Abstract
According to Cancer Research UK currently available estimates, 14.1 million new lung cancer cases were diagnosed and a staggering 8.2 million people worldwide died from lung cancer in 2012. EGFR and c-Met are two tyrosine kinase receptors most commonly overexpressed or mutated in Non-small Cell Lung Cancer (NSCLC) resulting in increased proliferation and survival of lung cancer cells. Tyrosine kinase inhibitors (TKIs), such as Erlotinib, approved by the FDA as first/second line therapy for NSCLC patients have limited clinical efficacy due to acquired resistance. In this manuscript, we investigate and discuss the role of epithelial mesenchymal transition (EMT) in the development of resistance against EGFR and c-Met TKIs in NSCLC. Our findings show that Zeb-1, a transcriptional repressor of E-Cadherin, is upregulated in TKI-resistant cells causing EMT. We observed that TKI-resistant cells have increased gene and protein expression of EMT related proteins such as Vimentin, N-Cadherin, β-Catenin and Zeb-1, while expression of E-Cadherin, an important cell adhesion molecule, was suppressed. We also confirmed that TKI-resistant cells display mesenchymal cell type morphology, and have upregulation of β-Catenin which may regulate expression of Zeb-1, a transcriptional repressor of E-Cadherin in TKI-resistant NSCLC cells. Finally, we show that down-regulating Zeb-1 by inducing miR-200a or β-Catenin siRNA can increase drug sensitivity of TKI-resistant cells.
Keywords: NSCLC, TKI resistance, EMT, β-Catenin, Zeb-1, miR-200a
1. Introduction
Growth factor receptors, namely Epidermal Growth Factor Receptor (EGFR) and Hepatocyte Growth Factor Receptor (HGFR or c-Met) have been observed to be highly over-expressed/activated in Non-small Cell Lung Cancer (NSCLC) [1]. Downstream signaling pathways, such as PI3K-AKT-mTOR and RAS-RAF-MEK-ERK, can be synergistically triggered upon co-activation of these receptors leading to enhanced cell proliferation and survival [2]. Several c-Met tyrosine kinase inhibitors (TKIs) are currently in clinical trials and may have the potential to benefit specific subsets of NSCLC patients on a clinical basis [3]. SU11274 used in this study is a c-Met targeting TKI that can significantly suppress cell survival and proliferation in c-Met-expressing NSCLC cells [1,2,4]. EGFR TKIs have also been shown to be clinically effective for treatment of locally advanced or metastatic NSCLC patients and many of them, such as erlotinib, gefitinib and afatinib, are approved by the FDA to treat NSCLC patients with mutated EGFR [5]. However, these TKIs have limited efficacy as NSCLC patients acquire resistance to these drugs within 9 to 14 months of treatment [6,7]. Resistance against c-Met and EGFR TKIs in NSCLC is currently poorly understood and further studies are needed.
Epithelial mesenchymal transition (EMT) is a process in which epithelial cells undergo phenotypic and morphological changes to acquire mesenchymal cell type characteristics [8]. Occurrence of EMT generally results in loss of tight junction proteins, such as E-Cadherin and Claudin, and upregulation of transcriptional repressors of tight junction proteins, such as ZEB1, Snail, Slug and Twist. It also results in morphological changes as the cells become elongated and loose cell polarity after undergoing EMT resulting in increased motility and invasiveness [8]. Occurrence of EMT, specifically in cancer cells, has been highly associated with poor prognosis and decreased overall survival. Previous investigations have shown that localization of β-Catenin to the nucleus can result in cellular transformations by means of EMT [9]. Our recent findings show that there is increased activation and nuclear accumulation of β-Catenin in TKI-resistant cells, which could be a potential regulator of TKI resistance [10].
EMT can be regulated by the microRNAs of the miR-200 family. There are five members in this family, miR-200a, miR-200b, miR-200c, miR-429 and miR-141, which are usually classified in two clusters based on their chromosomal locations [11]. The miR-200 family plays an important role in regulating Zeb-1 and induction of these microRNAs in mesenchymal cells can suppress expression of Zeb-1 thereby possibly reversing EMT [11].
The role of EMT in inducing resistance to c-Met TKIs such as SU11274 is not clearly understood. In this study, we compared induction of EMT in NSCLC cells resistant to erlotinib and SU11274, which are TKIs against EGFR and c-Met, respectively. This study demonstrates for the first time that SU11274-resistant NSCLC cells undergo EMT by upregulation of β-Catenin similar to erlotinib-resistant cells. For the purpose of this study, we used model NSCLC cell lines, H2170 and H358. We developed TKI-resistant cell strains of these cell lines by growing them in increasing concentration of SU11274 and erlotinib in culture media as described earlier [2] and studied proteins involved in induction of EMT and mechanism of resistance. Finally, we attempted to reverse the EMT process and increase the sensitivity of resistant cells to SU11274 and erlotinib by knockdown of β-Catenin or induction of miR-200a mimics.
2. Material and methods
2.1 Tyrosine Kinase Inhibitors and Growth factor Ligands
Erlotinib hydrochloride (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy) quinazolin-4-amine; C22H23N3O4•HCl) was obtained from LC laboratories (Woburn, MA) and SU11274 ((3Z)-N-(3-Chlorophenyl)-3-({3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl}methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide; C28H30ClN5O4S) was obtained from Sigma Aldrich (St. Louis, MO). The TKIs were reconstituted in Dimethyl Sulfoxide (DMSO) at a concentration of 20mM and then stored in aliquots at −20°C. Epidermal Growth Factor (EGF) and Hepatocyte Growth Factor (HGF) were obtained from Peprotech (Rocky Hill, NJ) and were reconstituted in PBS containing 0.1% BSA to obtain a concentration of 15ng/μl and 2ng/μl, respectively, and then were stored in aliquots at −20°C.
2.2 Antibodies
Rabbit monoclonal antibodies for Vimentin, E-Cadherin, N-Cadherin, Zeb-1 (as a part of the EMT antibody sampler kit), anti-Rabbit IgG and anti-mouse IgG HRP linked secondary antibodies were obtained from Cell Signaling (Danvers, MA). Mouse monoclonal antibody for β-Actin was purchased from Sigma Aldrich and for active-β-Catenin was obtained from EMD Millipore (Billerica, MA). All the primary antibodies were diluted according to the manufacturer’s instruction in TBST with 1% BSA. Secondary antibodies were prepared at a dilution of 1:1000 in TBST with 1% blocking grade milk from Bio-Rad (Hercules, CA). Antibody against E-Cadherin conjugated with Alexa-Fluor 647 for flow cytometry was purchased from BD Biosciences (Franklin Lakes, NJ).
2.3 Cell Lines
H2170 and H358 NSCLC cell lines (both with wt EGFR) were purchased from American Type Culture Collection (ATCC) (Rockville, MD) and were cultured according to ATCC’s instructions. Cells were cultured in increasing drug concentrations of erlotinib or SU11274 to obtain EGFR/c-Met TKI-resistant cells as discussed earlier [2]. Both these cell lines do not have known secondary mutations such as T790M or D761Y which could cause Erlotinib resistance [2].
2.4 Immunoblotting
H2170/H358 parental and TKI-resistant cells were cultured as described previously [10]. Cells were then treated with TKIs (10μM erlotinib/SU11274 in serum free RPMI media with 0.5% BSA) for 24 hr and then with ligand (15ng/ml EGF for 2.5 minutes or 40ng/ml HGF for 7.5 minutes in serum free media). Cell lysates were prepared, electrophoresed and transferred on to nitrocellulose membranes as described previously [10]. Membranes were probed with antibodies for EMT related proteins. Immunoblots were developed using Pierce ECL Substrate chemiluminescence kit (Thermo Fisher Scientific, IL) and modulations in protein expression were calculated by densitometric analysis using NIH ImageJ software.
2.5 Immunofluorescence
H2170 parental and TKI-resistant cells were seeded in 8-well chamber slide, fixed, permeabilized, blocked and incubated with primary antibody overnight at 4°C as described previously [10]. The next day, primary antibody was removed and cells were incubated for 1 hr at room temperature in the dark with anti-mouse/rabbit IgG secondary antibody conjugated with DyLight 488 [1:250] (Thermo Fisher Scientific) and Hoechst nuclear staining dye [1:10,000] (Life Technologies, Carlsbad, CA) diluted in 1× PBS with 1% BSA. Cells were observed using the Olympus Fv10i Fluoview confocal microscope and images were captured. Average fluorescence intensity of staining was quantitatively measured using the Olympus Fluoview image analysis software.
2.6 Real-Time PCR
All the primers used for qPCR experiments (Table-1) were purchased from Integrated DNA Technologies (Coralville, Iowa). H2170 parental and TKI-resistant cells were cultured in 35mm petri-dishes in serum free media as described previously [10], after which total RNA was collected using the PureLink RNA mini kit from Life Technologies. RNA was quantified and qPCR was performed as described previously [10]. The expression of each gene was analyzed in triplicate and the Ct values were normalized with GAPDH. The fold changes were calculated using 2(−ΔΔCt) method.
Table 1.
List of primers used for qPCR experiment with their sequences.
| Gene | Sequence | Melting Point | DNA Bases |
|---|---|---|---|
| E-Cadherin | F: GAACAGCACGTACACAGCCCT | 59.8°C | 21 |
| R: GCAGAAGTGTCCCTGTTCCAG | 58.0°C | 21 | |
| N-Cadherin | F: GACGGTTCGCCATCCAGAC | 58.4°C | 19 |
| R: TCGATTGGTTTGACCACGG | 55.4°C | 19 | |
| β-Catenin | F: TGGATGGGCTGCCTCCAGGTGAC | 65.5°C | 23 |
| R: ACCAGCCCACCCCTCGAGCCC | 58.9°C | 21 | |
| Zeb-1 | F: GCAGGTGAGCAACTGGGAAA | 58.1°C | 20 |
| R: ACAAGACACCGCCGTCATTT | 57.4°C | 20 | |
| Vimentin | F: TGTCCAAATCGATGTGGATGTTTC | 55.7°C | 24 |
| R: TTGTACCATTCTTCTGCCTCCTG | 56.8°C | 23 | |
| GAPDH | F: TTGCCAATGACCCCTTCA | 54.4°C | 18 |
| R: CGCCCCACTTGATTTTGGA | 55.8°C | 19 |
2.7 Flow Cytometry
H2170 parental and TKI-resistant cells (5×105 cells) were seeded in a 100mm petri-dishes and allowed to grow and adhere for 48 hr, after which growth medium was removed and cells were washed with PBS prior to treatment with Accutase Cell Detachment solution (Affymetrix, eBioscience, San Diego, CA) for 5 minutes in an incubator or until all the cells detached. Cells were collected in a 15ml tube, pelleted using a centrifuge and re-suspended in flow cytometry buffer (PBS+2mM EDTA+0.5% BSA). Cells were again pelleted and probed with fluorophore labeled antibody for 10 minutes on ice. Cells were then washed with flow cytometry buffer to remove excess antibody and then suspended in FACS buffer for analysis using BD FACScalibur flow cytometer.
2.8 siRNA Transfection
Cells were seeded a day before the transfection in media with 10% FBS only (without antibiotics). Cells were then transfected with either control or β-Catenin siRNA (Cell Signaling) using Dharmafect-2 (GE Life Sciences) following manufacturer’s protocol. Cell lysates were collected for Western blotting after 48, 72 and 96 hr of transfection.
2.9 miRNA Transfection
Cells were seeded a day before transfection in complete RPMI media (with FBS and antibiotics). Cells were then transfected with miR-200a mimics (miR-200a-3p, UAACACUGUCUGGUAACGAUGU + miR-200a-5p, CAUCUUACCGGACAGUGCUGGA; in 1:1 ratio) using HiPerFect transfection reagent (QIAGEN) following manufacturer’s protocol. Cell lysates were collected after 72 hr of transfection with microRNA for immunoblotting. For cell viability assay, the transfection media was removed after 10 hr and media containing drug (erlotinib/SU11274) was added for 72 hr.
2.10 MTT Cell Viability Assay
Cells were seeded at 8000 cells per well in a 96-well plate and were allowed to adhere and grow for 24 hr, after which they were transfected with β-Catenin siRNA or miRNA as described above. Cells were incubated in media containing erlotinib or SU11274 for 72 hr at 37°C, following which MTT cell viability assay was performed as described previously [10].
2.11 Statistical Analysis
All experiments were performed in triplicate and the quantitative data was analyzed for statistical significance using student’s t-test at a 95% confidence interval. A p-value of <0.05 was considered to be statistically significant for all the experiments.
3. Results
3.1 Modulations in expression of EMT related proteins in TKI-resistant NSCLC cells
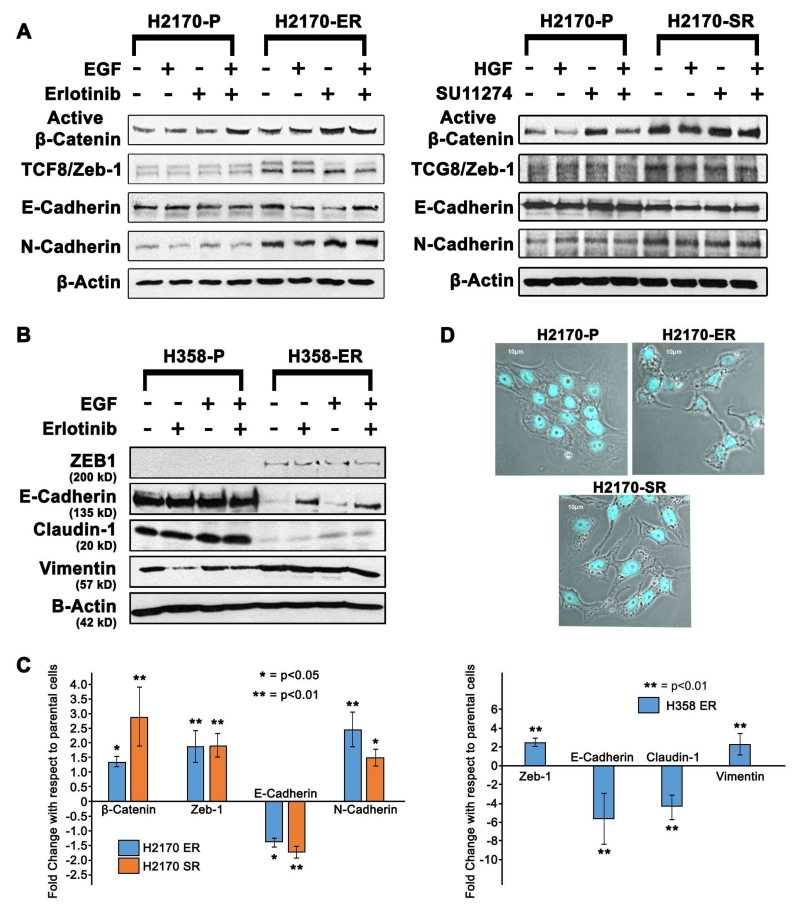
Expression of key EMT related proteins such as E-Cadherin, N-Cadherin, Zeb-1 and active β-Catenin was analyzed in H2170 cell line in the presence and absence of ligands (EGF or HGF) and/or TKIs (erlotinib or SU11274). The results indicate that N-Cadherin, active β-Catenin and Zeb-1 were upregulated between 1.5-fold and 2.5-fold while E-Cadherin was downregulated by 1.5-fold in the H2170-Erlotinib resistant (H2170-ER) cells when compared to the H2170-Parental (H2170-P) cells after similar treatments (p<0.05) (Fig. 1A). Similarly, we observed that N-Cadherin, active β-Catenin and Zeb-1 were upregulated 1.8-fold to 2.8-fold, and E-Cadherin was downregulated by 1.8-fold in H2170-SU11274 resistant (H2170-SR) cells when compared to H2170-P cells after similar treatments (Fig. 1A). Due to the diverse nature of NSCLC cells, we also analyzed H358 cells for modulations in expression of EMT related proteins. In H358-Erlotinib resistant (H358-ER) cells, we observed upregulation of EMT inducing proteins Vimentin and Zeb-1 by 2-fold each, and downregulation of E-Cadherin by 5-fold and Claudin-1 by 4-fold when compared to the H358-Parental (H358-P) cells (p<0.01) (Fig. 1B). The fold changes were calculated by densitometric analysis using ImageJ software and the average fold change for each protein is represented as bar graphs (Fig. 1C).
Fig 1.
Modulations in EMT related proteins in parental and TKI-resistant NSCLC cells 1.25×105 cells were seeded in 35 mm dishes and treated with EGF (15 ng/ml for 2.5 minutes) or HGF (40 ng/ml for 7.5 minutes) and/or erlotinib (10 μM for 24 hr) or SU11274 (10μM for 24 hr). (A, B) The immunoblotting results show that Vimentin, N-Cadherin, β-Catenin and Zeb-1 were up-regulated, while E-Cadherin was downregulated in the H2170/H358-ER and H2170-SR cells when compared to the H2170/H358-P cells with the same treatments. (C) The fold changes were calculated by densitometric analysis using ImageJ software and are shown as bar graphs. (D) Phase contrast confocal microscopic images of H2170 cells stained with DAPI. H2170-ER/SR cells show EMT characteristics as these cells are more elongated, spindle shaped and non-polar compared to H2170-P cells.
3.2 Increase in Vimentin filaments and loss of E-Cadherin in TKI-resistant H2170 cells
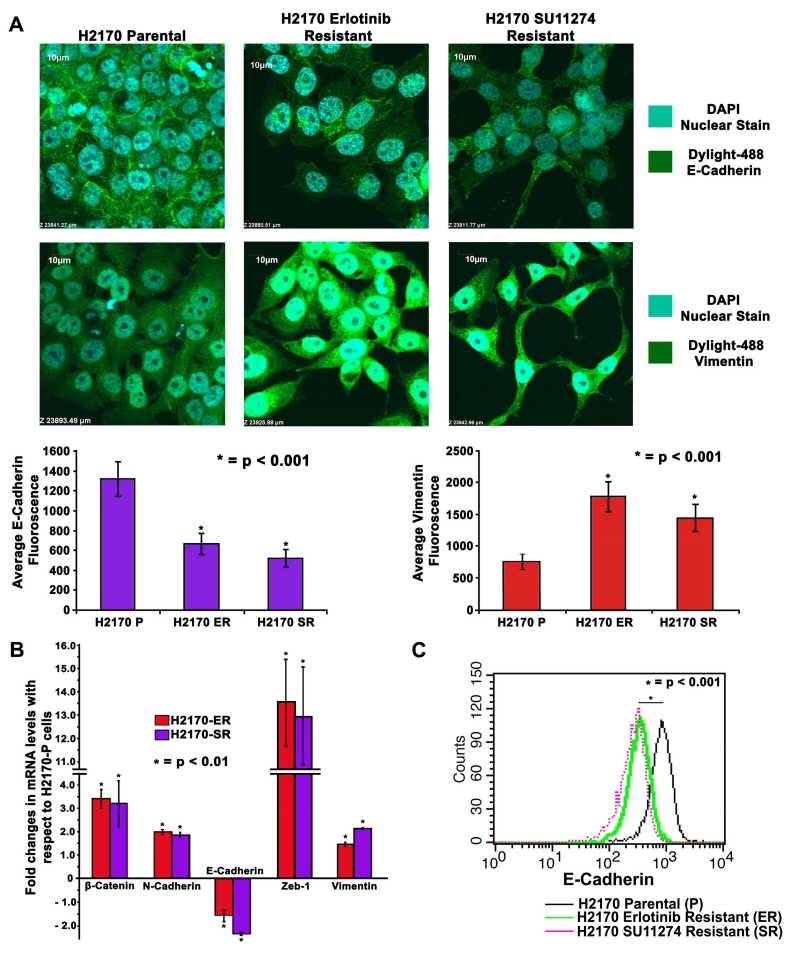
Immunofluorescence was used to specifically analyze morphological change and study expression of Vimentin and E-Cadherin in H2170 cells. We observed a change in the morphology of TKI-resistant cells which were more elongated and spindle shaped showing typical mesenchymal characteristics compared to parental cells (Fig. 1D). The results indicate that in H2170-ER and H2170-SR cells there was loss of E-Cadherin by 2.0-fold and 2.5-fold and gain of Vimentin filaments by 2.3-fold and 2.0-fold, respectively, as measured by fluorescence intensity in comparison to the H2170-P cells (p<0.001) (Fig. 2A).
Fig 2.
Changes in cell morphology and modulations in expression of EMT related proteins in TKI-resistant NSCLC cells (A) 2×104 cells per well in an 8-well chamber slide were plated, fixed and probed with Vimentin and E-Cadherin antibodies. DAPI was used as a nuclear stain while Dylight-488 conjugated secondary antibodies were used for detection of Vimentin and E-Cadherin. Images were captured using an Olympus fv10i confocal microscope and fluorescence quantification was performed using the Olympus Fluoview image analysis software. Vimentin filaments were found to be upregulated and E-Cadherin was observed to be downregulated in H2170-ER and H2170-SR cells when compared to H2170-P cells. (B) 2.5×105 cells were seeded in 35mm dishes and allowed to grow for 24 hr, and kept in serum free medium for 24 hr. Total RNA was collected and quantified. Subsequently, 1μg of total RNA was used to obtain cDNA to determine expression of genes of interest using qPCR. β-Catenin, N-Cadherin, Zeb-1 and Vimentin were found upregulated while E-Cadherin was found downregulated in H2170 TKI-resistant cells when compared to parental cells. The data was normalized with GAPDH and graphically represented relative to expression of respective genes in H2170-P cells. (C) H2170-P, ER and SR cells were collected using Accutase cell detachment media and then probed with E-Cadherin antibody conjugated with Alexa Fluor 647. The pellet was then washed and re-suspended in Flow cytometry buffer (PBS + 0.5% BSA + 5mM EDTA). Equal numbers of cells were analyzed for all the cell types and histogram for fluorescence intensity was plotted. The results show that there is a loss in E-Cadherin signal in H2170-ER and H2170-SR cells when compared to H2170-P cells.
3.3 Increased gene expression of EMT related proteins in TKI-resistant H2170 cells
Real-time PCR results indicate that the mRNA levels of β-Catenin were 3.4-fold higher in H2170-ER cells and 3.2-fold higher in H2170-SR cells when compared to H2170-P cells (p<0.01) (Fig. 2B). We also observed 2.0-fold and 1.85-fold upregulation of N-cadherin, and 1.5-fold and 2.2-fold upregulation of Vimentin in H2170-ER and H2170-SR cells, respectively, when compared to H2170-P cells (p<0.01). The mRNA levels of Zeb-1 were 13.5-fold and 12.9-fold higher in H2170-ER and H2170-SR cells, respectively, in comparison to H2170-P cells (p<0.01). Furthermore, we observed 1.7-fold and 2.4-fold downregulation of E-Cadherin in H2170-ER and H2170-SR cells in comparison with H2170-P cells (p<0.01) (Fig. 2B). These results further support our claim that TKI-resistant NSCLC cells undergo EMT.
3.4 Loss of membranous E-Cadherin in TKI-resistant H2170 cells as analyzed by FACS
Flow cytometry was performed to analyze the levels of membranous E-Cadherin in H2170-P, H2170-ER and H2170-SR cells. The results indicate that the fluorescence intensity of E-Cadherin was 2.5-fold and 2.9-fold lower in H2170-ER and H2170-SR cells, respectively, when compared to H2170-P cells (p<0.001) (Fig. 2C). Downregulation of E-Cadherin in TKI-resistant cells may be associated with EMT.
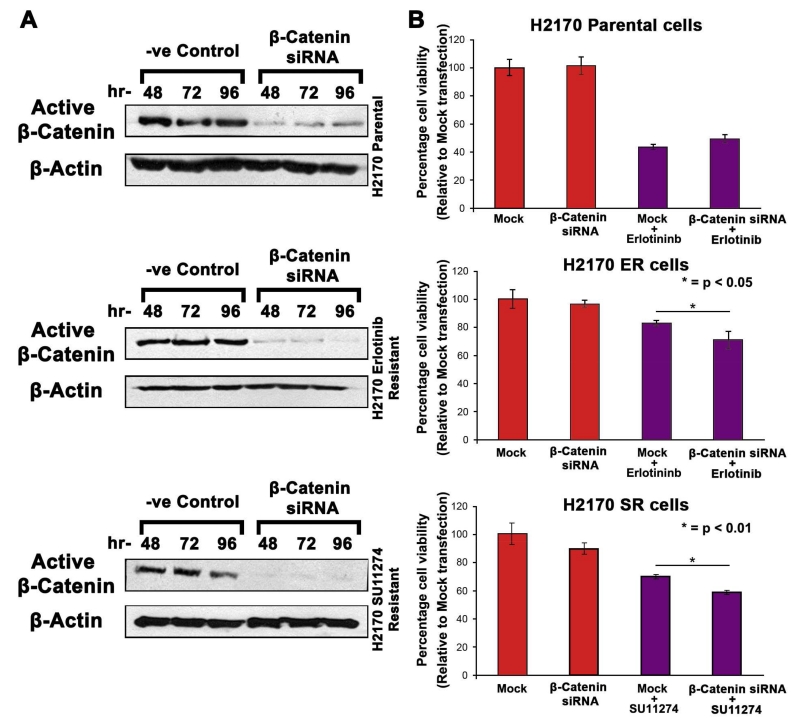
3.5 Effect of β-Catenin knockdown on H2170 cells
The results of β-Catenin siRNA transfection experiment showed that β-Catenin knockdown was successful and was most efficient at 96 hr (p<0.05) (Fig. 3A). Subsequently, we performed cell viability assays to assess the sensitivity of TKI-resistant cells to erlotinib and SU11274 after β-Catenin knockdown. We observed that the H2170-ER cells treated with 2.5μM erlotinib after β-Catenin knockdown have an increase in cell death by 15% when compared to cells treated with 2.5μM erlotinib after mock transfection (p<0.05). Similarly, in H2170-SR cells we observed an increase in cell death by 16% in β-Catenin siRNA treated cells in comparison to mock transfected cells, when treated with 5μM SU11274 (p<0.01). In contrast, this increase in cell death was not observed in H2170-P cells after β-Catenin knockdown and was exclusive to TKI-resistant cells (Fig. 3B).
Fig 3.
Effect of β-Catenin Knockdown on TKI-resistant NSCLC cells (A) 1.25×105 cells were plated in 35mm dishes and were then allowed to adhere and grow for 24 hr. Cells were then transfected with either mock or β-Catenin siRNA using Dharmafect-2 and cell lysates were collected after 48, 72 and 96 hr. Immunoblotting was performed to analyze protein expression which showed downregulation of β-Catenin in H2170-ER and H2170-SR cells when compared to the mock transfected cells. (B) 1×104 cells were seeded per well in a 96-well plate and allowed to adhere and grow for 24 hr. Cells were then transfected with either mock or β-Catenin siRNA using Dharmafect-2. The transfection media was removed after 10 hr and then the cells were treated with either erlotinib (2.5 μM) or SU11274 (5 μM) for 72 hr. The results from the MTT assay showed that the knockdown of β-Catenin increased erlotinib efficacy in ER cells and SU11274 efficacy in SR cells.
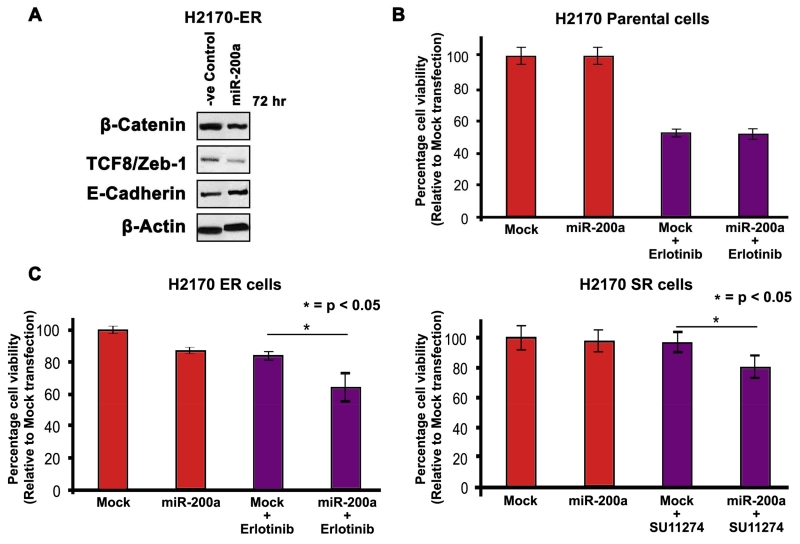
3.6 Effect of miR-200a induction in H2170 cells
The microRNAs from the miR-200 family are known to regulate Zeb-1 in epithelial cells. To analyze the effects of EMT on drug resistance, we induced miR-200a mimics in H2170 cells. Western blotting results showed significant downregulation of EMT related proteins such as β-Catenin and Zeb-1 in H2170-ER cells after 72 hr of miR-200a induction when compared to mock transfected cells. We also observed significant upregulation of cell adhesion molecule ECadherin in the H2170-ER cells after 72 hr of miR-200a mimic transfection (p<0.05) (Fig. 4A). Further, we analyzed the effect of miR-200a induction on drug sensitivity of TKI-resistant cells. We performed a cell viability assay to analyze the effects of erlotinib and SU11274 on H2170-ER and H2170-SR cells, respectively, after miR-200a induction. Induction of miR-200a along with drug treatment in H2170-P cells did not increase cell death induced by the inhibitors (Fig. 4B). However, miR-200a induction with 2.5μM erlotinib or 5μM SU11274 increased the cell death by 24% (p<0.05) and 17% (p<0.05) in H2170-ER and H2170-SR cells, respectively (Fig. 4C).
Fig 4.
Effects of miR-200a induction on H2170 parental and TKI-resistant cells (A) 1.25×105 cells were plated in 35mm dishes and allowed to adhere and grow for 24 hr. Cells were then transfected with either mock or miR-200a mimics using HiPerFect and cell lysates were collected after 72 hr. Immunoblotting was performed to analyze protein expression which showed downregulation of β-Catenin and TCF8/Zeb-1, and upregulation of E-Cadherin in H2170-ER and H2170-SR cells when compared to the mock transfected cells. (B) 1×104 cells were seeded per well in a 96-well plate and allowed to adhere and grow for 24 hr. Cells were then transfected with either mock or miR-200a mimics using HiPerFect. The transfection media was removed after 10 hr and then the cells were treated with erlotinib (2.5 μM) or SU11274 (5 μM) for 72 hr. The results from the MTT assay show that the induction of miR-200a increased efficacy of erlotinib and SU11274 in H2170-ER and H2170-SR cells, respectively.
4. Discussion
In this study, we demonstrated the occurrence of EMT in NSCLC cells resistant to SU11274, a c-Met inhibitor and compared it to erlotinib resistant cells. The results from our recently published study demonstrated increased levels of nuclear β-Catenin and over-activation of Wnt pathway in H2170 TKI-resistant (ER/SR) cells [10]. The present study indicates that increased expression of Zeb-1 in TKI-resistant NSCLC cells, leads to the occurrence of EMT which may be a result of activation of the Wnt pathway. To determine the mechanism, we demonstrated that knock down of important EMT proteins, such as β-Catenin and Zeb-1, can increase drug sensitivity in SU11274 or erlotinib-resistant cells.
Some of the most common characteristics of EMT is that the cells undergo morphological changes, lose cell adhesion, polarity and gain migratory and invasive properties [8]. According to our Western blotting results, we observed upregulation of Zeb-1 in TKI-resistant cells when compared to the parental cells. β-Catenin upon nuclear localization interacts with TCF4 and induces expression of Zeb-1, a transcriptional repressor of E-Cadherin [9]. Correspondingly, we observed downregulation of E-Cadherin in TKI-resistant cells in comparison to the parental cells. E-Cadherin is an integral part of the adherent junctions and forms an adhesion complex with (α,β,γ) catenins in epithelial cells to maintain cell adhesion [12]. The loss of E-Cadherin or repression of its transcription leads to loss in its adhesion properties and steers catenins in the cell cytosol [12]. As a result, excess β-Catenin present in the cell cytosol will either localize to the nucleus for transcriptional activity or will interact with active GSK-3β, which phosphorylates β-Catenin and marks it for ubiquitinylation, resulting in its degradation [13].
We also observed that N-Cadherin, an important EMT marker which regulates motility, was upregulated in both SU11274 and erlotinib-resistant cells when compared to parental cells. The morphological changes due to the loss of E-Cadherin and gain of Vimentin in H2170-ER and SR cells when visualized by immunofluorescence were indicative of the mesenchymal cell type characteristic. TKI-resistant cells displayed altered cell growth properties shown by the tendency of the cells to grow independently on the cell culture surface compared to parental cells which grew in clusters, indicating loss of adhesion and increase in motility in resistant cells. Furthermore, we confirmed the decline in levels of membranous E-Cadherin in H2170-ER and SR cells when compared to the H2170-P cells using flow cytometry. We observed increased mRNA levels of N-Cadherin, β-Catenin, Zeb-1 and Vimentin, and decreased mRNA levels for E-Cadherin in the H2170-ER and SR cells when compared to H2170-P cells further validating induction of EMT.
Since Zeb-1 and miR-200a function in a feedback loop and are capable of regulating each other during EMT [14], we attempted to restore the epithelial features by inducing miR-200a mimics in both SU11274 and erlotinib-resistant H2170 cells. Western blotting results indicated that β-Catenin and Zeb-1 were downregulated and E-Cadherin was restored in the miR-200a transfected cells when compared to the mock transfected cells. These results establish the relationship between occurrence of EMT and upregulation of β-Catenin in these cells.
Finally, the results from cell viability assays after miR-200a induction or β-Catenin knockdown indicated an increase in drug sensitivity in H2170-ER/SR cells. We observed that knockdown of β-Catenin or induction of miR-200a mimics followed by erlotinib or SU11274 treatment resulted in decreased cell viability of TKI-resistant cells, while no significant changes in cell viability were observed in H2170-P cells with similar treatments. This indicates that EMT plays an important role in development of resistance to TKIs such as SU11274 and erlotinib in H2170 cells which can be partially overcome by inhibition of key EMT regulating proteins such as β-Catenin and Zeb-1. Results obtained from β-Catenin knockdown by siRNA and Zeb-1 downregulation by miR-200a mimics confirms that nuclear accumulation of β-Catenin and upregulation of expression of Zeb-1 may lead to EMT in TKI-resistant cells.
Overall, this study concludes that SU11274-resistant NSCLC cells undergo EMT due to increase in expression of Zeb-1 which represses the transcription of E-Cadherin. Similar results were also obtained in NSCLC cells resistant to erlotinib. Modulations in these signaling molecules lead to EMT in TKI-resistant NSCLC cells. We also showed that treatment of TKI-resistant NSCLC cells with β-Catenin siRNA or miR-200a mimics can increase the sensitivity of the TKI-resistant cells towards SU11274 and erlotinib. Future studies can lead to the development of novel therapeutic strategies involving miR-200a for the treatment of NSCLC patients with TKI resistance. Additionally, studies with combination therapies targeting both β-Catenin and Zeb-1 may be effective in completely overcoming EMT mediated TKI resistance in NSCLC.
Supplementary Material
Highlights.
Resistance to TKIs in NSCLC cells is mediated via modulation in EMT related proteins.
EMT may induce c-Met mediated TKI resistance, similar to EGFR TKI resistance.
Role of β-catenin and cadherins in TKI resistance was validated by FACS and qPCR.
Knockdown of β-catenin or Zeb-1 can increase TKI sensitivity in TKI-resistant cells.
Targeting key EMT related proteins may overcome TKI resistance in NSCLC.
Acknowledgements
We would like to thank Dr. Marie C. Nlend from Thermo Fisher Scientific, Rockford, IL for her guidance and critical suggestions through the work presented in this manuscript. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R21CA158965-01A1 (http://www.nih.gov) to Neelu Puri. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest
The authors have no conflicts of interest in this work.
References
- [1].Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fong JT, Jacobs RJ, Moravec DN, et al. Alternative signaling pathways as potential therapeutic targets for overcoming EGFR and c-Met inhibitor resistance in non-small cell lung cancer. PLoS One. 2013;8:e78398. doi: 10.1371/journal.pone.0078398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stone SRA, Rastogi I, et al. c-Met: A Potential Target for Current Non-Small-Cell Lung Cancer Therapeutics. Chemotherapy. 2014;3:136. [Google Scholar]
- [4].Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- [5].Burotto M, Manasanch EE, Wilkerson J, et al. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20:400–410. doi: 10.1634/theoncologist.2014-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sanchez-Tillo E, de Barrios O, Siles L, et al. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011;108:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Botting GM, Rastogi I, Chhabra G, et al. Mechanism of Resistance and Novel Targets Mediating Resistance to EGFR and c-Met Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS One. 2015;10:e0136155. doi: 10.1371/journal.pone.0136155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- [14].Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.