Abstract
Background
Higher rates of obesity and heart failure have been observed in African Americans, but associations with mortality are not well described. We examined intermediate-and long-term clinical implications of obesity in African Americans and associations between obesity and all-cause mortality, heart failure, and heart failure hospitalization.
Methods and Results
We conducted a retrospective analysis of a community sample of 5292 African Americans participating in the Jackson Heart Study between September 2000 and January 2013. The main outcomes were associations between BMI and all-cause mortality at 9 years and heart failure hospitalization at 7 years using Cox proportional hazards models and interval development of heart failure (median 8 years follow-up) using a modified Poisson model. At baseline, 1406 (27%) participants were obese and 1416 (27%) were morbidly obese. With increasing BMI, the cumulative incidence of mortality decreased (P = .007), whereas heart failure increased (P < .001). Heart failure hospitalization was more common among morbidly obese participants (9.0%; 95% CI, 7.6–11.7) than among normal-weight patients (6.3%; 95% CI, 4.7–8.4). After risk adjustment, BMI was not associated with mortality. Each 1-point increase in BMI was associated with a 5% increase in the risk of heart failure (HR, 1.05; 95% CI, 1.03–1.06; P < .001) and the risk of heart failure hospitalization for BMI greater than 32 kg/m2 (HR, 1.05; 95% CI, 1.03–1.07; P < .001).
Conclusions
Obesity and morbid obesity were common in a community sample of African Americans, and both were associated with increased heart failure and heart failure hospitalization.
Introduction
The association of obesity with heart failure (HF) has not been well described in African Americans (AAs), who have higher rates of both obesity and HF than white populations.[1, 2] The relationship between obesity in AAs and mortality also remains unclear. Some studies have suggested a paradoxical lower risk of death in AAs with obesity compared with other populations with obesity.[3–9] To better understand associations between obesity and health outcomes among AAs, we examined relationships between BMI and all-cause mortality, prevalent HF at interval follow-up examinations, and HF hospitalization among more than 5000 participants in the Jackson Heart Study.[10–14] We hypothesized that obesity would be common in this cohort and that higher BMI would be associated with greater risks of mortality, prevalent HF, and hospitalization for HF.
Methods
Data Sources
The Jackson Heart Study is a prospective, community-based, observational study initiated in 2000 to investigate risk factors for cardiovascular disease in AAs.[10] A strength of the study is that it is the largest cohort to date specifically examining African Americans and their risks for cardiovascular disease. All participants provided written informed consent, and study protocols were approved by local institutional review boards. The institutional review board of the Duke University Health System approved our study and the use of study data. Participants completed 3 study visits: exam 1 between September 2000 and March 2004, exam 2 between October 2005 and December 2008, and exam 3 between February 2009 and January 2013. The details of data collected and visit procedures have been described previously.[15] The Jackson Heart Study cohort surveillance system collects follow-up data on all participants, including deaths from 2000 through 2011 and HF hospitalizations from 2005 through 2011.[16]
Study Population
For all outcomes, we included participants who completed exam 1 and had BMI measured. For the analysis of HF hospitalizations, we limited the cohort to participants who survived to January 1, 2005, when HF hospitalization surveillance began. For the assessment of all prevalent HF outcomes, we excluded participants with baseline HF at exam 1. We also required that participants completed exam 2 for the assessment of prevalent HF at exam 2, and we required completion of exam 3 for the assessment of prevalent HF at exam 3 (Supplemental Figure 1).
Body Mass Index
The study variable of interest was baseline BMI. Height and weight were determined with the participant wearing an examination gown without shoes.[17] We assessed BMI on a continuous scale per 1 point and categorically as follows: normal weight (less than 25 kg/m2), overweight (25 to less than 30 kg/m2), obese (30 to less than 35 kg/m2), and morbidly obese (35 kg/m2 or greater).
Outcomes
The primary outcomes were all-cause mortality, prevalent HF at exam 2, prevalent HF at exam 3, and hospital admission for HF. Methods for identification of all-cause mortality in the cohort have been described previously.[16] We assessed all-cause mortality within 9 years after the exam 1 visit date based on a median follow-up time of 9 years and 75th percentile of 10 years. Since HF history was not collected at the 3 clinical exams, we derived prevalent HF at each exam using the modified Gothenburg criteria developed and validated in the ARIC data set and as recently applied to the Jackson Heart Study cohort.[18, 19] Among participants without prevalent HF at exam 1, we assessed prevalent HF at exam 2 (median follow-up, 4.8 years from exam 1; range, 3.4–8.2 years) and prevalent HF at exam 3 (median follow-up, 8.0 years from exam 1; range, 6.4–12.2 years). We also assessed the cumulative incidence of HF hospitalization between 2005 and 2011 among study participants who survived to January 1, 2005, when HF hospitalization surveillance began. (Median and 75th percentile follow-up was 7 years.) Potential HF hospitalizations in the cohort were identified and adjudicated as described previously.[16]
Covariates
Variables from the baseline clinical exam included demographic characteristics, medical history, physical examination measurements, medications, laboratory test results, and cardiac test results. Medical history was based on either direct clinical examination, self-reported disease history, or health behaviors. For variables with less than 5% missingness, we imputed continuous variables to the overall median value, dichotomous variables to “no,” and multichotomous variables to the most frequent categorical value. For variables with greater than 5% missingness (medication variables), we treated the missing values as a separate category.
Statistical Analysis
We describe exam 1 baseline characteristics of the study population by BMI category using frequencies with percentages for categorical variables and medians with interquartile ranges or means with SDs for continuous variables. We tested for differences between groups using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables.
We calculated the cumulative incidence of all-cause mortality and HF hospitalization by BMI category using Kaplan-Meier estimates, and we tested for differences between groups using log-rank tests. For all survival analyses, we censored data at the time of participant loss to follow-up, or the end of study event surveillance follow-up (December 31, 2011). For HF hospitalization, we also censored data for participants at the time of death. For prevalent HF at exam 2 and prevalent HF at exam 3, we used frequencies with percentages, calculated exact CIs for binomial proportions, and tested for differences between groups using Fisher exact tests.
We assessed the unadjusted and adjusted associations between continuous and categorical BMI and outcomes. We used Cox proportional hazards models for mortality and HF hospitalization outcomes. To examine associations with prevalent HF at exam 2, we used a modified Poisson model with an offset parameter to adjust for log of participant time between exam 1 and exam 2.[20, 21] We explored both linear and nonlinear functional forms for BMI, including polynomials, restricted cubic splines, and linear splines. To explore the middle and tails of the BMI distribution in our restricted cubic spline analyses, we selected 5 BMI knots at the 5th (22 kg/m2), 35th (28 kg/m2), 50th (30 kg/m2), 65th (33 kg/m2) and 95th (45 kg/m2) percentiles and chose the population median of 30 kg/m2 as the reference category (hazard ratio, 1.00). To simplify interpretation, information gleaned from restricted cubic splines was then used to develop linear spline models with knots selected according to optimal model fit determined by the minimum Akaike information criterion.
We conducted 5 sensitivity analyses. First, we tested for interactions between BMI (categorical and continuous) and sex in all of multivariable models, because of previously observed associations with BMI and HF by sex.[22] Second, we repeated models replacing BMI with waist circumference as an alternative measure of obesity. Third, we excluded participants with a BMI less than 18.5 kg/m2 (underweight participants with a potentially increased risk profile as compared with a BMI 18.5 to less than 25 kg/m2) and repeated the unadjusted and multivariable models. Fourth, we performed additional analyses with the addition of a categorical variable for average hours of self-reported weekly physical activity in the past year (none; <1 hour; 1–<2 hours; 2–<3 hours; 3–<4 hours; ≥4 hours). Finally, we explored potential metabolic and/or inflammatory mediators (ie, hypertension, diabetes and glucose, serum triglyceride, and C-reactive protein) on the causal pathway between BMI and outcomes. For each outcome, we ran 5 models to compare the effect of BMI after adjustment for age, sex, and the potential mediators and to assess possible over-adjustment bias.
We used a 2-tailed α level of .05 to establish statistical significance, and we report 95% CIs. We used SAS version 9.3 (SAS Institute Inc) for all analyses.
Results
Of 5301 participants who completed exam 1, we excluded 9 (0.1%) who had missing BMI data (Supplemental Figure 1). The analysis sample for all-cause mortality thus consisted of 5292 participants. For the analysis of HF hospitalization, we included all 5184 participants who survived to 2005. Table 1 shows the baseline characteristics of the 5292 participants who completed exam 1, stratified by categories of BMI. In the overall cohort, 769 (15%) had normal weight, 1701 (32%) were overweight, 1406 (27%) were obese, and 1416 (27%) were morbidly obese. Relatively few patients in the lowest BMI category had a BMI less than 18.5 kg/m2 (n = 26/769; 3%). Morbidly obese participants were the youngest group, and female sex and baseline HF were more common with increasing BMI.
Table 1.
Baseline Characteristics of the Study Population
| Variable | Normala | Overweighta | Obesea | Morbidly Obesea | P Value |
|---|---|---|---|---|---|
| No. of participants | 769 | 1701 | 1406 | 1416 | |
| Age, median (IQR) | 55.2 (43.9–66.5) | 58.1 (46.6–65.9) | 56.3 (46.6–64.9) | 53.5 (44.1–63.0) | < .001 |
| Men, No. (%) | 356 (46.3) | 777 (45.7) | 489 (34.8) | 308 (21.8) | < .001 |
| Medical history, No. (%) | |||||
| Atrial fibrillation | 1 (0.1) | 9 (0.5) | 5 (0.4) | 3 (0.2) | .32 |
| Chronic lung disease | 61 (7.9) | 105 (6.2) | 90 (6.4) | 125 (8.8) | .02 |
| Diabetes mellitus | 58 (7.5) | 298 (17.5) | 349 (24.8) | 445 (31.4) | < .001 |
| Heart failure | 23 (3.0) | 71 (4.2) | 106 (7.5) | 196 (13.8) | < .001 |
| Hypertension | 357 (46.4) | 975 (57.3) | 896 (63.7) | 954 (67.4) | < .001 |
| Myocardial infarction | 36 (4.7) | 92 (5.4) | 87 (6.2) | 73 (5.2) | .46 |
| Current smoker | 179 (23.3) | 214 (12.6) | 154 (11.0) | 143 (10.1) | < .001 |
| Previous smoker | 138 (17.9) | 349 (20.5) | 286 (20.3) | 246 (17.4) | .08 |
| Stroke | 33 (4.3) | 71 (4.2) | 67 (4.8) | 62 (4.4) | .88 |
| Physical examination, median (IQR) | |||||
| Weight, kg | 66.0 (60.3–71.5) | 79.8 (72.9–86.6) | 91.5 (84.4–99.6) | 110.0 (100.5–124.0) | < .001 |
| Waist circumference, cm | 82.0 (76.0–87.0) | 94.0 (88.0–99.0) | 103.0 (97.0–109.0) | 116.5 (107.0–127.0) | < .001 |
| Neck circumference, cm | 35.0 (33.0–38.0) | 38.0 (35.0–40.0) | 39.0 (36.0–42.0) | 40.0 (38.0–43.0) | < .001 |
| Systolic blood pressure, mm Hg | 122.0 (112.0–136.0) | 124.0 (114.0–136.0) | 125.0 (116.0–137.0) | 126.0 (116.0–137.0) | < .001 |
| Laboratory test results, median (IQR) | |||||
| eGFR, mL/min/1.73 m2 | 88.3 (77.6–99.4) | 85.3 (75.6–94.8) | 85.9 (75.1–96.6) | 86.5 (75.9–97.3) | < .001 |
| Glucose, mg/dL | 88.0 (83.0–93.0) | 91.0 (85.0–99.0) | 93.0 (87.0–103.0) | 94.0 (87.0–107.0) | < .001 |
| Hemoglobin A1c, % | 5.4 (5.1–5.7) | 5.6 (5.3–6.0) | 5.7 (5.4–6.2) | 5.8 (5.5–6.5) | < .001 |
| Triglycerides, mg/dL | 74.0 (52.0–103.0) | 90.0 (63.0–123.0) | 98.0 (73.0–138.0) | 94.0 (72.0–134.0) | < .001 |
| HDL cholesterol, mg/dL | 55.0 (45.0–67.0) | 49.0 (41.0–60.0) | 48.0 (40.0–57.0) | 49.0 (41.0–57.0) | < .001 |
| LDL cholesterol, mg/dL | 119.0 (95.0–140.0) | 124.0 (103.0–150.0) | 124.0 (103.0–148.0) | 124.0 (101.0–147.0) | < .001 |
| High-sensitivity C-reactive protein, mg/dL | 0.1 (0.0–0.3) | 0.2 (0.1–0.4) | 0.3 (0.1–0.6) | 0.5 (0.3–0.9) | < .001 |
| Uric acid, mg/dL | 4.8 (3.8–5.7) | 5.3 (4.2–6.4) | 5.5 (4.5–6.7) | 5.7 (4.8–6.8) | < .001 |
| Baseline echocardiography and ECG | |||||
| Ejection fraction, mean (SD), % | 61.6 (7.3) | 61.7 (7.8) | 62.1 (7.4) | 62.4 (7.3) | .09 |
| Ejection fraction, median (IQR), % | 65.0 (55.0–65.0) | 65.0 (55.0–65.0) | 65.0 (55.0–65.0) | 65.0 (55.0–65.0) | .09 |
| LVEF < 40%, No. (%) | 8 (1.0) | 20 (1.2) | 10 (0.7) | 11 (0.8) | .51 |
| Left ventricular hypertrophy, No. (%) | 25 (3.3) | 103 (6.1) | 106 (7.5) | 188 (13.3) | < .001 |
| Left ventricular dimension, median (IQR), cm | 47.4 (44.3–49.9) | 48.3 (45.3–50.8) | 48.3 (45.8–50.9) | 48.9 (46.5–51.9) | < .001 |
| Medications, No. (%) | |||||
| β-Blocker | 49 (6.4) | 165 (9.7) | 160 (11.4) | 157 (11.1) | .001 |
| Missing | 57 (7.4) | 113 (6.6) | 108 (7.7) | 105 (7.4) | .70 |
| Calcium channel blocker | 84 (10.9) | 310 (18.2) | 290 (20.6) | 304 (21.5) | < .001 |
| Missing | 54 (7.0) | 103 (6.1) | 110 (7.8) | 96 (6.8) | .28 |
| Diuretic | 99 (12.9) | 323 (19.0) | 339 (24.1) | 459 (32.4) | < .001 |
| Missing | 52 (6.8) | 107 (6.3) | 99 (7.0) | 88 (6.2) | .79 |
| Statin | 53 (6.9) | 202 (11.9) | 194 (13.8) | 152 (10.7) | < .001 |
| Missing | 58 (7.5) | 108 (6.3) | 107 (7.6) | 105 (7.4) | .49 |
| Insulin | 14 (1.8) | 71 (4.2) | 102 (7.3) | 139 (9.8) | <.001 |
| Missing | 59 (7.7) | 113 (6.6) | 112 (8.0) | 103 (7.3) | .54 |
| Loop diuretic | 15 (2.0) | 58 (3.4) | 74 (5.3) | 159 (11.2) | < .001 |
| Missing | 58 (7.5) | 118 (6.9) | 114 (8.1) | 101 (7.1) | .63 |
| ACE inhibitor or ARB | 86 (11.2) | 243 (14.3) | 247 (17.6) | 307 (21.7) | < .001 |
| Missing | 56 (7.3) | 99 (5.8) | 106 (7.5) | 88 (6.2) | .20 |
| Anti-platelet agent | 10 (1.3) | 28 (1.6) | 24 (1.7) | 17 (1.2) | .63 |
| Missing | 59 (7.7) | 118 (6.9) | 116 (8.3) | 109 (7.7) | .59 |
| Digoxin | 12 (1.6) | 20 (1.2) | 20 (1.4) | 27 (1.9) | .41 |
| Missing | 57 (7.4) | 118 (6.9) | 114 (8.1) | 111 (7.8) | .63 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; and LVEF, left ventricular ejection fraction.
Normal indicates BMI < 25 mg/dL; overweight, BMI 25 to < 30 mg/dL; obese, 30 to < 35 mg/dL; and morbidly obese, ≥ 35 mg/d
Figure 1 shows the cumulative incidence of death within 9 years and HF hospitalization within 7 years after exam 1, stratified by categories of BMI. The observed incidence of mortality decreased as BMI increased (P = .007). Supplemental Table 1 shows the frequency of prevalent HF at exam 2 (3 to 8 years after exam 1) and exam 3 (6 to 12 years after exam 1). We observed a significant difference in prevalent HF between groups at both exams 2 and 3, with increasing frequency of HF observed as BMI increased (P < .001 for both comparisons). At exam 2, the number of participants having developed HF in each BMI category were as follows: 11 in the normal weight, 34 in the overweight, 41 in the obese, and 81 in the morbidly obese. At exam 3, 12 normal weight participants developed HF, 43 participants in the overweight category, 61 in the obese, and 98 in the morbidly obese. There was a significant difference in HF hospitalization between groups (P = .008), with the highest rate in morbidly obese participants of 9.0% (95% CI, 7.6–11.7) and the lowest rate in obese participants of 5.9% (95% CI, 4.8–7.4).
Figure 1.
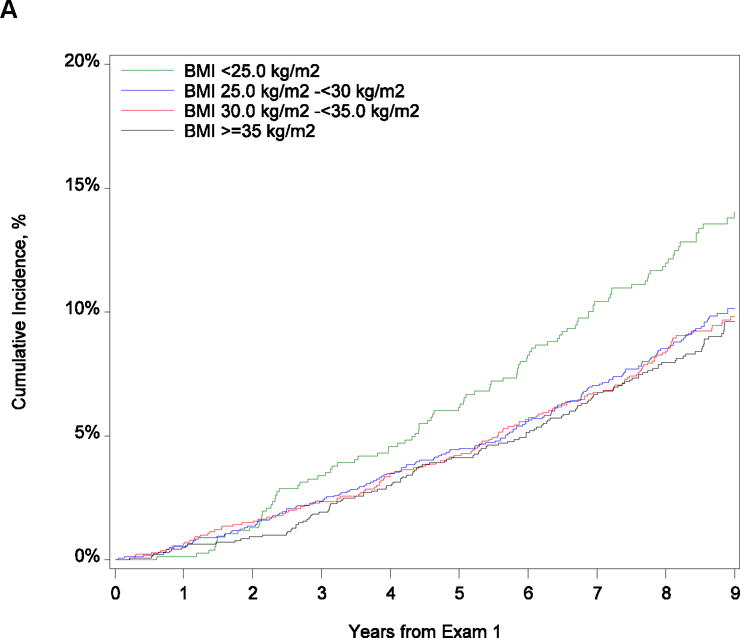
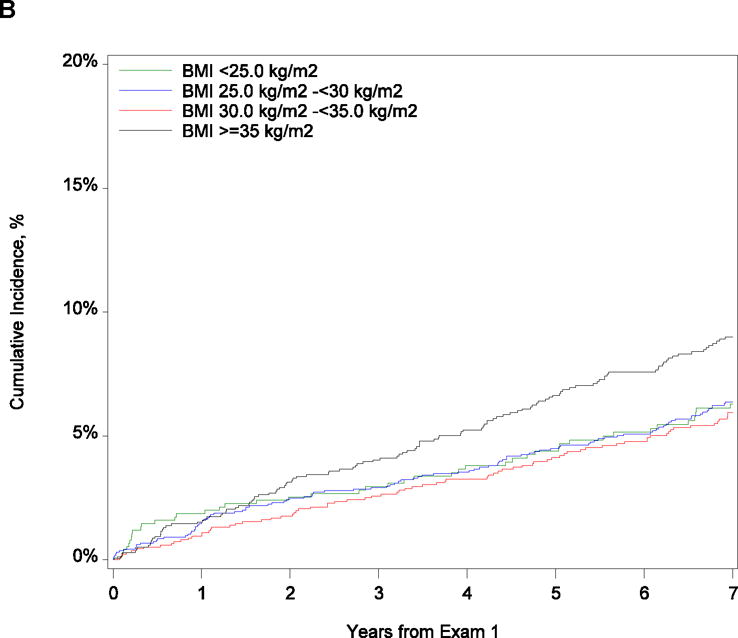
Cumulative Incidence of (A) Mortality at 9 Years and (B) Heart Failure Hospitalization at 9 Years by Body Mass Index Category
Table 2 shows the associations between BMI and the outcomes of all-cause mortality, prevalent HF at exam 2, prevalent HF at exam 3, and HF hospitalization. In both unadjusted and adjusted analyses of all-cause mortality, increasing BMI per 1 point was not associated with a statistically significant greater risk (P = .08 and P = .41). We further explored the adjusted association of continuous BMI and all-cause mortality using restricted cubic splines with the population median BMI of 30 kg/m2 as the reference category (Supplemental Figure 2A). The Wald chi-square test for nonlinear association was significant (P < .001). We also performed the unadjusted and adjusted analyses of associations between linear BMI splines and all-cause mortality with knots selected according to optimal model fit (Figure 2A; Supplemental Table 2). For each 1-point increase in BMI up to 27 kg/m2, the adjusted hazard of all-cause mortality decreased by 9%. However, there was no statistically significant association with mortality per 1 point increase in BMI greater than 27 kg/m2 (P = .22). In the categorical comparison with normal-weight participants, the multivariable adjusted risk of all-cause mortality was 34% lower in the overweight group, 30% lower in the obese group, and 34% lower in the morbidly obese group.
Table 2.
Associations Between BMI and All-Cause Mortality, Heart Failure, and Heart Failure Hospitalization
| Outcomea | Unadjusted Estimate | P Value | Adjusted Estimateb | P Value |
|---|---|---|---|---|
| All-cause mortality | ||||
| BMI as a continuous variable (n = 5292) | 0.99 (0.98–1.00) | .08 | 0.99 (0.98–1.01) | .41 |
| BMI by category | ||||
| < 25 kg/m2 (n = 769) | 1.00 [Reference] | 1.00 [Reference] | ||
| 25 to < 30 kg/m2 (n = 1701) | 0.70 (0.55–0.90) | .006 | 0.66 (0.51–0.86) | .002 |
| 30 to < 35 kg/m2 (n = 1406) | 0.68 (0.53–0.89) | .004 | 0.70 (0.52–0.93) | .01 |
| ≥ 35 kg/m2 (n = 1416) | 0.66 (0.51–0.85) | .002 | 0.66 (0.49–0.89) | .007 |
| Prevalent heart failure at exam 2 | ||||
| BMI as a continuous variable (n = 3896) | 1.06 (1.04–1.07) | < .001 | 1.06 (1.04–1.08) | < .001 |
| BMI by category | ||||
| < 25 kg/m2 (n = 554) | 1.00 [Reference] | 1.00 [Reference] | ||
| 25 to < 30 kg/m2 (n = 1317) | 1.29 (0.66–2.53) | .45 | 1.38 (0.72–2.65) | .33 |
| 30 to < 35 kg/m2 (n = 1061) | 1.95 (1.01–3.77) | .046 | 1.68 (0.87–3.24) | .12 |
| ≥ 35 kg/m2 (n = 964) | 4.26 (2.29–7.94) | < .001 | 3.74 (2.00–7.03) | < .001 |
| Prevalent heart failure at exam 3 | ||||
| BMI as a continuous variable (n = 3559) | 1.05 (1.04–1.07) | < .001 | 1.05 (1.03–1.06) | < .001 |
| BMI by category | ||||
| < 25 kg/m2 (n = 496) | 1.00 [Reference] | 1.00 [Reference] | ||
| 25 to < 30 kg/m2 (n = 1204) | 1.48 (0.78–2.77) | .23 | 1.53 (0.83–2.83) | .18 |
| 30 to < 35 kg/m2 (n = 969) | 2.59 (1.41–4.77) | .002 | 2.27 (1.23–4.21) | .009 |
| ≥ 35 kg/m2 (n = 890) | 4.53 (2.51–8.16) | < .001 | 3.97 (2.20–7.17) | < .001 |
| Heart failure hospitalization | ||||
| BMI as a continuous variable (n = 5184) | 1.03 (1.01,1.04) | < .001 | 1.02 (1.01,1.04) | .007 |
| BMI by category | ||||
| < 25 kg/m2 (n = 755) | 1.00 [Reference] | 1.00 [Reference] | ||
| 25 to < 30 kg/m2 (n = 1664) | 1.01 (0.71–1.44) | .96 | 0.79 (0.54–1.14) | .20 |
| 30 to < 35 kg/m2 (n = 1376) | 0.93 (0.64–1.35) | .71 | 0.68 (0.46–1.02) | .06 |
| ≥ 35 kg/m2 (n = 1389) | 1.44 (1.02–2.04) | .04 | 0.97 (0.66–1.44) | .89 |
Abbreviations: BMI, body mass index; HR, hazard ratio; RR, risk ratio.
Estimates for all-cause mortality and heart failure hospitalization are expressed as hazard ratio (95% CI). Estimates for prevalent heart failure are expressed as risk ratio (95% CI).
The multivariable models were adjusted for age, sex, prior myocardial infarction, hypertension, prior stroke, diabetes mellitus, chronic lung disease, smoking status, systolic blood pressure, pulse, sodium, estimated glomerular filtration rate, hemoglobin, glucose, high-sensitivity C-reactive protein, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, left ventricular ejection fraction, left ventricular hypertrophy, left ventricular diameter, beta-blocker, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, statin, antiplatelet agent, and missing medication status. The multivariable all-cause mortality and heart failure hospitalization models were also adjusted for heart failure based on modified Gothenburg criteria at exam 1.
Figure 2.
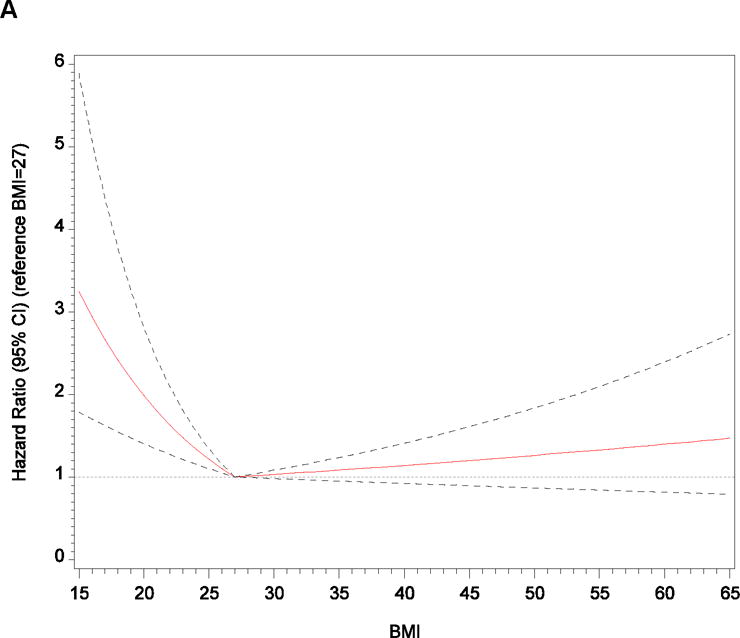
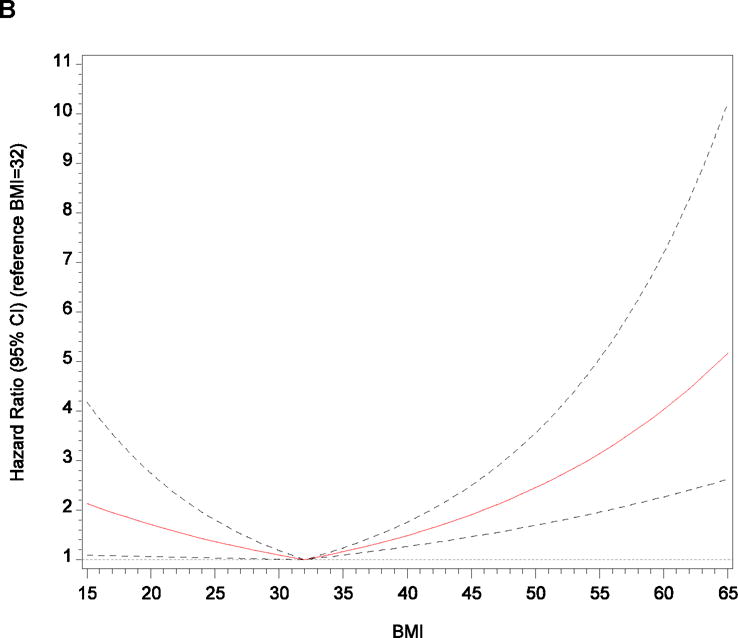
Adjusted Relationships Between Body Mass Index and (A) All-Cause Mortality and (B) Heart Failure Hospitalization After Application of Linear Splines
Abbreviation: BMI, body mass index.
Note: We used information gleaned from restricted cubic spline exploratory analyses to develop linear spline models with knots selected according to optimal model fit determined by the minimum Akaike information criterion. We selected knots at body mass index of 27 kg/m2 for all-cause mortality and 32 kg/m2 for heart failure hospitalization, which we then used as reference categories (ie, hazard ratio of 1.00) for linear spline graphs.
In multivariable adjusted analyses of prevalent HF, each 1-point increase in BMI was associated with a 6% increase in HF measured at exam 2 and a 5% increase at exam 3. In restricted cubic spline analyses, the Wald chi-square test for nonlinearity was not significant for HF at exam 2 (P = .14; Supplemental Figure 2B) or HF at exam 3 (P = .06; Supplemental Figure 2C). In the adjusted categorical comparison to participants with normal BMI, only morbidly obese participants had a higher risk of HF at exam 2 (P < .001). At exam 3, both obese (P = .009) and morbidly obese (P < .001) participants had higher risks of HF compared with participants with normal BMI.
After multivariable adjustment, each 1-point increase in BMI was associated with a 2% higher hazard of HF hospitalization (P = .007). We further explored the adjusted association of continuous BMI and HF hospitalization using restricted cubic splines (Supplemental Figure 2D). The graph suggests an increased hazard of HF hospitalization per increasing BMI only above the median, and the Wald chi-square test for overall nonlinear association was significant (P = .003). We also examined the unadjusted and adjusted associations between linear BMI splines and HF hospitalization (Figure 2B; Supplemental Table 2). For each 1-point increase in BMI up to 32 kg/m2, the adjusted hazard of HF hospitalization decreased by 4% (P = .03). However, with BMI greater than 32, each 1-point increase in BMI was associated with a 5% higher hazard of HF hospitalization (P < .001). In the unadjusted categorical comparison to normal-weight participants, only morbidly obese participants had a higher hazard of HF hospitalization (P = .04), but the association did not remain significant after multivariable adjustment.
In sensitivity analyses, we observed no significant interaction between sex and BMI. Substitution of waist circumference for BMI did not change the direction or magnitude of observed associations with outcomes. Exclusion of participants with a BMI less than 18.5 kg/m2 also did not change the direction of the observed associations with outcomes (Supplemental Table 3). Addition of self-reported physical activity as a covariate similarly led to nearly identical results as in the original models. Finally, the addition of potential metabolic and/or inflammatory mediators to age- and sex-adjusted models resulted in minimal change in the adjusted associations between continuous BMI and outcomes (Supplemental Table 4).
Discussion
Main findings
In this analysis of a community-based sample of AAs, obesity and morbid obesity were common; obesity and morbid obesity were not independently associated with greater all-cause mortality; there was a 5% increase in long-term risk of prevalent HF with every 1 kg/m2 increase in BMI among obese and morbidly obese participants; and there was a 5% increase in risk for HF hospitalization with each 1-point increase in BMI greater than 32 kg/m2. These findings underscore the significance of the obesity epidemic for AAs, with concerning links to the development of both HF and HF hospitalization. Prevention of obesity in AAs should thus remain a primary public health focus.
All-cause mortality
Increasing BMI was not associated with increased all-cause mortality in our study and, in fact, was observed to be associated with a 9% lower risk for each additional point of BMI up to 27 kg/m2. This breakpoint did not follow the boundaries of traditional BMI categories, such that even some participants in the overweight BMI range had relatively lower risk of death compared to those with normal weight. Previous analyses of large AA cohorts have also paradoxically observed a similar neutral or protective association between BMI and all-cause mortality.[3–9] However, in a recent pooled analysis of these studies, obesity was associated with an increased risk for all-cause mortality in AAs.[23] Our discordant findings between BMI and mortality may relate to the previously described “obesity paradox,” in which individuals with established cardiovascular disease, in particular HF, have better survival relative to their normal-weight counterparts.[24] The mechanisms of the obesity paradox are still under exploration, but result from study design (conditioning on a population with prevalent disease) or physiology (obese individuals having a “metabolic reserve” that helps in tolerating the catabolic effects of systemic disease).[25–29] Because the JHS is community-based, the selection bias that produces potentially distorted associations between BMI and mortality when restricting to disease-specific populations is not present. Therefore, these results suggest that racial differences may exist with regard to the prognostic utility of BMI values. Further data are necessary to confirm these findings and to explore potential underlying mechanisms.
Prevalent heart failure
Though BMI was not associated with increased all-cause mortality, the observation of increased HF and HF hospitalization in obese and morbidly obese AAs are important findings. Previous data exploring associations between obesity and HF have primarily been comprised of white populations, and our analysis represents one of the largest to date to assess these associations in AAs.[22, 30–32] The association of obesity and HF in AAs is of particular concern given that nearly one-third of participants were morbidly obese and represented the youngest group in our cohort. This increased risk for HF in the morbidly obese was seen at an earlier time point (exam 2) and persisted to follow up at exam 3. However, the association of obesity with increased prevalent HF was only observed after longer follow-up at exam 3.
In our analysis the overall cohort was predominantly female, with female gender becoming much more common as BMI increased (78.2% [n=1,108] of the morbidly obese). Importantly, we observed no differential effect of BMI on the risk of HF by gender. An increased risk for HF by obese females was suggested previously by results from the Framingham cohort, which again was predominantly white.[22] An increased risk for HF in obese AA women may also have been anticipated with our data, as those AAs (not stratified by body habitus) developing HF from the ARIC cohort were more likely to be women.[33] While gender differences in the risk of HF in AAs may exist, our study suggests that risk associated with BMI is the same for AA men and women.
Moreover, obesity may exert structural and hemodynamic changes that lead to the development of clinical HF.[25] Recent data suggest that obese patients without concomitant metabolic syndrome or those who are “metabolically healthy” may be at decreased risk for HF.[34, 35] To further investigate such potential mediators in the causal pathway between obesity and HF, we performed simplified models separately examining candidate variables of interest. We observed no significant changes in the magnitude and direction of associations of BMI with any of our outcomes in these models. Increasing BMI in AAs was confirmed as an independent risk factor for HF, not attenuated by markers of impaired metabolism or inflammation.
Heart failure hospitalization
Obesity has also been previously shown to be associated with an increased risk for HF hospitalization in the ARIC cohort and a clinical trial population with impaired glucose tolerance or metabolic syndrome.[36, 37] Obesity, in turn, is a risk factor for longer lengths of stay and increased costs during an episode of acute HF.[38, 39] Morbidly obese AAs in our cohort were relatively younger, potentially compounding the downstream burden associated with higher risk of repeated HF hospitalizations. Of note, the increased risk for HF hospitalization was only observed in the range of BMI greater than 32 kg/m2, whereas AA participants in traditional overweight and a subset of the obese (30 to 32 kg/m2) BMI categories had relatively lower risk compared with normal-weight participants. This observation does not detract from our overall findings, given that over 40% of Jackson Heart Study participants had BMI greater than 32 kg/m2, but perhaps warrants further appraisal of definitions of BMI categories in AAs. HF hospitalizations represent a major event in the HF disease course, and identification of possible reducible risks such as obesity in patient populations remains important.[40]
Clinical and public health implications
Our data highlight the concerning relationship between obesity and HF and HF hospitalization in AAs, with an incremental observed risk with progressive increases in BMI over specific thresholds. Obesity remains disproportionally high in AAs and obesity prevention and treatment in this population should remain a central focus.[41] Difficulties however remain in finding optimal approaches with regard to addressing the obesity epidemic in AAs.[42] Moreover, further research is also required to define those obese persons with perhaps differential risk (eg with central vs. visceral adiposity) for long-term mortality, HF, and HF hospitalization. The mechanisms underlying the observed “obesity paradox” in cardiovascular disease are complex and require further clarity.
Limitations
This was a retrospective analysis from a community cohort of AAs in the southern United States. Other measured and unmeasured variables may have influenced the results. As an example, physical activity level or fitness have been shown to impact the association of obesity with HF and survival.[43, 44] Our definition of HF may not have captured all patients with clinical HF; however, our definition has previously been used and validated in the ARIC and Jackson Heart Study data sets. In addition, consistent clinical data (eg, follow-up echocardiograms) was unavailable to define the type of HF (ie HF with preserved ejection fraction vs. HF with reduced ejection fraction). The discontinuity between clinical data collection at exam 1 (between 2000 and 2004) and the start of HF hospitalization surveillance (2005) may have resulted in an underestimation of the effect of BMI on HF hospitalization. Finally, BMI was examined at a single time point and BMI may have been dynamic during study participation. These subsequent changes in BMI (either increased or decreased) may have affected the observed associations with outcomes. However, the distribution of BMI categories at exams 2 and 3 was similar to baseline, and the median net change in BMI from baseline was 0.35 kg/m2 at exam 2 and 0.48 kg/m2 at exam 3.
Conclusions
Obesity and morbid obesity are common in African Americans and were associated with increased prevalent HF and HF hospitalization. Obesity remains a major public health concern and should be considered as an important risk factor for long-term morbidity in African American populations and a potential target for intervention.
Supplementary Material
Highlights.
The relationship between obesity and health outcomes such as mortality and heart failure (HF) in African Americans was further explored from the Jackson Heart Study
Increasing body mass index (BMI) was not associated with increased risk for all-cause mortality in African Americans
However, increasing BMI was associated with increasing risk for prevalent HF and HF hospitalization in African Americans
Obesity remains a major public health concern and should be considered as an important risk factor for long-term morbidity in African American populations and a potential target for intervention
Acknowledgments
Funding Source: This work was supported by grant R01HL117305 from the National Heart, Lung, and Blood Institute. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute on Minority Health and Health Disparities, or the National Institutes of Health.
Disclosures: Dr Krishnamoorthy reported working on projects funded by research grants to the Duke Clinical Research Institute from the NIH, Novartis, Maquet, Daiichi Sankyo, and Eli Lilly, and support to attend educational conferences from HeartWare, Thoratec, and Medtronic. Dr Eapen reported serving on advisory boards for Amgen, Cytokinetics, and Novartis; serving as a consultant for Amgen; and receiving an honorarium from Janssen. Dr Hernandez reported receiving research support from Bristol-Myers Squibb, GlaxoSmithKline, Janssen, and Novartis; and receiving honoraria from Amgen, Bristol-Myers Squibb, Janssen, and Novartis. Dr Mentz reported receiving research support from Amgen, AstraZeneca, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Otsuka, and ResMed; receiving honoraria from Thoratec; and serving on an advisory board for Luitpold Pharmaceuticals, Inc. No other authors reported financial disclosures.
Additional Contributions: We are grateful for the contributions of the Jackson Heart Study participants and data collection staff. Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
References
- 1.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Archives of internal medicine. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity. 2009;17:169–76. doi: 10.1038/oby.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. The New England journal of medicine. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 4.Boggs DA, Rosenberg L, Cozier YC, Wise LA, Coogan PF, Ruiz-Narvaez EA, et al. General and abdominal obesity and risk of death among black women. The New England journal of medicine. 2011;365:901–8. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. The New England journal of medicine. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SS, Signorello LB, Cope EL, McLaughlin JK, Hargreaves MK, Zheng W, et al. Obesity and all-cause mortality among black adults and white adults. American journal of epidemiology. 2012;176:431–42. doi: 10.1093/aje/kws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durazo-Arvizu R, Cooper RS, Luke A, Prewitt TE, Liao Y, McGee DL. Relative weight and mortality in U.S. blacks and whites: findings from representative national population samples. Annals of epidemiology. 1997;7:383–95. doi: 10.1016/s1047-2797(97)00044-6. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Wilkens LR, Murphy SP, Monroe KR, Henderson BE, Kolonel LN. Body mass index and mortality in an ethnically diverse population: the Multiethnic Cohort Study. European journal of epidemiology. 2012;27:489–97. doi: 10.1007/s10654-012-9695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis JP, Araneta MR, Wingard DL, Macera CA, Lindsay SP, Marshall SJ. Overall obesity and abdominal adiposity as predictors of mortality in u.s. White and black adults. Annals of epidemiology. 2009;19:134–42. doi: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HA., Jr The Jackson Heart Study: an overview. Ethnicity & disease. 2005;15:S6-1–3. [PubMed] [Google Scholar]
- 11.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Progress in cardiovascular diseases. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Progress in cardiovascular diseases. 2014;56:409–14. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Lavie CJ, Ventura HO. The Obesity Paradox in Heart Failure: Is it All About Fitness, Fat, or Sex? JACC Heart Fail. 2015;3:927–30. doi: 10.1016/j.jchf.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. The American journal of the medical sciences. 2004;328:131–44. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Keku E, Rosamond W, Taylor HA, Jr, Garrison R, Wyatt SB, Richard M, et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethnicity & disease. 2005;15:S6-62–70. [PubMed] [Google Scholar]
- 17.Taylor HA, Jr, Coady SA, Levy D, Walker ER, Vasan RS, Liu J, et al. Relationships of BMI to cardiovascular risk factors differ by ethnicity. Obesity. 2010;18:1638–45. doi: 10.1038/oby.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery CL, Mills KT, Chambless LE, Chang PP, Folsom AR, Mosley TH, et al. Long-term association between self-reported signs and symptoms and heart failure hospitalizations: the Atherosclerosis Risk In Communities (ARIC) Study. European journal of heart failure. 2010;12:232–8. doi: 10.1093/eurjhf/hfp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mentz RJ, Greiner MA, DeVore AD, Dunlay SM, Choudhary G, Ahmad T, et al. Ventricular conduction and long-term heart failure outcomes and mortality in african americans: insights from the jackson heart study. Circulation Heart failure. 2015;8:243–51. doi: 10.1161/CIRCHEARTFAILURE.114.001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslow NE, Day NE. Statistical methods in cancer research. Volume II–The design and analysis of cohort studies. IARC scientific publications. 1987:1–406. [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SS, Park Y, Signorello LB, Patel AV, Boggs DA, Kolonel LN, et al. A pooled analysis of body mass index and mortality among African Americans. PloS one. 2014;9:e111980. doi: 10.1371/journal.pone.0111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie M Carl J, Alpert, MD Martin A, Arena, PHD, PT Ross, Mehra, MBBS Mandeep R, Milani, MD Richard V, Ventura, MD Hector O. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. J Am Coll Cardiol HF. 2013:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. Journal of the American College of Cardiology. 2014;64:2281–93. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TJ. The obesity paradox in heart failure: weighing the evidence. Journal of the American College of Cardiology. 2014;64:2750–2. doi: 10.1016/j.jacc.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira I, Stehouwer CD. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. Journal of hypertension. 2012;30:2271–5. doi: 10.1097/HJH.0b013e32835b4fe0. [DOI] [PubMed] [Google Scholar]
- 29.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA: the journal of the American Medical Association. 2011;305:822–3. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of internal medicine. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 31.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Folsom AR, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circulation Heart failure. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) European heart journal. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 33.Gupta DKSA, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart Failure with Preserved Ejection Fraction in African-Americans – The Atherosclerosis Risk in Communities (ARIC) Study. JACC Heart Fail. 2013;1:156–63. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. Journal of the American College of Cardiology. 2011;58:1343–50. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 36.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. Journal of the American College of Cardiology. 2012;60:1640–6. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong YW, Thomas L, Sun JL, McMurray JJ, Krum H, Hernandez AF, et al. Predictors of incident heart failure hospitalizations among patients with impaired glucose tolerance: insight from the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research study. Circulation Heart failure. 2013;6:203–10. doi: 10.1161/CIRCHEARTFAILURE.112.000086. [DOI] [PubMed] [Google Scholar]
- 38.Lee CS, Chien CV, Bidwell JT, Gelow JM, Denfeld QE, Masterson Creber R, et al. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: an analysis of the U.S. Nationwide inpatient sample. BMC cardiovascular disorders. 2014;14:73. doi: 10.1186/1471-2261-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziaeian B, Sharma PP, Yu TC, Johnson KW, Fonarow GC. Factors associated with variations in hospital expenditures for acute heart failure in the United States. American heart journal. 2015;169:282–9.e15. doi: 10.1016/j.ahj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. Journal of the American College of Cardiology. 2014;63:1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 41.Kumanyika SK. Obesity in minority populations: an epidemiologic assessment. Obesity research. 1994;2:166–82. doi: 10.1002/j.1550-8528.1994.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 42.Kumanyika SK, Whitt-Glover MC, Haire-Joshu D. What works for obesity prevention and treatment in black Americans? Research directions. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2014;15(Suppl 4):204–12. doi: 10.1111/obr.12213. [DOI] [PubMed] [Google Scholar]
- 43.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clinic proceedings Mayo Clinic. 2013;88:251–8. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. The American journal of cardiology. 2015;115:209–13. doi: 10.1016/j.amjcard.2014.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


