Abstract
Background
Vitamin D is hypothesized to reduce risk for tooth loss via its influence on bone health, inflammation, and the immune response. We examined the association between plasma 25-hydroxyvitamin D (25[OH]D) concentrations and the prevalence and 5-year incidence of tooth loss in a cohort of postmenopausal women.
Methods
Participants underwent oral examinations at study baseline (1997–2000) and follow-up (2002–2005) to determine the number of missing teeth and the 5-year incidence of tooth loss, respectively. At both visits women self-reported reasons for each missing tooth. At baseline, 152 women reported no history of tooth loss and 628 were categorized as reporting a history of tooth loss due to periodontal disease (n=70) or caries (n=558) (total n=780). At follow-up, 96, 376, 48, and 328 women were categorized into the aforementioned categories as reasons for incident tooth loss (total n=472). Logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for tooth loss by category of baseline 25(OH)D (nmol/L) concentrations. Models were adjusted for age, income, smoking status, frequency of dental visits, waist circumference and recreational physical activity. P for trend was estimated using continuous concentrations of 25(OH)D.
Results
Among women with 25(OH)D ≥50 (adequate vitamin D status) compared to <50 nmol/L (deficient/inadequate), the adjusted ORs (95% CI) was 1.24 [0.82–1.87], p-trend=0.049 for the history (prevalence) of tooth loss due to periodontal disease or caries and 1.07 [0.62–1.85], p-trend=0.111 for the incidence of tooth loss due to periodontal disease or caries. No statistically significant association was observed between 25(OH)D and the history or incidence of tooth loss due to periodontal disease. An increased odds of the history of tooth loss due to caries was observed with increasing concentrations of 25(OH)D (p-trend=0.045), but was not confirmed in prospective analyses.
Conclusion
In this cohort of postmenopausal women, the data do not support an association between vitamin D status and tooth loss.
Keywords: Tooth Loss, Periodontitis, Dental Caries, Vitamin D, Women’s Health, Postmenopause
While nationally representative data revealed that the prevalence of edentulism has been decreasing in the United States (US) over the past several decades, nearly 23% of adults aged 65 and older were identified as edentulous.1 Edentulism has a negative impact on quality of life, nutritional status, and overall health, and thus represents a serious ongoing public health concern.2, 3 Periodontal disease, together with caries, remains the primary cause of edentulism among the elderly.4, 5
Vitamin D has been hypothesized to prevent development of periodontal disease,6 caries,7 and tooth loss.8–10 Vitamin D’s essential role in calcium homeostasis11, along with its anti-inflammatory12 and anti-microbial properties,13 may protect against alveolar bone loss and subsequent tooth loss. Previous research on vitamin D and tooth loss is limited. The few studies that have examined the association suggest that adequate vitamin D status may prevent tooth loss.8–10 To the best of our knowledge, there have been no studies of the association between the blood biomarker for vitamin D status, 25-hydroxyvitamin D (25[OH]D), and specific reasons for tooth loss (caries versus periodontal disease), in older postmenopausal women who may be particularly vulnerable to both vitamin D insufficiency and advanced periodontitis.
Using data from the Osteoporosis and Periodontal Disease Study (OsteoPerio), an ancillary study of the Women’s Health Initiative (WHI) Observational Study (OS), we investigated the association between plasma 25(OH)D concentrations and the history of tooth loss (prevalence) assessed at baseline (1997–2000) and the 5-year incidence of tooth loss assessed at follow-up (2002–2005). The outcomes assessed were tooth loss due to periodontal disease, caries, or either.
MATERIALS AND METHODS
Study Sample
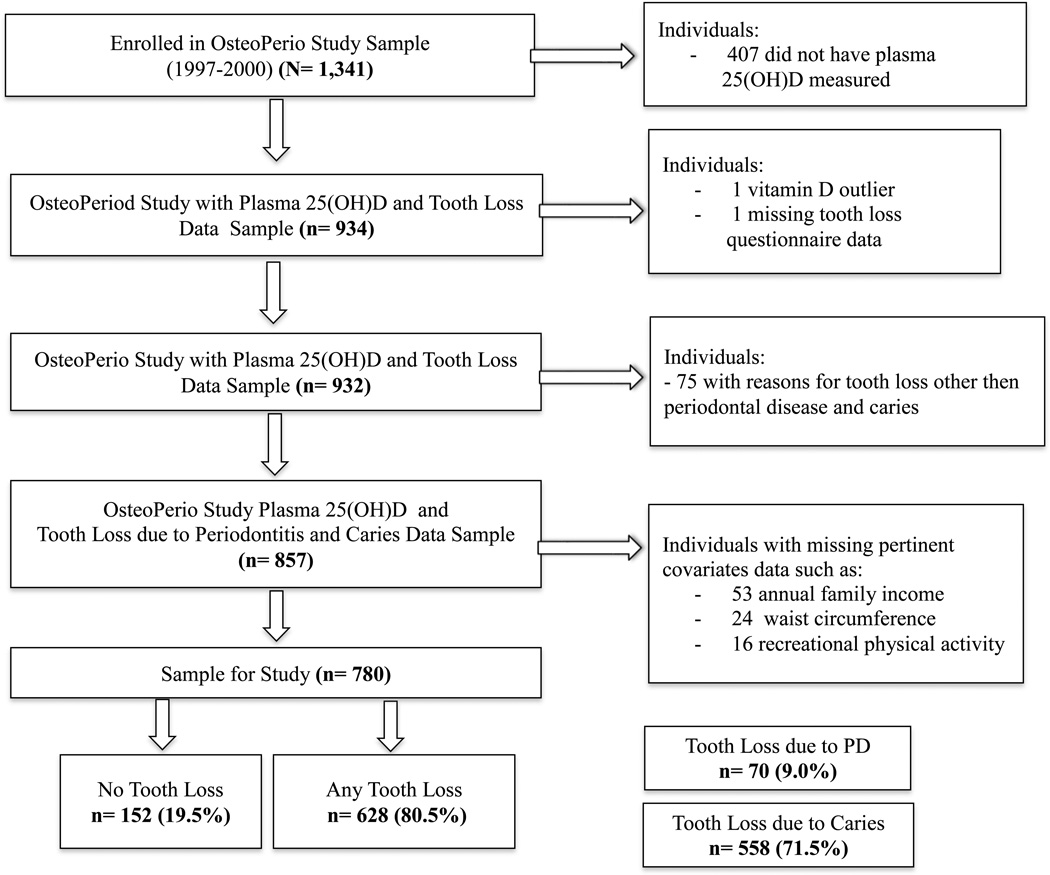
The OsteoPerio study is an ancillary study of the WHI OS of postmenopausal women.14, 15 Participants were enrolled from the Buffalo, NY WHI clinic center from 1997–2000 to examine the association between osteoporosis and periodontal disease.16 The OsteoPerio baseline data collection coincided with the WHI OS third year visit. There were 1,362 participants.16 Women were excluded if they had inadequate oral X-rays (n=16) or incomplete study questionnaires (n=5). Of the remaining 1,341 participants, 407 were excluded because they lacked plasma samples for 25(OH)D assessment and one women with an extremely high 25(OH)D value (530 nmol/L) was excluded.
Sample for Cross-Sectional Study of History of Tooth Loss (Prevalence)
A cross-sectional analysis was conducted to examine the association between baseline 25(OH)D concentrations and history of tooth loss assessed at baseline. Of the remaining 933 participants, one woman was excluded due to missing baseline data on tooth loss. Seventy-five women were excluded because they self-reported a history of tooth loss for reasons other than periodontal disease or caries. An additional 77 women were excluded because of missing pertinent covariate data: annual family income (n=53), waist circumference (n=24) and recreational physical activity (n=16). This left 780 women of whom 152 had no history of tooth loss due to any reasons and 628 had a history of tooth loss due to either periodontal disease or caries. There were 70 women with a history of at least one tooth lost due to periodontal disease (with or without teeth lost to caries) and 558 with a history of at least one tooth lost due to caries (with no teeth lost due to periodontal disease) (Figure 1).
Figure 1.
Flowchart of study participants. There are 152 women who self-reported never losing any teeth (women retaining all 28 natural teeth, excluding third molars). There are 628 women who self-reported tooth loss due to periodontal disease or caries. There are 70 women who self-reported tooth loss due to periodontal disease (with or without tooth loss due to caries) and there are 558 women who self-reported tooth loss due to caries (with no tooth loss due to periodontal disease).
Sample for Prospective Study of Incident Tooth Loss
A prospective analysis was conducted to examine the association between baseline 25(OH)D concentrations and the incidence of tooth loss from baseline to the 5-year follow-up. Of the 933 participants with 25(OH)D concentrations at baseline, 702 attended the OsteoPerio Study follow-up exam. Women were excluded from the prospective analysis if they were missing follow-up data on self-reported reasons for tooth loss (n=3) or because they self-reported reasons for incident tooth loss other than periodontal disease or caries (n=187). An additional 40 women were excluded because of missing pertinent covariate data: annual family income (n=25), waist circumference (n=14) and recreational physical activity (n=8). This left 472 women, of whom 96 had no incident tooth loss due to any reasons and 376 had incident tooth loss due to either periodontal disease or caries. There were 48 women with at least one incident tooth lost over follow-up due to periodontal disease (with or without incident tooth loss to caries) and 328 with at least one incident tooth lost due to caries (with no teeth lost due to periodontal disease)
For cross-sectional and prospective analyses examining associations between vitamin D intake and tooth loss, women were further excluded for missing data on either dietary or supplemental vitamin D intake at study baseline resulting in slightly smaller analytic samples than those with 25(OH)D (n=774 for the cross-sectional analyses and n=470 for the prospective analyses).
The University of Buffalo Institutional Review Board approved the study protocol and all study participants have signed informed consent.
Assessment of vitamin D status
Fasting blood samples were collected from participants at the OsteoPerio baseline visit, processed and stored at −80°C. Samples were assessed for 25(OH)D by competitive chemiluminescence assay§, as previously described.17 We adjusted 25(OH)D concentrations for the season of blood draw. Residuals were computed from the regression of 25(OH)D on the day of the year of blood draw and added to the sample mean for 25(OH)D.6 We used season-adjusted 25(OH)D concentrations in our analyses.
The estimated average daily dietary intake of vitamin D (IU/day), calcium (mg/day), and sugar (g/day) over the past three months was assessed from a previously validated, self-administered modified Block food frequency questionnaire (FFQ)18 given at the WHI OS year 3 follow-up (1997-200) which coincided with OsteoPerio baseline. Vitamin D and calcium supplement intake over the past 30 days was assessed at OsteoPerio baseline, including information on brand name, dosage, quantity, frequency and duration of intake. Total vitamin D and calcium intake was estimated by summing daily intake from dietary and supplement sources.
Oral Health Exam
An oral health exam, including radiographs, was performed on each participant. Trained and calibrated dental examiners assessed the number of teeth present and recorded the self-reported reasons for each tooth missing (excluding third molars). Reasons for missing teeth could include caries, periodontal disease, fracture/accident, root canal, orthodontic reasons, unerupted, congenitally missing, implant, retained root, or unable to determine. For these analyses, women were defined as having no tooth loss if they reported no missing teeth due to any reasons (retained all 28 natural teeth, excluding third molars) and as having any tooth loss if they reported losing at least one tooth due to periodontal disease or caries. Women were defined as having tooth loss due to periodontal disease if they reported losing at least one tooth due to periodontal disease, but could have reported a tooth lost to caries. Women were defined as having tooth loss due to caries if they reported losing at least one tooth due to caries, but no teeth lost due to periodontal disease. Validation of self-reported reasons for tooth loss has been reported in a previous publication.19
Covariates Assessment
At the OsteoPerio clinic visit the women’s height and weight was measured according to standardized protocols. Body mass index (BMI) (kg/m2) was calculated. Women completed self-reported questionnaires to assess age at visit, race/ethnicity, education level, marital status, annual household income and lifestyle information regarding smoking habits, alcohol intake, diet and physical activity.16, 20 Personal and family health history data and information on history of medication use, such as hormone therapy and bone drug use was collected. Oral health behavior data, such as frequency of tooth brushing, frequency of dental visits and frequency of flossing were obtained via a self-reported questionnaire.
Statistical Analyses
Guided by the Institute of Medicine’s (IOM) report on vitamin D and calcium,21 we divided the study population into four vitamin D status categories using 25(OH)D concentrations in nmol/L (<30, 30 to <50, 50 to <75, and ≥75) and two broader categories (<50 and ≥50). The IOM considers 25(OH)D concentrations <30 nmol/L as deficient vitamin D status and concentrations <50 nmol/L as deficient or inadequate vitamin D status.21 Vitamin D intake (IU/day) was categorized as <400, 400 to <600 and ≥600 as well as two broader categories (<600 and ≥600). The IOM’s Recommended Dietary Allowance (RDA) for vitamin D is 600 IU/day.21
We investigated if potential periodontal disease risk factors were associated with 25(OH)D concentrations and history of tooth loss at baseline. We used t-tests and ANOVAs to test if means of continuous variables differed across categories of 25(OH)D and tooth loss and Chi-square and Fischer’s exact tests to investigate if the distribution of categorical variables varied by category of 25(OH)D and tooth loss.
Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the history of any tooth loss, tooth loss due to periodontal disease, and tooth loss due to caries by the four categories of 25(OH)D, with the referent category of deficient vitamin D status (<30 nmol/L), and by the three categories of total vitamin D intake, with the referent category of <400 IU/day. Because our prospective analyses were limited by sample size, we estimated the ORs and 95% CIs for the incidence of any tooth loss, tooth loss due to periodontal disease, and tooth loss due to caries by the two categories of 25(OH)D, with the referent category of deficient or inadequate vitamin D status (<50 nmol/L), and by the two categories of vitamin D intake, with the referent category of <600 IU/day. To compare the results between the cross-sectional and prospective analyses, we also repeated the cross-sectional analyses with the same dichotomized exposure categories used in the prospective analyses. P for trend was estimated using continuous concentrations of 25(OH)D or total intake of vitamin D.
We investigated whether certain periodontal disease risk factors were confounding the crude associations between vitamin D status and the history of any tooth loss, tooth loss due to periodontal disease, and tooth loss due to caries. Factors associated with both vitamin D status and tooth loss at p-values ≤0.20 were considered as potential confounders in multivariable models. Potential confounders were added to the crude models separately to assess their influence on the ORs in a step-wise fashion and included in the multivariable model if they changed the OR 10% or more. We adjusted for the most inclusive group of confounders from multivariable models built for the odds of any tooth loss, tooth loss due to periodontal disease, and tooth loss due to caries. Age, annual family income, smoking status, frequency of dental visits, and waist circumference influenced ORs the greatest (>10%). We also adjusted for recreational physical activity as it appeared to confound the model, but not to the extent as the other noted covariates. The same model was used for our cross-sectional analyses of history of tooth loss and vitamin D intake as well as for all prospective analyses. For all analyses, P-values (two-tailed) of <0.05 were considered statistically significant.
We explored if the association between any tooth loss and 25(OH)D concentrations was modified by total calcium intake in either the cross-sectional or prospective analyses. Vitamin D status was defined as deficient/inadequate [25(OH)D <50 nmol/L] and adequate [25(OH)D ≥50 nmol/L]. Categories of calcium intake were chosen based on the IOM’s Recommended Dietary Allowance (RDA)21 of 1200 mg/day for females 51 years and older: <1200 and ≥1200 mg/day. We examined the odds of any tooth loss by the joint effects of total calcium intake and vitamin D status using calcium intake <1200 mg/day and 25(OH)D<50 nmol/L as the referent group. If the p-value for the interaction product term added to the multivariable model was <0.10 we considered the multiplicative interaction statistically significant.
We performed all analyses by using a statistical software program.**
RESULTS
In our sample 65.8% (n=513) of women had adequate vitamin D status (≥50 nmol/L) and 9.0% (n=70) were deficient (<30 nmol/L) (Table 1). Women with deficient compared to adequate status were, on average, older had lower annual family income and were less likely to be Caucasian. The highest percentage of current smokers was in vitamin D deficient women. On average, women with deficient status had larger BMIs, waist circumferences, and waist to hip ratios, reported less recreational physical activity, lower vitamin D intake, and a lower alcohol consumption. There was a greater proportion of never users of hormone therapy among women with deficient vitamin D status than in women with inadequate or adequate vitamin D status. The greatest percentage of bone drugs users was among women with adequate vitamin D status (≥50 to <75 nmol/L) and the lowest among those with inadequate status. Women with deficient vitamin D status were more likely to have osteoporosis and women with deficient or inadequate status were more likely to have diabetes, as compared to women with adequate status. Women with deficient vitamin D status were also less likely to visit the dentist and brush their teeth as frequently, and had, on average, a lower number of teeth present compared to women with adequate status. Results of participant’s characteristics by history of tooth loss are shown in Supplemental Table 1.
Table 1.
Characteristics by clinically defined categories of plasma 25(OH)D concentrations (nmol/L) at study baseline (1997–2000): The Women’s Health Initiative Osteoporosis and Periodontal Disease (OsteoPerio) Study (n=780)
| Vitamin D status defined by categories of 25(OH)D | |||||
|---|---|---|---|---|---|
| Characteristic | Deficient (< 30) n = 70 (9.0%) |
Inadequate (30 to < 50) n = 197 (25.3%) |
Adequate (50 to < 75) n = 338 (43.3%) |
Adequate (≥ 75) n = 175 (22.4%) |
p-value* |
| Demographic Factors | |||||
| Age (years), mean ± SD† | 68.3 ± 7.3 | 66.9 ± 7.2 | 67.1 ± 7.1 | 65.3 ± 7.1 | 0.007 |
| Race/Ethnicity, n (% Caucasian) | 66 (94.3) | 187 (94.9) | 333 (98.5) | 172 (98.3) | 0.028 |
| Education, n (%) | 0.237 | ||||
| High school | 20 (29.4) | 45 (22.8) | 74 (22.0) | 27 (16.0) | |
| College | 31 (45.6) | 86 (43.7) | 157 (46.7) | 77 (45.6) | |
| Post-graduate | 17 (25.0) | 66 (33.5) | 105 (31.3) | 65 (38.5) | |
| Annual Family Income, n (%) | 0.015 | ||||
| < 35, 000 | 37 (52.9) | 86 (43.7) | 139 (41.1) | 56 (32.0) | |
| ≥ 35, 000 | 33 (47.1) | 111 (56.4) | 199 (58.9) | 119 (68.0) | |
| Lifestyle Factors | |||||
| Smoking Status, n (%) | <0.001 | ||||
| Never | 35 (50.0) | 109 (55.3) | 177 (52.4) | 94 (53.7) | |
| Past | 26 (37.1) | 81 (41.1) | 156 (46.2) | 76 (43.4) | |
| Current | 9 (12.9) | 7 (3.6) | 5 (1.5) | 5 (2.9) | |
| Pack Years of Smoking, n (%) | 0.065 | ||||
| Never | 35 (50.0) | 109 (56.8) | 177 (53.6) | 94 (54.3) | |
| Light (< 8) | 7 (10.0) | 20 (10.4) | 61 (18.5) | 28 (16.2) | |
| Moderate (8 to <26) | 9 (12.9) | 33 (17.9) | 47 (14.2) | 29 (16.7) | |
| Heavy (≥ 26) | 19 (27.1) | 30 (15.6) | 45 (13.6) | 22 (12.7) | |
| Body Mass Index (kg/m2), mean ± SD | 28.1 ± 5.7 | 28.5 ± 5.3 | 26.2 ± 4.6 | 25.4 ± 4.8 | <0.0001 |
| Waist Circumference (cm), mean ± SD | 89.4 ± 13.6 | 89.0 ± 12.5 | 83.5 ± 11.4 | 80.1 ± 11.2 | <0.0001 |
| Waist to Hip Ratio, mean ± SD | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | <0.0001 |
| Recreational Physical Activity, (MET hrs/wk)‡, n (%) | <0.0001 | ||||
| None | 23 (32.9) | 35 (17.8) | 42 (12.4) | 21 (12.0) | |
| ≤ 10 | 27 (38.6) | 74 (37.6) | 115 (34.0) | 50 (28.6) | |
| > 10 | 20 (28.6) | 88 (44.7) | 181 (53.6) | 104 (59.4) | |
| Vitamin D Intake (IU/day), mean ± SD | |||||
| Dietary Intake | 172.2 ± 120.9 | 202.1 ± 140.7 | 217.3 ± 144.6 | 203.2 ± 119.4 | 0.081 |
| Supplemental Intake | 103.4 ± 183.0 | 237.3 ± 244.9 | 354.9 ± 281.2 | 425.6 ± 308.0 | < 0.0001 |
| Total Intake§ | 277.1 ± 221.1 | 440.8 ± 281.3 | 571.2 ± 320.1 | 628.8 ± 319.9 | < 0.0001 |
| Alcohol Consumption (servings/week), mean ± SD | 2.8 ± 5.2 | 2.6 ± 4.8 | 3.0 ± 4.3 | 4.0 ± 5.2 | 0.035 |
| Hormone Therapy Use, n (%) | 0.003 | ||||
| Never | 36 (51.4) | 71 (36.0) | 105 (31.1) | 47 (26.9) | |
| Past | 14 (20.0) | 43 (21.8) | 71 (21.0) | 31 (17.7) | |
| Current | 20 (28.6) | 83 (42.1) | 162 (47.9) | 97 (55.4) | |
| Current Bone Drugs Use¶, n (%Yes) | 6 (8.6) | 10 (5.1) | 39 (11.6) | 12 (6.9) | 0.054 |
| Comorbid Conditions | |||||
| Worst site T-score, n (%) | 0.003 | ||||
| Normal (T≥−1.0); | 11 (15.7) | 31 (15.7) | 49 (14.5) | 32 (18.3) | |
| Low bone density (−2.5<T<−1.0) | 17 (24.3) | 91 (46.2) | 163 (48.2) | 88 (50.3) | |
| Osteoporosis (T≤−2.5) | 42 (60.0) | 75 (38.1) | 126 (37.3) | 55 (31.4) | |
| Self-reported History of Diabetes, n (%Yes) | 5 (7.1) | 18 (9.1) | 10 (3.0) | 3 (1.7) | 0.001 |
| Self-reported History of Thyroid Disease, n (% Yes) | 19 (27.1) | 36 (18.3) | 74 (21.9) | 33 (18.9) | 0.373 |
| Oral Health Measures | |||||
| Days Since Last Dental Cleaning, n (% >90 days) | 46 (65.7) | 121 (61.4) | 212 (62.7) | 106 (60.6) | 0.884 |
| Frequency of Dental Visits, n (%) | 0.016 | ||||
| Never or only with a Problem | 15 (21.4) | 20 (10.2) | 26 (7.7) | 12 (6.9) | |
| Once a year | 9 (12.8) | 32 (16.2) | 46 (13.6) | 25 (14.3) | |
| More than once a year | 46 (65.7) | 145 (73.6) | 266 (78.7) | 138 (78.9) | |
| Frequency of Flossing, n (%) | 0.145 | ||||
| Not every day | 13 (18.6) | 43 (21.8) | 50 (14.8) | 36 (20.7) | |
| Once a week | 4 (5.7) | 17 (8.6) | 36 (10.7) | 19 (10.9) | |
| More than once a week | 27 (38.6) | 63 (32.0) | 100 (29.6) | 40 (23.0) | |
| Everyday | 26 (37.1) | 74 (37.6) | 152 (45.0) | 79 (45.4) | |
| Frequency of Tooth Brushing, n (%) | 0.013 | ||||
| Not every day | 2 (2.9) | 3 (1.5) | 1 (0.3) | 1 (0.6) | |
| One time a day | 24 (34.3) | 57 (28.9) | 64 (18.9) | 42 (24.0) | |
| Two times a day | 31 (44.3) | 104 (52.8) | 200 (59.2) | 88 (50.3) | |
| More than two times a day | 13 (18.6) | 33 (16.8) | 73 (21.6) | 44 (25.1) | |
| Number of Teeth Present, mean ± SD | 20.5 ± 7.3 | 23.2 ± 5.3 | 23.3 ± 5.3 | 23.9 ± 4.1 | < 0.0001 |
P-values from ANOVA for continuous variables and Chi-square and Fisher’s exact test for categorical variables.
SD: standard deviation.
MET: metabolic equivalents of task (1 MET is equivalent to a metabolic rate consuming 1 kilocalorie per kilogram of body weight per hour).
Dietary and supplemental vitamin D intake together.
Current bone drug use include women taking bone drugs Alendronate or Calcitonin.
Cross-Sectional Analysis of Vitamin D Status and History of Tooth Loss
The adjusted OR [95% CI] for the history of any tooth loss was 1.53 [0.67, 3.50] among women with an adequate (25[OH]D ≥75 nmol/L) compared to deficient (25[OH]D <30 nmol/L) vitamin D status (Table 2) and 1.24 [0.82–1.87] for women with an adequate (25[OH]D ≥50 nmol/L) compared to deficient or inadequate (25[OH]D <50 nmol/L) vitamin D status. The p for trend for was 0.049. We observed no association between vitamin D status and tooth loss due to periodontal disease (adjusted OR [95% CI] =1.10 [0.32, 3.76] for adequate [25[OH]D ≥75 nmol/L] compared to deficient [25[OH]D <30 nmol/L] vitamin D status with a p-for trend=0.189) (Table 2). Similar to the results for any tooth loss, we observed an increased odds for tooth loss due to caries (adjusted OR [95% CI]=1.72 [0.74, 4.00] for adequate [25[OH]D ≥75 nmol/L] compared to deficient [25[OH]D <30 nmol/L] vitamin D status with a p-for trend=0.045). Results were consistent when we estimated the odds of the history of tooth loss due to periodontal disease or caries among women with 25(OH)D concentrations defined as adequate (25[OH]D ≥50 nmol/L) compared to deficient or inadequate (25[OH]D <50 nmol/L) (data not shown). Adjustment of the caries analyses for sugar intake, a potential risk factor for caries,22, 23 did not influence results (data not shown). The association between 25(OH)D and history any tooth loss was not modified by total calcium intake (Supplemental Table 2).
Table 2.
Odds ratios (OR) and 95% confidence intervals (95% CIs) for prevalent tooth loss by vitamin D status determined at study baseline (1997–2000): The Women’s Health Initiative Osteoporosis and Periodontal Disease (OsteoPerio) Study
| Vitamin D status defined by categories of 25(OH)D (nmol/L) (n=780) | |||||
|
Deficient (<30) OR (95% CI) |
Inadequate (30 to <50) OR (95% CI) |
Adequate (50 to <75) OR (95% CI) |
Adequate (≥75) OR (95% CI) |
p-for trend* | |
| Any Tooth Loss† | |||||
| N outcome/total | 59/70 | 156/197 | 267/338 | 146/175 | |
| Unadjusted model | 1.0 | 0.71 (0.34–1.47) | 0.70 (0.35–1.41) | 0.94 (0.44–2.00) | 0.779 |
| Age adjusted model | 1.0 | 0.77 (0.37–1.61) | 0.75 (0.37–1.51) | 1.15 (0.53–2.48) | 0.361 |
| Multivariable model‡ | 1.0 | 0.81 (0.37–1.74) | 0.88 (0.42–1.87) | 1.53 (0.67–3.50) | 0.049 |
| Tooth Loss due to Periodontal Disease§ | |||||
| N outcome/total | 11/22 | 14/55 | 30/101 | 15/44 | |
| Unadjusted model | 1.0 | 0.34 (0.12–0.96) | 0.42 (0.17–1.08) | 0.52 (0.18–1.47) | 0.799 |
| Age adjusted model | 1.0 | 0.37 (0.13–1.08) | 0.46 (0.17–1.24) | 0.73 (0.24–2.19) | 0.626 |
| Multivariable model‡ | 1.0 | 0.39 (0.12–1.26) | 0.60 (0.20–1.80) | 1.10 (0.32–3.76) | 0.189 |
| Tooth Loss due to Caries‖ | |||||
| N outcome/total | 48/59 | 142/183 | 237/308 | 131/160 | |
| Unadjusted model | 1.0 | 0.79 (0.38–1.67) | 0.77 (0.38–1.55) | 1.04 (0.48–2.23) | 0.714 |
| Age adjusted model | 1.0 | 0.86 (0.40–1.83) | 0.83 (0.40–1.71) | 1.26 (0.57–2.76) | 0.341 |
| Multivariable model‡ | 1.0 | 0.93 (0.43–2.05) | 1.02 (0.47–2.19) | 1.72 (0.74–4.00) | 0.045 |
|
Vitamin D status defined by categories of total vitamin D intake (IU/day) (n=774) |
|||||
|
< 400 OR (95% CI) |
400 to < 600 OR (95% CI) |
≥ 600 OR (95% CI) |
p-for trend* | ||
| Any Tooth Loss¶ | |||||
| N outcome/total | 236/284 | 145/179 | 242/311 | ||
| Unadjusted model | 1.0 | 0.87 (0.53–1.41) | 0.71 (0.47–1.08) | 0.019 | |
| Age adjusted model | 1.0 | 0.88 (0.54–1.44) | 0.76 (0.50–1.1.5) | 0.029 | |
| Multivariable model‡ | 1.0 | 1.01 (0.61–1.67) | 0.79 (0.52–1.21) | 0.037 | |
| Tooth Loss due to Periodontal Disease# | |||||
| N outcome/total | 30/78 | 16/50 | 24/93 | ||
| Unadjusted model | 1.0 | 0.75 (0.36–1.59) | 0.56 (0.29–1.07) | 0.056 | |
| Age adjusted model | 1.0 | 0.76 (0.35–1.65) | 0.62 (0.32–1.22) | 0.106 | |
| Multivariable model‡ | 1.0 | 0.87 (0.37–2.04) | 0.53 (0.25–1.12) | 0.080 | |
| Tooth Loss due to Caries** | |||||
| N outcome/total | 206/254 | 129/163 | 218/287 | ||
| Unadjusted model | 1.0 | 0.88 (0.54–1.45) | 0.74 (0.49–1.12) | 0.028 | |
| Age adjusted model | 1.0 | 0.91 (0.55–1.50) | 0.78 (0.51–1.19) | 0.040 | |
| Multivariable model‡ | 1.0 | 1.03 (0.62–1.72) | 0.82 (0.53–1.27) | 0.048 | |
Logistic regression was used to calculate p for linear trend using continuous 25(OH)D plasma concentrations or total vitamin D intake.
There are 780 women in this analysis, of whom 628 had any history of tooth loss (due to periodontal disease, caries, or both), and 152 had no history of tooth loss due to any reasons.
Multivariable model: adjusted for age, annual family income, smoking status, frequency of dental visits, waist circumference and recreational physical activity.
There are 222 women in this analysis, of whom 70 had a history of tooth loss due to periodontal disease (with or without a history of tooth loss due to caries), and 152 had no history of tooth loss due to any reasons.
There are 710 women in this analysis, of whom 558 had a history of tooth loss due to caries and no history of tooth loss due to periodontal disease, and 152 had no history of tooth loss due to any reasons.
There are 774 women in this analysis, of whom 623 had any history of tooth loss (due to periodontal disease, caries, or both), and 151 had no history of tooth loss due to any reasons.
There are 221 women in this analysis, of whom 70 had a history of tooth loss due to periodontal disease (with or without a history of tooth loss due to caries), and 151 had no history of tooth loss due to any reasons.
There are 704 women in this analysis, of whom 553 had a history of tooth loss due to caries and no history of tooth loss due to periodontal disease, and 151 had a history of tooth loss due to any reasons.
We also examined the association between the history of any tooth loss and vitamin D status defined by total intake of vitamin D. Adjusted analyses showed a lower, but not statistically significant, odds of a history of any tooth loss, tooth loss due to periodontal disease, and tooth loss due to caries among women with high (≥600 IU) compared to low (<400 IU) total vitamin D intake. Analyses using continuous intake of total vitamin D showed a statistically significantly lower odds of the history of any tooth loss and tooth loss due to caries with increasing vitamin D intake (p-trend=0.037 and 0.048, respectively).
Prospective Analysis of Vitamin D Status and Incident Tooth Loss
There was no statistically significant association observed between vitamin D status defined by 25(OH)D or total vitamin D intake and the incidence of any tooth loss, tooth loss due to periodontal disease or tooth loss due to caries (Table 3). The association between 25(OH)D and incidence of any tooth loss was not modified by total calcium intake (Supplemental Table 2).
Table 3.
Odds ratios (OR) and 95% confidence intervals (95% CIs) for incident tooth loss by vitamin D status determined at study baseline (1997–2000)): The Women’s Health Initiative Osteoporosis and Periodontal Disease (OsteoPerio) Study
| Vitamin D status defined by categories of 25(OH)D (nmol/L) (n=472) | |||
|
Deficient/Inadequate (<50) OR (95% CI) |
Adequate (≥50) OR (95% CI) |
p-for trend* | |
| Any Tooth Loss† | |||
| N outcome/total | 126/156 | 250/316 | |
| Unadjusted model | 1.0 | 0.90 (0.56–1.46) | 0.765 |
| Age adjusted model | 1.0 | 0.93 (0.57–1.51) | 0.515 |
| Multivariable model‡ | 1.0 | 1.07 (0.62–1.85) | 0.111 |
| Tooth Loss due to Periodontal Disease§ | |||
| N outcome/total | 13/43 | 35/101 | |
| Unadjusted model | 1.0 | 1.22 (0.57–2.64) | 0.671 |
| Age adjusted model | 1.0 | 1.33 (0.60–2.96) | 0.368 |
| Multivariable model‡ | 1.0 | 1.61 (0.67–3.89) | 0.099 |
| Tooth Loss due to Caries‖ | |||
| N outcome/total | 113/143 | 215/281 | |
| Unadjusted model | 1.0 | 0.87 (0.53–1.41) | 0.804 |
| Age adjusted model | 1.0 | 0.89 (0.54–1.47) | 0.586 |
| Multivariable model‡ | 1.0 | 1.01 (0.58–1.77) | 0.142 |
| Vitamin D status defined by categories of total vitamin D intake (IU/day) (n=470) | |||
|
< 600 OR (95% CI) |
≥ 600 OR (95% CI) |
p-for trend* | |
| Any Tooth Loss¶ | |||
| N outcome/total | 224/276 | 151/194 | |
| Unadjusted model | 1.0 | 0.82 (0.52–1.28) | 0.292 |
| Age adjusted model | 1.0 | 0.86 (0.54–1.36) | 0.367 |
| Multivariable model‡ | 1.0 | 0.90 (0.56–1.44) | 0.567 |
| Tooth Loss due to Periodontal Disease# | |||
| N outcome/total | 26/78 | 22/65 | |
| Unadjusted model | 1.0 | 1.02 (0.51–2.05) | 0.838 |
| Age adjusted model | 1.0 | 1.35 (0.64–2.86) | 0.566 |
| Multivariable model‡ | 1.0 | 0.99 (0.43–2.28) | 0.956 |
| Tooth Loss due to Caries** | |||
| N outcome/total | 198/250 | 129/172 | |
| Unadjusted model | 1.0 | 0.79 (0.50–1.25) | 0.246 |
| Age adjusted model | 1.0 | 0.82 (0.51–1.31) | 0.304 |
| Multivariable model‡ | 1.0 | 0.87 (0.54–1.42) | 0.534 |
Logistic regression was used to calculate p for linear trend using continuous 25(OH)D plasma concentrations or total vitamin D intake.
There are 472 women in this analysis, of whom 376 had any incident tooth loss (due to periodontal disease, caries, or both), and 96 had no incident tooth loss due to any reasons.
Multivariable model: adjusted for age, annual family income, smoking status, frequency of dental visits, waist circumference and recreational physical activity.
There are 144 women in this analysis, of whom 48 had incident tooth loss due to periodontal disease (with or without incident tooth loss due to caries), and 96 had no incident tooth loss due to any reasons.
There are 424 women in this analysis, of whom 328 had incident tooth loss due to caries and no incident tooth loss due to periodontal disease, and 96 had no incident tooth loss due to any reasons.
There are 470 women in this analysis, of whom 375 had any incident tooth loss (due to periodontal disease, caries, or both), and 95 had no incident tooth loss due to any reasons.
There are 143 women in this analysis, of whom 48 had incident tooth loss due to periodontal disease (with or without incident tooth loss due to caries), and 95 had no incident tooth loss due to any reasons.
There are 422 women in this analysis, of whom 327 had incident tooth loss due to caries and no incident tooth loss due to periodontal disease, and 95 had no incident tooth loss due to any reasons.
DISCUSSION
In this study of postmenopausal women, our data do not support a protective association between 25(OH)D and the prevalence of, or 5-year incidence of, tooth loss due to periodontal disease or caries. Further, our results were not modified by total calcium intake. We originally hypothesized that having adequate vitamin D status, as defined by 25(OH)D concentrations, would reduce risk of tooth loss due to periodontal disease through its anti-inflammatory, immunologic and bone related properties,12, 13, 24–27 however, our study results do not support this hypothesis.
Previous studies do support a role of vitamin D in preventing tooth loss.8–10 Results from a 3-year randomized, placebo controlled supplementation trial in elderly individuals demonstrated that calcium and vitamin D supplementation was associated with lower odds of self-reported tooth loss.8 This study was limited by its small sample size (N=145) and inability to separate the effects of calcium from vitamin D supplementation. A 20-year cohort study of male health professions found a significant inverse association between a predicted 25(OH)D score and incident, self-reported tooth loss.9 Predictive scores for 25(OH)D do not strongly correlated with vitamin D status from 25(OH)D measures and may reflect a healthy lifestyle score rather than vitamin D status.28 The prospective, population-based Study of Health in Pomerania10 showed that higher serum 25(OH)D concentrations were associated with reduced risk for tooth loss, assessed by dental examiners. This study was conducted in Germany where foods are not fortified for vitamin D.29 They had a greater prevalence of participants with 25(OH)D<50 nmol/L (60% versus 34% in our study). In these studies,8–10 reasons for tooth loss, which could have provided additional understanding of the association between the vitamin D and tooth loss, were not assessed.
We hypothesized that adequate vitamin D status would prevent against tooth loss due to caries based on its anti-microbial properties,7, 30 however, in our study we observed an increased odds of the history of tooth loss due to caries in women with adequate compared to deficient vitamin D status (p-for trend=0.045). Adjustment of our multivariable analyses for recreational physical activity and waist circumference, which are strong predictors of vitamin D status,28 may have led to over-adjustment, and further strengthened rather than attenuated the observed direct association with tooth loss due to caries.
A recent, systematic meta-analyses of studies on vitamin D and different health outcomes suggested a possible protective association between 25(OH)D and dental caries in children.31 Literature on the association between vitamin D and risk of dental caries in adult populations is scarce. Previous studies from the 1950’s observed inverse associations between sun exposure, as a proxy for vitamin D status, and caries, but these studies were limited to males.32, 33 We speculate that our observed increased odds of the history of tooth loss due to caries was spurious and hypothesize that we did not observe a protective association because tooth loss due to caries likely occurred at an earlier stage in life than the postmenopausal period.31 We acknowledge that vitamin D status during the postmenopausal period may not strongly correlate with status at an earlier stage in life. We also propose that the observed increased odds of the history of any tooth loss is explained by the large proportion of women who reported a history of tooth loss due to caries. Of the 628 women defined as having a history of any tooth loss, 558 (88.9%) reported a history of losing teeth due to caries. This may be due to over-reporting of tooth loss due to caries as suggested by our self-reported tooth loss validation study.19 At the same time, our prospective analysis of the association between vitamin D status and incident tooth loss due to caries found no statistically significant associations and does not support the association observed in the cross-sectional analysis.
We also explored the association between tooth loss and total vitamin D intake from foods and supplements. Our cross-sectional analyses found an inverse relationship between total vitamin D intake and the history of any tooth loss and tooth loss due to caries. Our prospective analyses did not confirm these findings, however the results did trend towards a decreased odds of any incident tooth loss or incident tooth loss due to caries with higher compared to lower total vitamin D intake. As these results differ from our findings with 25(OH)D, it is possible that they reflect a protective effect of healthy diets or supplement intake on tooth loss among postmenopausal women, rather than current vitamin D status.
Taken together, our study results correlate with other recent findings within the Buffalo OsteoPerio Study where an inverse association between 25(OH)D and gingival bleeding, an acute measure of periodontal inflammation has been observed,6 and no association between 25(OH)D and alveolar crestal height, a chronic measure of oral bone loss, or changes in alveolar crestal height over time has been reported.6, 34 Our study’s findings in the OsteoPerio study suggest that vitamin D may be more influential during the earlier, acute phase of periodontal disease than in prevention of chronic periodontal disease and subsequent tooth loss. It is also possible that our follow-up time was not long enough to observe an association between vitamin D status and incident tooth loss.
Our study may be restricted by a lack of a sufficient sample size to detect a more precise relationship between vitamin D status and tooth loss due to periodontal disease. Additionally, we had a low percentage of women with deficient vitamin D status (9% of 780). The OsteoPerio sample is primarily Caucasian, well-educated women with higher socioeconomic status and good self-reported oral hygiene practices and overall health, limiting the generalizability to other populations. The self-report of the reasons for tooth loss may have led to misclassification. At the same time, standardized protocols were utilized to minimize measurement error in data collection. These data were obtained from the participants during a comprehensive, standardized oral examination by trained oral health examiners. It is unlikely that misclassification of tooth loss or reasons for tooth loss was differential by vitamin D status.
Important strengths of our study include the use of plasma 25(OH)D, a biomarker which reflects vitamin D from all sources including sunlight, diet and supplements. Our study sample is a relatively large group of postmenopausal women, who are at risk for both vitamin D insufficiency and advanced periodontitis. We also had extensive information on potential confounders of the association between vitamin D status and tooth loss. We were able to explore the association between vitamin D status and reason for tooth loss, which was not previously done in other studies, and we were able to examine these associations at baseline and prospectively over 5 years of follow-up.
CONCLUSION
The results of our presented study suggest that, in a sample of US postmenopausal women, vitamin D status, assessed by 25(OH)D concentrations or vitamin D intake, is not associated with tooth loss due to periodontal disease or caries.
Supplementary Material
Acknowledgments
This research is supported by NIH grants 1R21DE020918 (awarded to AE Millen) and 1R01DE13505 (awarded to Jean Wactawski-Wende) from the National Institute of Dental and Craniofacial Research (NIDCR) and a grant awarded to Jean Wactawski-Wende from the Department of Defense (DAMD179616319).
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Conflict of Interest Statement:
No authors have financial disclosures or conflicts of interest to report.
Key findings: No association was observed between vitamin D status and tooth loss in the Buffalo OsteoPerio Study cohort of postmenopausal women.
Liaison 25-OH Vitamin D Total Assay, DiaSorin, Stillwater, MN.
Version 9.2 for Windows, SAS Institute, Inc., Cary, NC.
REFERENCES
- 1.Dye BA, Li X, Beltran-Aguilar ED. Selected oral health indicators in the United States, 2005–2008. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Emami E, de Souza RF, Kabawat M, Feine JS. The impact of edentulism on oral and general health. Int J Dent. 2013;2013:498305. doi: 10.1155/2013/498305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B, Liang J, Plassman BL, Remle C, Luo X. Edentulism trends among middle-aged and older adults in the United States: comparison of five racial/ethnic groups. Community Dent Oral Epidemiol. 2012;40:145–153. doi: 10.1111/j.1600-0528.2011.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver RC, Brown LJ. Periodontal diseases and tooth loss. Periodontol 2000. 1993;2:117–127. doi: 10.1111/j.1600-0757.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 5.Phipps KR, Stevens VJ. Relative contribution of caries and periodontal disease in adult tooth loss for an HMO dental population. J Public Health Dent. 1995;55:250–252. doi: 10.1111/j.1752-7325.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 6.Millen AE, Hovey KM, LaMonte MJ, et al. Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J Periodontol. 2013;84:1243–1256. doi: 10.1902/jop.2012.120445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant WB. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol. 2011;3:193–198. doi: 10.4161/derm.3.3.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ, Dietrich T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014;17:844–852. doi: 10.1017/S1368980013000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan Y, Samietz S, Holtfreter B, et al. Prospective study of serum 25-hydroxy Vitamin D and tooth loss. J Dental Res. 2014;93:639–644. doi: 10.1177/0022034514534985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 12.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 17.Meng JE, Hovey KM, Wactawski-Wende J, et al. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2012;21:916–924. doi: 10.1158/1055-9965.EPI-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Mai X, Wactawski-Wende J, Hovey KM, et al. Associations between smoking and tooth loss according to the reason for tooth loss: the Buffalo OsteoPerio Study. J Am Dent Assoc. 2013;144:252–265. doi: 10.14219/jada.archive.2013.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMonte MJ, Hovey KM, Genco RJ, Millen AE, Trevisan M, Wactawski-Wende J. Five-year changes in periodontal disease measures among postmenopausal females: the Buffalo OsteoPerio study. J Periodontol. 2013;84:572–584. doi: 10.1902/jop.2012.120137. [DOI] [PubMed] [Google Scholar]
- 21.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academy Press; 2011. pp. 1–14. Summary. [Google Scholar]
- 22.Moynihan PJ, Kelly SA. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2013;93:8–18. doi: 10.1177/0022034513508954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta P, Gupta N, Pawar AP, Birajdar SS, Natt AS, Singh HP. Role of sugar and sugar substitutes in dental caries: a review. ISRN Dent. 2013;2013:519421. doi: 10.1155/2013/519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebolt CF. Effect of vitamin D and calcium on periodontitis. J Periodontol. 2005;76:1576–1587. doi: 10.1902/jop.2005.76.9.1576. [DOI] [PubMed] [Google Scholar]
- 25.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 26.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17:348–352. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]
- 27.Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–557. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown J, Sandmann A, Ignatius A, Amling M, Barvencik F. New perspectives on vitamin D food fortification based on a modeling of 25(OH)D concentrations. Nutr J. 2013;12:151. doi: 10.1186/1475-2891-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K, Elias PM, Oda Y, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunning JM. The influence of latitude and distance from seacoast on dental disease. J Dent Res. 1953;32:811–829. doi: 10.1177/00220345530320061001. [DOI] [PubMed] [Google Scholar]
- 33.Hadjimarkos DM. Geographic variations of dental caries in Oregon. VII. Caries prevalence among children in the Blue Mountains region. J Pediatr. 1956;48:195–201. doi: 10.1016/s0022-3476(56)80165-0. [DOI] [PubMed] [Google Scholar]
- 34.Millen AE, Andrews CA, LaMonte MJ, et al. Vitamin D status and 5-year changes in periodontal disease measures among postmenopausal women: the Buffalo OsteoPerio Study. J Periodontol. 2014;85:1321–1332. doi: 10.1902/jop.2014.130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.