Abstract
Purpose
Acid-suppression therapy is known to decrease the systemic exposure of erlotinib. The erlotinib prescribing information also recommends staggering dosing with a histamine-2 receptor antagonist (H2RA) and avoiding concurrent use of a proton-pump inhibitor (PPI). This retrospective analysis evaluated the frequency of concurrent acid-suppression therapy in oncology patients receiving erlotinib and its association with outcomes.
Methods
All patients prescribed erlotinib within UC San Diego Health System between February 26, 2011 and February 28, 2014 were assessed for eligibility, and for survival outcomes and adverse events.
Results
Of the 76 patients in the analysis, 24 were prescribed both a PPI and an H2RA with erlotinib therapy (31.6%). The two patient groups, with (n=24) and without PPI/H2RA (n=52), were similar in clinical characteristics and erlotinib dose. One patient received an H2RA therapy alone and was excluded from the analysis; no one received PPI therapy alone. Patients receiving erlotinib alone had a longer median progression-free survival (PFS) compared to patients with concurrent PPI/H2RA therapy (11.0 months vs. 5.3 months; P=0.029). Overall survival (OS) and incidence of rash and/or diarrhea did not correlate with use of acid-suppression therapy.
Conclusion
Nearly one-third of subjects received acid-suppression therapy. Patients treated with erlotinib and PPI/H2RA therapy had shorter PFS, but similar OS and adverse event profile compared to those who did not receive acid-suppression.
Keywords: oncology, erlotinib, proton-pump inhibitor, histamine-2 receptor antagonist, survival
INTRODUCTION
The average oncology patient is often on multiple therapeutic agents for comorbid conditions as diverse as depression, heart disease, pain, etc. in addition to their anti-cancer treatment regimen. A prior study showed that physicians routinely prescribe a median of eight medications to treat comorbid conditions in cancer patients [1]. There is thus the potential for drug-drug interactions that may alter the safety or efficacy of therapy. Acid-suppression therapy is common in the oncology patient population and up to one-third of cancer patients received acid-suppression treatment in the United States between 1999 to 2011 [2]. However, the increase in gastric pH with acid suppression has the potential to decrease absorption of other medications, which may have pH-dependent solubility. The prescribing information for several oral kinase inhibitors, including, but not limited to, bosutinib [3], dasatinib [4], and palbociclib [5] state that concurrent use with acid-suppression therapy can result in reduced drug concentrations.
Erlotinib is an oral epidermal growth factor receptor tyrosine kinase inhibitor (TKI). In the United States, it is approved for the treatment of non-small cell lung cancer (NSCLC) and pancreatic cancer [6]. The current erlotinib prescribing information recommends avoidance of proton pump inhibitors (PPIs) if possible. When treatment with a histamine-2 receptor antagonist (H2RA) is required, erlotinib must be taken 10 hours after H2RA dosing and at least two hours before the next dose of the H2RA [6]. These recommendations were based on a study done in healthy volunteers [7] and there is conflicting evidence on efficacy and safety outcomes in the cancer patient population [8,9]. Thus, the true effect of acid-suppression on survival and safety outcomes in oncology patients taking erlotinib is incompletely elucidated. Although erlotinib should not be taken with PPIs and administration should be staggered with H2RA administration, it may not be possible to adhere to dosing and administration recommendations in all clinical scenarios. If acid-suppression is administered and lowers erlotinib exposure, it is conceivable that both benefits and adverse events are attenuated. This study aimed to determine the frequency of acid-suppression therapy use with erlotinib and measure its potential association with progression-free survival (PFS), overall survival (OS), and incidence of adverse events in patients receiving erlotinib as standard of care.
MATERIALS AND METHODS
The current study evaluated a subset of patients on the UC San Diego PREDICT study (Profile Related Evidence Determining Individualized Cancer Therapy Study) [10] conducted in accordance with the UC San Diego Health System Institutional Review Board guidelines. Patients on the PREDICT protocol were screened through pharmacy records to identify those who had been prescribed erlotinib through the UC San Diego Health System between February 26, 2011 and February 28, 2014. Patients who received erlotinib as part of combination chemotherapy, never started erlotinib therapy, or had a duration of erlotinib therapy ≤14 days were excluded from the study. Data was extracted from the UC San Diego Health System electronic health records (Epic Systems Corp, Verona, Wisconsin) primarily through chart review of oncology physician notes written as part of standard of care and medication prescription information, but also from hospital records and telephone encounters. Data included erlotinib prescription dates and doses, PFS, OS, PPI and H2RA prescriptions, age, sex, weight, date of cancer diagnosis, cancer type, and adverse events (rash and diarrhea of any grade). PFS was calculated as the difference in months from the start date of erlotinib therapy to the date of physician-assessed progression or death. OS was calculated as the difference in months from the start date of erlotinib therapy to the date of death. At the time of analysis, patients without progression (for PFS) or still alive (for OS) were censored as of that date.
Concurrent acid-suppression therapy was defined as the prescription of a PPI or H2RA while on erlotinib therapy. Patients who were prescribed acid-suppression therapy within the date range of erlotinib therapy or within 90 days of the initiation or discontinuation of erlotinib therapy were included in the concurrent acid-suppression group. Average daily erlotinib dose was calculated using the average prescribed daily dose ordered through the electronic medication order system and did not take into account skipped doses or drug holidays not recorded in the electronic health record.
The difference in adverse events between patients with and without concurrent acidsuppression therapy was assessed using Fisher’s exact test. Median PFS and OS were analyzed using the Kaplan-Meier method. The Cox proportional hazards log-rank statistic was used to compare PFS and OS between patients with and without concurrent PPI therapy using the survfit and coxph functions in the ‘survival’ package [11] in RStudio version 0.98.1103 and R version 3.2.0 [12]. All statistical analyses were completed using R/RStudio.
RESULTS
Study population
Ninety-four patients were identified through the preliminary screen as having been prescribed erlotinib between February 26, 2011 and February 28, 2011 (Figure 1). Of these patients, 17 were excluded because they either received erlotinib as part of combination therapy or never initiated erlotinib therapy despite having a prescription record in the electronic health record. Fifty-two patients (68.4%) received erlotinib therapy in the absence of H2RA and PPI administration and 24 (31.6%) patients received erlotinib with both PPI and H2RA prescriptions during the course of therapy. There was insufficient sample size to evaluate the effects of H2RA therapy alone (n=1) or PPI therapy alone (n=0); thus these categories were excluded from the analysis. Medication prescriptions and physician notes did not provide sufficient detail to determine if therapy with PPI and H2RA was sequential or concurrent and the degree of overlap. There was also insufficient detail to determine if H2RA dosing was staggered with erlotinib dosing as recommended in the erlotinib prescribing information such that erlotinib was taken 10 hours after H2RA dosing and at least 2 hours before the next dose of the H2RA [6].
Figure 1.
Consort Diagram. Patients taking erlotinib therapy between February 26, 2011 and February 28, 2014 were identified through pharmacy records. Patients who received erlotinib less than 14 days or as part of combination therapy were excluded from the analysis.
Both groups of patients (those with and without PPI/ H2RA) were predominately composed of individuals with lung cancer (approximately 90%) and received the same median daily erlotinib dose (150 mg). Baseline demographics, including gender, weight, age, and disease characteristics, including cancer type, metastatic sites, and prior lines of therapy, were balanced between patients with and without concurrent acid-suppression therapy (Table 1).
Table 1.
Baseline patient and disease characteristics
| Erlotinib alone | Erlotinib + Acid Suppression (PPI/H2RA) |
P* | |
|---|---|---|---|
| Total | 52 | 24 | |
| Male sex, № (%) | 31 (59.6) | 12 (50.0) | 0.46 |
| Weight (kg), median(range)† | 66 (35–102) | 66 (40–106) | 0.72 |
| Age (years) , median (range)‡ | 67 (46–90) | 66 (33–84) | 0.085 |
| Prescribed daily erlotinib dose (mg), mean; median (range)§ | 140; 150 (50–150) | 141; 150 (87–150) | 0.82 |
| Cancer type, № (%) | |||
| Lung | 50 (94.3) | 20 (83.3) | 0.27 |
| Head & neck | 2 (3.8) | 2 (8.3) | |
| Other | 1 (1.9) | 2 (8.3) | |
| Metastatic sites, № (%) | |||
| Brain | 17 (32.7) | 7 (29.2) | 1.0 |
| Other | 29 (55.7) | 15 (62.5) | 0.62 |
| Lines of chemotherapy before erlotinib, № (%) | 0.64 | ||
| 0 | 22 (42.3%) | 8 (33.3%) | |
| 1 | 19 (36.5%) | 8 (33.3%) | |
| 2 | 6 (11.5%) | 3 (12.5%) | |
| 3 | 4 (7.7%) | 3 (12.5%) | |
| ≥4 | 1 (1.9%) | 2 (8.3%) | |
| EGFR mutation status | 0.71 | ||
| Activating mutation | 30 (57.7%) | 13 (54.2%) | |
| Wild-type | 11 (21.2%) | 5 (20.8%) | |
| Unknown status | 10 (19.2%) | 7 (29.2%) |
Abbreviation: EGFR: epidermal growth factor receptor.
Fisher’s exact test used for testing significance of binary variables and Welch’s two sample t-test for continuous variables.
Weight values were not available for 18 patients.
Age at first erlotinib dose.
Prescribed daily dose does not take into account skipped doses or drug holidays not recorded in the electronic medication order entry system.
Outcomes
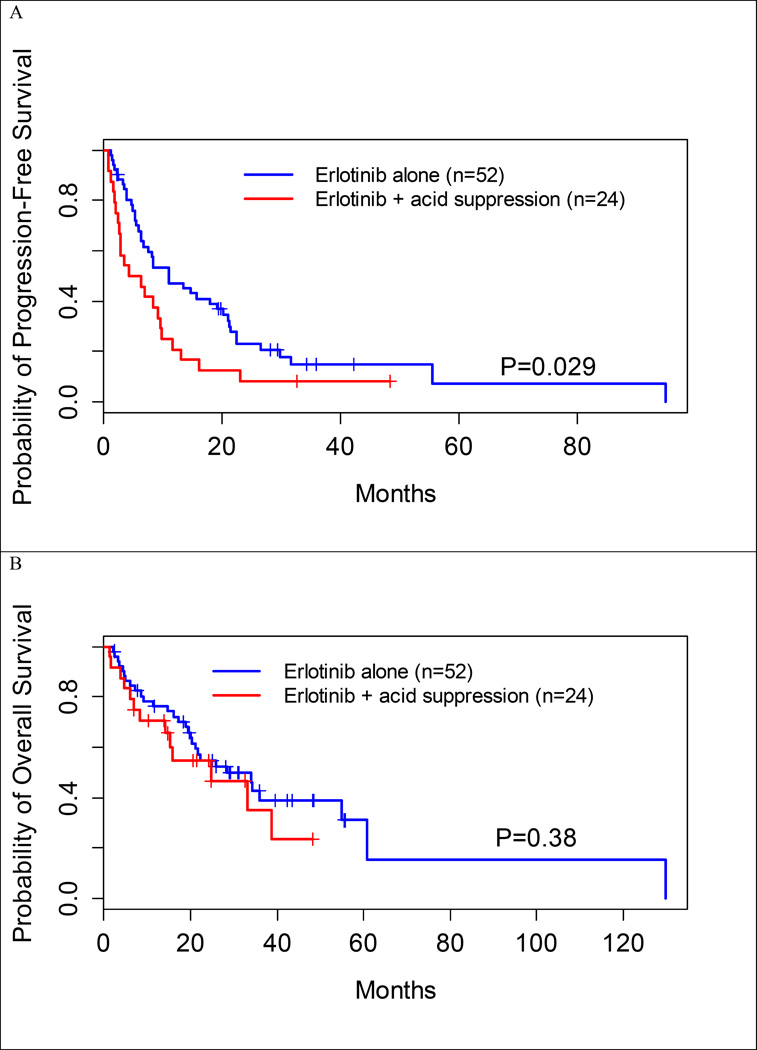
Patients receiving erlotinib in the absence of acid-suppression had a longer median PFS than those receiving concurrent acid-suppression therapy (11.0 vs. 5.3 months, P=0.029) (Table 2, Figure 2A). Median OS was longer in patients receiving erlotinib alone compared to those with concurrent acid-suppression therapy (28.5 vs. 24.7 months, P=0.38), but this was statistically insignificant (Table 2, Figure 2B).
Table 2.
Comparison of progression-free survival (PFS) and overall survival (OS) between patients with and without acid-suppression therapy (PPI/H2RA)
| n | Progression-free survival (months)* |
P† | Overall survival (months)* |
P† | ||
|---|---|---|---|---|---|---|
| Concurrent acid- suppression therapy |
Erlotinib alone | 52 | 11.0 [6.8, 21.0] | 0.029 | 28.5 [20.4, not reached] |
0.38 |
| Erlotinib + acid- suppression |
24 | 5.3 [2.8, 9.7] | 24.7 [14.3, not reached] |
Abbreviations: H2RA=histamine-2 receptor antagonist; PPI=proton pump inhibitor.
Median [95% confidence interval]
Log-rank test.
Figure 2.
Kaplan-Meier curves. A. Progression-free survival is significantly improved for patients taking erlotinib in the absence of acid suppression therapy with H2RA and PPI therapy (11.0 vs. 5.3 months, P=0.029). B. No significant differences in overall survival were seen with the use of concurrent acid-suppression therapy (28.5 vs. 24.7 months, P=0.38).
Safety outcomes
Adverse events were not significantly different in the presence or absence of PPI and H2RA (Table 3). Rash occurred in 69.8% of patients on erlotinib alone as compared to 83.3% of patients taking concurrent acid suppression therapy (P=0.21). Diarrhea occurred in 54.7% of patients on erlotinib alone as compared to 45.8% of patients taking concurrent acid suppression therapy (P=0.52). Rash and diarrhea occurred in the same patients for 43.4% of patients on erlotinib alone and 45.8% of patients taking concurrent acid suppression (P=0.84). For the study population, adverse events (rash, diarrhea, and concurrent rash/diarrhea) did not show significant differences with sex, cancer type (lung vs. non-lung origin), or age (greater than or less than the median of 66 years) (Table 3).
Table 3.
Incidence of adverse events in patients on erlotinib with and without concurrent acid suppression (PPI/H2RA)
| n | Rash n (%) |
P† | Diarrhea n (%) |
P† | Rash & diarrhea n (%) |
P† | ||
|---|---|---|---|---|---|---|---|---|
|
Concurrent PPI |
Erlotinib alone |
52 | 37 (71.5%) | 0.21 | 29 (55.7%) | 0.52 | 23 (44.2%) | 0.84 |
| Erlotinib + acid- suppression |
24 | 20 (83.3%) | 11 (45.8%) | 11 (45.8%) | ||||
| Sex | Male | 33 | 28 (84.8%) | 0.052 | 17 (51.5%) | 0.98 | 16 (48.5%) | 0.43 |
| Female | 43 | 28 (65.1%) | 22 (51.2%) | 17 (39.5%) | ||||
| Cancer type | Lung | 69 | 51 (73.9%) | 0.89 | 35 (50.7%) | 0.30 | 29 (42.0%) | 0.99 |
| Not lung | 7 | 5 (71.4%) | 5 (71.4%) | 4 (57.1%) | ||||
| Age* | Age > 66 years |
40 | 27 (67.5%) | 0.20 | 23 (57.5%) | 0.26 | 17 (42.5%) | 0.86 |
| Age ≤ 66 years |
36 | 29 (80.6%) | 16 (44.4%) | 16 (44.4%) | ||||
Abbreviations: PPI: proton pump inhibitor; H2RA: histamine-2 receptor antagonist.
Median age is 66 years old
Calculated using Fisher’s exact test
DISCUSSION
Erlotinib is a small molecule tyrosine kinase inhibitor targeting epidermal growth factor receptor (EGFR). It is FDA approved for treatment of NSCLC and pancreatic cancer [6]. In NSCLC, erlotinib is recommended as first-line therapy in patients with advanced, recurrent, or metastatic non-squamous disease who have known active sensitizing EGFR mutations regardless of performance status [13]. In pancreatic cancer, erlotinib is recommended in combination with gemcitabine as an option for patients with locally advanced or metastatic disease and good performance status [14].
While the FDA-approved prescribing information for erlotinib indicates that concurrent acid-suppression results in decreased bioavailability and subsequent systemic exposure, it is known that acid-suppression therapy, in the form of either a PPI or H2RA, is used in up to one-third of cancer patients [2]. In a healthy volunteer study, concurrent administration of erlotinib with the PPI omeprazole decreased erlotinib exposure by 46% and maximum concentration by 61%. When erlotinib was administered 2 hours following a 300 mg dose of the H2RA ranitidine, the erlotinib exposure was reduced by 33% and maximum concentration by 54%. When erlotinib was administered with ranitidine 150 mg twice daily (at least 10 hours after the previous ranitidine evening dose and 2 hours before the ranitidine morning dose), the erlotinib exposure and maximum concentration decreased by 15% and 17%, respectively [6,7]. Despite the prescribing information recommendation, concurrent PPI/H2RA with erlotinib was administered to approximately one-third of our patients. The aqueous solubility of erlotinib is pH-dependent, with increased solubility at pH levels below 5 and maximal solubility occurring at a pH level of approximately 2 [7]. There is typically a higher degree of reduced bioavailability and systemic exposure with PPIs compared to H2RAs for oral kinase inhibitors, consistent with the greater efficacy of PPIs [3–6,15]. The prescribing information recommendations were based on a study done in healthy volunteers [7] and the true effect of acid-suppression on survival and safety outcomes in oncology patients taking erlotinib is not clear.
The current study examined 76 oncology patients at UC San Diego receiving erlotinib therapy. The median PFS was 6 months less for patients using concurrent acid-suppression therapy as compared to those taking erlotinib alone and this difference was statistically significant. The OS was shorter in these patients as well, but the difference was not statistically significant. No change in rates of adverse events was observed. The majority of patients in the study has lung cancer (94.3% in the erlotinib alone group and 83.3% in the erlotinib with acid-suppression group). The 11.0 month PFS rate seen with erlotinib alone is consistent with that described in prior studies of patients with lung cancer receiving erlotinib as first-line therapy [16]. The incidence rates of rash and diarrhea were similar to those reported in the erlotinib prescribing information [6]. While it is possible that the difference in PFS was due to diminished erlotinib concentrations with concurrent acid-suppression therapy, any differences in drug exposure between these groups were not associated with alterations in primary drug toxicities of rash or diarrhea. It is conceivable that the small numbers of patients precluded finding statistically significant results for some of these parameters.
Prior retrospective studies have had discrepant results when evaluating the effect of concurrent acid-suppression therapy (PPI or H2RA) on PFS, OS, and adverse event incidence (rash, diarrhea, and/or infection) in a NSCLC population [8,9]. Hilton et al utilized data from the phase III BR.21 study in 485 patients with NSCLC on erlotinib as second- or third-line therapy [9,16]. This study found no significant differences in PFS or OS with acid-suppression therapy (either PPI or H2RA therapy). Patients on concurrent acid-suppression therapy had a similar frequency of rash, but higher frequency of diarrhea compared to patients receiving erlotinib alone. In contrast, the retrospective study described by Chu et al found a difference in PFS and OS with acid suppression therapy (either PPI or H2RA) for 507 NSCLC patients on erlotinib therapy. There was a greater incidence of rash for the patients without acid-suppression therapy, but diarrhea was not significantly different. This study utilized patient records from a single centralized institution in Canada and the central database was used to document prescription medications in Alberta, Canada and represented patients primarily treated with erlotinib as second-line therapy [8]. Both studies considered PPI or H2RA therapy as acid-suppression therapy and neither distinguished between H2RA and PPI therapy in evaluating outcomes. In contrast, in our study, all patients received both PPI and H2RA therapy in the course of erlotinib therapy.
The current study had several limitations. Given the retrospective study design and dependence on pharmacy records, medication adherence, intensity and duration of each type of acid-suppression therapy could not be determined. It is also unclear if patients attempted to stagger erlotinib with acid reduction therapy as recommended in the prescribing information. Drug levels were not available to determine relationships between the decrease of systemic erlotinib exposure with acid-suppression therapy and therapeutic outcomes. Furthermore, the degree of toxicity (grade 3 and 4 versus less toxicity) could not be determined given the limited information available in clinic notes.
In conclusion, the current study demonstrates frequent use of acid-suppression therapy (PPI and H2RA) in combination with erlotinib and provides evidence of a potential association of this combination with reduction in PFS. These data suggest that the reduction in erlotinib plasma concentrations expected with acid-suppression therapy may have important clinical relevance. This study supports the recommendation that the concurrent use of PPI therapy with erlotinib should be avoided.
Acknowledgments
The authors would like to thank Andrew Chang and Sariah Liu for their assistance with study start up.
Conflict of Interest
Lisa H. Lam is a postdoctoral fellow with funding supported by Pfizer Global Research and Development and the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego. Edmund V. Capparelli has consultant funds from Gilead Sciences, Alexion Pharmaceuticals, The Medicines Company, and Cempra. Razelle Kurzrock has research funding from Guardant, Sequenom, Foundation Medicine, Merck Serono, Pfizer, and Genentech, consultant funds from Sequenom, Actuate Therapeutics and X-Biotech, and an ownership interest in CureMatch Inc. and Novena Inc.
References
- 1.Borad MJ, Curtis KK, Babiker HM, et al. The impact of concomitant medication use on patient eligibility for phase I cancer clinical trials. J Cancer. 2012;3:345–353. doi: 10.7150/jca.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smelick GS, Heffron TP, Chu L, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10(11):4055–4062. doi: 10.1021/mp400403s. [DOI] [PubMed] [Google Scholar]
- 3.Bosulif (bosutinib) [prescribing information] New York, NY: Pfizer, Inc; 2016. [Accessed April 19, 2016]. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=884. [Google Scholar]
- 4.Sprycel (dasatinib) [prescribing information] Princeton, NJ: Bristol-Myers Squibb Company; 2015. [Accessed April 19, 2016]. Available at: http://packageinserts.bms.com/pi/pi_sprycel.pdf. [Google Scholar]
- 5.Ibrance (palbociclib) [prescribing information] New York, NY: Pfizer, Inc; 2016. [Accessed April 19, 2016]. Available at: labeling.pfizer.com/ShowLabeling.aspx?id=2191. [Google Scholar]
- 6.Tarceva (erlotinib) [prescribing information] Northbrook, IL: OSI Pharmaceuticals, LLC; 2015. [Accessed April 19, 2016]. Available at: http://www.gene.com/download/pdf/tarceva_prescribing.pdf. [Google Scholar]
- 7.Kletzl H, Giraudon M, Ducray PS, et al. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs. 2015;26(5):565–572. doi: 10.1097/CAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 8.Chu MP, Ghosh S, Chambers CR, et al. Gastric Acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clin Lung Cancer. 2015;16(1):33–39. doi: 10.1016/j.cllc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Hilton JF, Tu D, Seymour L, et al. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer. 2013;82(1):136–142. doi: 10.1016/j.lungcan.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 10.University of California, San Diego. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 April 21]. Study of Personalized Cancer Therapy to Determine Response and Toxicity (UCSD_PREDICT) Available at: https://clinicaltrials.gov/show/NCT02478931. NLM Identifier: NCT02478931. [Google Scholar]
- 11.Therneau T. A Package for Survival Analysis in S. version 2.38. 2015 Available at: http://CRAN.R-project.org/package=survival.
- 12.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available at: http://www.R-project.org/ [Google Scholar]
- 13.National Comprehensive Cancer Network. [Accessed April 21, 2016];NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 4. 2016 Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 14.National Comprehensive Cancer Network. [Accessed April 21, 2016];NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Version 1. 2016 Available at: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 15.DeVault KR, Castell DO. American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100(1):190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]