Abstract
Objective
To identify neuromuscular impairments most predictive of unfavorable mobility outcomes in late-life.
Design
Longitudinal cohort study.
Setting
Research clinic.
Participants
Community-dwelling primary care patients aged ≥65 years (N=391) with self-reported mobility modifications, randomly selected from a research registry.
Intervention(s)
Not applicable.
Main Outcome Measure(s)
Categories of decline in and persistently poor mobility across baseline, 1 and 2 years of follow-up in the Lower-Extremity Function scales of the Late-Life Function and Disability Instrument. The following categories of impairment were assessed as potential predictors of mobility change: strength (leg strength), speed of movement (leg velocity, reaction time, rapid leg coordination), ROM (knee flexion/knee extension/ankle ROM), asymmetry (asymmetry of leg strength and knee flexion/extension ROM measures), and trunk stability (trunk extensor endurance, kyphosis).
Results
The largest effect sizes were found for baseline weaker leg strength (OR [95% CI]: 3.45 [1.72-6.95]), trunk extensor endurance (2.98 [1.56-5.70]), and slower leg velocity (2.35 [1.21-4.58]) predicting a greater likelihood of persistently poor function over 2 years. Baseline weaker leg strength, trunk extensor endurance, and restricted knee flexion motion also predicted a greater likelihood of decline in function (1.72 [1.10-2.70], 1.83 [1.13-2.95], 2.03 [1.24-3.35]).
Conclusion
Older adults exhibiting poor mobility may be prime candidates for rehabilitation focused on improving these impairments. These findings lay the ground work for developing interventions aimed at optimizing rehabilitative care and disability prevention and highlight the importance of both well-recognized (leg strength) and novel impairments (leg velocity, trunk extensor muscle endurance).
Keywords: Mobility limitations, muscle strength, range of motion, older adults, rehabilitation
Limitations in mobility activities like walking, climbing stairs, and getting up from a chair, are highly prevalent among older adults, affecting approximately 25% of adults aged 70 years and older and 50% of adults aged 80 years and older.1 Such activities are indicative of adverse outcomes in late-life, including development of disability, nursing home admission, and mortality.2 Since mobility limitations pose a significant threat to the health and independence of older adults, studies informing treatment strategies are vital.
Rehabilitation providers strive to apply evidence-based approaches to treat mobility problems; however, significant knowledge gaps exist for treating older adults with mobility limitations.3 No consensus exists on optimal types of exercise that should be prescribed and evidence is lacking on which treatable physical impairments are most responsible for changes in mobility.3 Establishing this evidence to guide effective and parsimonious approaches to care is especially crucial, given the constraints of both time and cost, coupled with the limited physical capacity of this patient population.
The Boston Rehabilitative Impairment Study of the Elderly (RISE) was designed to investigate key research issues identified by geriatric rehabilitation experts such as identifying underlying neuromuscular predictors of poor and declining mobility.3,4 Cross-sectional findings from Boston RISE show that lower-extremity strength and range of motion (ROM) are important for mobility, but also that less commonly recognized impairments in leg velocity, trunk extensor endurance, and strength asymmetry may play a key role.4,5 Longitudinal investigation is needed to determine the relationship between potential neuromuscular targets for rehabilitation and unfavorable outcomes in late-life mobility. The aim of this study was to identify the neuromuscular impairments associated with unfavorable mobility outcomes across baseline, 1 and 2 years of follow-up. Based on previous cross-sectional findings,4 we hypothesized that impairments in leg strength, leg velocity, trunk extensor endurance, asymmetry, and range of motion (ROM) would predict unfavorable mobility outcomes longitudinally.
Methods
Boston RISE is a prospective longitudinal cohort study designed to investigate which combinations of neuromuscular impairments are most responsible for changes in function and mobility. Study methods have been described previously in detail.4,6 Briefly, participants aged ≥65 years who were at risk for mobility decline7,8 were recruited from a registry of 9 different primary care practices located across the greater Boston area from December 2009 to January 2012. Eligibility included difficulty or task modification with walking one-half mile and/or climbing 1 flight of stairs7 and no moderate or severe dementia (Mini-Mental State Examination score <18) or severe mobility limitation (Short Physical Performance Battery score <4).9,10 Targeted recruitment was used to approximate ethnic/racial representation of older adults residing within a 10-mile radius of the facility. Methods were approved by the Spaulding Rehabilitation Hospital Institutional Review Board and written consent was obtained from all participants. Out of 430 participants who completed baseline visits, analyses included 391 with the outcome measure at baseline and either or both follow-up assessments (n=8 died, n=8 withdrew due to illness, n=23 withdrew or were lost to follow-up). We compared baseline characteristics between participants with and without follow-up assessments. Participants without follow-up assessments were less likely to have had postgraduate schooling (10.3% vs. 26.1%, p=0.03), and had worse baseline leg strength (8.6 ± 2.4 vs. 9.5 ± 2.5 N/kg, p=0.03), average reaction time (274.6 ± 70.2 vs. 246.2 ± 48.6 ms p<0.02), trunk extension endurance (71.0 ± 53.2 vs. 97.9 ± 58.6 s, p=0.01), gait speed (0.81 ± 0.20 vs. 0.92 ± 0.21 m/s, p<0.003), Short Physical Performance Battery score (7.8 ± 2.4 vs. 8.8 ± 2.2, p<0.01), and advanced lower-extremity function score (35.9 ± 17.1 vs. 42.4 ± 14.3, p=0.008). They did not differ from participants with follow-up assessments by age, sex, race, body mass index, number of comorbidities, leg strength, rapid leg coordination, knee or ankle ROM, asymmetry of leg strength or ROM measures, kyphosis, or basic lower-extremity function score.
Neuromuscular and mobility assessments were conducted by research assistants who were trained based on standardized materials developed for the study. Training sessions included lectures, demonstrations of techniques, and practice with other staff and senior volunteers. Co-investigators assisted with the training process relevant to their areas of expertise. Research assistants underwent a formalized certification process in which they were required to demonstrate competence in performing data collection during supervised pilot administrations of the protocols. Recertification and additional training, if indicated, took place every 6 months throughout the data collection. Due to limited staffing, the same examiner often assessed the neuromuscular predictors and mobility outcomes and, therefore, was not blinded.
Outcome
We measured patient-reported mobility across baseline, 1, and 2 years of follow-up during clinic visits or by phone (when participants were unable to return). We included 2 subdomains of the Late Life Function and Disability Instrument (LLFDI), which assesses limitations in 25 physical tasks applicable to daily life.11 Basic Lower-Extremity Function includes activities involving standing, stooping, and basic walking tasks. Advanced Lower-Extremity Function includes activities involving higher levels of physical ability and endurance, such as walking several blocks or standing up from the floor. Both subscales are transformed to a score from 0-100, with higher scores indicating better function. Evidence supports the test-retest reliability (ICC=0.91-0.98) and psychometric properties of the LLFDI, with known-groups analyses confirming the ability of the test to discriminate among groups of older adults with different levels of function, minimal floor and ceiling effects,11 high predictive validity for poor self-rated health, hospitalizations, and disability, and moderate to high responsiveness to change over 2 years.12 The minimal detectable change with 90% confidence (MDC90) for the LLFDI has been established previously in this cohort. A change of 4.4 points for Basic Function and 6.3 for Advanced Function is required to indicate true change beyond measurement error on the LLFDI.12
Neuromuscular impairments
Neuromuscular impairments measured at baseline were selected based on evidence linking them to mobility status and their potential amenability to rehabilitation.13-15 Five categories of neuromuscular impairments cross-sectionally associated with mobility were identified previously4: strength (leg strength), speed of movement (leg velocity, reaction time, rapid leg coordination), ROM (knee flexion/knee extension/ankle ROM), asymmetry (asymmetry of leg strength and knee flexion/extension ROM measures), and trunk stability (trunk extensor endurance, kyphosis). We use the term impairments to describe worse function in these neuromuscular attributes on a continuous scale.
Leg strength and power were measured on each leg with a pneumatic leg press machine using previously published protocols.4,16 Peak power was recorded as the highest power out of five trials performed with each leg at 40% and 70% of the one repetition maximum. Leg press power has demonstrated excellent test-retest reliability within community-dwelling older adults (ICC=0.85-0.93).16 Leg velocity was calculated by dividing peak power by force at peak power. Reaction time was measured using a device developed and validated by Lord et al. and has demonstrated moderate test-retest reliability (ICC=0.69).17 Rapid leg coordination was measured as time to complete 10 repetitions of placing the heel of one foot just below the opposite knee and then back to the floor while seated.18 Leg coordination has shown good test-retest reliability (r>0.8).18 Knee and ankle ROM were measured with a goniometer.19 Knee flexion and extension ROM have demonstrated excellent test-retest reliability (flexion ICC=0.90-0.99; extension ICC=0.86-0.98)19 and ankle dorsiflexion and plantarflexion ROM have demonstrated moderate to excellent reliability (dorsiflexion ICC=0.64-0.99; plantarflexion ICC=0.47-0.99).20 Strength asymmetry was calculated by dividing the higher value side by the lower value. Knee ROM difference was calculated by subtracting the higher value side by the lower value. Trunk extensor muscle endurance was measured with the participant lying prone on a specialized plinth positioned 45° from vertical using a previously published protocol.15 Excellent test-retest reliability of trunk endurance has been previously reported within a subset of our study sample (r=0.88–0.91).15 Kyphosis was measured using a reliable (r=0.78) and valid flexicurve method.21,22
Adjustment variables
Age, sex, race, education, and baseline conditions and characteristics known and hypothesized to be associated with mobility were assessed for inclusion in the models. Body mass index defined obesity/overweight status.23 Depressive symptoms were defined by a Patient Health Questionnaire (PHQ-9) score >5.24 The Digit Symbol Substitution Test (DSST) measured executive function.25 Sensory loss was measured using the Semmes-Weinstein monofilament test.26 Visual impairment was defined as inability to read the 20/50 line of the Snellen eye chart.27 Number of comorbidities was measured using a validated questionnaire that included heart disease, hypertension, lung disease, diabetes, ulcer/stomach disease, kidney disease, liver disease, anemia/other blood diseases, cancer, depression, osteoarthritis/rheumatoid arthritis, and back pain.28
Statistical Analysis
A 5-level categorical variable was created for each the Basic and Advanced Lower-Extremity Function components of the LLFDI to capture longitudinal mobility across baseline, year 1, and year 2: persistently poor, decline in, persistently average, improvement in, and persistently high function. “Decline” and “improvement” were defined as a decrease or increase ≥MDC90 between any two time points. If both decline and improvement occurred, the amount of change from the first time point to the last was used as the tie breaker. “Persistently poor function” and “persistently high function” were defined as scores at the last follow-up (year 2 or 1) within the lowest and the highest quartiles, respectively, with no change ≥MDC90 during the study. “Persistently average function” was defined as function at the last follow-up between the highest and lowest quartiles with no change ≥MDC90. Participants were included if they had the LLFDI at baseline and either follow-up (n=46 had outcome at year 1 but not year 2; n=1 had outcome at year 2 but not year 1). Latent class growth modeling was performed to confirm the optimal number of change groups for both basic and advanced function.29 Bayesian Information Criterion value and model convergence showed that models with 5 groups had the best fit.29 In order to establish the temporal relationship between the impairments and mobility limitations, an important criterion for causation, we performed a sensitivity analysis excluding participants with poor function at baseline. The cut point for poor function was defined by the upper limit of the lowest quartile score for the study population at the last follow-up.
Frequencies and proportions were reported for categorical variables and means, SD, minimum, and maximum values were reported for continuous variables. Multinomial logistic regression models were built using the 5-level categorical dependent variables for basic and advanced function with persistently high function as the reference group. All models were adjusted for age and sex. Baseline neuromuscular impairments were standardized by dividing by the SD for the study sample. Both impairments and adjustment variables that were associated with the outcome (alpha=0.1) were entered into the model. Then impairments that were not significant (alpha=0.05) and adjustment variables that were not significant and did not alter the relationship between an impairment and outcome by ≥20% were removed. Analyses were performed using impairment data that underwent multiple weighted imputation of missing values.30 We used likelihood ratio tests, c-statistics,31 and pseudo R2 values to evaluate the fit of our models. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
During the 2-year follow-up period, of 391 participants, 41 (10.5%) experienced persistently poor basic function, 26 (6.7%) had persistently poor advanced function, 136 (34.8%) declined in basic function, 134 (34.3%) declined in advanced function, 37 (9.4%) had persistently high basic function, and 48 (12.3%) had persistently high advanced function. Baseline characteristics are presented in Table 1. As reported previously, age, sex, and racial distributions of the study population are consistent with the 2004 census for older adults within the study recruitment area.4 Table 2 shows distributions for neuromuscular impairments. Leg strength asymmetry had the most missing data at 14%. Subsequent results are presented using imputed impairment data.30
Table 1. Participants' Baseline Characteristics (n=391).
| Variable | n | Mean ± SD or % | Min | Max |
|---|---|---|---|---|
| Age (years) | 391 | 76.5 ± 7.1 | 65.0 | 96.0 |
| Women | 261 | 66.8% | ||
| Nonwhite | 66 | 16.9% | ||
| Education | ||||
| <High school | 46 | 11.8% | ||
| High school | 113 | 28.9% | ||
| College graduate | 130 | 33.3% | ||
| Postgraduate | 102 | 26.1% | ||
| BMI (kg/m2) | 390 | 29.4 ± 6.2 | 18.4 | 55.7 |
| <25 | 94 | 24.1% | ||
| 25-30 | 156 | 40.0% | ||
| >30 | 140 | 35.9% | ||
| Chronic Conditions (0-12) | 391 | 4.0 ± 1.9 | 0 | 11 |
| Habitual gait speed (m/s) | 391 | 0.92 ± 0.21 | 0.31 | 1.50 |
| SPPB score (0-12) | 391 | 8.8 ± 2.2 | 4.0 | 12.0 |
SPPB = Short Physical Performance Battery.
Table 2. Neuromuscular Impairmentsa among Participants (n=391).
| Impairment Category | Variable | n | Mean ± SD or % | Min | Max |
|---|---|---|---|---|---|
| Strength | Leg strength (N/kg) | 351 | 9.5 ± 2.5 | 3.1 | 20.3 |
| Speed | Leg velocity (m/s) | 345 | 1.0 ± 0.3 | 0.2 | 1.9 |
| Average reaction time (ms) | 391 | 246.2 ± 48.6 | 177.5 | 648.4 | |
| Rapid leg coordination (s) | 375 | 11.1 ± 3.1 | 5.5 | 28.3 | |
| ROM | Maximal knee flexion (deg) | 386 | 124.6 ± 13.7 | 58.0 | 149.0 |
| Maximum knee extension (deg) | 386 | 9.5 ± 5.9 | -3.0 | 25.0 | |
| Ankle range of motion | 390 | ||||
| Impaired | 281 | 72.1% | |||
| Non-impaired | 109 | 28.0% | |||
| Asymmetry | Leg strength asymmetry (ratio) | 338 | 1.2 ± 0.3 | 1.0 | 4.3 |
| Knee flexion difference (deg) | 386 | 5.5 ± 7.1 | 0 | 51.0 | |
| Knee extension difference (deg) | 382 | 3.1 ± 3.1 | 0 | 16.0 | |
| Trunk Stability | Kyphosis (height/length*100 of flexicurve instrument) | 391 | 10.5 ± 3.0 | 3.3 | 21.7 |
| Trunk extensor endurance (s) | 368 | 97.9 ± 58.6 | 0 | 150 |
Non-imputed data used. ROM = range of motion. Deg = degrees.
Multinomial logistic regression models met criteria for good fit (likelihood ratio tests: p-value<0.001; c-statistics ≥ 0.7;31,32 pseudo R2 from 0.2-0.433). Models were adjusted for age, sex, overweight/obesity status, and number of comorbidities. Additional covariates that were tested but did not meet criteria for inclusion were: race, education, DSST score, visual impairment, depression, and sensory loss. Figures 1 and 2 show the neuromuscular impairments predictive of unfavorable outcomes in basic and advanced lower-extremity function, respectively. Odds are presented for every SD of change in the predictor in the more impaired direction. None of the neuromuscular impairments significantly predicted decline in basic function. Weaker leg strength, slower leg velocity, poorer trunk extensor endurance, and restricted knee flexion ROM were each associated with around a 2 times greater likelihood of persistently poor basic function. Weaker leg strength, slower leg velocity, poorer trunk extensor endurance were each associated with up to a 2 times greater likelihood of decline in advanced function, and 2-3.5 times greater likelihood of persistently poor advanced function. Findings from the sensitivity analysis excluding participants with poor advanced function at baseline (n=25, 6%) were consistent with the main analysis (results not shown). Excluding participants with poor basic function at baseline (n=32, 8%), the effect sizes within the sensitivity analysis were mostly consistent with those from the main analysis; however, they did not achieve statistical significance.
Figure 1. Neuromuscular impairmentsa predictive of unfavorable outcomes in basic lower-extremity function.
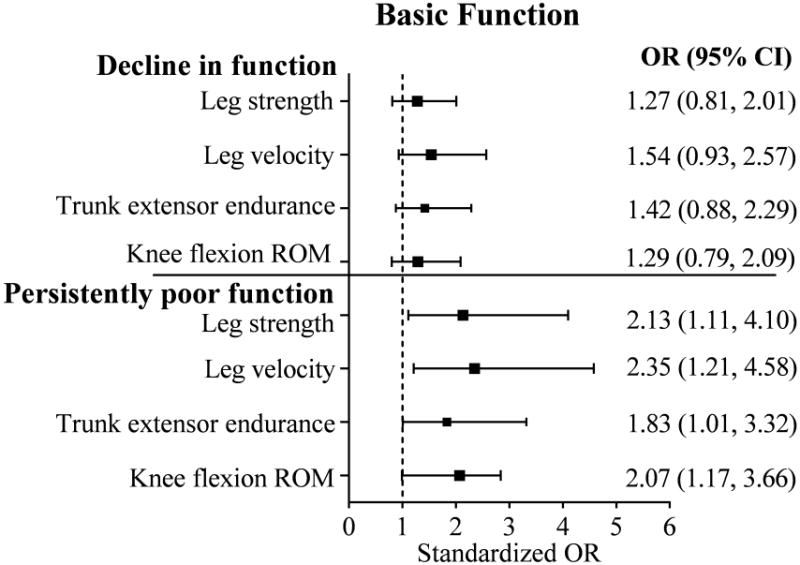
aImputed data used for neuromuscular impairments. Odds are presented for every one SD worse impairment (decrease in attribute). Total N=391; n = 136 with decline in function; n = 41 with persistently low function; n = 37 with persistently high function (reference group). Minimal Detectable Change (MDC90) = 4.4. Multivariable logistic regression model adjusted for age, sex, overweight/obesity status, and number of comorbidities. Additional covariates tested but not included in the model were not significant and did not alter the relationship between the predictors and outcome by ≥20%: race, education, Digit Symbol Substitution Test score, visual impairment, depression, and sensory loss. OR = odds ratio. CI = confidence interval.
Figure 2. Neuromuscular impairmentsa predictive of unfavorable outcomes in advanced lower-extremity function.
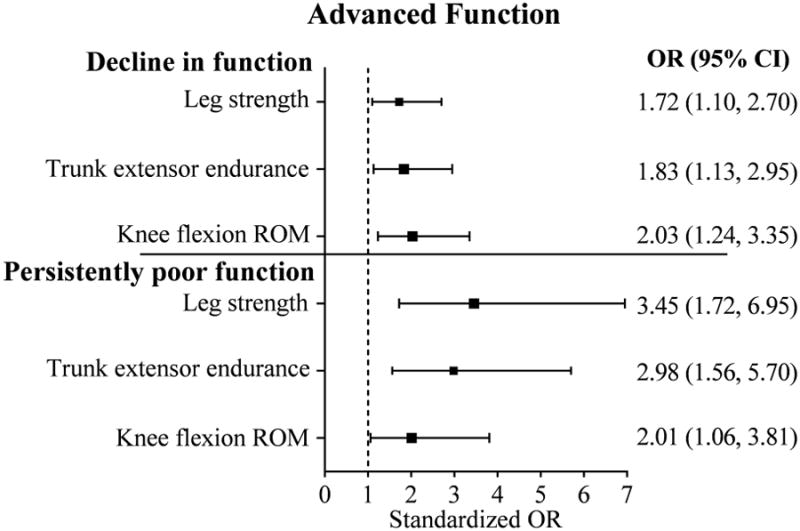
aImputed data used for neuromuscular impairments. Odds are presented for every one SD worse impairment (decrease in attribute). Total N=391; n = 134 with decline in function; n = 26 with persistently low function; n = 48 with persistently high function (reference group). Minimal Detectable Change (MDC90) = 6.3. Multivariable logistic regression model adjusted for age, sex, overweight/obesity status, and number of comorbidities. Additional covariates tested but not included in the model were not significant and did not alter the relationship between the predictors and outcome by ≥20%: race, education, Digit Symbol Substitution Test score, visual impairment, depression, and sensory loss. OR = odds ratio. CI = confidence interval. ROM = range of motion.
Discussion
Within this study of older primary care patients, four modifiable neuromuscular impairments were associated with poor mobility outcomes over 2 years of follow-up. These were impairments in leg strength, leg velocity, trunk extensor endurance and knee flexion ROM. Impairments had the strongest associations with persistently poor function, which may suggest that they are especially relevant among those with existing and persisting limitations in mobility. Importantly, two of these impairments, leg velocity and trunk extensor endurance have not been previously emphasized in the rehabilitative care of older adults. These findings provide important new evidence suggesting potential targets for treating both persistent and incident mobility problems among older patients.
Consistent with our original hypothesis, we identified four out of five categories of neuromuscular impairments predictive of unfavorable outcomes in mobility, with the exception of side to side asymmetries in strength and in ROM. Leg strength, trunk extensor endurance, and knee flexion ROM were predictive of unfavorable basic and advanced lower-extremity function longitudinally. Leg velocity predicted unfavorable basic function, exclusively. It is unclear why leg velocity was predictive of basic but not advanced function longitudinally. Possibly, leg velocity may have an effect on basic function up to a certain threshold above which faster velocity is not required for more advanced functioning.
A novel aspect of this study is that we compared several underemphasized impairments with several well-established impairments for their longitudinal impact on mobility. Importantly, we showed that impairments in leg velocity and trunk extensor endurance currently not prioritized among older adults, contribute to poor mobility over time.5 Leg velocity is a major component of muscle power that distinguishes it from strength. Muscle power, which is the combination of force and velocity, has been increasingly recognized as an important contributor to mobility in older adults.13,34,35 Findings from this study support that impairments in both leg velocity and strength are important to address in mobility limited older adults. Trunk extensor endurance has been hypothesized to be a mechanism by which kyphosis influences mobility and fall-related injuries.36 While we did not find a relationship between kyphosis and mobility, trunk extensor endurance had a strong and consistent association with decline in and persistently poor lower-extremity function over time, suggesting that it should be a central target to explore in the development of new rehabilitative interventions. Previous studies examining the effects of kyphosis on mobility have not included assessment of trunk extensor endurance.36,37
The neuromuscular impairments examined in our analysis tended to be stronger predictors of persistently poor lower-extremity function than decline in function, with greater effect sizes and a greater number of significant relationships. This may be because the impairments identified are stronger predictors of chronic (persistent) rather than acute (short-term) mobility limitations, which could have other contributors, such as acute illness or injury. Longer and more frequent study follow-up is needed to address this hypothesis. These findings also suggest that impairments amenable to rehabilitative care are most predictive of mobility in older adults who may already have existing limitations. Patients with more severe mobility limitations may therefore still benefit from treatment that prioritizes these four impairments. Our findings were observed after adjustment for age, sex, overweight/obesity status, and number of comorbidities. We additionally tested the effects of various additional conditions and characteristics known to be associated with mobility (education, cognition, visual impairment, depression, and sensory loss) and found that none had any material impact on the findings. Moreover, on average, our participants manifested four comorbidities measured using a validated index.28 Our findings suggest that these four impairments in leg strength, leg velocity, knee flexion ROM, and trunk extensor endurance are common and lead to poor mobility over time, independent of various other influential factors including comorbidity. While we recognize that individualization is beneficial for effective rehabilitative care, our findings lay a foundation for standardizing assessments to include all four impairments.
Our sensitivity analysis, in which we excluded participants with poor function at baseline, showed findings consistent with the main analysis for advanced function, illustrating a temporal relationship with neuromuscular impairments preceding persistently poor and declining advanced function among this study population. For basic function, effect sizes from the sensitivity analysis were mostly consistent with those from the main analysis, although they did not reach statistical significance. A likely explanation for this discrepancy is lack of statistical power within the sensitivity analysis due to the small number of participants within the persistently poor group. While our effect sizes suggest temporal relationships between the neuromuscular impairments and persistently poor basic function, a larger sample size may be needed to confirm these relationships.
There are a number of notable strengths within this study and analysis. First, Boston RISE is the most extensive comparison of neuromuscular impairments amenable to rehabilitative intervention to date, examining both established and underemphasized impairments. Second, by design, our study sample consists of older primary care patients at risk for mobility decline. These at-risk individuals would likely be the main recipients of mobility screening within the mandated Medicare Annual Wellness visits who would be referred to rehabilitative care. Our findings can help inform assessments and interventions that ultimately help rehabilitation providers care for these patients. Additionally, we modeled change in mobility using five different change groups. This approach allowed us to account for some of the heterogeneity that occurs with mobility progression in late-life.38 The difference in effect sizes that we found for the decline in and persistently poor lower-extremity function categories highlight the importance of this approach. However, it is possible that some of the variation in mobility was not fully captured by these change groups. A larger sample size is needed to assess this. Finally, our study had very good retention, with 91% of participants receiving an outcome assessment at follow-up.
An important future direction is to use these findings to inform comparative effectiveness trials. For example, landmark results from the Lifestyle Interventions and Independence for Elders (LIFE) study have shown that structured physical activity can effectively reduce major mobility disability in at-risk individuals.39 Yet it remains unknown whether exercises targeted at improving specific impairments particularly among those most at risk for disability could lead to more optimal benefits.
Study Limitations
Although targeted recruitment within this study resulted in demographic distributions consistent with the 2004 census for older adults living within the recruitment area,6 Boston RISE is not a population-based study, and findings may not generalize to community-dwelling older adults living in other geographical regions. Missing data occurred due to a small number of participants who were unable to perform some of the impairment tests at baseline and due to loss to follow-up. To address the missing impairment data, we performed weighted multiple imputation.30 Still, some lower-extremity function groups had small numbers, which were further decreased within the sensitivity analysis. Participants with missing follow-up data who were not included in this analysis did have worse impairment in some neuromuscular attributes and poorer advanced lower-extremity function at baseline. Future studies should include analytic methods that are able to examine death as a competing risk. Since the same study staff may have performed both the neuromuscular and mobility assessments, they were not blinded, and therefore some expectation bias may have occurred. Although, extensive training of study staff was undertaken to standardize all measurement procedures and minimize bias.
Some participants who experienced a decline in lower-extremity function may have gone on to experience persistently poor function; longer follow-up is needed to assess this. Only baseline impairments were included in this analysis; however, our assessments may mirror rehabilitative care in which a clinician targets impairments assessed within a single initial evaluation. In addition, this study focused on unfavorable mobility outcome categories: decline in and persistently poor lower-extremity function. Investigating predictors of favorable mobility outcomes was beyond the scope of our original study aims. Future studies designed to address the impact of these neuromuscular attributes on improvement and maintenance of mobility are needed.
Conclusions
In summary, our study identified four key impairments amenable to rehabilitation that were predictive of unfavorable mobility outcomes in older primary care patients. These impairments were leg strength, leg velocity, knee flexion range of motion, and trunk extensor muscle endurance. Impairments had the strongest associations with persistently poor mobility. Identification of leg velocity and trunk extensor endurance as potentially significant contributors to mobility limitation, in particular, may have important implications for clinical care and will inform future studies of interventions.
Acknowledgments
This work was supported by the National Institute on Aging (R01 AG032052-03); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966-01 to JB); and the National Center for Research Resources in a grant to the Harvard Clinical and Translational Science Center (1 UL1 RR025758-01). Rachel E. Ward is supported by the National Institute on Disability and Rehabilitation Research (H133P120001). Marla K. Beauchamp is supported by a fellowship from the Canadian Institutes of Health Research. Alan M. Jette declares stock in CREcare, LLC, a small business that disseminates outcome tools including the Late-Life Function and Disability Instrument. An abstract was accepted and an oral presentation was given on the results from these data at the Gerontological Society of America 67th Annual Meeting in Washington, DC in November, 2014.
List of abbreviations
- Boston RISE
Boston Rehabilitative Impairment Study of the Elderly
- DSST
Digit Symbol Substitution Test
- LIFE Study
Lifestyle Interventions and Independence for Elders Study
- LLFDI
Late Life Function and Disability Instrument
- MDC90
minimal detectable change with 90% confidence
- PHQ-9
Patient Health Questionnaire-9
- ROM
range of motion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao Y, McGee DL, Cao G, Cooper RS. Recent changes in the health status of the older U.S. population: findings from the 1984 and 1994 supplement on aging. Journal of the American Geriatrics Society. 2001 Apr;49(4):443–449. doi: 10.1046/j.1532-5415.2001.49089.x. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE. Mobility performance: a high-tech test for geriatricians. The journals of gerontology Series A, Biological sciences and medical sciences. 2003 Aug;58(8):712–714. doi: 10.1093/gerona/58.8.m712. [DOI] [PubMed] [Google Scholar]
- 3.Lee LW, Siebens HC. Geriatric Rehabilitation. In: LoCicero J, Rosenthal RA, editors. A Supplement to New Frontiers in Geriatrics Research. New York, NY: The American Geriatrics Society; 2007. pp. 301–334. [Google Scholar]
- 4.Bean JF, Latham NK, Holt N, Kurlinksi L, Ni P, Leveille S, Percac-Lima S, Jette A. Which neuromuscular attributes are most associated with mobility among older primary care patients? Archives of physical medicine and rehabilitation. 2013 Dec;94(12):2381–2388. doi: 10.1016/j.apmr.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute on Aging. Exercise and physical activity: your everyday guide from the National Institute on Aging. 09-4258. Bethesda: National Institute on Aging; 2009. [Google Scholar]
- 6.Holt NE, Percac-Lima S, Kurlinski LA, Thomas JC, Landry PM, Campbell B, Latham N, Ni P, Jette A, Leveille SG, Bean JF. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Archives of physical medicine and rehabilitation. 2013 Feb;94(2):347–355. doi: 10.1016/j.apmr.2012.08.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. The journals of gerontology Series A, Biological sciences and medical sciences. 2000 Jan;55(1):M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Young Y, Rubin G, Bandeen-Roche K. Self-reported preclinical disability identifies older women with early declines in performance and early disease. Journal of clinical epidemiology. 2001 Sep;54(9):889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 9.Bean JF, Kiely DK, LaRose S, Leveille SG. Which impairments are most associated with high mobility performance in older adults? Implications for a rehabilitation prescription. Archives of physical medicine and rehabilitation. 2008 Dec;89(12):2278–2284. doi: 10.1016/j.apmr.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 11.Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, Ashba J. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. The journals of gerontology Series A, Biological sciences and medical sciences. 2002 Apr;57(4):M217–222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 12.Beauchamp MK, Jette AM, Ward RE, Kurlinski LA, Kiely D, Latham NK, Bean JF. Predictive Validity and Responsiveness of Patient-Reported and Performance-Based Measures of Function in the Boston RISE Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2014 Dec 15; doi: 10.1093/gerona/glu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Archives of physical medicine and rehabilitation. 2004 Jul;85(7 Suppl 3):S31–42. doi: 10.1016/j.apmr.2004.03.010. quiz S43-34. [DOI] [PubMed] [Google Scholar]
- 14.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age and ageing. 2002 Mar;31(2):119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 15.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM & R : the journal of injury, function, and rehabilitation. 2009 Oct;1(10):916–924. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging clinical and experimental research. 2007 Jun;19(3):194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 17.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical therapy. 2003 Mar;83(3):237–252. [PubMed] [Google Scholar]
- 18.Shahar A, Patel KV, Semba RD, Bandinelli S, Shahar DR, Ferrucci L, Guralnik JM. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Movement disorders : official journal of the Movement Disorder Society. 2010 Sep 15;25(12):1909–1915. doi: 10.1002/mds.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins MA, Riddle DL, Lamb RL, Personius WJ. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in a clinical setting. Physical therapy. 1991 Feb;71(2):90–96. doi: 10.1093/ptj/71.2.90. discussion 96-97. [DOI] [PubMed] [Google Scholar]
- 20.Martin RL, McPoil TG. Reliability of ankle goniometric measurements: a literature review. Journal of the American Podiatric Medical Association. 2005 Nov-Dec;95(6):564–572. doi: 10.7547/0950564. [DOI] [PubMed] [Google Scholar]
- 21.Milne JS, Lauder IJ. Age effects in kyphosis and lordosis in adults. Annals of human biology. 1974 Jul;1(3):327–337. doi: 10.1080/03014467400000351. [DOI] [PubMed] [Google Scholar]
- 22.Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane database of systematic reviews. 2009;(2):CD003288. doi: 10.1002/14651858.CD003288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arteriosclerosis, thrombosis, and vascular biology. 1996 Dec;16(12):1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 24.Zuithoff NP, Vergouwe Y, King M, Nazareth I, van Wezep MJ, Moons KG, Geerlings MI. The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a crosssectional study. BMC family practice. 2010;11:98. doi: 10.1186/1471-2296-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karp JF, Reynolds CF, 3rd, Butters MA, Dew MA, Mazumdar S, Begley AE, Lenze E, Weiner DK. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006 Sep-Oct;7(5):444–452. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes research and clinical practice. 2001 Nov;54(2):115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis) Transactions of the American Ophthalmological Society. 2009 Dec;107:311–324. [PMC free article] [PubMed] [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and rheumatism. 2003 Apr 15;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.Andruff H, Carraro N, Thompson A, Gaudreau P. Latent Class Growth Modeling: A Tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 30.Carpenter J, Kenward M, White I. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Stat Methods Med Res. 2007;16:259–275. doi: 10.1177/0962280206075303. [DOI] [PubMed] [Google Scholar]
- 31.Hand DJ, Till RJ. A Simple Generalisation of the Area Under the ROC Curve for Multiple Class Classification Problems. Machine Learning. 2001;45:171–186. [Google Scholar]
- 32.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 33.McFadden D. Quantitative Methods for Analyzing Travel Behaviour on Individuals: Some Recent Developments. In: Hensher D, Stopher P, editors. Bahvioural Travel Modelling. London: Croom Helm; 1979. pp. 279–318. [Google Scholar]
- 34.Mayson DJ, Kiely DK, LaRose SI, Bean JF. Leg strength or velocity of movement: which is more influential on the balance of mobility limited elders? American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2008 Dec;87(12):969–976. doi: 10.1097/PHM.0b013e31818dfee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-based versus patient-reported physical function: what are the underlying predictors? Physical therapy. 2011 Dec;91(12):1804–1811. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eum R, Leveille SG, Kiely DK, Kiel DP, Samelson EJ, Bean JF. Is kyphosis related to mobility, balance, and disability? American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2013 Nov;92(11):980–989. doi: 10.1097/PHM.0b013e31829233ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katzman WB, Vittinghoff E, Ensrud K, Black DM, Kado DM. Increasing kyphosis predicts worsening mobility in older community-dwelling women: a prospective cohort study. Journal of the American Geriatrics Society. 2011 Jan;59(1):96–100. doi: 10.1111/j.1532-5415.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005 Mar 15;161(6):575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 39.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. Jama. 2014 Jun 18;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]


