Abstract
Purpose
The average delay from first seizure to diagnosis of psychogenic non-epileptic seizures (PNES) is over 7 years. The reason for this delay is not well understood. We hypothesized that a perceived decrease in seizure frequency after starting an anti-seizure medication (ASM) may contribute to longer delays, but the frequency of such a response has not been well established.
Methods
Time from onset to diagnosis, medication history and associated seizure frequency was acquired from the medical records of 297 consecutive patients with PNES diagnosed using video-electroencephalographic monitoring. Exponential regression was used to model the effect of medication trials and response on diagnostic delay.
Results
Mean diagnostic delay was 8.4 years (min 1 day, max 52 years). The robust average diagnostic delay was 2.8 years (95% CI: 2.2-3.5 years) based on an exponential model as 10 to the mean of log10delay. Each ASM trial increased the robust average delay exponentially by at least one third of a year (Wald t=3.6, p=0.004). Response to ASM trials did not significantly change diagnostic delay (Wald t=−0.9, p=0.38).
Conclusion
Although a response to ASMs was observed commonly in these patients with PNES, the presence of a response was not associated with longer time until definitive diagnosis. Instead, the number of ASMs tried was associated with a longer delay until diagnosis, suggesting that ASM trials were continued despite lack of response. These data support the guideline that patients with seizures should be referred to epilepsy care centers after failure of two medication trials.
Keywords: Antiepileptic drugs, non-epileptic seizures, diagnostic delay, evidence based medicine
1. Introduction
Based on published reports, the average delay from first seizure to definitive diagnosis of psychogenic non-epileptic seizures (PNES) is over 7 years [1]. PNES often appear behaviorally similar to epileptic seizures, which commonly leads to a mistaken diagnosis of epileptic seizures (ES) because the prevalence of ES is much higher than that of PNES [2]. Key barriers to diagnosis include providers unfamiliar with PNES and limited access to care due to insurance or social support [3, 4]. Understanding the reasons for this diagnostic delay are critical because, prior to accurate diagnosis, patients do not receive appropriate treatment while incurring direct and indirect annual costs similar to patients with medication resistant seizures, estimated at 20,995 euros [5, 6]. Patients who are diagnosed earlier with PNES have an improved long-term seizure prognosis [7-9] and cost reduces substantially after diagnosis [10]. Treatment for ES can involve anti-seizure medications (ASMs), the ketogenic diet, neurostimulators and surgery, whereas standard treatment for PNES without co-morbid ES addresses underlying psychological distress with cognitive behavioral inspired therapy and sometimes psychoactive medications, but not ASMs [11-13]. Approximately 10% of patients with PNES have comorbid ES, although this frequency varies substantially among reports [2, 13, 14].
A definitive diagnosis of PNES is based upon simultaneous video and electroencephalographic recordings (VEEG) [15]. Patients are referred to tertiary care centers for epilepsy where their evaluation can include VEEG when their seizures are resistant to ASM treatment, or when their history and seizure semiology is suggestive of non-epileptic seizures (NES) [16]. At our center, 50% of patients admitted for differential diagnosis of seizure-like episodes have PNES without co-morbid epilepsy, whereas 6% of patients admitted for epilepsy surgery evaluation have PNES alone [14]. Once referral has occurred, the time to diagnosis is short. Prior to referral, more than half of patients with PNES have been tried on at least one ASM [2, 17]. The reported efficacy and duration of efficacy of ASM in patients with PNES has not been well established [18].
We hypothesize that positive responses to trials of ASMs contribute to the long delay in definitive diagnosis. In addressing this theory, we characterized the pre-referral treatment course of a large population of patients with PNES, which also contributes to the understanding of the natural history of PNES from onset to diagnosis. To our knowledge, this has not been discussed in the literature since 1990 [19].
2. Methods
We reviewed the medical records of all 1,126 patients admitted to the UCLA adult epilepsy VEEG monitoring unit from January 2006 until April 2014, and identified 297 patients with PNES who were diagnosed as not also having epileptic seizures or physiologic non-epileptic seizure-like events. Patients with PNES who had one or more other seizures manifestations that were not recorded during VEEG were excluded because the unrecorded seizure(s) could be epileptic or physiologic. We performed retrospective chart review for all 297 identified patients with only PNES to determine age at diagnosis, age of seizure onset, and initial response in seizure frequency to each ASM. Delay to diagnosis was calculated as the difference between age at diagnosis and age of seizure onset. For delays less than 3 months, delay was recorded to the nearest day. For delays less than 1 year, the delay was recorded to the nearest month. An ASM treatment response was defined as 50% or more reduction in seizure frequency reported for the length of time needed to determine a frequency decrease, which was defined as a seizure-free period at least three times longer than their pre-treatment inter-seizure period. The three times longer interval was based on the International League Against Epilepsy (ILAE) definition of the period to observe to determine treatment response [16]. The 50% or more criterion was chosen because it is used as a clinically relevant outcome measure used in randomized clinical trials of ASMs for epilepsy; however, the measure did not replicate the clinical trials’ use of blinded, prospective assessment over the same time period for all participants [20]. We use precise language to differentiate response to an ASM from success of an ASM trial: success and failure is based upon seizure freedom, not reduction in seizure frequency. To compare the response rate of medications for PNES, we used Fisher-exact statistics.
Delay to diagnosis was modeled using exponential regression. When describing delay alone, we report raw averaged and robust average. The robust average reduces the contribution of outliers with very long delays by averaging the log of delay. For regressions, the log of delay to diagnosis was modeled against linear effects of number of ASM trials and number of successful ASM trials controlling for sex. Exponential regression was used because delay to diagnosis was distributed exponentially over the population and is understood theoretically as a waiting time.
All patients consented for the use of their records in research, and the UCLA Institutional Review Board approved this study. This work is consistent with Declaration of Helsinki. De-identified raw data and code for this study is available at http://www.brainmapping.org/MarkCohen/research.html.
3. Results
Population demographics for the 297 patients with PNES are summarized in Table 1. Diagnostic delay was recorded in 268 patients (90%), with a raw mean of 8.4 years (95% CI 7.0-9.8 years). Of the 297 patients, 258 (87%) patients had 894 cumulative trials of ASMs prior to the diagnosis of PNES. The remaining 39 (13%) patients had not been treated with an ASM prior to assessment. The average delay to diagnosis for patients who took two or fewer ASMs was 5.9 years (95% CI 4.3-7.6 years). The robust average delay from first presentation at our center to diagnosis was 43 days (95% CI 33-55 days).
Table 1.
Ages and delays are in years unless otherwise specified. Robust average diagnostic delay was calculated as exp(mean[log(delay)]).
| Percent Female | Onset Age | Assessment Age | Diagnostic Delay | ASM Trials | ASM Responses | |
|---|---|---|---|---|---|---|
| Min | 1 day | 12 | 1 day | 0 | 0 | |
| 95 CI LB | 68 | 29 | 37 | 2.2 | 2.78 | 0.17 |
| Robust Average | 73 | 31 | 39 | 2.8 | 3.04 | 0.26 |
| 95 CI UB | 78 | 33 | 41 | 3.4 | 3.30 | 0.35 |
| Max | 85 | 88 | 52 | 12 | 6 | |
Abbreviations: Anti-Seizure Medication (ASM), Confidence Interval (CI), Lower Bound (LB), Upper Bound (UB).
Of 354 medication trials with a detailed post-treatment seizure frequency, 10% (35/354) of ASM trials were associated with a period of seizure freedom whereas 30% (109/354) were associated with a reduction in seizure frequency by the criterion described above. No medication was significantly more or less likely to result in a response (Fisher exact tests, minimum pairwise p>0.09). A clinically relevant response to at least one ASM was reported in 17% (44/258) of patients who tried at least one ASM. The response rate for more than one ASM is as follows: 7.7% (20/258) to at least two, 2.4% (7/258) to at least three, 1% (3/258) to at least four, 0.8% (2/258) to at least five, and 0.4% (1/258) to six. The frequency with which patients responded to each ASM is illustrated in Supplemental Figure 1. Patients who reported a response to at least one ASM had significantly fewer ASM trials prior to referral than patients who did not respond to any ASM (2.9 vs 4.0 trials, respectively; t-test unequal variances, p=0.005).
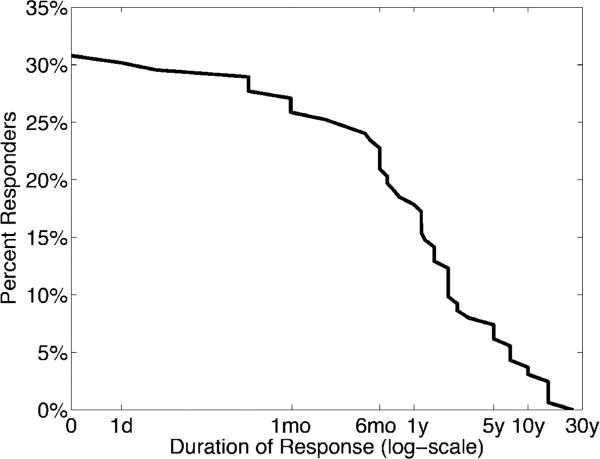
In total, 53 years of ASM trials were reported, with a median of 1 year per cumulative treatment. Of the 109 successful trials, 30 patients also reported the duration of response for 50 trials (46%). Figure 1 illustrates the survival curve of medication response. The robust average duration of response was 2.0 years (median 14 months), if the patient responded initially (95% confidence interval: 1.6-2.5 years); the difference between the median and expectation reflect the heavy right tail of the exponential distribution that causes the expectation to be higher than the median.
Figure 1. Duration of response to ASMs in patients with PNES.
If a patient initially responded, the robust average duration of response was 2.0 years. Abbreviations: day (d), month (mo), year (y).
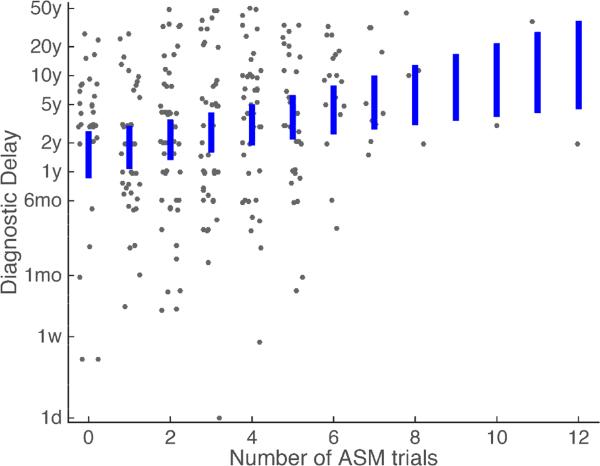
Sex, number of ASM trials and number of responses to ASM trials explained a significant amount of the variation in diagnostic delay using exponential regression (likelihood ratio test, D 39.9, df=3, p=10−8). However, there was significant variation in diagnostic delay not explained by these factors (Figure 2). The robust average delay in a patient with no ASM trials was 1.6 years (95% CI: 0.9-2.7 years). Increasing ASM trials significantly increased the diagnostic delay (0.18 log years per trial, Wald t=3.6, p=0.004). Sex and increasing number of responses to ASM trials did not affect diagnostic delay significantly (Sex: 0.04 log years, Wald t=0.15, p=0.88; Successful ASMs: −0.14 log years per successful trial, Wald t=−0.87, p=0.38).
Figure 2. Diagnostic delay with respect to number of ASM trials.
Blue bars reflect the 95% confidence interval of the robust average diagnostic delay using our regression model. Each gray dot reflects a patient, with uniform noise added in the horizontal direction for visualization. Abbreviations: Anti-Seizure Medications (ASM), day (d), week (w), month (mo), year (y).
4. Discussion
The delay to diagnosis of psychogenic seizures often is long, and has not been investigated as comprehensively as the delay to diagnosis of epilepsy [1]. New, and objective, methods to identify patients at risk for psychogenic seizures early after presentation are necessary to improve the accuracy of treatment and thereby improve quality of life and reduce cost of treatment. Our results suggest that the number of ASM trials is associated with an exponential increase in the delay to diagnosis. Because the clinical goal for all patients with seizures should be seizure freedom, providers should consider modifying a patient's ASM regimen when the regimen fails to result in seizure freedom, irrespective of if the patient's seizure frequency is reduced [16]. Health care providers should reconsider the epilepsy diagnosis whenever switching ASMs by re-evaluating the patient's risk for psychogenic seizures. Patients at risk for PNES should be referred to an epilepsy center for more definitive diagnostic assessment. This proposed practice is not novel because it mirrors the conventional ILAE recommendation for referral to an epilepsy center for evaluation when two adequate doses of appropriately chosen ASMs fail to produce seizure freedom [16]. If this same criterion were applied to patients with psychogenic seizures, then the average delay to diagnosis would be shortened to 5.9 years from 8.4 years, resulting in a potential cost savings of 52,000 euros per patient [5]. Based on published reports about long-term prognosis [7-9], such a change also could have a huge impact upon seizure outcomes, morbidity, and quality of life.
Our data show that 17% of patients with PNES reported that their seizure frequency decreased by at least 50% when treated with at least one ASM. Such responses had a robust average duration of two years. Although most patients did not have this response to ASMs, the 17% are a substantial minority. Therefore, a history of treatment response to an ASM is not a reliable factor to exclude the diagnosis of PNES [18]. This response did not have a significant effect upon overall diagnostic delay, suggesting that providers were not influenced by seizure control in response to ASMs when assessing risk for PNES.
Our sample size did not enable sufficient power to study the effect of individual ASMs, but no ASM had evidence for greater or lesser effectiveness, including ASMs with possible psychotropic effects (lorazepam, clonazepam, diazepam, primidone, carbamazepine, oxcarbazepine, valproate, gabapentin, pregabalin, lamotrigine, levetiracetam, phenytoin, and topiramate)[21]. These data do not support the possibility that ASM treatment may benefit psychogenic seizure control. Currently, standard treatment of PNES is based on randomized clinical trials validating cognitive behavioral-inspired therapy that identifies the patients’ stressors and provides alternative coping strategies [11, 12].
A large portion of the variation in delay was not explained by the number of ASM trials. In four cases for example, patients had seizures for more than ten years without a prescription for an ASM or a diagnosis of psychogenic seizures. One patient had PNES for 52 years with only four ASM trials, and their prescription of two ASMs had not changed for 30 years prior to accurate diagnosis. In the other extreme, the shortest delay was one day, because the patient developed seizures after hydromorphone injection while in the intensive care unit for gastrointestinal bleeding. The patient was treated immediately with three ASMs due to concern for status epilepticus and VEEG was obtained. After discussion of the diagnosis with the patient, seizure control was achieved. These outliers exemplify the need for additional investigations to identify other factors associated with long delays, especially as the patient with a 52-year history of PNES has been seizure free for 10 years after psychotherapy.
The use of systemic chart review, rather than structured patient interviews, to identify treatment response introduces a bias to the results. A structured interview would provide greater consistency in data acquisition and potentially decrease the number of gaps in the data; however, we found in pilot structured interviews that patients often did not remember treatment responses as clearly as was documented from prior clinical encounters. As such, the accuracy from chart review may have been greater than that of structured interviews. Of course, a prospective ascertainment would be superior. Nevertheless, our results are consistent with what would typically be obtained during a first neurology encounter. Therefore, our results have clinical relevance to such encounters.
5. Conclusion
Our results identify that successive trials of ASMs was associated with an exponentially longer delay to diagnosis of PNES. This reinforces the clinical guideline that all patients with medication resistant seizures warrant evaluation at an epilepsy center with VEEG, and other advanced diagnostic and treatment modalities if two adequate trials of appropriately chosen ASMs have failed to produce seizure freedom [16]. When treatment fails, following the same treatment direction by using ASMs delays the initiation of the most appropriate and effective treatment. The observation that 30% of ASM trials resulted in clinically significant reductions in psychogenic seizure frequency is a novel result and worthy of consideration when treating medication resistant seizures, but contrary to our hypothesis, this response did not contribute to diagnostic delay.
Supplementary Material
Acknowledgements
The authors thank Kirk Shattuck, Marc Nuwer, and Edward P. Lau for organization support, access to the data, and technical support. This work was supported by the UCLA-California Institute of Technology Medical Scientist Training Program (NIH T32 GM08042), the Neuroimaging Training Program (NIH T90 DA022768, R90 DA022768 & R90 DA023422 to MSC), the William M. Keck Foundation, research grants to JE (NS03310 & NS080181), and the UCLA Departments of Psychiatry & Biobehavioral Sciences and Biomathematics.
Abbreviations
- ASM
Anti-seizure medication
- ES
Epileptic seizures
- ILAE
International League Against Epilepsy
- NA
Not available
- PNES
Psychogenic non-epileptic seizures
- UCLA
University of California, Los Angeles
- VEEG
Video-electroencephalography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Conflicts & Ethical Publication:
Drs. Engel, Stern and Kerr have clinical responsibilities that include the diagnosis and treatment of patients with epilepsy and non-epileptic seizures. The remaining authors have no declared conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Reuber M, Fernandez G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology. 2002;58:493–495. doi: 10.1212/wnl.58.3.493. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson P, Looper KJ. Psychogenic nonepileptic seizures: a current overview. Epilepsia. 2012;53:1679–1689. doi: 10.1111/j.1528-1167.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- 3.Pretorius C. Barriers and facilitators to reaching a diagnosis of PNES from the patients' perspective: Preliminary findings. Seizure. 2016;38:1–6. doi: 10.1016/j.seizure.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Shneker BF, Elliott JO. Primary care and emergency physician attitudes and beliefs related to patients with psychogenic nonepileptic spells. Epilepsy Behav. 2008;13:243–247. doi: 10.1016/j.yebeh.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, Dubinsky S, Newmark ME, Leibson C, So EL, Rocca WA. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–351. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Magee JA, Burke T, Delanty N, Pender N, Fortune GM. The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav. 2014;33:45–48. doi: 10.1016/j.yebeh.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Walczak TS, Papacostas S, Williams DT, Scheuer ML, Lebowitz N, Notarfrancesco A. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia. 1995;36:1131–1137. doi: 10.1111/j.1528-1157.1995.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones SG, TJ OB, Adams SJ, Mocellin R, Kilpatrick CJ, Yerra R, Lloyd JH, Velakoulis D. Clinical characteristics and outcome in patients with psychogenic nonepileptic seizures. Psychosom Med. 2010;72:487–497. doi: 10.1097/PSY.0b013e3181d96550. [DOI] [PubMed] [Google Scholar]
- 9.LaFrance WC, Jr., Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology. 2006;66:1620–1621. doi: 10.1212/01.wnl.0000224953.94807.be. [DOI] [PubMed] [Google Scholar]
- 10.Razvi S, Mulhern S, Duncan R. Newly diagnosed psychogenic nonepileptic seizures: health care demand prior to and following diagnosis at a first seizure clinic. Epilepsy Behav. 2012;23:7–9. doi: 10.1016/j.yebeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LH, Chalder T, Chigwedere C, Khondoker MR, Moriarty J, Toone BK, Mellers JD. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74:1986–1994. doi: 10.1212/WNL.0b013e3181e39658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFrance WC, Jr., Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54(Suppl 1):53–67. doi: 10.1111/epi.12106. [DOI] [PubMed] [Google Scholar]
- 13.Perez DL, LaFrance WC. Nonepileptic seizures: an updated review. CNS Spectr. 2016:1–8. doi: 10.1017/S109285291600002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr WT, Anderson A, Lau EP, Cho AY, Xia H, Bramen J, Douglas PK, Braun ES, Stern JM, Cohen MS. Automated diagnosis of epilepsy using EEG power spectrum. Epilepsia. 2012;53:e189–192. doi: 10.1111/j.1528-1167.2012.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaFrance WC, Jr., Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–2018. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- 16.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Seneviratne U, Briggs B, Lowenstern D, D'Souza W. The spectrum of psychogenic non-epileptic seizures and comorbidities seen in an epilepsy monitoring unit. J Clin Neurosci. 2011;18:361–363. doi: 10.1016/j.jocn.2010.07.120. [DOI] [PubMed] [Google Scholar]
- 18.Alessi R, Valente KD. Psychogenic nonepileptic seizures: should we use response to AEDS as a red flag for the diagnosis? Seizure. 2014;23:906–908. doi: 10.1016/j.seizure.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Lempert T, Schmidt D. Natural history and outcome of psychogenic seizures: a clinical study in 50 patients. J Neurol. 1990;237:35–38. doi: 10.1007/BF00319665. [DOI] [PubMed] [Google Scholar]
- 20.French JA, Wang S, Warnock B, Temkin N. Historical control monotherapy design in the treatment of epilepsy. Epilepsia. 2010;51:1936–1943. doi: 10.1111/j.1528-1167.2010.02650.x. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger AB. Psychotropic effects of antiepileptic drugs. Neurology. 2006;67:1916–1925. doi: 10.1212/01.wnl.0000247045.85646.c0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.