en/
- 23.R Development Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2011. Available: http://www.r-project.org/ [Google Scholar]
- 24.Leandro ACCS, Rocha MA, Lamoglia-Souza A, VandeBerg JL, Rolla VC, Bonecini-Almeida M da G. No association of IFNG+874T/A SNP and NOS2A-954G/C SNP variants with nitric oxide radical serum levels or susceptibility to tuberculosis in a Brazilian population subset. BioMed Res Int. 2013;2013: 901740 10.1155/2013/901740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller BLA, Ramalho DM de P, Santos PFG dos, Mesquita EDD, Kritski AL, Oliveira MM. Inflammatory and immunogenetic markers in correlation with pulmonary tuberculosis. J Bras Pneumol Publicaçaäo Of Soc Bras Pneumol E Tisilogia. 2013;39: 719–727. 10.1590/S1806-37132013000600011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lio D, Marino V, Serauto A, Gioia V, Scola L, Crivello A, et al. Genotype frequencies of the +874T—>A single nucleotide polymorphism in the first intron of the interferon-gamma gene in a sample of Sicilian patients affected by tuberculosis. Eur J Immunogenetics Off J Br Soc Histocompat Immunogenetics. 2002;29: 371–374. [DOI] [PubMed] [Google Scholar]
- 27.Ansari A, Talat N, Jamil B, Hasan Z, Razzaki T, Dawood G, et al. Cytokine gene polymorphisms across tuberculosis clinical spectrum in Pakistani patients. PloS One. 2009;4: e4778 10.1371/journal.pone.0004778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvaraj P, Alagarasu K, Harishankar M, Vidyarani M, Nisha Rajeswari D, Narayanan PR. Cytokine gene polymorphisms and cytokine levels in pulmonary tuberculosis. Cytokine. 2008;43: 26–33. 10.1016/j.cyto.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 29.Vidyarani M, Selvaraj P, Prabhu Anand S, Jawahar MS, Adhilakshmi AR, Narayanan PR. Interferon gamma (IFNgamma) & interleukin-4 (IL-4) gene variants & cytokine levels in pulmonary tuberculosis. Indian J Med Res. 2006;124: 403–410. [PubMed] [Google Scholar]
- 30.Bream JH, Ping A, Zhang X, Winkler C, Young HA. A single nucleotide polymorphism in the proximal IFN-gamma promoter alters control of gene transcription. Genes Immun. 2002;3: 165–169. 10.1038/sj.gene.6363870 [DOI] [PubMed] [Google Scholar]
- 31.Jabot-Hanin F, Cobat A, Feinberg J, Grange G, Remus N, Poirier C, et al. Major Loci on Chromosomes 8q and 3q Control Interferon γ Production Triggered by Bacillus Calmette-Guerin and 6-kDa Early Secretory Antigen Target, Respectively, in Various Populations. J Infect Dis. 2016;213: 1173–1179. 10.1093/infdis/jiv757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Zheng T, Lan Q, Foss F, Kim C, Chen X, et al. Cytokine polymorphisms in Th1/Th2 pathway genes, body mass index, and risk of non-Hodgkin lymphoma. Blood. 2011;117: 585–590. 10.1182/blood-2010-07-295097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Ma A, Wang Q, Han X, Cai J, Schouten EG, et al. Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus. PloS One. 2013;8: e80122 10.1371/journal.pone.0080122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dots represent missing data.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.