Abstract
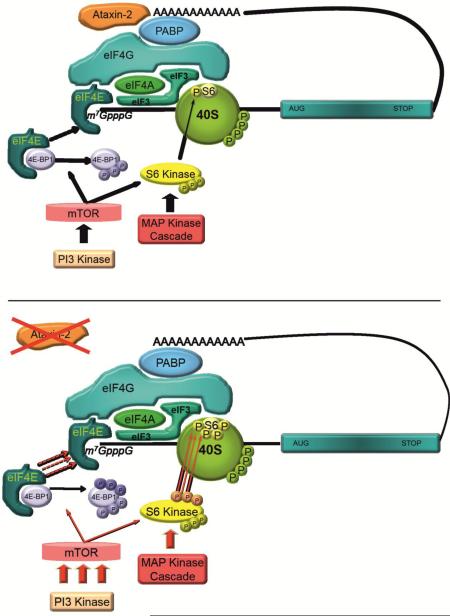
Ataxin-2 is a cytoplasmic protein, product of the ATXN2 gene, whose deficiency leads to obesity, while its gain-of-function leads to neural atrophy. Ataxin-2 affects RNA homeostasis, but its effects are unclear. Here, immunofluorescence analysis suggested that ataxin-2 associates with 48S pre-initiation components at stress granules in neurons and mouse embryonic fibroblast, but is not essential for stress granule formation. Coimmunoprecipitation analysis showed associations of ataxin-2 with initiation factors, which were concentrated at monosome fractions of polysome gradients like ataxin-2, unlike its known interactor PABP. Mouse embryonic fibroblasts lacking ataxin-2 showed increased phosphorylation of translation modulators 4E-BP1 and ribosomal protein S6 through the PI3K/mTOR pathways. Indeed, human neuroblastoma cells after trophic deprivation showed a strong induction of ATXN2 transcript via mTOR inhibition. Our results support the notion that ataxin-2 is a nutritional stress-inducible modulator of mRNA translation at the pre-initiation complex.
Graphical Abstract
Introduction
The ataxin-2 gene (ATXN2) was found to underlie Spinocerebellar Ataxia type 2 (SCA2) and was localized to chromosome 12q24.1 [1-4]. SCA2 is a neurodegenerative disease, which usually manifests with a cerebellar syndrome or alternatively with a Parkinsonian midbrain syndrome, while later stages also affect the brainstem, spinal cord and thalamus [5-11]. The unstable expansion of a polyglutamine (polyQ) domain in the disease protein leads to a toxic gain-of-function pathogenesis, through the accumulation and aggregation of ataxin-2 plus the sequestration of crucial interactor molecules such as the poly(A)-binding protein, PABP [12, 13]. The human ATXN2 gene contains a (CAG) repeat of normally 22-23 units within exon 1; the presence of more than 31 triplets usually causes the clinically manifest multi-system neurodegeneration known as SCA2 with autosomal dominant inheritance, while alleles with 27 to 33 triplets have reduced penetrance and contribute to the risk of motor neuron disease [14-18]. Conversely, the loss-of-function of ataxin-2 leads to obesity, dyslipidemia and insulin resistance in mouse, and also in humans some gene variants at the ATXN2 chromosomal locus have been associated with hypertension and diabetes mellitus in several genome-wide association studies [19-21].
Overall, the cellular function of ataxin-2 remains unclear. This 140-kDa cytoplasmic protein is localized at the rough endoplasmic reticulum (rER) and is expressed in select neuron populations of the brain and in peripheral cells such as hepatocytes [22-24]. Several lines of evidence implicate ataxin-2 in growth receptor regulation and RNA metabolism [25-28]. Recent expression profiling efforts in Atxn2-knockoout mouse tissues demonstrated a selective downregulation of mitochondrial matrix components global proteome profiles and a preferential downregulation of calcium homeostasis factors together with an upregulation of mRNA translation factors in global transcriptomics [29-31]. The structure of ataxin-2 contains a PAM2 motif which mediates association with the poly(A)-binding protein PABP [12, 13, 32], thought to promote ataxin-2 association with polyribosomes [33]. Ataxin-2 structure also contains two globular domains named Lsm (‘Like Sm’ domain) and LsmAD (Lsm-associated domain), that were shown to mediate direct interaction with RNA [33, 34].
During cellular stress, ataxin-2 relocalizes with PABP and RNA from the rER to cytosolic foci of ribonucleoprotein (RNP) quality control, which were named stress granules (SG) and are usually identified by the presence of the marker protein TIA-1 [28]. In such periods when the high demand for damage-repair is at odds with low availability of energy, the eukaryotic cells reprogram their translational machinery to allow the selective expression of proteins required for viability, e.g., immediate early genes encoding molecular chaperones [35]. SG are composed largely of stalled 48S pre-initiation complexes and contain mRNA, small ribosomal subunits, eIF3, eIF4E, eIF4G, and PABP as their core components [36]. Furthermore, they contain TIA-1, an RNA-binding protein that relocalizes from the nucleus during stress and promotes the assembly of cytosolic SG, associating with mRNA subsets and repressing their translation [37].
The yeast ortholog of ATXN2, Pbp1, is also an interactor of poly(A) binding protein and modulates RNA processing, relocalizing to SG in periods of cell stress [38, 39]. Recent reports showed a role of Pbp1 in the regulation of mTOR signals that control translation [40, 41] and implicated it in the stress-dependent translational regulation of precursor proteins destined to mitochondria [42].
To gain insight into its cellular functions, we now analyzed ataxin-2 together with other stress granule components regarding colocalizations, cosedimentation, protein interactions and phosphorylation changes, using rat primary neurons, mouse embryonal fibroblasts (MEFs) from ataxin-2 knock-out (KO) animals, as well as human HEK-293 and SH-SY5Y neuroblastoma cells.
Results
Immunofluorescence studies show ataxin-2 presence in SG of cultured rat neurons
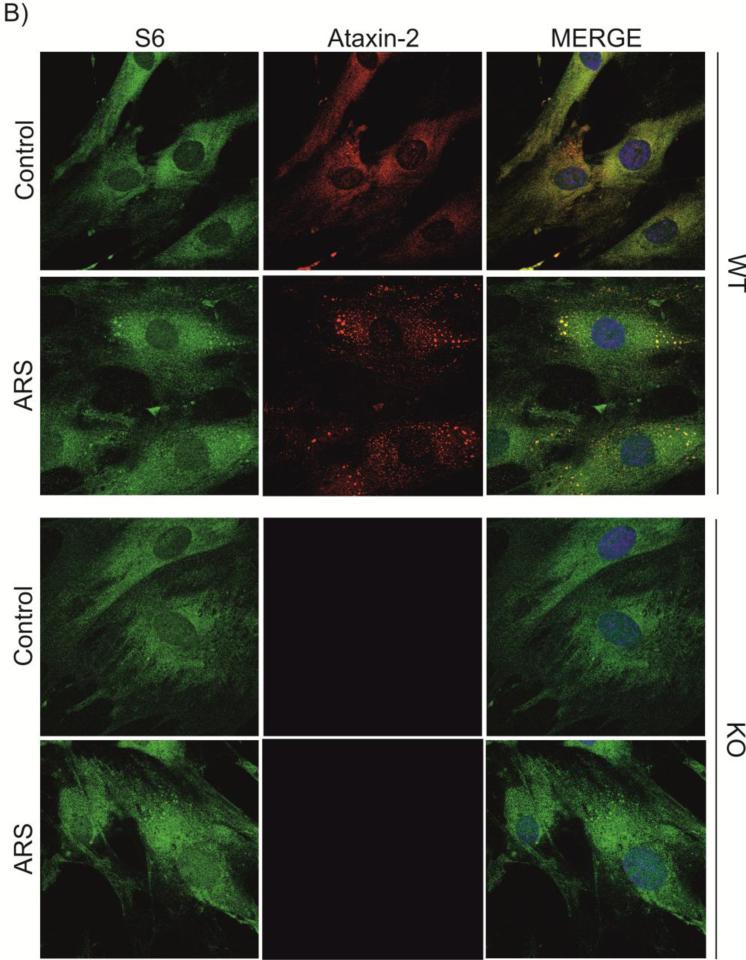
It has been reported that ataxin-2 is present in SG in different cell lines like COS-1 and HEK-293 [13, 28], but neurons have not been studied. Given the importance of ataxin-2 for neuronal function, we analyzed whether ataxin-2 is also present in SGs in rat hippocampal E18 neuron cultures. Consistent with earlier reports, in untreated cells the localization of ataxin-2 was predominantly in the cytoplasm [43] while TIA-1 as a stress granule marker was primarily nuclear [44] (Figure 1A, upper panels). When cells were stressed with arsenite for 45 min, TIA-1 was mobilized to the cytoplasm and localized in SG, together with ataxin-2 (Figure 1A, lower panels). Besides TIA-1, other established stress granule markers such as PABP, RPS6 (small ribosomal subunit 6), eIF4G, eIF4A1 and eIF3B (translation initiation factors) [36] colocalized with ataxin-2 after arsenite treatment (Figure 1 B-E and Supplementary Figure 1), confirming the association of ataxin-2 with SG under stress. Stress conditions also triggered the colocalization of PABP and TIA-1 in SGs (Figure 1F), demonstrating that TIA-1 and PABP were adequate neuronal SG markers in these experiments. In untreated cells, ataxin-2 also colocalized with PABP, RPS6, eIF4G, eIF4A1 (Figure 1, B-E) and eIF3B (Supplementary Figure 1) in the cytoplasm, suggesting that ataxin-2 is in proximity of these components of the translation pre-initiation complex under normal conditions.
Figure 1. Ataxin-2 colocalizes with TIA-1, PABP, RPS6, eIF4G and eIF4A1 in SG of hippocampal neuron cultures.
Hippocampal neurons after 7 days of culture (DIV7) were either left untreated (Control) or were stressed (ARS for arsenite, 0.5 mM for 45 min). The expression and subcellular localization of TIA-1 (A, F), PABP (B, F), RPS6 (C), eIF4G (D), eIF4A1 (E), and ataxin-2 (A-E) was monitored by immunofluorescence. Where indicated, the colocalization of two proteins was studied (Merge). DAPI staining was included to visualize the nuclei.
The absence of ataxin-2 does not impair the relocalization of TIA-1 to cytoplasm in stressed MEFs
Previous studies indicated that siRNA-mediated knock-down of ataxin-2 impaired SG assembly, reducing their number and size [28]. To gain a deeper understanding of this process, we used mouse embryonic fibroblasts (MEFs) derived from wild-type (WT) or ataxin-2-knock-out (KO) mice. Ataxin-2 and TIA-1 showed precise colocalization in SG of WT-MEFs (Figure 2A), as in the rat neuron cultures mentioned above (Figure 1A). When KO-MEFs were used, TIA-1 still redistributed to SG (Figure 2B). These results suggest that ataxin-2 is not necessary for TIA-1 redistribution to SG.
Figure 2. The absence of ataxin-2 in MEFs does not impair TIA-1 redistribution from nucleus to cytosolic SG.
MEFs from WT and KO were either left untreated (Control) or were stressed (ARS for arsenite, 0.5 mM for 45 min). The subcellular localization of TIA-1 was monitored by immunofluorescence. Where indicated, the colocalization of two proteins was studied (Merge). DAPI staining was included to visualize the nuclei.
The absence of ataxin-2 does not prevent the formation of RNP foci in stressed MEFs
In order to test whether ataxin-2 is essential for the assembly of RNP foci in the cytoplasm of stressed cells, we monitored SG formation using other cytosolic SG markers like PABP, RPS6, eIF4G, eIF4A1 and eIF3B. In WT-MEF cultures, ataxin-2 and PABP colocalized in untreated and stress conditions (Figure 3A, upper panel). In KO-MEFs, PABP still accumulated in stress granules (Figure 3A, lower panel), suggesting that SGs could assemble in absence of ataxin-2. This result was confirmed by studying the distribution of RPS6, eIF4G and eIF4A1 (Figure 3B-E), which localized at SGs despite the absence of ataxin-2 (for eIF3B see Supplementary Figure 2). In fact, in all cells treated with arsenite, SG formation was observed with the different markers used. Taken together, these results indicate that ataxin-2 is not essential for the assembly of cytosolic RNP foci.
Figure 3. The absence of ataxin-2 does not prevent the formation of SG.
MEFs from WT and KO were either left untreated (Control) or were stressed (ARS for arsenite, 0.5 mM for 45 min). The subcellular localization of PABP (A), RPS6 (B), eIF4G (C, E), eIF4A1 (D, E), and ataxin-2 (A-D) was monitored by immunofluorescence. Where indicated, the colocalization of two proteins was studied (Merge). DAPI staining was included to visualize the nuclei.
Coimmunoprecipitation experiments show ataxin-2 to associate with TIA-1 in stressed cells and to be part of the pre-initiation complex of translation also in unstressed cells
The findings on the subcellular localization of ataxin-2 prompted biochemical validation analyses. Given the limited amounts of commercially available rat neurons and the slow growth of primary fibroblasts, the following immunoprecipitation studies were performed in HEK-293 cell lysates from either untreated or arsenite-treated cells. Under both conditions ataxin-2 antibody coimmunoprecipitated ataxin-2 and TIA-1 (Figure 4A), indicating that ataxin-2 and TIA-1 may associate within the same complex. A similar observation was already reported for nuclear TDP-43, which relocalizes to the cytosol in stressed neurons and interacts with ataxin-2 via RNA-binding in an indirect manner [15]. It may be noteworthy that ataxin-2 can also shuttle to the nucleus and act on transcription in some cell contexts [45].
Figure 4. Ataxin-2 coimmunoprecipitates with TIA-1 and the translation pre-initiation complex.
HEK-293 cells were left untreated or stressed with 0.5 mM arsenite for 45 min. Ataxin-2 was immunoprecipitated from cell homogenates with the 12SCA2-2B6 monoclonal antibody against ataxin-2 (IP ATXN2). Immunoprecipitates were analyzed by PAGE and western blotting with detection by the relevant antibodies. (A) Similar amounts of TIA-1 were coimmunoprecipitated with ataxin-2 in unstressed and stressed cells. (B) Similar amounts of several constituents of the translation pre-initiation complex were coimmunoprecipitated with ataxin-2 in unstressed and stressed cells.
To validate the association of ataxin-2 with components of the cytosolic translation pre-initiation complex under normal conditions, ataxin-2 was immunoprecipitated in either untreated or arsenite-treated HEK-293 cell lysates, and immunoblot analysis was performed for eIF4G, eIF3B, eIF4A1 and RPS6 (Figure 4B). As shown, ataxin-2 forms a complex with these proteins under normal conditions. These associations may be direct protein interactions as between ataxin-2 and PABP, or they could be also indirect via RNA given that ataxin-2 contains an LsmAD domain. Although immunoprecipitation experiments cannot be quantified accurately, more of the ataxin-2-eIF4G complex was found under stress conditions (Figure 4B). These results indicate that one major physiological function of ataxin-2 involves the constitutive association with components of the 48S pre-initiation translation complex.
Polysome gradients show ataxin-2 and initiation factors enriched in monosome fractions
To test the relative distribution of ataxin-2 and initiation factors within the translation machinery of cells, we analyzed the assembly profile of ribosomes and actively translating polysomes in WT and KO MEFs. In WT MEFs, the peaks of unassembled 40S and 60S peaks were relatively small, and a large assembled 80S complex together with prominent peaks in the well-defined polysome tail were detected (Figure 5A). In KO MEFs, the peak height of the assembled 80S ribosomes was reduced, as were the peak heights and area of the polysomal fraction in the gradient (Figure 5B). This reduction of optical density signals, which represent nucleotides from ribosomes and polysomes in the high molecular weight fractions that reflect active translation, coincided with a slight reduction of PABP and RPS6 in the same fractions (Figure 5C, 5D). These reductions may simply be due to less vigorous growth of the clonal fibroblast lines under study and may be unrelated to ataxin-2 effects on translation. However, they certainly coincide with our previously published observation that the incorporation rate of radioactively labelled amino acids is diminished by 34% in Atxn2-KO MEFs [31]. It can be concluded safely from these data that ataxin-2 is not a prerequisite for ribosome assembly. Immunoblot analysis of the composition of different fractions from the polysome profiles of WT MEFs detected ataxin-2, eIF3B and eIF4A1 in the first several fractions (ataxin-2 was most enriched at the cytoplasmic peak and the 40S subunit fraction, while the initiation factors were most enriched in the 80S fraction) (Figure 5C), whereas PABP and RPS6 were present at all molecular weights including the polysomal fractions. In KO MEFs, no major changes of the protein expression profile in the fractions was observed, although there was a slight decrease in the amount of PABP, eIF3B and RPS6 at the polysomal tail fraction (Figure 5D). Although this reduction in polysome levels may be due to the native cellular state differences such as smaller KO cells, the equal distribution of PABP from monosomes to polysomes contrasts with the enrichment of ATXN2 in the monosome 40S fraction. These data are compatible with a scenario where ataxin-2 acts mostly in the 40S fraction at the pre-initiation stage of translation, quite in contrast to its known interactor PABP which is also necessary at the elongation stage for polysomes.
Figure 5. Polysome gradients reveal ataxin-2 distribution and KO effects.
(A, B) Polysome profiles on sucrose gradients: Lysates from WT-MEFs (A) and KO-MEFs (B) were layered on a 10-ml continuous sucrose gradient (10% to 50% sucrose). The profile of optical density representing the nucleotide content of the 40S, 60S, 80S ribosome components and polysomes are indicated.
(C, D) Western blot analyses of the distribution of ataxin-2 and pre-initiation complex protein components among fractions from polysomal profiles in WT-MEFs (C) and KO-MEFs (D).
Lack of ataxin-2 increases phosphorylation of RPS6 and 4E-BP1 through the PI3K/mTOR pathway
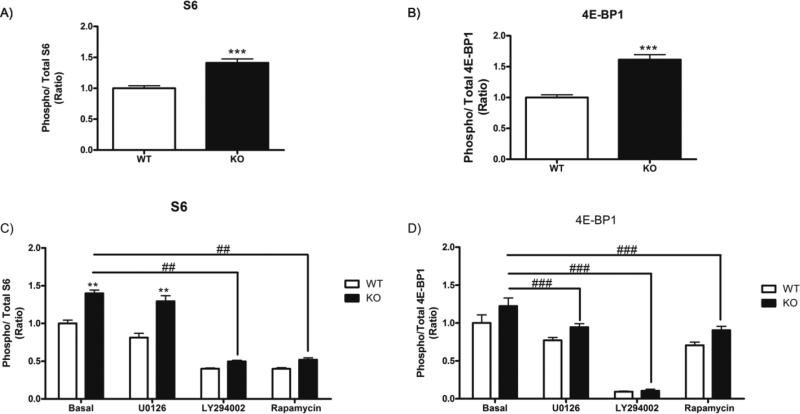
To elucidate the role of ataxin-2 for initiation factors beyond the established change in phosphorylation of RPS6, we went on to study also the phosphorylation of the initiation inhibitor protein 4E-BP1 (eukaryotic initiation repressor 4E-binding protein 1) in MEF cultures after 24 hours serum deprivation through an ELISA approach. A previous report from our team showed the ratio of phosphorylated RPS6 versus total RPS6 increased in serum-starved Atxn2-KO MEFs both at the basal state and after insulin administration [31]. As before, we now found elevated RPS6 phosphorylation in KO-MEFs (Figure 6A). In addition, the phosphorylation of 4E-BP1 was significantly increased in the absence of ataxin-2 (Figure 6B). These reductions are probably an ataxin-2 effect, but might alternatively be due to growth variability of the clonal fibroblast lines under study.
Figure 6. The absence of ataxin-2 induces increased phosphorylation of the ribosomal protein S6 and of the translation initiation repressor 4E-BP1, mainly through activation of the PI3K and the mTOR pathway.
Phosphorylation of RPS6 (A) and 4E-BP1 (B) in WT- and KO-MEFs after 24 h of serum deprivation was measured using an ELISA kit, with the graphs illustrating the ratio of phospho-RPS6 versus total-RPS6 levels and of phospho-4E-BP1 versus total 4E-BP1. (C-D): Thirty min before cell harvest, MEF cultures were treated with the MEK inhibitor U0126, the PI3K inhibitor LY294002 or the mTOR inhibitor rapamycin; 5 WT- and 5 KO-MEF lines were tested in each case for RPS6 phosphorylation (C) and 4E-BP1 phosphorylation (D), with each experiment being performed three times (* p<0.05, ** p<0.01, *** p<0.005; * significant between WT-MEFs and KO-MEFs; # significant between KO-MEF different treatments). The relative results are presented with arbitrary units.
The phosphorylation of RPS6 and 4E-BP1 can be induced by two well-studied signaling pathways: the Ras-MAPK pathway with MEK phosphorylating and activating ERK1/2 protein kinases, and the PI3K/Akt/mTOR pathway [46]. In order to define which of these pathways is implicated in the phosphorylation of RPS6 and 4E-BP1, MEFs were treated either with U0126 as a MEK inhibitor, or LY294002 as a PI3K inhibitor, or rapamycin as an inhibitor of mTOR. In the case of RPS6, the phosphorylation decreased significantly after incubation with LY294002 or rapamycin, an effect substantially enhanced in the absence of ataxin-2, whereas treatment with U0126 had no effect (Figure 6C). These results indicate that the increased RPS6 phosphorylation in the absence of ataxin-2 is due to the activation of the PI3K/mTOR pathway. In the case of 4E-BP1 phosphorylation, all three inhibitors resulted in a significant reduction of the phosphorylation excess, with LY294002 having the most potent effect (Figure 6D). Thus, in serum-deprived cells with absence of ataxin-2 an elevation of RPS6 and 4E-BP1 phosphorylation was observed, mainly through the PI3K as well as the mTOR pathway, modifying the regulatory control over the pre-initiation complex.
Nutrient and serum deprivation of neural cells induces ataxin-2 transcriptionally
In view of the importance of the PI3K and mTOR phosphorylation cascades to reflect the availability of trophic signals and nutrient abundance [47], we decided to subject neural cells to nutrient and serum deprivation while analysing the response of ataxin-2 levels. Human SH-SY5Y neuroblastoma cells were either treated with RPMI containing 10% FCS and 2g/L glucose or incubated with Hank's Balanced Salt Solution (HBSS) to starve them for serum, amino acids and glucose. The cells were cultured for 2 to 72 h and again analyzed for ATXN2 expression, normalized against the transcript levels of the housekeeping gene TBP. A significant ATXN2 mRNA induction was observed between 2 h and 16 h in a phasic manner, reaching above 2-fold factors at 12 h and 16 h (Figure 7A). Very similar time courses of phasic transcriptional induction with maximal values at 12 h have previously been observed for PINK1 and PARKIN as modulators of selective autophagy of dysfunctional mitochondria [48] and indeed the proteins ATXN2 and PARKIN are known to interact [49]. The initiation of autophagy via the ULK1 complex is a process known to be under control of PI3K-AKT-mTOR signaling [50]. To unravel the mechanism behind this triggering of ATXN2 levels, SH-SY5Y cells were incubated in various media containing drugs that modulate PI3K-AKT-mTOR signaling and the autophago-lysosomal pathway. For this purpose, cells were exposed for 16 h to control medium (RPMI + FCS) or to starvation medium (HBSS - FCS) either supplemented with 0.05% vehicle (DMSO), 5 nM bafilomycin A1 (BAF), 0.5 μM rapamycin (RAP) or 25 μM LY294002 (LY). Vehicle (DMSO) treatment had no impact on ATXN2 mRNA levels. Only the mTOR inhibitor rapamycin was able to trigger a significant elevation of ATXN2 (Figure 7B). Serum and nutrient deprivation of SH-SY5Y cells resulted in a stronger ATXN2 induction than rapamycin alone, an effect that could not be exacerbated by any PI3K-AKT-mTOR inhibitor (black bars in Figure 7B). Thus, nutrient and trophic deprivation stress preferentially through the mTOR pathway contributes to the transcriptional induction of ATXN2.
Figure 7. Nutrient and serum starvation elicits a transcriptional induction of ATXN2 via the mTOR pathway.
(A) Human SH-SY5Y neuroblastoma cells were cultured in RPMI medium containing 10% FCS (RPMI + FCS) or in HBSS without nutrients (HBSS - FCS). At the indicated time points the ATXN2 transcript levels were quantified by qPCR and normalized to non-starvation (RPMI + FCS) at time 2 h. Student's t-test was used to calculate significance between starved and non-starved cells. (B) SH-SY5Y cells either treated with control (RPMI + FCS, white bars) or starvation medium (HBSS – FCS, black bars) with 0.05% DMSO, 5 nM bafilomycin A1 (BAF), 0.5 μM rapamycin as mTOR pathway antagonist (RAP), 25 μM LY294002 (LY) or nothing (CON) were incubated for 16 h prior to mRNA analysis by qPCR (* p<0.05, ** p<0.01, *** p<0.005).
Discussion
Our study found that ataxin-2 expression levels increase in response to nutrient and trophic stress in a mTOR-signaling dependent manner; consequently, the absence of ataxin-2 leads to adaptive changes in the PI3K/mTOR-driven phosphorylation of RPS6 and 4E-BP as regulatory components of the 48S pre-initiation complex of mRNA translation. In polysome gradients ataxin-2 cosediments preferentially with the 40S fraction of ribosomes, an observation that contrasts with the even distribution of its interactor protein PABP among monosomes and polysomes. Colocalization and coimmunoprecipitation studies showed ataxin-2 to associate with various initiation factors These data are in agreement with our interpretation that ataxin-2 responds to stress due to limited nutrient availability and controls translation repression at the pre-initiation stage.
Ataxin-2 was previously known to bind to RNA and PABP [33, 51]. Nuclear RNA splicing and RNA surveillance, ribosomal biogenesis as well as cytosolic mRNA translation and RNA degradation are part of posttranscriptional regulation and are key aspects of gene expression under control of the mTOR pathway. The process of protein synthesis (mRNA translation) involves the sequential decoding of the mRNA into protein, performed on the ribosome, and is conventionally divided into initiation, elongation, and termination [52-54]. At the initiation step, the mRNA 5’cap is bound by eIF4F, a protein complex formed by the eukaryotic initiation factors eIF4G, eIF4E and eIF4A. The molecular scaffold protein eIF4G interacts with the poly(A)-binding protein (PABP), which binds to the poly(A) tail of the mRNA, circularizing the mRNA together with other proteins as part of the 48S pre-initiation complex and stimulating mRNA translation [55, 56]. Another integral component of the pre-initiation complex is the ribosomal protein S6, whose phosphorylation downregulates translation initiation and cell proliferation, while augmenting cell size and affecting glucose homeostasis [57]. It has been demonstrated that ataxin-2 interacts directly with PABP in Drosophila melanogaster and mouse brain [12, 33], colocalizes with the rER and cosediments with RPS6 in mouse brain [58]. We report now that ataxin-2 indeed colocalizes with PABP also in rat hippocampal neuron cultures and murine embryonal fibroblasts, but also colocalizes with eIF4A1, eIF3B and eIF4G (Figure 1) and shows more similar cosedimentation with the initiation factors than with PABP and RPS6 (Figure 5). Furthermore, coimmunoprecipitation experiments in HEK-293 cells revealed that ataxin-2 forms a complex with eIF4G, eIF4A1, eIF3B and RPS6 (Figure 4B), indicating that ataxin-2 associates with the 48S pre-initiation complex of translation.
Under environmental stress, global cap-dependent protein synthesis is stalled and most components of this pre-initiation complex (i.e. the small ribosomal subunit plus translation initiation factors eIF3/eIF4G/eIF4A1 and PABP) together with the associated transcripts are relocalized to cytosolic RNP foci in the presence of normally nuclear RNA-binding proteins that possess homotypic aggregation properties, such as TIA-1 and TIAR, in a process that forms SG. We confirm that ataxin-2 is present at the SG as a consequence of its association with the pre-initiation complex components PABP, eIF4G, eIF4A1, eIF3B and RPS6. Previous studies found that ataxin-2 knock-down decreased the redistribution of TIA-1 from the nucleus to SGs and concluded that SG formation is impaired [28]; by contrast, our data show that discrete cytosolic foci containing TIA-1, PABP, eIF4G, eIF4A1, eIF3B and RPS6 still form after arsenite treatment in the absence of ataxin-2. Given that ATXN2 has strong sequence homology to another human protein named Ataxin-2-like (ATXN2L), which can also localize to SG [59], we have to admit that the role of ATXN2 for SG might be rescued by ATXN2L and that the knockdown or knockout of ATXN2 alone might be insufficient to understand all its roles for SG and translation control. The current view is that SG can only form once TIA-1 has relocalized to the cytoplasm with its prion-like domain serving as an aggregation seed and with its RNA-binding RRM domains constituting the necessary docking structure for the association of translation-competent mRNA with SG [37]. Our evidence suggests that ataxin-2 is not necessary for the assembly of RNA foci after stress.
Phosphorylation signals are important regulators of mRNA translation, targeting initiation factors and ribosomal components to control this rate-limiting step in translation [60]. The phosphorylation state of these factors is altered during development, environmental stress (heat shock, starvation, or heme deprivation), or viral infection. Two of these factors are the ribosomal protein S6 and the initiation repressor 4E-BP1. Ribosomal protein S6 and 4E-BPs (eIF4E inhibitory proteins) are phosphorylated in response to a wide variety of mitogens, growth factors, and tyrosine kinases [60, 61]. Phosphorylation of 4E-BP1 controls cap-dependent translation of mRNAs with extensive secondary structure [62]. Phosphorylation of RPS6 is a determinant of translation initiation, cell proliferation, cell size, and glucose homeostasis [57]. Interestingly, mice unable to phosphorylate RPS6 (rpS6P−/−) suffer from diminished levels of pancreatic insulin, hypoinsulinemia, and impaired glucose tolerance, showing increased transcript levels but decreased protein levels of insulin receptor [63]. The same phenotype was observed in S6K1-deficient mice, which also displayed diminished pancreatic ß-cell size [64]. It is well known that 4E-BP1 is a mediator of adipose tissue development and energy homeostasis in mammals, and that mice lacking 4E-BP1 presented adipose tissue reduction [65]. We now observed in Atxn2-KO MEFs after serum deprivation that the phosphorylation of RPS6 and 4E-BP1 remains strikingly upregulated by the PI3K/mTOR pathway. In contrast to 4E-BP1-deficient and S6K1-deficient mice, Atxn2-KO mice present with increased insulin levels in pancreas and blood serum, abdominal obesity and hepatosteatosis [20]. Jointly, all evidence confirms a role of ataxin-2 in the control of translation by PI3K/mTOR signals that reflect the abundance of nutrients and trophic stimuli.
This scenario is consistent with our findings that ATXN2 expression is transcriptionally induced by starvation and by the mTOR inhibitor rapamycin. Previous studies of the ATXN2 promoter indicated an important role of ETS1 [66], a transcription factor known to control several cytokines/chemokines and known to be modulated by growth factors and various stressors [67]. The expression of ATXN2 was also found to be under control of ataxin-2 itself and its interacting protein ZBRK1 [45], a transcriptional repressor involved in the responses to DNA damage [68]. Thus, the regulation of ATXN2 transcription had not been clearly linked to nutrient abundance signalling via mTOR, but our data are well compatible with recent reports on the ataxin-2 ortholog in Saccharomyces cerevisiae, Pbp1, having a role in the mTOR pathway. This yeast protein like mammalian ataxin-2 relocalizes to stress granules [38], interacts with the poly(A)-binding protein [69] and interacts also with Lsm12 at ribosomes, a protein of unknown function but predicted to contain a N-terminal Lsm domain [70]. Recent reports showed that Pbp1 modulates mTORC1 (mechanistic target of rapamycin complex 1) signalling by sequestration of this kinase complex to stress granules, and is itself regulated by phosphorylation cascades under control of Snf1, the yeast ortholog of mammalian AMPK1, that are triggered in periods of low cellular energy [40, 41, 71]. Thus, our mammalian data that implicate ataxin-2 in mTOR signalling are in excellent agreement with recent yeast data, which revealed Pbp1 to act as stress-triggered modulator of mTORC1.
In conclusion, the evidence presented here indicates that mammalian ataxin-2 responds to nutritional stress, modulates the initiation repressor 4E-BP1 via PI3K/mTOR signalling and associates with several components of the pre-initiation complex also during stress periods.
Materials and Methods
Cell culture and arsenite stress treatment
E18 primary Sprague-Dawley rat hippocampal neurons were obtained from BioCat GmbH, Genlantis, Germany and cultured following the supplier's instructions: in Neurobasal medium, B27 serum-free supplement and glutamax (Invitrogen) on poly-D-lysine (Sigma) coated coverslips. After seven days, neurons were stressed and fixed and stained with the indicated antibodies.
Mouse embryonic skin fibroblasts (MEFs) with constitutive deletion of exon 1 of the Sca2 gene resulting in absence of ataxin-2 as described elsewhere [20] were isolated around embryonal day 20. Skin pieces were minced in 3-cm Petri dishes and underwent enzymatic digestion at 37 °C for 30 min in Falcon tubes with 1 ml HyQTase (Hyclone). The digested tissue was passed through a 160 μm Nitex filter into 3 cm Petri dishes, and the enzymes neutralized with DMEM + 15% Bovine Growth Serum (HyClone). The resulting suspension was centrifuged at 450 × g for 5 min, and the cells were plated at a density of 1 × 106 cells/cm2 in culture flasks. The fibroblasts were placed in an incubator at 37 °C supplemented with 5% CO2, and medium was changed daily for the first three days. After reaching near-confluence, cells were detached with HyQTase and replated at a density of 1 × 106 cells/cm2 in 6 cm plates every third day.
Cells growing on glass coverslips were stressed by exposure to 0.5 mM sodium arsenite for 45 min, rinsed with phosphate-buffered saline (PBS) and fixed (see below).
SH-SY5Y cells were purchased from The European Collection of Cell Cultures (Sigma-Aldrich, Taufkirchen, Germany) and cultured in Roswell Park Memorial Institute (RPMI) 1,640 medium containing 2 g/l D-glucose, 2 mM l-glutamine and 10% FCS.
Primary antibodies
Antibodies against eIF4G, eIF3B eIF4A1 and TIA-1 were obtained from Santa Cruz Biotechnology, Inc. Anti–PABP was purchased from Abcam (UK) and Sigma-Aldrich (Germany). Anti-RPS6 was obtained from Cell Signaling Technology, Inc. Monoclonal mouse anti-ataxin-2 antibody from BD Transduction Laboratories was used for immunodetection, while a newly generated monoclonal antibody 12SCA2-2B6 directed against the C-terminal epitope SNAEHKRGPEVc (amino acids 841-851 of mouse ataxin-2) generated at the EuroSCA core facility at IGBMC Strasbourg (Dr. Yvon Trottier) was employed in Co-IP experiments. Mouse anti-GAPDH antibody was purchased from Calbiochem.
Immunofluorescence microscopy
Cells were fixed with 4% paraformaldehyde (PFA) in PBS at room temperature for 15 min. After washing with PBS, cells were blocked with 5% ChemiBLOCKER (Chemicon) in PBS/0.5% Triton X-100 for 30 min at room temperature. All antibodies were diluted in PBS (1:100). Cy™2-conjugated AffiniPure Donkey anti-rabbit IgG, Cy™3-conjugated AffiniPure Donkey anti-mouse IgG and Cy™2-conjugated AffiniPure Donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc.) were used as secondary antibodies (1:500). All immunostaining experiments were run in parallel reactions without primary or secondary antibodies to control for non-specific fluorescence. All images were acquired on a Zeiss LSM 510 using a Plan Apochromat 63x objective, NA 1.4 and were arranged with Adobe Photoshop® (v7.0). For image acquisition we employed the 405 nm laser with LP420 filter to detect DAPI, 488 laser with LP 505 filter for Cy2 and 543 laser with BP 560-615 filter for Cy3.
Coimmunoprecipitation from HEK-293 cells with and without arsenite stress
Cells were incubated with or without 0.5 mM sodium arsenite for 45 min and were lysed in cell lysis buffer A [10 mM HEPES-NaOH, pH 7.5, 10 mM KCl, 5 mM MgCl2, 50 mM NaF, 2 mM DTT, 5% Igepal CA-630, 0.5 mM PMSF, 25 μl/ml protease inhibitor cocktail (Sigma), 10 μl/ml phosphatase inhibitor (Sigma), and 10 μl/ml RNaseOUT (Invitrogen)]. Cell lysates were incubated with appropriate amounts of antibodies for at least 1 hr at 4 °C. Antibody-protein complexes were precipitated with Protein A/G Plus Agarose (#SC-2003, Santa Cruz) at 4 °C for 2 h. The agarose beads were sedimented by centrifugation and extensively washed with ice-cold cell lysis buffer A. The beads were boiled in SDS sample buffer, and the supernatant was analyzed by western blotting.
Characterization of new monoclonal anti-ataxin-2 antibodies
For antibody generation, six different antigenic peptides were synthesized (Biogenes/Berlin), coupled to ovalbumine via a cysteine residue, used to immunize mice and to generate monoclonal hybridoma cells; among these, 23 showed specific signals by ELISA at the antibody core facility of IGBMC, Illkirch, France, and were characterized further. For immunoblot analysis, 100 μg of wildtype and Sca2-KO mouse brain extract and 20 μg of lysates from HeLa cells overexpressing Myc-Ataxin-2(22Q) and (79Q) were analyzed in parallel with the hybridoma supernatants. The same extracts were probed with the BD Biosciences antibody (#611378) in comparison. For immunoprecipitation, 150 μl of mouse brain cytosolic fraction prepared as previously described were incubated overnight with 15 μl of hybridoma supernatant at 4 °C with rotation. Sixteen h later, 20 μl of a 50/50 slurry of Protein A/G Plus Agarose (#SC-2003, Santa Cruz) was added for one hour. The agarose was sedimented using 1000 × g for 1 min at 4 °C and was washed three times with ice cold lysis buffer (50 mM Hepes, pH 7.4, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 150 mM NaCl, 5% glycerin, 0.1% NP-40, Complete™ protease-inhibitor tablet from Roche, 1 mM PMSF). Afterwards the agarose was resuspended in 50 μl 2X loading buffer and heated at 95 °C for 5 min. 10 μl of the supernatant was analyzed using SDS-PAGE and western blot. Detection in western blot was carried out using the commercial antibody against ataxin-2 from BD Biosciences.
Polysomal profiling and protein extraction from sucrose gradient fractions
Polysomal profiles were performed according to Sivan et al. [72] with modifications. Specifically, WT and KO MEFs were cultured in 100 mm tissue culture dishes until they reached 100% confluence, incubated for 15 min with 100 μg/ml cycloheximide (CHX), harvested, and stored at −80 °C. Prior to analysis, the cells from 8-10 plates were resuspended in 0.45 ml of LBA buffer [18 mM Tris, pH 7.5, 50 mM KCl, 10 mM MgCl, 10 mM NaF, 1.4 μg/ml pepstatin, 2 μg/ml leupeptin, EDTA-free protease inhibitor cocktail (Complete; Roche), 70 μg/ml CHX, 1.25 mM dithiothreitol, and 200 μg/ml heparin], homogenized with 15 strokes using a dounce homogenizer, and Triton X-100 and deoxycholate were added to a final concentration of 1.2% each for lysis of 5 min on ice. Twenty five optical density units (260nm) were loaded on each 10-50% (w/v) sucrose gradient made with a Biocomp Gradient master. Following centrifugation for 100 min at 40,000 rpm in a Beckman SW40 rotor, the sucrose gradients were processed on an ISCO fractionator with constant OD254 reading and 0.5 ml fractions were collected. Protein was extracted by precipitation using chloroform-methanol. The protein samples were diluted in Laemmli sample buffer and analyzed by western blot.
RPS6 and 4E-BP1 phosphorylation assays
MEF cell cultures were incubated in serum-free medium for 24 h before the experiment. Then cells were washed with cold PBS, and lysed with cell lysis buffer from Cell Signaling Technology (#9803), scraped and sonicated briefly. Then the extracts were centrifuged for 10 min at 14,000 × g at 4 °C. The supernatants were aliquoted and stored at −80 °C until used. For the experiments with inhibitors, cells were treated 30 min before harvest with 10 μM MEK inhibitor U0126 (Promega), 50 μM PI3 kinase inhibitor LY294002 (Cell Signaling Technology) and 20 nM mTOR inhibitor rapamycin (Calbiochem). Phosphorylation assays were performed by using PathScan®Phospho-S6 Ribosomal Protein (Ser235/236) Sandwich ELISA kit (#7205) and PathScan®Phospho-4E-BP1 (Thr37/Thr46) Sandwich ELISA kit (#7216) (Cell Signaling Technology) from the aliquots collected. For the analysis of Total-S6 or Total-4E-BP1 protein levels, PathScan®Total-S6 Ribosomal Protein Sandwich ELISA kit (#7225) and PathScan®Total-4E-BP1 Sandwich ELISA kit (#7179) were used (Cell Signaling Technology). The analyses of the ELISAs were performed on the Tecan Infinite M200 plate reader.
Starvation experiments
As previously described [48], SH-SY5Y neuroblastoma cells were starved of trophic factors by incubating them in their normal growth medium (RPMI 1640 containing 2 g/L D-glucose and 2 mM l-glutamine) but without FCS (serum starvation). The starvation effect was maximized by incubating SH-SY5Y cells in HBSS (Invitrogen, nutrient starvation). HBSS contains 1.26 mM CaCl2, 0.493 mM MgCl2 × 6H2O, 0.407 mM MgSO4 × 7H2O, 5.33 KCl, 0.441 KH2PO4, 4.17 mM NaHCO3, 137.93 mM NaCl, 0.338 mM Na2HPO4, 0.0266 mM Phenol Red, and 5.56 mM (1 g/L) D-glucose but no amino acids.
To analyze mRNA levels by real-time reverse transcriptase quantitative PCR (qPCR), 0.5 × 106 SH-SY5Y cells were seeded in a six well, always 20 h prior to experimental start. After medium was removed, the cells were washed with PBS and covered with 2 ml of control medium or starvation medium as indicated in the figure captions. To address the potential mechanisms underlying the starvation-dependent control of ATXN2 transcription, SH-SY5Y cells were either treated with control RPMI or HBSS starvation medium, supplemented with 0.05% DMSO, 5 nM bafilomycin A1, 0.5 μM rapamycin as mTOR pathway antagonist, 25 μM LY294002 or nothing and were incubated for 16 h at 37 °C, 95%, air and 5% CO2 prior to mRNA analysis by qPCR.
RNA isolation, cDNA synthesis, and quantitative real-time PCR
Again as previously described [48], supernatant medium was collected and spun down (5 min, 500×g, 4 °C). Cell pellets were combined with adherent cells, and both were washed twice with PBS. Lysis of cells and isolation of total RNA was carried out utilizing the RNeasy mini kit (QIAGEN, Hilden, Germany). For first-strand synthesis, 1 μg of total RNA was digested with DNase I and reverse transcribed with SuperScript III reverse transcriptase utilizing oligo(dT)20 and random primers (all Invitrogen, Karlsruhe, Germany). Transcript changes of 30 ng cDNA per sample were analyzed in a 20 μl reaction volume with qPCR in a StepOnePlus Real-Time PCR System and the appropriate TaqMan gene expression assays (all Applied Biosystems, Darmstadt, Germany): ATXN2 (Hs00268077_m1). The mean of expression changes was normalized to the mean of TATA box-binding protein (TBP: Hs99999910_m1) as an internal “housekeeping” control. Relative expression changes were calculated with the 2−ΔΔCt method [73] utilizing Microsoft Excel 2007 software, whereupon the ΔCt of the corresponding control served as calibrator. The resulting 2−ΔΔCt values of n experiments (indicated in the figures) were averaged for the appropriate time and treatment.
Statistical analyses
The Graph-Pad software package (version 4.03, GraphPad Software Inc., San Diego, California, USA) was used to perform unpaired Student's t-tests when normal distribution and equal variances were fulfilled, or the non-parametric Mann-Whitney test, one-way ANOVA, two-way ANOVA and to represent data with bar graphs, illustrating mean values and standard errors of the mean. Significant differences were highlighted with asterisks (* p<0.05; ** p < 0.01; *** p < 0.005).
Supplementary Material
Highlights.
Ataxin-2 transcript is induced by starvation via mTOR signals
Its deficiency increases mTOR/PI3K phosphorylation signals controlling translation
The protein localizes and immunoprecipitates with translation preinitiation factors
It sediments with 40S ribosomal subunits, unlike its interactor PABP
Its absence during cell stress does not prevent stress granule formation
Acknowledgement
I.L.-B. was a fellow of the Alexander von Humboldt Foundation. This work was supported by the EU project EuroSCA [LSHM-CT-2004-005033], the DFG [AU96/9-1,11-1,13-1], the German Ministry of Education, Research and Technology [0313128B] and the DFG SFB 645 [B3]. M.G. was supported by the NIA-IRP, NIH [National Institute on Aging-Intramural Research Program, National Institutes of Health]. N.K. was supported by the NIH [RO1 AI 033600].
We are grateful to Birgitt Meseck-Selchow and Bianca Scholz for experimental assistance. We thank Dr. P. Anderson for the TIA-1 expression vectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Gispert S, Twells R, Orozco G, Brice A, Weber J, Heredero L, Scheufler K, Riley B, Allotey R, Nothers C, et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993;4:295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- 2.Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, Weber C, Mandel JL, Cancel G, Abbas N, Durr A, Didierjean O, Stevanin G, Agid Y, Brice A. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- 3.Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, Starkman S, Orozco-Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 4.Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, Tashiro K, Ishida Y, Ikeuchi T, Koide R, Saito M, Sato A, Tanaka T, Hanyu S, Takiyama Y, Nishizawa M, Shimizu N, Nomura Y, Segawa M, Iwabuchi K, Eguchi I, Tanaka H, Takahashi H, Tsuji S. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14:277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- 5.Estrada R, Galarraga J, Orozco G, Nodarse A, Auburger G. Spinocerebellar ataxia 2 (SCA2): morphometric analyses in 11 autopsies. Acta Neuropathol. 1999;97:306–310. doi: 10.1007/s004010050989. [DOI] [PubMed] [Google Scholar]
- 6.Rub U, Schols L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf HW, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Lastres-Becker I, Rub U, Auburger G. Spinocerebellar ataxia 2 (SCA2) Cerebellum. 2008;7:115–124. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- 8.Rub U, Del Turco D, Del Tredici K, de Vos RA, Brunt ER, Reifenberger G, Seifried C, Schultz C, Auburger G, Braak H. Thalamic involvement in a spinocerebellar ataxia type 2 (SCA2) and a spinocerebellar ataxia type 3 (SCA3) patient, and its clinical relevance. Brain. 2003;126:2257–2272. doi: 10.1093/brain/awg234. [DOI] [PubMed] [Google Scholar]
- 9.Gierga K, Burk K, Bauer M, Orozco Diaz G, Auburger G, Schultz C, Vuksic M, Schols L, de Vos RA, Braak H, Deller T, Rub U. Involvement of the cranial nerves and their nuclei in spinocerebellar ataxia type 2 (SCA2) Acta Neuropathol. 2005;109:617–631. doi: 10.1007/s00401-005-1014-8. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez-Perez L, Seifried C, Santos-Falcon N, Abele M, Ziemann U, Almaguer LE, Martinez-Gongora E, Sanchez-Cruz G, Canales N, Perez-Gonzalez R, Velazquez-Manresa M, Viebahn B, von Stuckrad-Barre S, Fetter M, Klockgether T, Auburger G. Saccade velocity is controlled by polyglutamine size in spinocerebellar ataxia 2. Ann Neurol. 2004;56:444–447. doi: 10.1002/ana.20220. [DOI] [PubMed] [Google Scholar]
- 11.Schols L, Reimold M, Seidel K, Globas C, Brockmann K, Hauser TK, Auburger G, Burk K, den Dunnen W, Reischl G, Korf HW, Brunt ER, Rub U. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain. 2015;138:3316–3326. doi: 10.1093/brain/awv255. [DOI] [PubMed] [Google Scholar]
- 12.Damrath E, Heck MV, Gispert S, Azizov M, Nowock J, Seifried C, Rub U, Walter M, Auburger G. ATXN2-CAG42 sequesters PABPC1 into insolubility and induces FBXW8 in cerebellum of old ataxic knock-in mice. PLoS Genet. 2012;8:e1002920. doi: 10.1371/journal.pgen.1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralser M, Albrecht M, Nonhoff U, Lengauer T, Lehrach H, Krobitsch S. An integrative approach to gain insights into the cellular function of human ataxin-2. J Mol Biol. 2005;346:203–214. doi: 10.1016/j.jmb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Sahba S, Nechiporuk A, Figueroa KP, Nechiporuk T, Pulst SM. Genomic structure of the human gene for spinocerebellar ataxia type 2 (SCA2) on chromosome 12q24.1. Genomics. 1998;47:359–364. doi: 10.1006/geno.1997.5131. [DOI] [PubMed] [Google Scholar]
- 15.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T, Li YR, Ingre C, Weber M, Grehl T, Gredal O, de Carvalho M, Meyer T, Tysnes OB, Auburger G, Gispert S, Bonini NM, Andersen PM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients. Human molecular genetics. 2011;20:1697–1700. doi: 10.1093/hmg/ddr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gispert S, Kurz A, Waibel S, Bauer P, Liepelt I, Geisen C, Gitler AD, Becker T, Weber M, Berg D, Andersen PM, Kruger R, Riess O, Ludolph AC, Auburger G. The modulation of Amyotrophic Lateral Sclerosis risk by ataxin-2 intermediate polyglutamine expansions is a specific effect. Neurobiol Dis. 2012;45:356–361. doi: 10.1016/j.nbd.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Lahut S, Omur O, Uyan O, Agim ZS, Ozoguz A, Parman Y, Deymeer F, Oflazer P, Koc F, Ozcelik H, Auburger G, Basak AN. ATXN2 and its neighbouring gene SH2B3 are associated with increased ALS risk in the Turkish population. PLoS One. 2012;7:e42956. doi: 10.1371/journal.pone.0042956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiehl TR, Nechiporuk A, Figueroa KP, Keating MT, Huynh DP, Pulst SM. Generation and characterization of Sca2 (ataxin-2) knockout mice. Biochem Biophys Res Commun. 2006;339:17–24. doi: 10.1016/j.bbrc.2005.10.186. [DOI] [PubMed] [Google Scholar]
- 20.Lastres-Becker I, Brodesser S, Lutjohann D, Azizov M, Buchmann J, Hintermann E, Sandhoff K, Schurmann A, Nowock J, Auburger G. Insulin receptor and lipid metabolism pathology in ataxin-2 knock-out mice. Human molecular genetics. 2008;17:1465–1481. doi: 10.1093/hmg/ddn035. [DOI] [PubMed] [Google Scholar]
- 21.Auburger G, Gispert S, Lahut S, Omur O, Damrath E, Heck M, Basak N. 12q24 locus association with type 1 diabetes: SH2B3 or ATXN2? World J Diabetes. 2014;5:316–327. doi: 10.4239/wjd.v5.i3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nechiporuk T, Huynh DP, Figueroa K, Sahba S, Nechiporuk A, Pulst SM. The mouse SCA2 gene: cDNA sequence, alternative splicing and protein expression. Human molecular genetics. 1998;7:1301–1309. doi: 10.1093/hmg/7.8.1301. [DOI] [PubMed] [Google Scholar]
- 23.Huynh DP, Del Bigio MR, Ho DH, Pulst SM. Expression of ataxin-2 in brains from normal individuals and patients with Alzheimer's disease and spinocerebellar ataxia 2. Ann Neurol. 1999;45:232–241. doi: 10.1002/1531-8249(199902)45:2<232::aid-ana14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.van de Loo S, Eich F, Nonis D, Auburger G, Nowock J. Ataxin-2 associates with rough endoplasmic reticulum. Exp Neurol. 2009;215:110–118. doi: 10.1016/j.expneurol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Nonis D, Schmidt MH, van de Loo S, Eich F, Dikic I, Nowock J, Auburger G. Ataxin-2 associates with the endocytosis complex and affects EGF receptor trafficking. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Drost J, Nonis D, Eich F, Leske O, Damrath E, Brunt ER, Lastres-Becker I, Heumann R, Nowock J, Auburger G. Ataxin-2 modulates the levels of Grb2 and SRC but not ras signaling. J Mol Neurosci. 2013;51:68–81. doi: 10.1007/s12031-012-9949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralser M, Nonhoff U, Albrecht M, Lengauer T, Wanker EE, Lehrach H, Krobitsch S. Ataxin-2 and huntingtin interact with endophilin-A complexes to function in plastin-associated pathways. Human molecular genetics. 2005;14:2893–2909. doi: 10.1093/hmg/ddi321. [DOI] [PubMed] [Google Scholar]
- 28.Nonhoff U, Ralser M, Welzel F, Piccini I, Balzereit D, Yaspo ML, Lehrach H, Krobitsch S. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007;18:1385–1396. doi: 10.1091/mbc.E06-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meierhofer D, Halbach M, Sen NE, Gispert S, Auburger G. Atxn2-Knock-Out mice show branched chain amino acids and fatty acids pathway alterations. Mol Cell Proteomics. 2016 doi: 10.1074/mcp.M115.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halbach MV, Gispert S, Stehning T, Damrath E, Walter M, Auburger G. Atxn2 Knockout and CAG42-Knock-in Cerebellum Shows Similarly Dysregulated Expression in Calcium Homeostasis Pathway. Cerebellum. 2016 doi: 10.1007/s12311-016-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fittschen M, Lastres-Becker I, Halbach MV, Damrath E, Gispert S, Azizov M, Walter M, Muller S, Auburger G. Genetic ablation of ataxin-2 increases several global translation factors in their transcript abundance but decreases translation rate. Neurogenetics. 2015;16:181–192. doi: 10.1007/s10048-015-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim NS, Kozlov G, Chang TC, Groover O, Siddiqui N, Volpon L, De Crescenzo G, Shyu AB, Gehring K. Comparative peptide binding studies of the PABC domains from the ubiquitin-protein isopeptide ligase HYD and poly(A)-binding protein. Implications for HYD function. J Biol Chem. 2006;281:14376–14382. doi: 10.1074/jbc.M600307200. [DOI] [PubMed] [Google Scholar]
- 33.Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Human molecular genetics. 2006;15:2523–2532. doi: 10.1093/hmg/ddl173. [DOI] [PubMed] [Google Scholar]
- 34.Neuwald AF, Koonin EV. Ataxin-2, global regulators of bacterial gene expression, and spliceosomal snRNP proteins share a conserved domain. J Mol Med. 1998;76:3–5. doi: 10.1007/s001090050184. [DOI] [PubMed] [Google Scholar]
- 35.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 37.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swisher KD, Parker R. Localization to, and effects of Pbp1, Pbp4, Lsm12, Dhh1, and Pab1 on stress granules in Saccharomyces cerevisiae. PLoS One. 2010;5:e10006. doi: 10.1371/journal.pone.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura Y, Irie K. Pbp1 is involved in Ccr4- and Khd1-mediated regulation of cell growth through association with ribosomal proteins Rpl12a and Rpl12b. Eukaryot Cell. 2013;12:864–874. doi: 10.1128/EC.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahara T, Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell. 2012;47:242–252. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 41.DeMille D, Badal BD, Evans JB, Mathis AD, Anderson JF, Grose JH. PAS kinase is activated by direct SNF1-dependent phosphorylation and mediates inhibition of TORC1 through the phosphorylation and activation of Pbp1. Mol Biol Cell. 2015;26:569–582. doi: 10.1091/mbc.E14-06-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Chen XJ. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature. 2015;524:481–484. doi: 10.1038/nature14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh DP, Del Bigio MR, Ho DH, Pulst SM. Expression of ataxin-2 in brains from normal individuals and patients with Alzheimer's disease and spinocerebellar ataxia 2. Ann Neurol. 1999;45:232–241. doi: 10.1002/1531-8249(199902)45:2<232::aid-ana14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Cande C, Vahsen N, Metivier D, Tourriere H, Chebli K, Garrido C, Tazi J, Kroemer G. Regulation of cytoplasmic stress granules by apoptosis-inducing factor. J Cell Sci. 2004;117:4461–4468. doi: 10.1242/jcs.01356. [DOI] [PubMed] [Google Scholar]
- 45.Hallen L, Klein H, Stoschek C, Wehrmeyer S, Nonhoff U, Ralser M, Wilde J, Rohr C, Schweiger MR, Zatloukal K, Vingron M, Lehrach H, Konthur Z, Krobitsch S. The KRAB-containing zinc-finger transcriptional regulator ZBRK1 activates SCA2 gene transcription through direct interaction with its gene product, ataxin-2. Human molecular genetics. 2011;20:104–114. doi: 10.1093/hmg/ddq436. [DOI] [PubMed] [Google Scholar]
- 46.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 47.Edinger AL. Controlling cell growth and survival through regulated nutrient transporter expression. Biochem J. 2007;406:1–12. doi: 10.1042/BJ20070490. [DOI] [PubMed] [Google Scholar]
- 48.Klinkenberg M, Gispert S, Dominguez-Bautista JA, Braun I, Auburger G, Jendrach M. Restriction of trophic factors and nutrients induces PARKIN expression. Neurogenetics. 2012;13:9–21. doi: 10.1007/s10048-011-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbach MV, Stehning T, Damrath E, Jendrach M, Sen NE, Basak AN, Auburger G. Both ubiquitin ligases FBXW8 and PARK2 are sequestrated into insolubility by ATXN2 PolyQ expansions, but only FBXW8 expression is dysregulated. PLoS One. 10:e0121089. doi: 10.1371/journal.pone.0121089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 53.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 55.Majumdar R, Chaudhuri J, Maitra U. Reconstitution of mammalian 48S ribosomal translation initiation complex. Methods Enzymol. 2007;430:179–208. doi: 10.1016/S0076-6879(07)30008-6. [DOI] [PubMed] [Google Scholar]
- 56.Derry MC, Yanagiya A, Martineau Y, Sonenberg N. Regulation of poly(A)-binding protein through PABP-interacting proteins. Cold Spring Harb Symp Quant Biol. 2006;71:537–543. doi: 10.1101/sqb.2006.71.061. [DOI] [PubMed] [Google Scholar]
- 57.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 58.van de Loo S, Eich F, Nonis D, Auburger G, Nowock J. Ataxin-2 associates with rough endoplasmic reticulum. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Kaehler C, Guenther A, Uhlich A, Krobitsch S. PRMT1-mediated arginine methylation controls ATXN2L localization. Exp Cell Res. 2015;334:114–125. doi: 10.1016/j.yexcr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 61.Frederickson RM, Sonenberg N. Signal transduction and regulation of translation initiation. Semin Cell Biol. 1992;3:107–115. doi: 10.1016/s1043-4682(10)80020-0. [DOI] [PubMed] [Google Scholar]
- 62.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 65.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 66.Scoles DR, Pflieger LT, Thai KK, Hansen ST, Dansithong W, Pulst SM. ETS1 regulates the expression of ATXN2. Human molecular genetics. 2012;21:5048–5065. doi: 10.1093/hmg/dds349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao CC, Tsai CY, Chang WC, Lee WH, Wang JM. RB.E2F1 complex mediates DNA damage responses through transcriptional regulation of ZBRK1. J Biol Chem. 2010;285:33134–33143. doi: 10.1074/jbc.M110.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mangus DA, Smith MM, McSweeney JM, Jacobson A. Identification of factors regulating poly(A) tail synthesis and maturation. Mol Cell Biol. 2004;24:4196–4206. doi: 10.1128/MCB.24.10.4196-4206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 2006;20:1294–1307. doi: 10.1101/gad.1422006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchan JR, Capaldi AP, Parker R. TOR-tured yeast find a new way to stand the heat. Mol Cell. 2012;47:155–157. doi: 10.1016/j.molcel.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Sivan G, Kedersha N, Elroy-Stein O. Ribosomal slowdown mediates translational arrest during cellular division. Mol Cell Biol. 2007;27:6639–6646. doi: 10.1128/MCB.00798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.