Abstract
Swarming behavior of neutrophils has been noticed in both sterile injury and infection models and the mechanisms are being unveiled. So far, no in vitro model has been established to study neutrophil swarming to microbes. In the current study, using live-cell imaging, we observed in vitro neutrophil swarming toward Cryptococcus neoformans, a fungal pathogen causing human meningoencephalitis. Complement C3 and CD11b expression are essential for neutrophils to form cell swarms surrounding C. neoformans. Leukotriene B4 (LTB4) was quickly released by neutrophils during their interactions with C. neoformans. Blockade of LTB4 synthesis inhibited the swarming response to C. neoformans. Importantly, blockade of LTB4 synthesis also significantly reduced neutrophil recruitment in the lung vasculature of mice infected intravenously with C. neoformans, demonstrating a critical role of LTB4 in intravascular neutrophil swarming during infection. Together, this is the first report of neutrophil dynamics of swarming toward a microorganism in vitro, mediated by complement and LTB4.
Keywords: neutrophils, Cryptococcus neoformans, swarming, complement, CD11b, LTB4
1. Introduction
As the most abundant innate immune cells, neutrophils not only play a fundamental role in defense against invading pathogens, but also serve as critical mediators of sterile inflammation in acute and chronic diseases [1]. To exert their functions, neutrophils must be recruited to sites of infection and inflammation. Decades ago, neutrophils have been observed to aggregate in the presence of chemotactic factors and mechanic stirring [2–4]. Recently, with the development of imaging techniques, much progress has been made on neutrophil dynamics during their migration to sites of infection and inflammation [5].
With the use of intravital microscopy, neutrophils have been recently shown to rapidly migrate toward sites of sterile tissue injury [6, 7] or infections [8–12]. As the dynamic of neutrophil accumulation is similar to swarming behavior of some insects, the behavior of neutrophils is commonly referred to as “neutrophil swarming” [5]. It has been recently shown that the lipid leukotriene B4 (LTB4), secreted by neutrophils, plays a central role in neutrophil activation and migration to formyl peptides [13]. More recently, in vivo imaging has revealed that both LTB4 and integrins are required for neutrophil swarming in the extravascular space of a damaged tissue [8]. However, the role of LTB4 in intravascular neutrophil swarming has not been determined in vivo [5].
Although neutrophil swarming has been recently described in vivo using both tissue injury models and infection models [8, 9, 11], this important behavior of neutrophils has been poorly addressed in vitro. Questions remain as to whether neutrophils swarm to pathogens in an in vitro system and how it happens.
Using live-cell imaging, we have recently established an in vitro model to study the dynamic interactions of neutrophils with C. neoformans, a human pathogenic fungus causing fatal meningoencephalitis [14]. Given the complexity of in vivo environment, in vitro models provide an alternative approach to dissect mechanisms underlying host-pathogen interactions, with the advantage of being able to precisely control experimental conditions. In the current study, we visualized the swarming of neutrophils toward C. neoformans in vitro and examined the underlying mechanisms.
2. Materials and methods
2.1. Animals and C. neoformans
C57BL/6 mice were purchased from National Cancer Institute (Frederick, MD, USA). C3−/− (JAX003640), CD11b−/− (JAX003991), and C5−/− (JAX000461) mice were purchased from the Jackson laboratory (Bar Harbor, ME, USA) and bred in the animal facility of the University of Maryland. For all experiments, eight- to ten-week-old mice were used. The animal use protocol was approved by the Institutional Animal Care and Animal Use Committee (IACUC) of the University of Maryland.
Encapsulated Cryptococcus neoformans H99 strain (serotype A, ATCC no. 208821) was used throughout the experiments. A single fungal colony was inoculated into Sabouraud’s dextrose broth and cultured to exponential phase at 32°C with gentle rotation (180 rpm) for 24 h. The yeast cells were washed twice with PBS and counted before use.
2.2. Neutrophil isolation and culture
Neutrophils were isolated from tibia and femur of adult mice using 69% and 78% Percoll (GE, Pittsburg, PA, USA) as previously described [15] and resuspended in Hanks’ balanced saline solution (HBSS) without calcium and magnesium. Neutrophils (5×105) were incubated with C. neoformans (5×104) in 96-well plates containing 200 μL RPMI 1640 in the presence of 40% fresh mouse plasma at 37°C. In some experiments, neutrophils were incubated with recombinant C5a (rC5a, 250 ng/ml, R&D Systems, Minneapolis, MN, USA) in the presence or absence of Zileuton (100 μM, 5-lipoxygenase inhibitor, Cayman chemicals, Ann Arbor, MI, USA). The supernatants and cells were collected for ELISA and qPCR, respectively.
2.3. Live cell imaging of neutrophil swarming
Live cell imaging was performed in a 35-mm glass-bottom dish (Thickness no. 1.5; MatTek, Ashland, MA, USA) containing 200 μl RPMI-1640 supplemented with 40% fresh mouse plasma. Neutrophils and yeast cells were added at an effect to target ratio of 10:1 (5×105:5×104). In some experiments, sytox orange (5 μM, Thermofisher scientific, Waltham, MA, USA) were added to detect cell viability. Neutrophil swarming was visualized using Zeiss LSM 510 system coupled with a CO2 module and a temperature control module (PECON, Germany) connected to a transparent chamber to maintain 5% CO2 and 37°C.
2.4. Animal infections
Mice were intravenously infected with 5×106 C. neoformans. In some experiments, mice were intranasally instilled with 2 μg LPS dissolved in 20 μl PBS as control. In other experiments, mice were intraperitoneally injected with Zileuton (dissolved in PBS containing 0.2% Tween 20) at the dose of 50 mg/kg body weight 30 min before infection [16].
2.5. ELISA
ELISA assay kits for LTB4 and mouse CXCL1 were purchased from R&D systems (Minneapolis, MN, USA) and the assays were performed according to manufacturer’s instructions.
2.6. qPCR
Total RNA was extracted from cultured neutrophils using PureLink RNA Mini Kit (Thermofisher scientific, Waltham, MA, USA). SuperScript IV First-Strand Synthesis System (Invitrogene) was used for cDNA synthesis. Primers used for qPCR were as follows: CXCL1C: CCGAAGTCATAGCCACACTCAA (forward), GCAGTCTGTCTTCTTTCTCCGTTAC (reverse); CXCL2: AGACAGAAGTCATAGCCACTCTCAAG (forward), CCTCCTTTCCAGGTCAGTTAGC (reverse); IL-1β: CTGCAGCTGGAGAGTGTGGAT (forward), TGTGCTCTGCTTGTGAGGTGCT (reverse); IL-6: TAGTCCTTCCTACCCCAATTTCC (forward), TTGGTCCTTAGCCACTCCTTC (reverse); TNF-α: CATCTTCTCAAAATTCGAGTGACAA (forward), TGGGAGTAGACAAGGTACAACCC (reverse); qPCR was performed on CFX96 Real-Time PCR Detection System (BioRad).
2.7. Flow cytometry
Leukocytes were purified from the lung or bone marrow of mice as previously described [17]. For flow cytometry staining, Fc receptors were blocked using anti-CD16/32 Abs (93; eBioscience). The cells were stained using APC-Cy7-anti-CD45 (30-F11, BioLegend), FITC-anti-Ly6G (1A8, BioLegend), PE-Cy7-anti-CD11b (M1/70, BioLegend), and Brilliant Violet 421-anti-CD11c (N418, BioLegend). Neutrophils were defined as CD45+ Ly6G+CD11b+CD11c−.
2.8. Statistical analysis
For single comparisons, an unpaired two-sided Student’s t-test was performed; for multiple comparisons, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used. In both cases, P <0.05 is considered significant.
3. Results
3.1. Complement C3 and CD11b are required for neutrophil swarming to C. neoformans
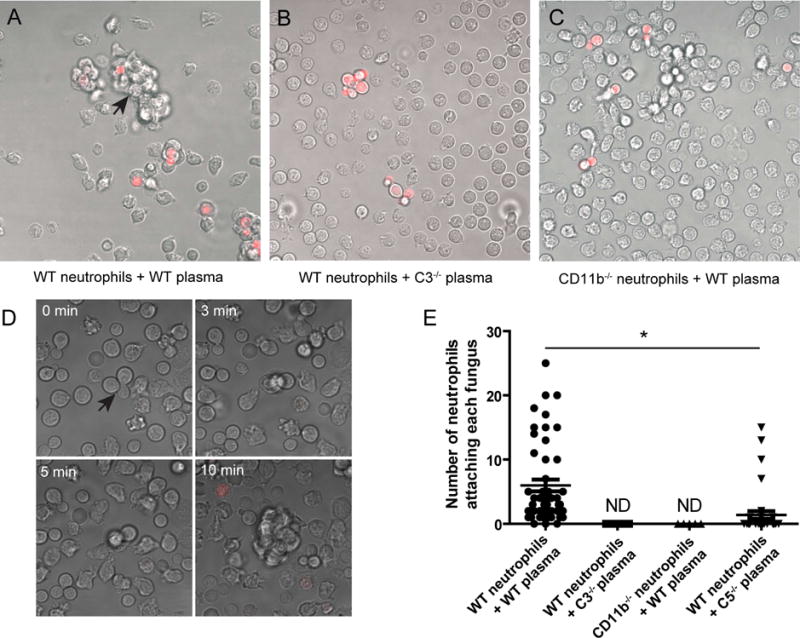
Using live-cell imaging, we observed frequently swarming of neutrophils toward C. neoformans in the presence of wild-type mouse plasma (Fig. 1A, Video S1). This swarming behavior is similar to a previous observation in a sterile tissue injury model [6]. The swarming response was completely absent in the presence of C3−/− mouse plasma (Fig. 1B), suggesting that complement activation is absolutely required for neutrophil swarming toward the organism. Since CD11b is essential for neutrophils to bind to C. neoformans [14], we next evaluate the role of CD11b in neutrophil swarming toward C. neoformans. Interestingly, although CD11b−/− neutrophils continuously pushed C. neoformans forward, no neutrophil clusters was observed even after prolonged incubation in the presence of wild-type mouse plasma (Fig. 1C, Video S2).
Fig. 1. Essential role of complement and CD11b in neutrophil swarming to C. neoformans.
(A and B) Live-cell imaging showing that neutrophil swarming to C. neoformans (red, labeled with TRITC) occurred in the presence of wild-type mouse plasma (A, arrow, see also Video S1), but not C3−/− mouse plasma (B). (C) No swarming to C. neoformans (red) was observed for CD11b−/− neutrophils in the presence of wild-type mouse plasma (see also Video S2). (D) Series of images showing that neutrophils swarm to C. neoformans (arrow) when co-cultured in the presence of C5−/− mouse plasma. Red: dead cells stained by sytox orange (see also Video S3). (E) Quantification of the number of neutrophils attaching each fungus 30 min after coincubation using live-cell imaging. Data are presented as means ± SEM. Data are representative of results from 2 independent experiments. *, p<0.05.
Since C. neoformans in C3−/− mouse plasma induces neither opsonization (C3b deposition) nor C5a release, we next incubated neutrophils with C. neoformans in the presence of C5−/− mouse plasma, which provides opsonization but does not induces C5a release. Although neutrophil swarming is less frequent than in wild type control, few neutrophils were seen casually encounter fungi via random movements and the subsequent interaction could also trigger neutrophil swarming (Fig. 1D, Video S3). The results indicated that C5a contributed to, but is not absolutely required for neutrophil swarming toward C. neoformans. We also quantified neutrophils attaching to each yeast cell (Fig. 1E). Nearly all yeast cells were attached by neutrophils in the presence of wild-type mouse plasma, but the number was significantly reduced in the presence of C5−/− mouse plasma (Fig. 1E). Neutrophil cluster was undetectable for CD11b−/− neutrophils or in the presence of C3−/− mouse plasma (Fig. 1E). Collectively, the results demonstrate that C3 and CD11b are essential for neutrophils to form cell swarms in response to C. neoformans.
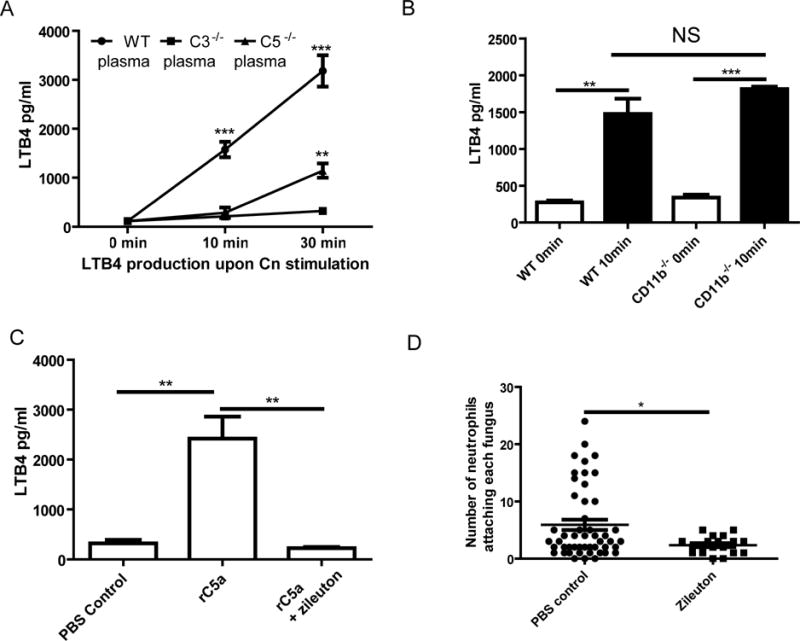
3.2. LTB4 is critically involved in neutrophil clustering around C. neoformans
According to previous reports, LTB4 can be quickly released by neutrophils upon formyl peptide stimulation [13] and plays an important role in neutrophil swarming in a sterile injury model [8]. In the current study, LTB4 was quickly released from neutrophils when incubated with C. neoformans (Fig. 2A). The production of LTB4 depended on complement activation as almost no LTB4 was detected when using C3−/− mouse plasma (Fig. 2A). LTB4 was secreted, to a less extent, by neutrophils at a later but not earlier time point when incubated with C. neoformans in the presence of C5−/− mouse plasma (Fig. 2A). Interestingly, the LTB4 secretion of CD11b−/− neutrophils was comparable to that of wild-type neutrophils (Fig. 2B).
Fig. 2. Contribution of LTB4 to neutrophil swarming.
(A) Secretions of LTB4 by neutrophils when cocultured with C. neoformans in the presence of wild-type, C3−/− or C5−/− mouse plasma. Comparisons were made to C3−/− mouse plasma. (B) Secretions of LTB4 by CD11b−/− neutrophils when cocultured with C. neformans in the presence of wild-type mouse plasma. (C) Secretions of LTB4 by neutrophils when incubated with rC5a for 10 min with or without the addition of Zileuton. (D) Quantification of the number of neutrophils attaching each fungus 30 min after coincubation in the presence of wild-type mouse plasma with or without the addition of Zileuton. Data are presented as means ± SEM. Data are representative of results from 2 independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
We further showed that rC5a was able to trigger secretions of LTB4 by neutrophils, and that the secretions of LTB4 could be abolished by Zileuton (Fig. 2C), an inhibitor of 5-lipoxygenase essential for leukotriene synthesis. Live-cell imaging showed that C. neoformans only attracted neighboring neutrophils, but not those at more distant sites, when Zileuton was added to the culture (Video S4). As a result, only small, rather than big, neutrophil clusters were formed around C. neoformans, demonstrating that Zileuton dramatically inhibited the swarming response (Fig. 2D, Video S4). Together, these data suggest that LTB4 is critically involved in neutrophil swarming toward C. neoformans.
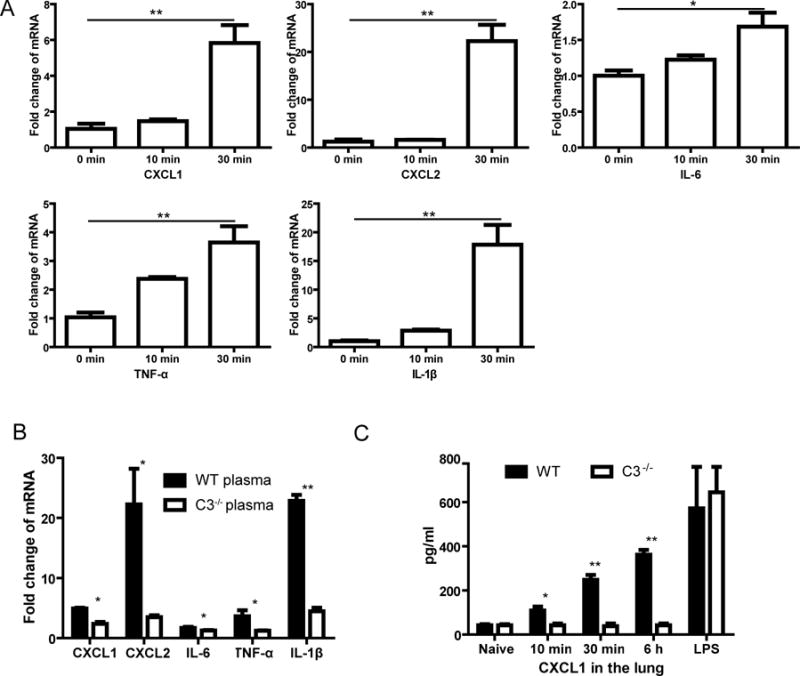
3.3. Enhanced expression of chemokines and proinflammatory cytokines by neutrophils during the swarming response
Cryptocococcal capsular polysaccharide can induce secretions of chemokines and proinflammatory cytokines by neutrophils [18]. In particular, chemokines may also contribute to neutrophil swarming. We next examined profiles of chemokines and proinflammatory cytokines during neutrophil swarming toward C. neoformans. mRNA levels of CXCL1, CXCL2, IL-1β, IL-6, and TNF-α were upregulated in neutrophils following incubation with C. neoformans (Fig. 3A). In addition, neutrophils had significantly higher expression of these chemokines and cytokines in the presence of wild-type mouse plasma, compared to C3−/− mouse plasma (Fig. 3B). There was also higher production of CXCL1 in the lung of wild-type mice infected with C. neoformans, compared to infected C3−/− mice (Fig. 3C). Since CXCL1 and CXCL2 are important chemokines for neutrophil attraction, we next inhibited their receptor CXCR2 expressed on neutrophils using SB225002 in vitro. However, such treatment did not affect neutrophil swarming to C. neoformans (data not shown).
Fig. 3. Upregulation of chemokines and proinflammatory cytokines during neutrophil swarming.
(A) mRNA expression levels of CXCL1, CXCL2, IL-6, TNF-α, and IL-1β in neutrophils following incubation with C. neoformans in the presence of mouse plasma. (B) Comparisons of mRNA expression levels of chemokines and proinflammatory cytokines in neutrophils 30 min after incubation with C. neoformans in the presence of wild-type or C3−/− mouse plasma. (C) Secretion of CXCL1 in the lung (homogenate of the lung tissues in 2 ml PBS) of wild-type or C3−/− mice (n=5) following intravenous injection with C. neoformans. Mice instilled intranasally with LPS were used as positive control. Data are presented as means ± SEM. Data are representative of results from 2 independent experiments. *, p<0.05; **, p<0.01.
3.4. LTB4 promotes intravascular accumulation of neutrophils in the lung following infection with C. neoformans
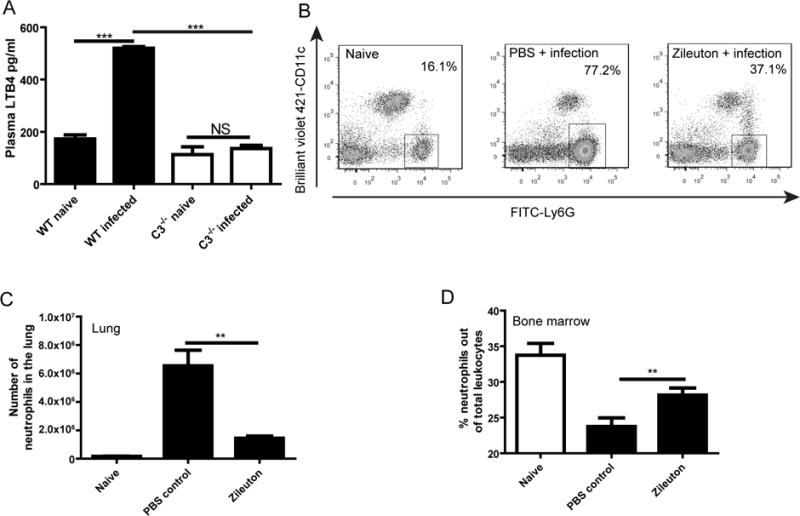
The swarm-like neutrophil behavior is reminiscent of our recent observation that neutrophils rapidly accumulated in the lung vasculature following intravenous infection with C. neoformans [17]. Although LTB4 is required for optimal extravascular swarming of neutrophils in vivo [8], the role of LTB4 in intravascular neutrophil swarming has not been determined [5]. Interestingly, LTB4 is rapidly detected in the blood after intravenous infection with C. neoformans in a complement C3 dependent manner (Fig. 4A). In this regard, we treated mice with Zileuton 30 min before intravenous infection with C. neoformans to inhibit LTB4 synthesis, as described previously [19]. Strikingly, Zileuton treatment significantly reduced neutrophil recruitment to the lung (Fig. 4B, 4C). Accordingly, significantly more neutrophils were retained in the bone marrow of infected mice treated with Zileuton (Fig. 4D). Together, these data demonstrate that LTB4 contributes to accumulation of neutrophils in the lung vasculature during C. neoformans infection.
Fig. 4. LTB4 enhances neutrophil accumulation in the lung.
(A) Plasma levels of LTB4 in wild-type and C3−/− mice (n=5) 30 min after intravenous infection with C. neoformans. (B) Representative dot plots showing the frequency of neutrophils (Ly6G+, gated on CD45+ cells) in the lung of mice treated with Zileuton or PBS 30 min after intravenous infection with C. neoformans. (C and D) The absolute number of neutrophils in the lung (C) and the percentage of neutrophils out of the total leukocytes in the bone marrow (D) of mice (n=5) treated with Zileuton or PBS 30 min after intravenous infection with C. neoformans. **, p<0.01; ***, p<0.001.
4. Discussion
Neutrophil swarming has substantial biological significance, because it enables neutrophils to accumulate efficiently at sites of infection and inflammation for pathogen clearance and tissue repair [5]. In this study, we found that neutrophils rapidly migrated to C. neoformans in vitro in the presence of mouse plasma. Initially, only neutrophils close to C. neoformans migrated to the organism, but later, neutrophils at more distant sites also migrated to the organism. As a result, neutrophil clusters were formed around the yeast cells. To our knowledge, this is the first report of neutrophil dynamics of swarming toward a microorganism in vitro, although neutrophils were previously seen to form cell swarms in vitro in the presence of formyl peptide [4, 13].
Neutrophil swarming to C. neoformans required complement C3 in our system. No interaction was seen between neutrophils and C. neoformans in the presence of C3−/− mouse plasma. It is known that cryptococcal capsule can efficiently activate complement system [20]. Thus, the inability of neutrophils to interact with C. neoformans was likely attributed to lack of opsonization of the organism (C3b deposition). Interestingly, less neutrophil clusters were observed in the presence of C5−/− mouse plasma, compared to wild-type mouse plasma. In the presence of C5−/− mouse plasma, C. neoformasn should be opsonized by C3b/iC3b, despite lack of C5a-C5aR signaling. Thus, opsonization was essential but not sufficient to trigger neutrophil swarming to C. neoformans. It appears that C5a-C5aR signaling guided neutrophils to migrate to the yeast cells. In the absence of C5a-C5aR signaling, neutrophils could casually encounter C. neoformans via random movements. Even so, the subsequent interactions could lead to attraction of other neutrophils to migrate to the organism.
In the current study, we found that CD11b−/− neutrophils continuously pushed C. neoformans forward, which is consistent with our previous observations [14]. However, CD11b−/− neutrophils were unable to form stable clusters surrounding C. neoformans. We have previously shown that the original expression of CD11b on neutrophils is essential for the cells to bind to C. neoformans [14]. Obviously, the binding of early arriving “pioneer” neutrophils to C. neoformans is critical for late recruited cells to form a stable cluster around C. neoformans. In this respect, recent findings demonstrated that CD11b−/− neutrophils failed to form stable clusters in damaged tissues in vivo, although neutrophils were recruited [8].
LTB4 has been shown to mediate neutrophil swarming in vitro in the presence of formyl peptides [4, 13]. It was reported that neutrophil-derived LTB4 is essential for neutrophil recruitment in inflammatory arthritis [21–23] and allergic skin inflammation [24]. More recently, intravital imaging demonstrated that LTB4 is required for neutrophil swarming at local sites of tissue injury [8]. Consistent with these observations, our data demonstrated that LTB4 was critically involved in neutrophil clustering around C. neoformans. However, blockade of LTB4 synthesis only inhibited the formation of big neutrophil clusters. It was proposed that, following tissue injury, initially rare scouting neutrophils migrated to the damage focus, followed by the attraction of waves of additional neutrophils [6]. Accordingly, we suggest that C. neoformans attracts “pioneer” neutrophils close to it via C5a-C5aR signaling, followed by massive migration of neutrophils from more distant sites through LTB4 secreted by the “pioneer” cells.
Inhibition of LTB4 has been shown to relieve inflammatory arthritis [21], acute lung injury [19] and allergic skin inflammation [24]. We have recently shown that intravenous infection with C. neoformans leads to massive accumulation of neutrophils in the lung vasculature [17]. In the current study, we showed that inhibition of LTB4 significantly reduced neutrophil recruitment in the lung, demonstrating that, for the first time, LTB4 promotes intravascular swarming of neutrophils in vivo in an infection model, a similar role of LTB4 described in extravascular swarming in interstitial tissue in a tissue injury model [8].
In summary, we have shown that neutrophils swarm toward C. neoformans in vitro, which is mediated by complement, CD11b, and LTB4. In addition, LTB4 also promotes neutrophil accumulation in the lung vasculature following C. neoformans infection in vivo. These data contribute significantly to our understanding of interactions of neutrophils with pathogens as well as neutrophil biology in general.
Supplementary Material
Video S1. Neutrophils swarm toward C. neoformans in the presence of wild-type mouse plasma.
Video S2. No neutrophil cluster was observed when CD11b−/− neutrophils were incubated with C. neoformans in the presence of wild-type mouse plasma.
Video S3. Swarming of neutrophils toward C. neoformans was occasionally seen in the presence of C5−/− mouse plasma.
Video S4. Blockade of LTB4 synthesis by Zileuton inhibits neutrophil swarming response to C. neoformans. Only neighboring neutrophils, but not those at more distant sites, swarmed to C. neoformans.
Highlights.
Neutrophils swarm toward Cryptococcus neoformans in vitro.
Complement and CD11b are essential for neutrophil swarming toward C. neoformans.
LTB4 is involved in neutrophil swarming in vitro and in lung vasculature.
Acknowledgments
We thank Dr. Yunsheng Wang for his technical assistance with confocal assistance with confocal microscopy and FACS analysis.
Funding: This work was supported by start-up funds from the University of Maryland (to M.S.) and supported in part by NIH grant AI115086A (to M.S.).
Abbreviations
- C3
complement component 3
- C5
complement component 5
- C5a
complement component 5a
- C5aR
complement component 5a receptor
- CXCL1
chemokine (C-X-C motif) ligand 1
- CXCL2
chemokine (C-X-C motif) ligand 2
- rC5a
recombinant complement 5a
- LTB4
Leukotriene B4
- TRITC
Tetramethylrhodamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
The authors declare no financial or commercial conflict of interest.
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation, Nature reviews. Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.O’Flaherty JT, Showell HJ, Becker EL, Ward PA. Neutrophil aggregation and degranulation. Effect of arachidonic acid. The American journal of pathology. 1979;95:433–444. [PMC free article] [PubMed] [Google Scholar]
- 3.O’Flaherty JT, Showell HJ, Ward PA, Becker EL. A possible role of arachidonic acid in human neutrophil aggregation and degranulation. The American journal of pathology. 1979;96:799–810. [PMC free article] [PubMed] [Google Scholar]
- 4.Rochon YP, Frojmovic MM. Regulation of human neutrophil aggregation: comparable latent times, activator sensitivities, and exponential decay in aggregability for FMLP, platelet-activating factor, and leukotriene B4. Blood. 1993;82:3460–3468. [PubMed] [Google Scholar]
- 5.Lammermann T. In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues. Journal of leukocyte biology. 2015 doi: 10.1189/jlb.1MR0915-403. [DOI] [PubMed] [Google Scholar]
- 6.Ng LG, Qin JS, Roediger B, Wang Y, Jain R, Cavanagh LL, Smith AL, Jones CA, de Veer M, Grimbaldeston MA, Meeusen EN, Weninger W. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. The Journal of investigative dermatology. 2011;131:2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 7.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 8.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latge JP, Brakhage AA, Gunzer M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS pathogens. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Developmental cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Zhang M, Liu G, Wu H, Zhu X, Zhou H, Shi M. Real-Time imaging of interactions of neutrophils with Cryptococcus neoformans demonstrates a crucial role of complement C5a-C5aR signaling. Infection and immunity. 2016;84:216–229. doi: 10.1128/IAI.01197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. Journal of leukocyte biology. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 16.Genovese T, Rossi A, Mazzon E, Di Paola R, Muia C, Caminiti R, Bramanti P, Sautebin L, Cuzzocrea S. Effects of zileuton and montelukast in mouse experimental spinal cord injury. British journal of pharmacology. 2008;153:568–582. doi: 10.1038/sj.bjp.0707577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun D, Zhang M, Liu G, Wu H, Li C, Zhou H, Zhang X, Shi M. Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. European journal of immunology. 2016 doi: 10.1002/eji.201546239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel TR. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infection and immunity. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Failla M, Genovese T, Mazzon E, Gili E, Muia C, Sortino M, Crimi N, Caputi AP, Cuzzocrea S, Vancheri C. Pharmacological inhibition of leukotrienes in an animal model of bleomycin-induced acute lung injury. Respiratory research. 2006;7:137. doi: 10.1186/1465-9921-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryotic cell. 2010;9:835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. The Journal of experimental medicine. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. The Journal of experimental medicine. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyoshi MK, He R, Li Y, Mondal S, Yoon J, Afshar R, Chen M, Lee DM, Luo HR, Luster AD, Cho JS, Miller LS, Larson A, Murphy GF, Geha RS. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity. 2012;37:747–758. doi: 10.1016/j.immuni.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Neutrophils swarm toward C. neoformans in the presence of wild-type mouse plasma.
Video S2. No neutrophil cluster was observed when CD11b−/− neutrophils were incubated with C. neoformans in the presence of wild-type mouse plasma.
Video S3. Swarming of neutrophils toward C. neoformans was occasionally seen in the presence of C5−/− mouse plasma.
Video S4. Blockade of LTB4 synthesis by Zileuton inhibits neutrophil swarming response to C. neoformans. Only neighboring neutrophils, but not those at more distant sites, swarmed to C. neoformans.


